Introduction
Fungal and oomycete plant pathogens cause destructive diseases in crops and pose real economic and food security threats [1]. These filamentous, eukaryotic organisms can also upset natural ecosystems when they spread invasively [2]. The capability of plant immune systems to detect and respond to pathogen effector proteins is a major determinant of disease susceptibility. Plant pathogen effector proteins that trigger host immunity are often encoded by conditionally detrimental genes that are under strong and contrasting selective pressures [3,4]. Pathogen effectors evolved to play a positive role in virulence by enabling growth and reproduction on host plants [5,6]. Nonetheless, effectors can meet their match with host immune receptors that recognize their presence, a result that ends badly for the pathogen. Such immunity-triggering proteins are known as avirulence (Avr) effectors, encoded by Avr genes.
Escaping Host Immunity
When an Avr effector triggers a host immune receptor, the pathogen’s survival depends upon generating variants that escape, suppress, or alter this recognition event in ways that allow the pathogen to grow and reproduce. This can be accomplished by numerous means. Transposon insertions or mutations to the DNA sequence encoding the Avr gene, or its complete loss, are commonly encountered gain of virulence mechanisms. This is well demonstrated in a study on the tomato leaf mold pathogen Cladosporium fulvum [7]. In fact, pathogen genomes have evolved configurations that position Avr effector genes in repetitive [8], transposon-rich [9], and teleomeric regions [10], or in dispensable segments [11], to aid mutation and recombination that results in gain of virulence. There are also ways to defeat immunity without loss or alteration of the DNA sequence of the offending Avr gene. This can occur through acquisition or evolution of an additional, epistatic effector that supresses the immune-triggering event caused by the Avr effector. Such scenarios are well documented in oomycete and fungal plant pathogens, for example, in the potato late blight pathogen Phytophthora infestans [12], the wheat powdery mildew pathogen Blumeria graminis [13], the tomato wilt pathogen Fusarium oxysporum [14], and the canola blackleg pathogen Leptosphaeria maculans [15]. This arms race can go through repeated iterations and lead to difficulties in untangling the molecular basis of host–pathogen compatibilities.
Beyond Mutation
Another way to escape immunity without changing the DNA sequence of the Avr gene is by altering its expression. This could simply result from shifting the DNA mutation to the Avr gene’s regulatory region or to an epistatic location in the genome that affects Avr gene transcription. Each of these mechanisms has been proposed to occur in the soybean root rot pathogen Phytophthora sojae [8,16]. Changes to Avr gene expression states that are not dependent on any DNA sequence alterations have also been postulated, based upon sequence-identical epialleles that differ in expression, as shown in Fig 1. The inheritance of Avr gene silencing in P. sojae shows unusual strain-specific patterns, including transgenerational effects [17], a phenomenon that is reminiscent of inter-nuclear silencing caused by ectopic expression of transgenes in P. infestans [18]. Although epigenetic inheritance of Avr gene expression states is controversial and difficult to prove conclusively, epigenetic switches that do not rely on DNA sequence changes offer the most plausible explanation for observations of reversible changes in virulence and Avr gene expression in clonally propagating cultures [19,20]. Virulence traits that are epigenetically reversible could impart a survival advantage to the pathogen by providing a means of recycling or re-deploying valuable effectors that are conditionally detrimental, in response to varying host immune capabilities [21,22]. Similar biological bet-hedging is observed in other successful pathogens. For example, knowledge of transcriptional control of antigenic variation in the malaria parasites (Plasmodium spp.) is comparatively far-advanced and provides another perspective on how pathogens recruit epigenetic systems that generate phenotypic variation in expression of immune-triggering effectors [23].
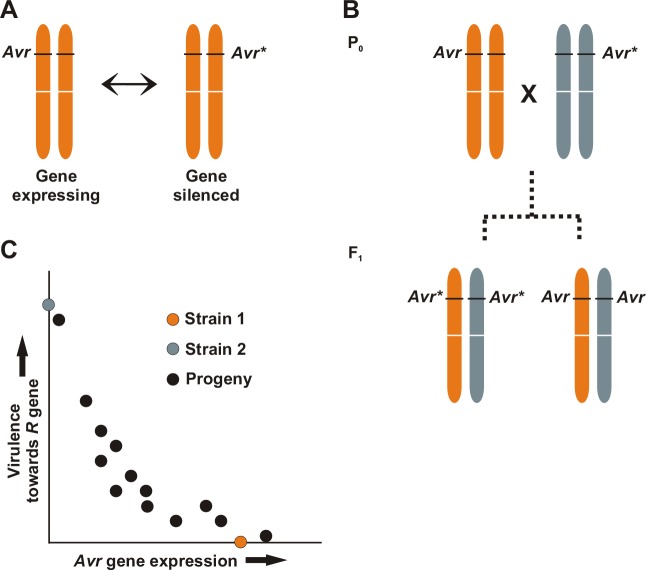
Fig 1. Avirulence (Avr) gene silencing in diploid oomycetes.
A, Immunity-triggering effectors encoded by Avr genes can spontaneously switch between active (Avr) and gene silenced (Avr*) expression states in clonally propagating cultures. The Avr gene locus is depicted on a diploid chromosome pair. B, Sexual crosses between expressing (Avr) and gene silenced (Avr*) alleles can result in varying outcomes and unusual inheritance patterns for Avr gene expression in progeny. Progeny can differ qualitatively for Avr gene expression. Epigenetic reprogramming and strain-specific epistatic loci likely play a role in determining the result. The Avr gene locus is depicted on a diploid chromosome pair. C, Sexual crosses between expressing (Avr) and gene silenced (Avr*) alleles can also result in quantitative variation for Avr gene expression in progeny and incomplete penetrance of the avirulence trait when tested against host plants with the corresponding resistance (R) gene. The conceptual illustrations in this figure are based upon observations of Avr1a, Avr1c, and Avr3a expression and inheritance in the oomycete plant pathogen Phytophthora sojae [8,17,19,27].
A Big Role for Small RNA
Mechanistically, there is evidence that effector gene silencing in P. sojae and P. infestans is intertwined with small RNA pathways. Characterization of small RNA molecules in Phytophthora species show that the majority fall into two discrete size classes: approximately 21 nt and 25 nt [24,25]. The two sizes of small RNA differentially target transposons and effector gene families and associate with separate Argonaute proteins [26]. The Avr3a effector genes in P. sojae and P. infestans are non-orthologous, but each are subject to silencing that is naturally occurring and strain-specific [17,25]. In P. sojae, silencing of PsAvr3a has been linked to the presence of 25 nt small RNA. Genetic crosses between PsAvr3a-silenced and non-silenced strains can generate F1 hybrid progeny with unpredictable and variable levels of PsAvr3a gene expression. Hybrids display strain-specific effects that likely result from epigenetic reprogramming and from interplay of conventional and epigenetic variation between parental strains [17,27].
The Challenges of Polyploidy
Gain of virulence mechanisms that rely on suppression of Avr triggered immunity or on epigenetic reprogramming of Avr gene transcription have the benefit of preserving the sequence of the Avr gene itself. For plant pathogens that are normally diploid or polyploid during their infective stages, such as the oomycetes, these mechanisms may additionally be favored because they can potentially offer an alternative to Avr gene dominance. Filamentous plant pathogens commonly exist in haploid, diploid, polyploid, or dikaryotic nuclear states during their host-infective stages. Sexual reproduction serves to re-assort alleles, but this could be limited by availability of complementary mating types for heterothallic species. A diploid, polyploid, or dikaryotic pathogen strain that possesses two or more functional copies of an Avr gene faces a different and arguably more difficult challenge to escape host immunity compared to a haploid organism possessing a single copy. This is because all copies of the Avr gene require gain of virulence changes. For example, consider a homozygous Avr gene in a diploid organism; a mutation to one allele results in heterozygosity for the Avr locus. This outcome does not achieve any phenotypic changes in virulence in the typical case of a dominant Avr gene. One way oomycete plant pathogens have evolved to compensate is through high frequency gene conversion or loss of heterozygosity [28–30]. Another adaptive solution is to regulate activity of the Avr gene through operationally dominant epistatic or epigenetic mechanisms or through stochastic switches. Studies of effector gene transcription in P. sojae and P. infestans offer evidence for these hypotheses [19,27,31,32]. Plant pathogens with dikaryotic infective stages, such as the cereal rusts, also have to counteract Avr gene dominance, so the oomycetes can be an instructive model in this instance. Certainly, it is intuitive that the ploidy status of the pathogen will influence the genetic-adaptive mechanisms that are employed to overcome host immunity. This could be especially pertinent in cases where gain of virulence relies on loss of function changes to effector genes that can either contribute to virulence or trigger host immune systems, in a conditional manner.
Place Your Bets
The proposal that there are core sets of pathogen effectors that are indispensable and essential to virulence has become a popular idea that has pervaded molecular plant pathology [33–35]. Part of the attraction of this hypothesis is that it could offer opportunities for development of more durable plant resistance [1]. However, there is a need for perspective, because in host–pathogen interactions, outcomes are conditional. A precept of plant pathology is the concept of the disease triangle, which states that disease only occurs when host, pathogen, and environmental conditions are all permissive [36]. Extending this concept to effector biology is reasonable and would predict that the set of core effectors required for successful infection and reproduction is malleable and qualified by the host and environment. For effectors with the potential to trigger host immunity, selective forces drive redundancy and diversity, as exemplified by the RXLR (arginine-x-leucine-arginine) class of effectors in oomycetes [4,37]. Perhaps a useful analogy for this interplay between pathogen effector and host immunity components is a card game, in which some cards may have more face value than others, but ultimate success depends upon the combination of cards in hand and those held by opponents.
Conclusion
The discovery of transcriptional and epigenetic variation of Avr gene expression in oomycetes provides an additional layer of knowledge as to how plant pathogens can escape host immune systems and adapt to changing selective pressures. The idea that epigenetic systems can enable effector recycling has been recently considered [21,22]. The hypothesis that epigenetic control of Avr gene expression is, in part, an adaptive response to n > 1 ploidy or nuclear states provides another rationale to explain the present set of observations. The prediction arising from this hypothesis is that epigenetic reprogramming of Avr gene expression will continue to be more frequently encountered as a gain of virulence mechanism in diploid, polyploid, and dikaryotic species compared to plant pathogens that are haploid during host infection.
Funding Statement
Research in the authors' laboratory is supported by grants from Agriculture and Agri-Food Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science 341: 746–751. 10.1126/science.1236011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gladieux P, Feurtey A, Hood ME, Snirc A, Clavel J, et al. (2015) The population biology of fungal invasions. Mol Ecol 24: 1969–1986. 10.1111/mec.13028 [DOI] [PubMed] [Google Scholar]
- 3. Thrall PH, Barrett LG, Dodds PN, Burdon JJ (2015) Epidemiological and Evolutionary Outcomes in Gene-for-Gene and Matching Allele Model s. Front Plant Sci 6: 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Win J, Chaparro-Garcia A, Belhaj K, Saunders DG, Yoshida K, et al. (2012) Effector Biology of Plant-Associated Organisms: Concepts and Perspectives. Cold Spring Harbor Symposia on Quantitative Biology LXXVII. [DOI] [PubMed] [Google Scholar]
- 5. Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, et al. (2015) Fungal effectors and plant susceptibility. Annu Rev Plant Biol 66: 513–545. 10.1146/annurev-arplant-043014-114623 [DOI] [PubMed] [Google Scholar]
- 6. Dong S, Raffaele S, Kamoun S (2015) The two-speed genomes of filamentous pathogens: waltz with plants. Curr Opin Genet Dev 35: 57–65. 10.1016/j.gde.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 7. Iida Y, van 't Hof P, Beenen H, Mesarich C, Kubota M, et al. (2015) Novel Mutations Detected in Avirulence Genes Overcoming Tomato Cf Resistance Genes in Isolates of a Japanese Population of Cladosporium fulvum. PLoS ONE 10: e0123271 10.1371/journal.pone.0123271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qutob D, Tedman-Jones J, Dong S, Kuflu K, Pham H, et al. (2009) Copy number variation and transcriptional polymorphisms of Phytophthora sojae RXLR effector genes Avr1a and Avr3a. PLoS ONE 4: e5066 10.1371/journal.pone.0005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ali S, Laurie JD, Linning R, Cervantes-Chavez JA, Gaudet D, et al. (2014) An immunity-triggering effector from the Barley smut fungus Ustilago hordei resides in an Ustilaginaceae-specific cluster bearing signs of transposable element-assisted evolution. PLoS Pathog 10: e1004223 10.1371/journal.ppat.1004223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orbach MJ, Farrall L, Sweigard JA, Chumley FG, Valent B (2000) A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell 12: 2019–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rep M, van der Does HC, Meijer M, van Wijk R, Houterman PM, et al. (2004) A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Molecular Microbiology 53: 1373–1383. [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Liu Z, Halterman DA (2012) Molecular determinants of resistance activation and suppression by Phytophthora infestans effector IPI-O. PLoS Pathog 8: e1002595 10.1371/journal.ppat.1002595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bourras S, McNally KE, Ben-David R, Parlange F, Roffler S, et al. (2015) Multiple Avirulence Loci and Allele-Specific Effector Recognition Control the Pm3 Race-Specific Resistance of Wheat to Powdery Mildew. Plant Cell 27: 2991–3012. 10.1105/tpc.15.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Houterman PM, Cornelissen BJC, Rep M (2008) Suppression of plant resistance gene-based immunity by a fungal effector—art. no. e1000061. PLoS Pathog 4: 61–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plissonneau C, Daverdin G, Ollivier B, Blaise F, Degrave A, et al. (2016) A game of hide and seek between avirulence genes AvrLm4-7 and AvrLm3 in Leptosphaeria maculans. New Phytol 209: 1613–1624. 10.1111/nph.13736 [DOI] [PubMed] [Google Scholar]
- 16. Shan WX, Cao M, Dan LU, Tyler BM (2004) The Avr1b locus of Phytophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Molecular Plant-Microbe Interactions 17: 394–403. [DOI] [PubMed] [Google Scholar]
- 17. Qutob D, Chapman PB, Gijzen M (2013) Transgenerational gene silencing causes gain of virulence in a plant pathogen. Nature communications 4: 1349 10.1038/ncomms2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van West P, Kamoun S, van 't Klooster JW, Govers F (1999) Internuclear gene silencing in Phytophthora infestans. Mol Cell 3: 339–348. [DOI] [PubMed] [Google Scholar]
- 19. Na R, Yu D, Chapman BP, Zhang Y, Kuflu K, et al. (2014) Genome re-sequencing and functional analysis places the Phytophthora sojae avirulence genes Avr1c and Avr1a in a tandem repeat at a single locus. PLoS ONE 9: e89738 10.1371/journal.pone.0089738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutherford FS, Ward EWB, Buzzell RI (1985) Variation in virulence in successive single-zoospore propagations of Phytophthora megasperma f.sp. glycinea . Phytopathology 75: 371–374. [Google Scholar]
- 21. Gijzen M, Ishmael C, Shrestha SD (2014) Epigenetic control of effectors in plant pathogens. Front Plant Sci 5: 638 10.3389/fpls.2014.00638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kasuga T, Gijzen M (2013) Epigenetics and the evolution of virulence. Trends in microbiology 21: 575–582. 10.1016/j.tim.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 23. Waters AP (2016) Epigenetic Roulette in Blood Stream Plasmodium: Gambling on Sex. PLoS Pathog 12: e1005353 10.1371/journal.ppat.1005353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fahlgren N, Bollmann SR, Kasschau KD, Cuperus JT, Press CM, et al. (2013) Phytophthora have distinct endogenous small RNA populations that include short interfering and microRNAs. PLoS ONE 8: e77181 10.1371/journal.pone.0077181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vetukuri RR, Asman AK, Tellgren-Roth C, Jahan SN, Reimegard J, et al. (2012) Evidence for small RNAs homologous to effector-encoding genes and transposable elements in the oomycete Phytophthora infestans. PLoS ONE 7: e51399 10.1371/journal.pone.0051399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Åsman A, Fogelqvist J, Vetukuri R, Dixelius C (2016) Argonaute 1 binds microRNA and small RNAs from effector genes and transposable elements. New Phytologist. [DOI] [PubMed] [Google Scholar]
- 27. Shrestha SD, Chapman P, Zhang Y, Gijzen M (2016) Strain specific factors control effector gene silencing in Phytophthora sojae . PLoS ONE: e0150530 10.1371/journal.pone.0150530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chamnanpunt J, Shan WX, Tyler BM (2001) High frequency mitotic gene conversion in genetic hybrids of the oomycete Phytophthora sojae. Proc Natl Acad Sci U S A 98: 14530–14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lamour KH, Mudge J, Gobena D, Hurtado-Gonzales OP, Schmutz J, et al. (2012) Genome sequencing and mapping reveal loss of heterozygosity as a mechanism for rapid adaptation in the vegetable pathogen Phytophthora capsici. Molecular plant-microbe interactions: MPMI 25: 1350–1360. 10.1094/MPMI-02-12-0028-R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tyler BM, Gijzen M (2014) The Phytophthora sojae genome sequence: Foundatoin for a revolution In: Dean RA, Lichens-Park A, Kole C, editors. Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens. Berlin Heidelberg: Springer-Verlag. [Google Scholar]
- 31. Cooke DE, Cano LM, Raffaele S, Bain RA, Cooke LR, et al. (2012) Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathog 8: e1002940 10.1371/journal.ppat.1002940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilroy EM, Breen S, Whisson SC, Squires J, Hein I, et al. (2011) Presence/absence, differential expression and sequence polymorphisms between PiAVR2 and PiAVR2-like in Phytophthora infestans determine virulence on R2 plants. The New phytologist 191: 763–776. 10.1111/j.1469-8137.2011.03736.x [DOI] [PubMed] [Google Scholar]
- 33. Bos JI, Armstrong MR, Gilroy EM, Boevink PC, Hein I, et al. (2010) Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proceedings of the National Academy of Sciences of the United States of America 107: 9909–9914. 10.1073/pnas.0914408107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Q, Han C, Ferreira AO, Yu X, Ye W, et al. (2011) Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. The Plant cell 23: 2064–2086. 10.1105/tpc.111.086082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hemetsberger C, Mueller AN, Matei A, Herrberger C, Hensel G, et al. (2015) The fungal core effector Pep1 is conserved across smuts of dicots and monocots. New Phytol 206: 1116–1126. 10.1111/nph.13304 [DOI] [PubMed] [Google Scholar]
- 36. Agrios GN (2005) Plant Pathology. Burlington, MA, USA: Elsevier Academic Press. [Google Scholar]
- 37. Anderson RG, Deb D, Fedkenheuer K, McDowell JM (2015) Recent Progress in RXLR Effector Research. Mol Plant Microbe Interact 28: 1063–1072. 10.1094/MPMI-01-15-0022-CR [DOI] [PubMed] [Google Scholar]