Abstract
Premenstrual syndrome (PMS) is a common disorder. Due to the knowledge lack of the precise etiology of this syndrome, different treatment methods are recommended, one of them is the use of medicinal herbs. This study aimed to investigate the effect of Valerian (纈草 xié cǎo) root extract on the intensity of PMS symptoms.
In this double-blind clinical trial, 100 female students of Islamic Azad University, Tonekabon Branch, Mazandaran Province, Iran, with PMS were randomly divided into groups receiving Valerian (scientific name: Valeriana officinalis) and placebo in 2013. The participants received 2 pills daily in the last seven days of their menstrual cycle for 3 cycles and recorded their symptoms.
The data collection tools included demographic information questionnaire, daily symptom severity questionnaire, and a provisional diagnosis of premenstrual syndrome questionnaire.
Data were compared previous, one, two, and three cycles after student's intervention using and analyzed by independent t-test, paired t-test, chi-squared test, and repeated measures ANOVA in SPSS 16.
A significant difference was seen in mean emotional, behavioral and physical premenstrual symptom severity in the intervention group before and after the intervention (P < 0.001). However, this difference was not statistically significant in the control group. The results of this study showed that Valerian root extract may reduce emotional, physical, and behavioral symptoms of premenstrual syndrome.
Keywords: Premenstrual syndrome, Valerian root extract, Mood symptoms, Behavioral symptoms, Physical symptoms
Graphical abstract
Valerian root extract may reduce emotional, physical, and behavioral symptoms of premenstrual syndrome (P value < 0.001).
1. Introduction
Premenstrual syndrome (PMS) is one of the most common difficulties in women at their reproductive age.1 PMS is known as the recurrent mood and physical symptoms which is generally in the luteal phase, and it remits in the follicular phase of the menstrual cycle.2, 3 There is a high incidence of PMS; about 80% of women reported mild premenstrual symptoms, 20%–50% reported moderate symptoms, and about 5% of women had severe symptoms.4, 5 Despite the high prevalence of premenstrual syndrome, causes of it have not been clear and several etiologies have been proposed (e.g., hormonal change, neurotransmitters, prostaglandins, diet, drugs, and lifestyle).6
Symptoms vary among individuals. The most common symptoms include fatigue, irritability, flatulence, breast tenderness, sensitive mood to alternation of sadness and anger, mood changes, and depression7 as well as have been reported anxiety disorders in a large proportion of patients with this syndrome.8 Premenstrual syndrome, causing disturbance in communication, disruption of the normal activities, lack of exercise and interest, and reduce the accuracy of the individual tasks. If the severity of symptoms is high, will affect lifestyle, convenience, and health of the person. This syndrome is a disease can change in the women's individual characteristics and behaviors. The result of this change in behavior has significant impact on the family. These effects include conflicts with spouse, child abuse and criminal behavior. Recurrent negative effects of the increased tension will be in the family, reduce the durability of family, disconnect between family members, and decrease participation in the family and social issues.9
A wide variety of strategies have been proposed for this syndrome. Women affecting by mild symptoms recommended education, supportive consultation and generally self-care measures, such as increasing exercise and adopting a healthy diet. Women suffering from severe symptoms can be a range of helpful medications.7 However, for many women, no change in lifestyle and use of medications are not entirely one satisfactory approach in premenstrual syndrome. Some women with moderate symptoms may be lifestyle changes to be insufficient and tend to use prescription medications for a long time, that all of them would have significant adverse effects.10
Due to the side effects of chemical drugs, except severe cases, chemical drugs consumption is not recommended. Today, complementary and herbal medicine are commonly used in the treatment of many chronic conditions such as PMS.11, 12, 13, 14, 15 New therapeutic approaches are valuable and special place. Valerian (纈草 xié cǎo) plant, scientific name: Valeriana officinalis and belonging to the Velerianceae family, it is known as the cat grass. Many compounds have been detected in extracts of this plant that most important of which are noted to include: Valproate, Isovalproate and Didovalproate. The sedative effects of Valerian attributed to Volatile oils consist of Valerenal and Valernik acids. The sedative effects of Valerian stated in the books of the ancient Greek physicians like Hippocrates and trials of this work confirms, using of it goes back in traditional medicine for thousands of years. This plant has known due to anticonvulsant effects, sedation, anti-hysteria, and remove heart palpitations.16 Valerian ability is known to create and promote relaxation in the central nervous system, reduce stress and anxiety, and enhance sleep in the worldwide. The Valerian capsule contains 530 mg pharmaceutical markets of Valerian plant root.17 In a study conducted in Germany, the Valerian herb was indicated to be effective in reducing depression and anxiety.14 The results of another study conducted in Brazil on Valerian showed 3 plants was effective for anxiety disorders and the need for further research.18 Meanwhile, in the other studies showed that Valerian was effective in reducing the primary dysmenorrhea.19 Also in all these studies not been mentioned any side effects.
Due to limited research on this herbal drug and as regards the definition of World Health Organization, Midwife as a member of the health team is responsible and has the numerous tasks including education, care for girls and women, and freedom from damage to them such as during menstruation the researcher came upon to research one of the most common side effect of the plant on menstrual period. So that in case of positive outcomes taken steps to promote physical and mental health of women.
2. Methods
2.1. Study type
The study was double-blinded clinical trial.
2.2. Ethical approval
In this study, the researcher after taking an introduced letter of Tehran University of Medical Sciences, registration in the clinical trial site (IRCT Code: 201211179463N5, Ethics Committee No. 91/D/130/3183), providing to the Tonekabon Islamic Azad University, explaining the purpose of the study and how doing it, received the agreement of Azad authorities. It is mentioned the Ethics Committee of Tehran University of Medical Sciences approved this study and all participants gave their informed consent.
2.3. Subjects and inclusion criteria
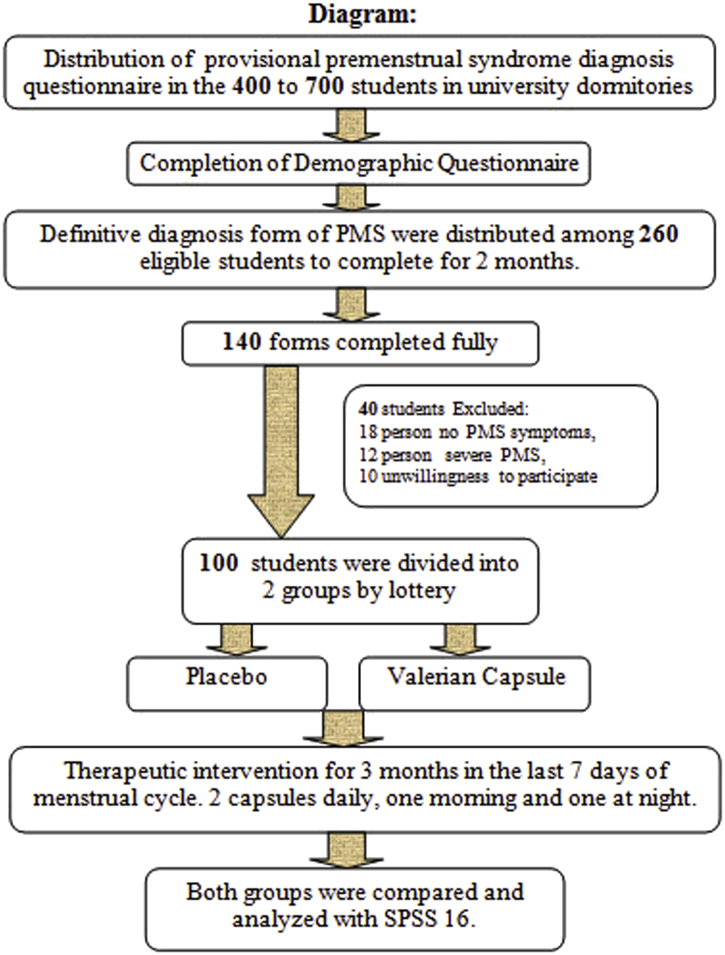
After obtaining a license sampling, researcher presented on consecutive days in the environment of study from March to end of August 2013, and provided the necessary explanation of research purposes in the students of university dormitory. Then the researcher expressed summarizes of the premenstrual syndrome (series of the mood and emotions, physical symptoms occur during the secretary menstrual cycle), how to recognize it, and assured the confidentiality of information. The provisional diagnosis of premenstrual syndrome questionnaire was provided in the 400 to 700 students living in university dormitories after explaining to do and expressed their willingness to participate in the study. Premenstrual syndrome identified based on the presence of symptoms for 3 months. These symptoms include depressed mood, sense of hopelessness, self-deprecation, anxiety, restlessness, irritability, depression, nervous tension, freak, lack of control over the actions and behavior, difficulty concentrating, confusion, dizziness, marked changes in appetite or desire for food, changes in sleep (hypersomnia and insomnia), crying for no reason, fatigue, physical symptoms such as breast tenderness or swelling, muscle or joint pain, swelling of the extremities, headaches, and bloated feeling or weight increasing. Of this number, 260 students qualified to participate in the study. Students were selected who have at least 5 symptoms of premenstrual syndrome. Demographic questionnaire was completed through the interviews and excluded students having conflict response to the inclusion criteria (18–35 year-old, single, regular menstrual cycles 21–35 days, the duration of 3–7 days during the last 6 months, no physical and mental well-known disease, insensitivity to herbal medicines, non-use of specific medications, stressful events during the 3 months before the study such as the death of close someone, parental divorce, economic problems in the family, an accident or adverse events, lack of enterprise in other similar researches), then record sheets for the final diagnosis of premenstrual syndrome were placed the eligible students before treatment completed them the first day of menstrual cycle for two cycles (the first day of bleeding calculated as the first day of the menstrual cycle and the students were asked o complete symptoms, according to the intensity of the feeling in the midday during each cycle daily). Record sheets included a 35-day table containing all of mood symptoms (anxiety, depression, crying for no reason, mood changes, irritability), physical symptoms (tenderness or swelling breast, muscle or joint pain, swelling of extremities, headache, and flatulence or weight gain), emotional, and behavioral symptoms (insomnia, fatigue, lack of energy and concentration, and bulimia). Also, it was explained to students how to complete the form in the first day of the menstrual cycle for two cycles. Zero was given in the absence of symptoms, mild symptoms that may not be barrier to daily activities given number one, moderate symptoms that interfere with daily activity number two, and severe symptoms that interfere with daily activities such as work and education number three.
Of these, 140 students completely filled forms. with respect to the exclusion criteria included: A number of students had, such as severe premenstrual syndrome, no wanting of students to continue taking the drug, the emergence of drug allergy symptoms, physical or emotional illness and need to take medicine, cessation of drug use for a week in the first cycle and irregular use of drugs in the second and third cycles for two days, understanding the physical and mental illness during the study, marriage during the study, death of relatives, and surgeries over the past 2 months excluded this study.
Forty students were excluded from the research: 18 people due to no premenstrual syndrome symptoms, 12 people suffering from severe syndrome, and 10 unwillingness to participate in the study.
The required sample size determined at the confidence level 95% and test power 80%, assuming that, the effect of Valerian (纈草 xié cǎo) root extract can decrease premenstrual syndrome score 3.3 (difference (d): The least differences which is valuable in terms of clinical research) compared with the control group, the sample size in each group was estimated 50 students.
2.4. Study protocol and medication
The first provisional diagnosis of premenstrual syndrome questionnaire, demographic questionnaires, and record sheets were collected every one of the students before treatment. Qualified student according to the inclusion criteria were tending to participate in the study and obtained informed consent. Each person was assigned a code and the criteria of premenstrual syndrome calculated from total scores of students (This means that score of 1–190 indicated mild, 191–380 moderate, and 381–570 severe form of premenstrual syndrome).
Then randomly were placed by lottery in the case and control groups. In that case, the letters A and B were written on cards and they were asked to choose one of the cards. If selected card A, they placed Valerian root extract group (Valerian Capsule or Case group) and if choose card B, included in the placebo group (Control group). This process continued until the full sample groups. The students and investigator were unaware of the drug and the 2 drugs (Valerian capsule containing Valerian root extract) and placebo prepared with the same shape and packaging then were encoded. Drug consumption explained (Twice a day, every morning and evening after meals, preferably used with a glass of water in the last seven days of the menstrual cycle for three cycles). Also Valerian record sheets for three menstrual cycles, questionnaires related to removal criteria sample and side effects were placed at the disposal of them.
Registration symptom forms (record sheets) were coded similar to student code and then each student was given three forms for three menstrual cycles. Researcher during the intervention ensured the proper use of drugs through phone calls and questions about how to do research and the completion of forms in the participants.
It is mentioned, drug was prepared under the supervision of pharmacologist also root of Valerian extract was prepared in capsule format and placebo was exactly the same as it. All of its expenses were covered by authors and all participants were given gifts after completing intervention.
2.5. Statistical analysis
After completing intervention, were collected registration form after three cycles, compared, and analyzed severity of mood or emotional, behavioral, and physical premenstrual syndrome symptoms in both groups before, one, two, and three months after intervention by SPSS 16.
3. Results
In this double-blind clinical Trial, the effect of Valerian (纈草 xié cǎo) root extracts (Valerian capsules) compared with placebo on the symptoms of premenstrual syndrome. The independent variable in this study was Valerian root extract and the dependent variables of premenstrual syndrome symptoms were symptoms of emotional, physical, and behavioral.
The research variables included: age, body mass index (BMI), education, age, occupation and economic status, age of menarche, duration of bleeding and menstrual cycle, self and family history of PMS. The intervention phase of the study, 100 participants (50 Valerian capsules group, and 50 patients in the placebo group) to end their collaboration. All samples involved in the study had mild or moderate PMS.
Results of Independent t-test and Chi-squared Test, Table 1, Table 2, indicated the significant difference did not exist in the mean and standard deviation of subjects in two groups, so were quite homogeneous.
Table 1.
Demographic status of students.
| Groups | Control |
Case |
P value∗ | |||
|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | N (%) | Mean ± SD | |||
| Age | 20–25 | 48 (96) | 21.68 ± 1.77 | 48 (96) | 21.46 ± 1.80 | 0.541 |
| 26–30 | 2 (4) | 2 (4) | ||||
| Education | Bachelor | 41 (82) | – | 45 (90) | – | 0.303 |
| Master degree | 1 (2) | 0 (0) | ||||
| Medicine | 8 (16) | 5 (10) | ||||
| Occupation/Job | Employed | 4 (8) | – | 1 (2) | – | 0.285 |
| Unemployed | 46 (92) | 44 (88) | ||||
| Socioeconomically status | Good | 25 (50) | – | 31 (62) | – | 0. 908 |
| Moderate | 2 (4) | 1 (2) | ||||
| Bad | 2 (4.4) | 7 (15.6) | ||||
| BMI | 17–21 | 23 (53.5) | 22.55 ± 4.36 | 22 (46.7) | 22.48 ± 3.93 | 0.273 |
| 22–26 | 15 (34.9) | 21 (44.7) | ||||
| 27–31 | 4 (9.3) | 3 (6.4) | ||||
| 32 or more | 1 (2.3) | 1 (2.1) | ||||
| Duration of PMS (Year) | 1–5 | 23 (69.7) | 4.54 ± 2.03 | 29 (70.7) | 4 ± 2.77 | 0.349 |
| 6–10 | 10 (30.3) | 12 (29.3) | ||||
| No responses | 17 (–) | 9 (–) | ||||
| Regular exercise program | Yes | 5 (10) | – | 4 (8) | – | 0.500 |
| No | 45 (90) | 46 (92) | ||||
| Total | 50 (100) | 50 (100) | ||||
*The P values were tested using independent t-test and chi-squared test.
Table 2.
Menstrual cycle status of students.
| Groups | Control |
Case |
P value∗ | |||
|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | N (%) | Mean ± SD | |||
| Age at menarche | 10–13 | 37 (74) | 12.85 ± 1.42 | 30 (60) | 13 ± 1.32 | 0.541 |
| 14–17 | 13 (26) | 20 (40) | ||||
| Duration of menstrual Cycles | 20–30 | 45 (90) | 29.90 ± 2.89 | 42 (84) | 29.18 ± 2.20 | 0.742 |
| 31–35 | 5 (10) | 8 (16) | ||||
| Having pain (dysmenorrhea) | Yes | 45 (90) | – | 44 (88) | – | 0.500 |
| No | 5 (10) | 6 (12) | ||||
| During of menstrual bleeding | 3–6 | 28 (56) | 5.82 ± 1.38 | 23 (46) | 6.18 ± 1.32 | 0.198 |
| 7–10 | 22 (44) | 27 (54) | ||||
| Having spotting | Yes | 13 (26) | – | 14 (28) | – | 0.500 |
| No | 37 (74) | 36 (72) | ||||
| Total | 50 (100) | 50 (100) | ||||
*The P values were tested using independent t-test and chi-squared test.
Most of the subjects in both groups were in the age group 20–25 years, the mean and standard deviation of BMI were used to control and Valerian groups, respectively, 22.55 ± 4.36 and 22.48 ± 3.99. Majority of the subjects had a bachelor education in the control and Valerian group.
Most of the subjects were unemployed in the control and Valerian groups. The economic situations in the most of the subjects were appropriate in the control and Valerian group. The mean and standard deviation of premenstrual syndrome duration were 4.54 ± 2.03 in the control and 4.35 ± 3.42 in Valerian groups. The majority of subjects had not a regular exercise program. The Most of the students had menarche age 10–13 years; the menstrual cycles of them were 20–30 days and duration of menstrual bleeding in 100% of students ranging from 3–10 days. The majority of the subjects stated that they had dysmenorrhea but did not have spotting in the cycles. Paired t-test results in Table 3 indicated that no statistically significant differences were in the severity of the premenstrual syndrome symptoms before and after the intervention in the control group (P = 0.051) but the significant difference was observed in the Valerian group (P = 0.001). Also results of independent t-test showed that statistically significant differences were in mean severity of premenstrual syndrome in both control and Valerian groups (P = 0.001).
Table 3.
Comparison of the severity premenstrual syndrome symptom scores previous and after intervention in two groups.
| Variable | Groups |
|||
|---|---|---|---|---|
| Control |
Case |
|||
| Previous intervention | After intervention (12 weeks) | Previous intervention | After intervention (12 weeks) | |
| Mean ± SD | 132.17 ± 30.31 | 125.06 ± 31.31 | 106.07 ± 49.50 | 41.30 ± 19.06 |
| Number | 50 | 50 | 50 | 50 |
| Paired t-test | P = 0.51 | P = 0.001 | ||
| Independent sample t-test | P = 0.001 | |||
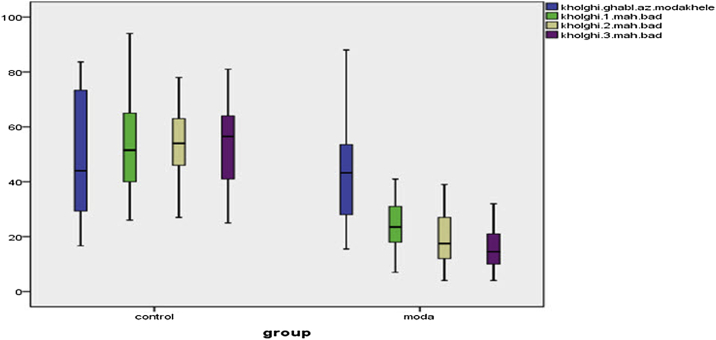
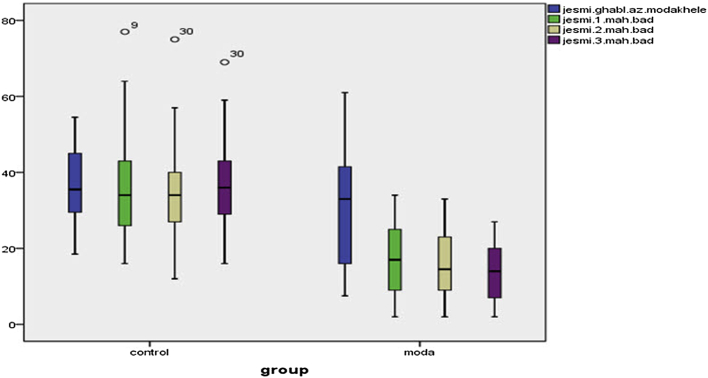
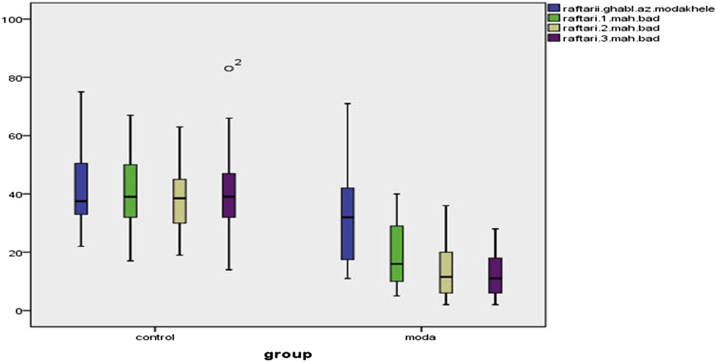
Paired t-test results in Table 4, Fig. 1, Fig. 2, Fig. 3 showed that no statistically significant differences were in mean severity of premenstrual syndrome (the emotional (P = 0.853), behavioral (P = 0.970) and physical (P = 0.552) symptoms) before and after the intervention in the control group, whereas significant differences found between the Valerian group (P = 0.000). Repeated measures ANOVA showed emotional, behavioral and physical symptoms of premenstrual syndrome before, 1, 2 and 3 months after intervention in the control group were not statistically significant, while in Valerian observed a significant difference (P = 0.0001).
Table 4.
Comparison of the emotional, physical, and behavioral symptoms of PMS previous and after intervention in two groups.
| Groups | Symptoms | Time | M ± SD | P value repeated measures ANOVA | Time | M ± SD | P value paired t-test |
|---|---|---|---|---|---|---|---|
| Control | Emotional | Previous intervention | 54.13 ± 11.88 | 0.472 | Previous intervention | 54.13 ± 11.88 | 0.853 |
| 1 month after intervention | 52.54 ± 15.36 | ||||||
| 2 month after intervention | 53 ± 12.65 | After intervention | 53.76 ± 14.80 | ||||
| 3 month after intervention | 53.76 ± 14.80 | ||||||
| Physical | Previous intervention | 36.73 ± 9.69 | 0.281 | Previous intervention | 36.73 ± 9.69 | 0.970 | |
| 1 month after intervention | 35.40 ± 12.38 | ||||||
| 2 month after intervention | 34.24 ± 12.42 | After intervention | 36.68 ± 10.52 | ||||
| 3 month after intervention | 36.68 ± 10.52 | ||||||
| Behavioral | Previous intervention | 41.31 ± 11.57 | 0.340 | Previous intervention | 41.31 ± 11.57 | 0.552 | |
| 1 month after intervention | 40 ± 11.92 | ||||||
| 2 month after intervention | 38.48 ± 11.37 | After intervention | 40.04 ± 12.66 | ||||
| 3 month after intervention | 40.04 ± 12.66 | ||||||
| Case | Emotional | Previous intervention | 42.87 ± 19.07 | 0.0001 | Previous intervention | 42.87 ± 19.07 | 0.000 |
| 1 month after intervention | 23.92 ± 9.01 | ||||||
| 2 month after intervention | 19.26 ± 9.82 | After intervention | 15.80 ± 7.47 | ||||
| 3 month after intervention | 19.66 ± 7.87 | ||||||
| Physical | Previous intervention | 30.75 ± 16.01 | 0.0001 | Previous intervention | 30.75 ± 16.01 | 0.000 | |
| 1 month after intervention | 12.24 ± 9.68 | ||||||
| 2 month after intervention | 15.74 ± 8.38 | After intervention | 13.14 ± 7.02 | ||||
| 3 month after intervention | 13.14 ± 7.02 | ||||||
| Behavioral | Previous intervention | 33.45 ± 16.73 | 0.0001 | Previous intervention | 33.45 ± 16.73 | 0.000 | |
| 1 month after intervention | 19.08 ± 10.42 | ||||||
| 2 month after intervention | 13.48 ± 9.00 | After intervention | 12.36 ± 7.22 | ||||
| 3 month after intervention | 12.36 ± 7.22 |
Fig. 1.
The difference between the control and intervention groups in the emotional symptom.
Fig. 2.
The difference between the control and intervention groups in the physical symptom.
Fig. 3.
The difference between the control and intervention groups in the behavioral symptom.
4. Discussion
This study aimed to investigate the effects of Valerian (纈草 xié cǎo) root extract on the symptoms of premenstrual syndrome. The severity of physical, emotional, and behavioral premenstrual syndrome symptoms before, one, two, and three months after taking the Valerian capsule showed significant differences between case and control groups (P = 0.0001). Findings of a research Jenabi et al. (2012) had done in order to compare the effects of Valerian and Mefenamic acid on dysmenorrheal symptoms, suggested that the total mean scores in two groups of Valerian and Mefenamic acid after 1 and 2 months of using them showed no significant difference (P > 0.05).20 This means that Valerian as Mefenamic acid was effective the pain of dysmenorrhea, which was consistent with our results. They improve the effectiveness of Valerian in pain, because of the root of this plant that has Valrnik acid. It should be noted that the Valerian is anti-spasticity, prevent the smooth muscle contraction of the uterus during menstruation by inhibiting the release of prostaglandins, and thereby leads to relief pain in women. The results of this study matched with the finding of a study by Mirabi and colleagues in 2009, was performed to evaluate the effects of Valerian root on primary dysmenorrhea and the results showed the effectiveness of the Valerian plant has been the improvement of dysmenorrhea.19 Also the results of the Taavoni and colleagues' study (2012) done to determine the effect of Valerian capsule on sleep disorders in postmenopausal women, demonstrated the positive therapeutic effects of Valerian on sleep in postmenopausal women.17 Because of dysmenorrhea and sleep disorders are one of the physical premenstrual syndrome symptoms, the alignment of the results is not unexpected this research study.
The finding of this study has been consistent with the results of a study by Diethard Müller and colleagues in (2003) were conducted to evaluate the effect of Valerian extract and St. John's wort for depression and anxiety, found Valerian and St. John's wort extract were effective in the treatment of depression and anxiety.21 In this study, symptoms of depression and anxiety after the intervention cannot be attributed only to the effects of Valerian, because in this study, along with Valerian extract, the St. John's Wort extract has been used in order to achieve therapeutic effects. The results of a study by Miyasaka et al. in Brazil (2009), was conducted to determine the impact of Valerian for anxiety disorders showed no significant difference in levels of anxiety symptoms in patients before and after treatment. The findings of this study indicated that Valerian taking were improved anxiety symptoms after the completion of intervention sessions.18 Differences in research population, the low volume of the study sample, less time of medication using in this study can be attributed to the non-alignment of the results of the above studies in comparison with the present study. The results of a study were performed by Rezaei et al. in 2010 to investigate the effects of sedative and anxiolytic Valerian extract in rates in comparison with diazepam, showed that a dose of 200 mg Valerian root extract meaningful improved pain and anxiety symptoms in the intervention group.16 Given that the anxiety is the emotional symptoms of premenstrual syndrome (PMS) the alignment of these research results are not unexpected.
Chemical composition of the plant can vary depending on the species. However, all it contains arginine, glutamine, alanine, and GABA. Different mechanisms of this plant action have been suggested that increase the GABA transmission and the effect on the serotonin.22 Proper functioning of the central nervous system is adjusted by receptors in the brain, called GABA receptors. Based on in vitro studies found that alerianV can connect these receptors to be lazy. Thus, the mechanism of action and its effectiveness is partly determined. It is an effective therapeutic effect on people with varying degrees of insomnia, Valrnik acid in Valerian extract is increased “GABA” concentration by inhibiting the catabolizes enzymes of GABA. GABA concentrations decreased neuronal irritability and reduced anxiety. Valrnik acid leads to decrease the onset sleep time, improve sleep disorder, avoid frequent waking during sleep, and ultimately improve the quality of sleep. Valrnik acid and Valproate are in the strongest combinations of Valerian root sedative. This plant can be used as an alternative to chemical medicines such as barbiturates and benzodiazepines, hypnotics, and anti-stress.18
Similar studies have not been done that; have examined the effect of this plant on premenstrual syndrome. Other herbal remedies have been used in the treatment of this syndrome.
A study by Khayat et al. is done (2013) to assess the effectiveness of ginger and curcumin on the severity of PMS. The mean scores of PMS symptoms before intervention was 110.2 ± 30.77 in ginger group, 106.7 ± 44.65 in the placebo group and not significantly different (P = 0.710).11 But the severity of PMS symptoms in both groups 1, 2 and 3 months after treatment were significantly different (P = 0.001). The results of this research to improve behavioral symptoms associated with premenstrual syndrome after taking Valerian root extract has been a new and unique. Because that has not been observed any study in behavioral symptom and in none of the study in this field has not been evaluated such a finding.
So in this field cannot be definitively concluded and definitive conclusions in this area requires further study.
5. Conclusion
Our girls and women are an important part of the family and society and their health depend on to provide health, cultural and economic needs of them. Premenstrual Syndrome is a common disease of women in the world and Iran, also the risk of this syndrome is higher in younger women. Because of this society groups plays a decisive role in motivation and shaping the character and behavior, attention to physical and spiritual health is important. The exact cause of this syndrome has not been correctly diagnosed, but due to its impact on the health and social costs, treatment is necessary. With this study aimed to investigate the effects of Valerian (纈草 xié cǎo) root extract (Valerian Capsule) was conducted on the severity of the premenstrual syndrome symptoms. The results of it suggest that the extract could reduce the severity of symptoms of premenstrual syndrome.
Disclosure
The authors report no real or perceived interest that relate to this article that could be construed a conflict of interest.
Acknowledgment
This study was financially supported by the Tehran University of Medical Sciences. The authors wish to express their sincere gratitude to the study participants without whom this study could not have been conducted.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Zahra Behboodi Moghadam, Email: behboodi@tums.ac.ir.
Elham Rezaei, Email: rezai520@yahoo.com.
Roghaieh Shirood Gholami, Email: roghaye.shiroodgholami@yahoo.com.
Masomeh Kheirkhah, Email: m_kheirkhah@iums.ac.ir.
Hamid Haghani, Email: haghani511@yahoo.com.
References
- 1.Freeman E.W., Sammel M.D., Lin H., Rickels K., Sondheimer S.J. Clinical subtypes of premenstrual syndrome and responses to sertraline treatment. Obstet Gynecol. 2011;118(6):1293–1300. doi: 10.1097/AOG.0b013e318236edf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford O., Lethaby A., Roberts H., Mol B.W.J. Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD003415.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Bakr I., Ez-Elarab H.S. Prevalence of premenstrual syndrome and the effect of its severity on the quality of life among medical students. Egypt J Community Med. 2010;28(2):19–30. [Google Scholar]
- 4.Pearlstein T., Steiner M. Premenstrual dysphoric disorder: burden of illness and treatment update. J Psychiatry Neurosci. 2008;33(4):291–301. [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmbhatt S., Sattigeri B.M., Shah H., Kumar A., Parikh D. A prospective survey study on premenstrual syndrome in young and middle aged women with an emphasis on its management. Int J Res Med Sci. 2013;1(2):69–72. [Google Scholar]
- 6.Sadler C., Smith H., Hammond J. Lifestyle factors, hormonal contraception, and premenstrual symptoms: the United Kingdom Southampton women's survey. J Womens Health (Larchmt) 2010;19(3):391–396. doi: 10.1089/jwh.2008.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adrianne B. The potential for dietary supplements to reduce Premenstrual Syndrome (PMS) symptoms. J Am Coll Nutr. 2000;19(1):3–12. doi: 10.1080/07315724.2000.10718907. [DOI] [PubMed] [Google Scholar]
- 8.Wallenstein G.V., Blaisdell-Gross B., Gajria K. Development and validation of the Premenstrual Symptoms Impact Survey (PMSIS): a disease-specific quality of life assessment tool. J Womens Health (Larchmt) 2008;17(3):439–450. doi: 10.1089/jwh.2007.0377. [DOI] [PubMed] [Google Scholar]
- 9.Kiani Asiabar A., Heidari M., Mohamadi Tabar S.H., Phaghihzade S. Prevalence, Signs, symptoms, and causes of premenstrual syndrome in employed women. Bimon Sci Res (Daneshvar) 2009;16(81):45–54. [In Persian] [Google Scholar]
- 10.Penelope M.B., WHNP M.S.N. Understanding and treating PMS/PMDD. Nursing. 2003;33(13):14–17. [Google Scholar]
- 11.Khayat S., Kheirkhah M., BehboodiMoghadam Z., Fanaei H., Kasaeian A., Javadimehr M. Effect of treatment with ginger on the severity of premenstrual syndrome symptoms. ISRN Obstet Gynecol. 2014:5. doi: 10.1155/2014/792708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yonkers K.A., O'Brien P.S., Eriksson E. Premenstrual syndrome. Lancet. 2008;371(9619):1200–1210. doi: 10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto T., Asakura H., Hayashi T. Does lavender aromatherapy alleviate premenstrual emotional symptoms? A randomized crossover trial. Biopsychosoc Med. 2013;7(12):1–8. doi: 10.1186/1751-0759-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha Filho E.A., Lima J.C., Pinho Neto J.S., Montarroyos U. Essential fatty acids for premenstrual syndrome and their effect on prolactin and total cholesterol levels: a randomized, double blind, placebo-controlled study. Reprod Health. 2011 Jan 17;8:2. doi: 10.1186/1742-4755-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Z., Chen R., Zhou Y. Treatment for premenstrual syndrome with Vitex agnus castus: a prospective, randomized, multi-center placebo controlled study in China. Maturitas. 2009;63(1):99–103. doi: 10.1016/j.maturitas.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Rezaei A., Pashazade M., Ahmadzade C.H., Jafari B., Jalilzade Hedayati M. Sedative and anxiolytic effects of Valerian extract compared to diazepam in rats. Q Med Plants. 2010;9(36):169–176. [In Persian] [Google Scholar]
- 17.Taavoni S., Ekbatani N., Kashaniyan M., Haghani H. Effect of Sedamin capsule on sleep disorder among menopausal women. J Gorgan Univ Med Sci. 2012;14(1):39–45. [In Persian] [Google Scholar]
- 18.Miyasaka L.S., Atallah Á.N., Soares B. Valerian for anxiety disorders. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD004515.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Doulatian M., Mirabi P., Mojab F., Alavi Majd H. The effect of Valerian plant on the intensity of primary dysmenorrhea. J Reprod Infertil. 2009;10(4):253–259. [In Persian] [Google Scholar]
- 20.Jenabi E., Asl Toghiri M., Hejrati P. Comparison of Valerian root analgesic effects and mefenamic acid in relieving primary dysmenorrhea. Iran J Obstet Gynecol Infertil. 2012;15(2):44–48. [In Persian] [Google Scholar]
- 21.Müller D., Pfeil T., Von den Driesch V. Treating depression comorbid with anxiety – results of an open, practice-oriented study with St John's wort WS® 5572 and Valerian extract in high doses. Phytomedicine. 2003;10(4):25–30. doi: 10.1078/1433-187x-00305. [DOI] [PubMed] [Google Scholar]
- 22.Kinrys G., Coleman E., Rothstein E. Natural remedies for anxiety disorders: potential use and clinical applications. Depress Anxiety. 2009;26(3):259–265. doi: 10.1002/da.20460. [DOI] [PubMed] [Google Scholar]