Abstract
Morinda citrifolia L. commonly known as noni is used by the pharmaceutical and cosmetic industries due to the plethora of pharmacological activities of its metabolites. In Mauritius, the fruits of M. citrifolia are used in folk medicine against a number of indications. The present study aimed at evaluating the antioxidant activity of ripe and unripe noni fruit at both biochemical and cellular levels. Using an array of established assay systems, the fruit antioxidant propensity was assessed in terms of its radical scavenging, iron reducing and metal chelating potentials. Ascorbic acid, total phenolic and total flavonoid contents of the fruits were also determined. The ascorbic acid content of ripe noni was 76.24 ± 1.13 mg/100 g while total phenolics of ripe and unripe fruit extracts were 748.40 ± 8.85 μg and 770.34 ± 2.27 μg GAE g−1 FW respectively. Both the ripe and unripe extracts of M. citrifolia were potent scavengers of nitric oxide, superoxide and hydroxyl radicals. The ferric reducing capacity ranged from 11.26 ± 0.33 to 11.90 ± 0.20 mM Fe2+ g−1 FW while the IC50 values for the iron (II) chelating power were 0.50 ± 0.01 and 1.74 ± 0.01 g FW/mL for the ripe and unripe fruit extracts respectively. Cellular studies additionally demonstrated that noni were able to dose-dependently counteract accumulation of reactive oxygen species (ROS)-induced oxidative stress, a potential obesogenic factor within human liposarcoma SW872 cells as well as significantly restore cell death within the concentration range of 0.106–0.813 g/mL. Results reported herein suggest noni as an interesting source of prophylactic antioxidants modulated by its polyphenol composition.
Keywords: Antioxidant activity, Folk medicine, Morinda citrifolia, Oxidative stress, Polyphenolics
Graphical abstract
1. Introduction
Concerns regarding oxidative stress (OS) are primarily due to its involvement in various pathophysiologies ranging from inflammation, reperfusion injury, atherosclerosis, cancer, osteoporosis, aging, fibrosis and cognitive function.1, 2, 3 This has prompted interest in antioxidant phytophenolic rich dietary sources and their putative protective effects on human health.4 Thus, over the past few decades, herbal and natural products from folk medicines have become increasingly popular globally because of their long standing use, efficacy and reduced toxicity.5
Morinda citrifolia L. commonly known as noni, belongs to the Rubiaceae family, and is indigenous to the tropical zones.6 Its wide array of secondary metabolites including more than 160 phytochemical compounds ranging from phenolic compounds, organic acids and alkaloids, is widely suggested to account for the reported prophylaxis of the plant extracts. Anthraquinones in particular damnacanthal, morindone, morindin, and aucubin, asperuloside and scopoletin have been prominently identified.7
These phenolics exhibit their antioxidative activity via several mechanisms of action inter alia: as reducing agents, singlet oxygen quenchers, hydrogen donating antioxidants, free radicals scavengers and metal ions chelators.8, 9 In addition, in view of their pluripharmacological properties, they can exert modulatory actions in cells by interacting with a wide range of cellular and molecular targets.9, 10
In the last decade, extensive research have credited noni with antioxidant,11 anti-microbial properties,12 anti-inflammatory,13 anticarcinogenic,14 antidiabetic activity,15 immune stimulating16 and analgesic activity.17 In Mauritius, noni fruits and leaves have ethnomedicinal applications against type 2 diabetes, hypercholesterolemia, hypertension and pain.18
In the light of the documented beneficial properties of noni, the evaluation of the phytophenolic richness and antioxidative properties of the locally cultivated noni fruit was carried out using several in-vitro assays and on human adipocytes SW872, a dual model of obesity and oxidative stress. Results reported herein support the traditional use of noni as a health enhancer in herbal and complementary medicine.
2. Methodology
2.1. Chemicals
Aluminium chloride was purchased from Surechem Products, United Kingdom, Nitrobluetetrazolium, Nicotinamide-adenine dinucleotide, ferrozine and Dulbecco's modified eagle's medium (DMEM) were bought from HiMedia laboratories, Mumbai (India). Moreover, quercetin was purchased from Sigma–Aldrich, India and deoxyribose from Fluka Analytical Laboratories, Germany. Fetal bovine serum, l-glutamine and penicillin–streptomycin were purchased from Sigma (USA).
2.2. Fruit source
M. citrifolia L. ripe and unripe fruits were collected from Grand-Bel-Air in the South East of Mauritius during the month of October 2013. The fruits were identified and authenticated at the Herbarium of Mauritius, Mauritius Sugar Industry Research Institute.
2.3. Vitamin C determination in whole fruits
Ascorbic acid content in M. citrifolia L. fruits was determined according to the AOAC 967.21 official method, using the 2, 6-dichloroindophenol titri-metric method. 50 g of each fruit sample was weighed and blended with 100 mL of distilled water. The mixture was filtered and was made up with distilled water up to 250 mL in a volumetric flask. To 5 mL of metaphosphoric acid solution, 2 mL of sample fruit juice was added and titrated with indophenol dye solution until a light rose – pink color persisted for more than 5 min. Results were expressed as mean mg ascorbic acid 100 g−1 fresh fruits of three replicates.
2.4. Phytophenolic analyses
2.4.1. Extraction
Pulps from the ripe and unripe fruit respectively were freeze dried. They were then extracted with 80% methanol (1:3 w/v) and allowed to macerate exhaustively at 4 °C prior to being concentrated in vacuo at 37 °C. Finally, the concentrated extract was lyophilized and the resulting powders were subsequently dissolved in deionized water or 80% methanol for further analyses.
2.4.2. Total phenolic content determination
The Folin-Ciocalteu assay assay adapted from Neergheen et al. (2006) was used to estimate the total phenolic content of the fruit extracts of M. citrifolia L.19 The results were expressed in terms of μg gallic acid equivalent (GAE) g−1 FW.
2.4.3. Determination of total flavonoid content
Total flavonoid content of fruit extracts were investigated using the spectrophotometric assay adapted from Zhishen et al. (1999).20 The results were expressed in terms of mg quercetin equivalent (QE) g−1 FW.
2.5. Determination of antioxidant capacities
2.5.1. Ferric reducing antioxidant power
The FRAP assay adapted from Benzie and Strain (1996) was modified to evaluate the reducing power of fruit extracts of M. citrifolia L.21 At low pH, ferric tripyridyltriazine complex is reduced to ferrous form, the resulting intense blue color being linearly related to the amount of reductant present. The FRAP reagent consisting of 2,2,6 tripyridyl-5-triazine (TPTZ, 10 mM) in 40 mM HCl and ferric chloride (20 mM) in 200 mL of sodium acetate buffer (pH 3.6, 0.25 M) was freshly prepared and warmed at 37 °C prior to analysis. To 180 μL FRAP reagent, 20 μL of the extracts was added in a 96 – well plate and was left to react for 6 min at ambient temperature. The absorbance was read at 593 nm (Synergy HT, BioTek instruments, USA). A calibration curve of ferrous sulphate was used and results were expressed in terms of mM Fe2+ g−1 FW.
2.5.2. Iron chelating activity
The method adapted from Neergheen-Bhujun et al. (2014). was used to assess the iron (II) chelating effect of the fruit extracts.22 The reacting mixture contained 200 μl of varied concentration of the ripe and unripe fruits extract (0.1–2.8 g/mL) and 50 μl FeCl2.4H2O (0.5 mM).The reacting mixture was then made up to 1 mL with distilled deionized water and incubated for 5 min at room temperature. After incubation, 50 μl of ferrozine (2.5 mM) was added and the purple coloration formed read at 562 nm. EDTA was used as a positive control. The percentage of chelating activity was calculated and results were expressed as mean IC50 (g FW mL−1).
2.5.3. Inhibition of deoxyribose damage
The hydroxyl scavenging activities of the extracts under study was determined using the deoxyribose assay adapted from Halliwell et al., 198723 and Aruoma 1994.24 The reaction mixture for the deoxyribose assay contained in a final volume of 1 mL the following reagents, order of addition indicated: 200 μL KH2PO4–KOH (100 mM), 200 μL FeCl3 (500 μM), 100 μL EDTA (1 mM), 100 μL sample of varied concentrations (0.1–2.8 g/mL), 200 μL deoxyribose (15 mM), 100 μL H2O2 (10 mM) and 100 μL ascorbic acid (1 mM).The reaction mixture was incubated at 37 °C for 1 h.
After incubation, 1 mL of 1% (w/v) thiobarbituric acid (TBA) was added to each mixture followed by the addition of 1 mL of 2.8% (w/v) trichloroacetic acid (TCA). The solutions were heated on a water bath at 80 °C for 20 min to develop the pink colored malondialdehyde–thiobarbituric acid: MDA–(TBA)2 adduct. The MDA–(TBA)2 chromogen was extracted into 3 mL butan-1-ol and its absorbance measured at 532 nm. Gallic acid was used as positive control and results were expressed as mean IC50 (g FW mL−1).
2.5.4. Nitric oxide radical inhibition assay
Nitric oxide was generated from sodium nitroprusside and was measured by the GriessIllosvoy reagent using 0.1% w/v naphthylethylene–diamine–dihydrochloride as described in Mandal et al., 2009.25 The reaction mixture contained 2 mL sodium nitroprusside, 0.5 mL phosphate saline buffer, pH 7.4 and 0.5 mL of plant extract (variable concentrations, 0.008–0.712 g/mL) and was allowed to incubate for 2½ h at 25 °C. Following incubation, 0.5 mL of the reaction mixture was added to 1 mL sulphanilic acid (0.33% in 20% glacial acetic acid) and the mixture was allowed to stand for 5 min 1 mL of naphthylethylene–diamine–dihydrochloride (0.1%) was then added to the mixture, vortexed and allowed to stand for further 30 min. The absorbance was read at 546 nm and the % inhibition was calculated as follows:
| % inhibition = [(Absc − Abss)/Absc] × 100, where, Absc = absorbance control, Abss = absorbance sample. |
2.5.5. Superoxide anion radical scavenging assay
The superoxide anion radical scavenging assay of the fruit extracts was measured according to a modified assay for microplate from Rummun et al. 2013.26 To 60 μL nitrobluetetrazolium (NBT) (156 μM), 60 μL β-nicotinamide-adenine dinucleotide (reduced disodium salt of NADH; 187 μM) and 30 μL of the plant extracts (variable concentrations, 0.030–1.626 g/mL) were added and the reaction was initiated by 60 μL phenazine methosulphate solution (PMS, 60 μM). The reaction mixture was allowed to incubate at 25 °C for 15 min and the absorbance was read at 560 nm. The superoxide radical scavenging was calculated as above.
2.6. Cell culture
Human adipocytes cells SW872 (ATCC® HTB-92) were used to evaluate cell viability and accumulation of intracellular reactive oxygen species. Cells were grown in high glucose Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/L streptomycin-penicillin and 0.5 μg/mL amphotericin B. Cells were maintained at 37 °C in an atmosphere of 5% CO2 and 95% humidity.
2.6.1. Cell viability assessment by MTT assay
SW872 cells were seeded in a 96-well culture plate at a cell density of 10 × 104 cells/well. After pre-treatment with noni extract for 24 h, cells were exposed to H2O2 (1 mM) or PBS for 30 min 20 μL 3 (4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide (MTT) (5 mg/mL) was added to each well and incubated for a further 2 h. After carefully removing the culture medium, formazan crystals were dissolved in 150 μL DMSO and the absorbance was read at 595 nm and 690 nm (Biotek Synergy HT, USA). Results were expressed as mean percentage (%) compared to a control (cells treated with PBS instead of noni extract).
2.6.2. Determination of reactive oxygen species (ROS) accumulation by DCF-DA
The level of ROS accumulated within SW872 cells exposed to H2O2 was determined using the fluorogenic dye 2′, 7′-dichlorofluorescein diacetate. After seeding cells overnight in a 96-well culture plate at a density of 10 × 104 cells/well, cells were treated with noni extracts for 24 h then stained with DCF-DA (10 μM) for a further 30 min. After incubation, cells were exposed to H2O2 (1 mM) for 30 min. Fluorescence was measured at 485 nm (excitation) and 535 nm (emission) (BioTek Synergy HT, USA). Results are expressed as mean percentage (%) compared to a control (cells treated with H2O2 only).
2.7. Data analysis
All graphs were generated using Microsoft Excel software (Version 2007) and GraphPad Prism (Version 6). ANOVA (single factor) was used to test for significant differences in mean values of the different extract for each assay as well as the LSD (Least Significant Difference) tests were carried out.
3. Results
The total phenolic content of the fruits were 748.40 ± 8.85 μg GAE g−1 FW and 770.34 ± 2.27 μg GAE g−1 FW with the highest content measured in the unripe fruits. No significant difference was observed between the samples. The highest level of flavonoids was measured in the unripe fruit (228.02 ± 0.37 μg QE g−1 FW) compared to a much lower amount (67.67 ± 1.55 μg QE g−1 FW) in ripe fruits. Moreover, noni ripe fruits were richer in vitamin C (76.24 ± 1.13 mg/100 g) than the unripe sample (53.19 ± 0.79 mg/100 g).
Comparable ferric reducing potentials were observed for both unripe (11.90 ± 0.20 mM Fe2+ g−1 FW) and ripe fruit extracts (11.26 ± 0.33 mM Fe2+ g−1 FW). The fruits also exhibited a dose-dependent effect against nitric oxide radical at the concentration range tested. The ripe and unripe fruits were both potent scavenger of the free radical. A calculated IC50 value of (0.009 ± 0.001 g FW/mL) was obtained for the ripe fruit whereas the unripe fruit exhibited 50% inhibition at a concentration of 0.013 ± 0.006 g FW/mL. Gallic acid was used as positive control with 50% inhibition at (0.480 ± 0.106 mg/mL). No significant differences were observed between the samples (p > 0.05).
A similar trend was observed for the superoxide anion radical scavenging activity at the concentration range tested. The ripe fruit was the most powerful superoxide radical scavengers with the lowest IC 50 value (0.107 ± 0.012 g FW/mL) whereas the unripe fruit exhibited 50% superoxide scavenging at 0.140 ± 0.035 g FW/mL. Gallic acid used as positive control (IC50 value of 0.170 ± 0.01 mg/mL) was more potent than both fruit samples. No significant difference was noted in the superoxide scavenging capacity between the samples (p > 0.05).
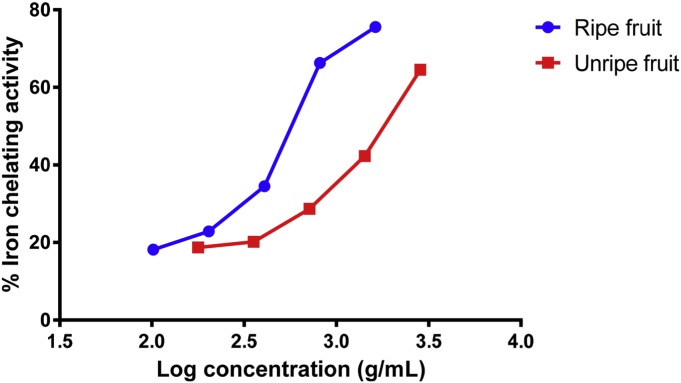
The results also indicated that the extracts at the concentration range tested (Fig. 1), demonstrated an ability to chelate Fe2+ in a dose-dependent manner. Calculated IC50 values were 0.500 ± 0.012 g FW/mL and 1.740 ± 0.006 g FW/mL for the ripe and unripe fruit respectively. EDTA was used as a positive control and it exhibited 50% inhibition at a concentration of 0.047 ± 0.012 mg/mL. Significant difference was recorded between the extracts (p < 0.05).
Fig. 1.
Fe2+ ions chelating activity of fruit extracts. Results are expressed as 50% chelating activity mean value ± standard deviation (g FW/mL) (n = 3).
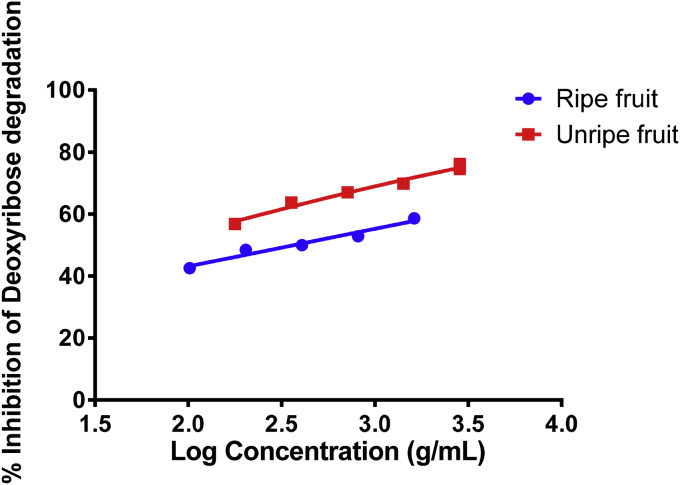
The samples under study were also potent inhibitors of deoxyribose degradation (Fig. 2). However, the unripe fruit extract was a more potent inhibitor of deoxyribose oxidation with the lowest IC50 value (0.060 ± 0.013 g FW/mL) compared to 0.370 ± 0.015 g FW/mL for the ripe fruits. Gallic acid was used a positive control and an IC50 value of 0.191 ± 0.001 mg/mL was recorded at 50% inhibition. A significant difference in scavenging capacity was observed between the extracts (p < 0.05).
Fig. 2.
Percentage inhibition of deoxyribose degradation of fruit extracts. Results are expressed as 50% inhibition of nitric oxide radical mean value ± standard deviation (g FW/mL) (n = 3).
3.1. Effect of noni fruit extracts on SW872 cell line viability
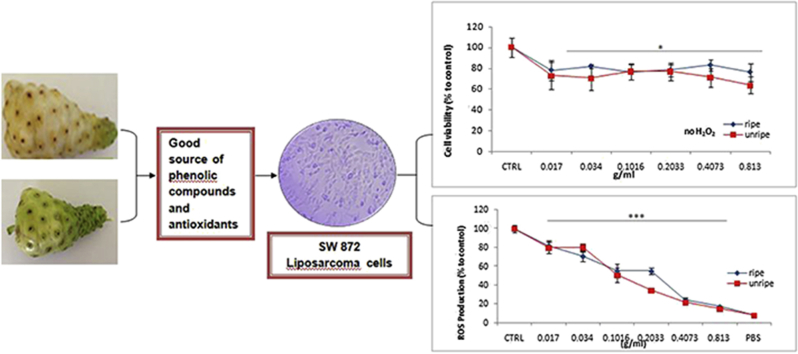
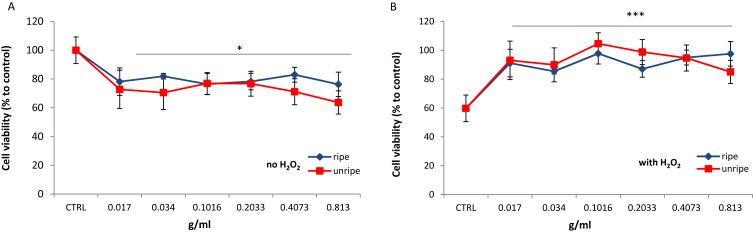
The MTT assay was used to evaluate the effect of noni extracts on the viability of SW872 adipocytes. Fig. 3(A) showed that the viability of adipocytes (25 × 104 cells/well) treated with extracts for 24 h decreased in a general dose–response manner. Viability of cells ranged between 63.7–76.9% and 76.2–82.9% for cells pretreated with ripe and unripe noni extracts respectively, where unripe extracts were noted to be slightly more cytotoxic than ripe. Extracts exceeding a dose of 0.81 g/mL seemed to be ideal for reducing the proliferation rate of human liposarcoma SW872 cells.
Fig. 3.
Effect of noni extracts on SW872 cell viability without (A) or with (B) the addition of 1 mM H2O2 as an inducer of oxidative stress. Viability was expressed as a percentage compared to a control treated with PBS or H2O2 only. Results are presented as the mean of two independent experiments performed in triplicate, where error bars represent ± standard deviation, *p < 0.05 and ***p < 0.001 vs. control.
The cell viability was observed to significantly drop to 59.7% (p < 0.001 vs. PBS control) upon exposure of SW872 to H2O2 indicating the cytotoxic effect of the latter. The effect of H2O2-induced oxidative stress was strongly attenuated upon pretreatment with noni extracts (Fig. 3(B)). Cell viability was found to increase in a general dose dependent manner reaching a maximum of 97.8% (ripe) and 104.6% (unripe) (vs. 59.7% H2O2 control), which represented highly significant increases of 63.6 and 75.1% in cell viability (p < 0.001). The data suggested that a dose of 0.102 g/mL is optimal and can exert a cytoprotective effect against the deleterious effects of H2O2.
3.2. Effect of noni fruit extracts on ROS production
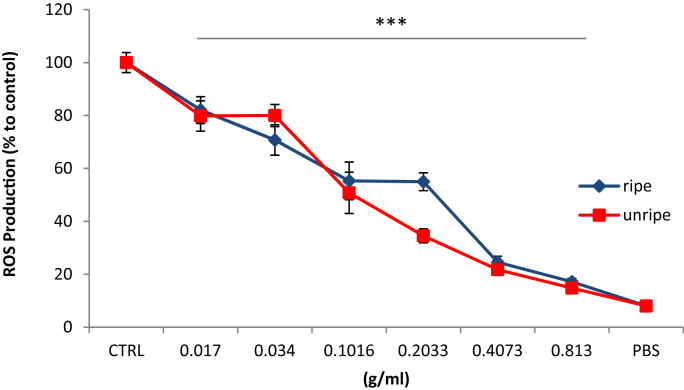
The antioxidant activity of noni is illustrated in Fig. 4, where intracellular ROS levels were quantified using the fluorescent dye DCFA. 2′, 7′-dichlorofluorescein diacetate (DCFH-DA) is a fluorogenic dye used to indicate the level of oxidative stress in-situ. Upon its reaction with ROS, DCFH-DA is oxidized into a highly fluorescent compound 2′, 7′-dichlorofluorescein (DCF) whose intensity correlates with the amount of ROS present within the cell. Therefore this sensitive assay can be used to evaluate the antioxidant or prooxidant activity of natural extracts in living biological system. The levels of ROS in SW872 pretreated with noni fruit extracts was observed to decline linearly in a dose–response manner, clearly demonstrating its strong free-radical scavenging activity. ROS levels decreased from 82.1% to 17.2% and 79.8% to 14.8% in ripe and unripe treated cells respectively, representing an overall decrease of 82.8% and 85.2% (p < 0.001). Unripe noni extracts seemed to exert a slightly stronger antioxidant potential in this cell line system.
Fig. 4.
A comparison of the free radical scavenging potential of noni extracts in SW872 cells challenged with 1 mM H2O2. ROS accumulation was expressed as a percentage compared to a control treated with H2O2 only. Results are presented as the mean of two independent experiments performed in triplicate, where error bars represent ± standard deviation, ***p < 0.001 vs. control.
4. Discussion
M. citrifolia L. or noni has several ethnopharmacological uses.27 Noni leaves and fruits are used as blood purifiers, antihelminthic agents, tonic supplements, against digestive disorders, hypertension tuberculosis, urinary tract dysfunctions, hypertension, diabetes, depression and as appetite stimulator. The leaves are utilized as poultice for broken bones, swelling joints, sprains, wounds, ulcers, boil and burns28; a number of these pathophysiologies being linked to a high state of oxidative stress. Moreover, products derived from noni fruits and leaves are commonly commercialized in the form of capsules, teas, and juice29 as adjunct therapy in nutritional programs. In view of its phytochemistry and functional properties, noni has been envisaged in the development of nutraceuticals and functional foods, and has emerged as a major product of the health and wellness industry.5
This study therefore aimed at evaluating the antioxidative properties and antiproliferative effects of M. citrifolia extracts on human liposarcoma SW872 cells as well as the phytophenolic richness of the latter. A comprehensive study of the phytochemical composition of the popular juice extracts in addition to their underlying health benefits mechanisms are deeply warranted in the light of its wide demand and utilization for the treatment of several ailments.
A study demonstrated that vitamin C is the most prominent vitamin in ripe noni fruit puree with a mean content of 1.13 mg/g30 and that 100 g of the fruit puree provides 251% of the daily recommended daily vitamin C requirement for adults.31 Moreover, the ascorbic acid content of M. citrifolia L. was investigated at different stages of maturity and noted that there was an increase in ascorbic acid as the fruits ripened. They reported an ascorbic acid content ranging from 4.58 ± 0.12 mg/100 g to 6.82 ± 0.01 mg/100 g from 9th week and 15th week respectively.31
Likewise, a study determined the ascorbic acid content of M. citrifolia L. and showed that the ripe fruits contained 155 mg/100 g.32 A previous study also reported the ascorbic acid content of immature (green), submature (white hard) and mature (ripe soft) fruits as 32.0 ± 7.2 mg/100 g, 224.3 ± 23.0 mg/100 g and 173.7 ± 41.0 mg/100 g, showing that the ripe fruits had less ascorbic acid than the white hard fruits.33 In the present investigation, noni ripe fruits were richer in vitamin C, (76.24 ± 1.13 mg/100 g) than the unripe sample (53.19 ± 0.79 mg/100 g) and the results were consistent with studies discussed above, except for the report of Yang et al. (2011).33
Phenolics in particular flavonoids were present in both the ripe and the unripe fruits but were more prominent in the ripe fruit. Similar findings were reported in a study carried out at different stages of maturity of the fruits and observed that the white hard noni (unripe) fruits had the highest total phenolics (284.8 ± 25.9 mg GAE/100 g) than the ripe soft fruits (225.3 ± 41.0 mg GAE/100 g).33 Our data showed that higher levels of phenolics were observed in the ripe (748.40 ± 8.85 μg GAE g−1 FW) and unripe fruits (770.34 ± 2.27 μg GAE g−1 FW). A similar trend was reported by Yang et al.33 in the unripe fruits. However, the different amount detected in the fruits under study can be ascribed to environmental factors as well as to the type and polarity of the extracting solvents, extraction time, temperature of extractions which influence recovery, yield and type of phenolics compounds present in extracts.34
A multi-method approach was used to assess the antioxidant potency of the extracts since no single method can predict the antioxidant efficiency of an extract.35 Thus, in this work 5 independent methods were used to provide a thorough mechanistic insight of the antioxidant actions of the extracts under study. According to a study, noni fruits exhibited good antioxidant activities, probably due to their flavonol contents and in particular kaempferol derivatives.4 In the present work, all the extracts showed considerable antioxidant potential with unripe fruit showing the highest reducing and iron chelating potential and the ripe fruit being a better scavenger of nitric oxide radical and superoxide radical. Moreover, the free radical scavenging activity of some medicinal plants revealed that ethanolic extracts of noni fruits had the highest nitric oxide radical scavenging activity (IC50 value: 0.596 mg/mL) while the water extracts of noni fruits had an IC50 value of 0.635 mg/mL.36
Superoxide anion radical is a weak oxidant playing an important role in the formation of ROS such as singlet oxygen, hydrogen peroxide and hydroxyl radical that are liable to induce oxidative damage in proteins, lipids and DNA in the body.37 Our data showed that the ripe fruit was the most powerful superoxide radical scavengers with the lowest IC 50 value. It was also reported that noni scavenged superoxide anion radical with an IC50 value of 1.37 ± 0.08 mg/mL which was consistent with the calculated IC50 value of the unripe fruit under study (1.4 mg/mL).4
Iron, a prooxidant transition metal is known to strongly trigger lipid peroxidation due to its high reactivity and the mechanism involves the formation of a ferrous state of iron which accelerates lipid oxidation by breaking down hydrogen and lipid peroxides to reactive free radicals via the Fenton reaction.35 A decrease in absorbance of the iron – ferrozine complex due to reduced complex formation at 562 nm indicated the chelating potential of the extracts under study. The extracts chelated Fe2+ in a dose-dependent manner but were not as potent as EDTA. The iron chelation of M. citrifolia extract has been documented in the literature. For instance, a study determined the ferrous iron chelating ability of M. citrifolia water extract and noted an IC50 value of 0.280 mg/mL which was more potent than the data obtained in this study.36
Hydroxyl radicals, produced in biological systems, have been of great concern due to their high reactivity as they lead to mutagenesis and inactivation of various proteins.38, 39 These radicals were generated via the Fenton reaction in vitro and the extent of deoxyribose degradation was measured.40, 41 Compared to the ripe fruit, the unripe fruit extract protected deoxyribose oxidation to a greater extent which can be partly explained by its hydroxyl radical scavenging ability.42 Similarly, the free radical scavenging activity of M. citrifolia extracts was investigated and noted that aqueous, ethanolic and ethyl acetate fractions can inhibit hydroxyl radicals in the Fenton reaction.42 Thus, varied activities noted in the different systems justify the use of a battery of assays to ascertain the antioxidant efficacies.
The rising incidence of cancer in both the developing and developed countries has prompted research towards dietary and medicinal plant extracts as prophylactics. Complementary and alternative medicine use is common among cancer patients and in many surveys, herbal medicines are among the most commonly used group of treatments. Thus, search for new chemopreventive, antioxidant agents and antitumor agents that are more effective but exhibiting less toxicity has rekindled interest in phytochemicals.14 In this study the effect of ripe and unripe noni fruit extracts on human liposarcoma SW872 cells was carried out. It was found that viability of adipocytes treated with extracts for 24 h decreased in a general dose–response manner. Unripe extracts of noni were noted to be slightly more cytotoxic than ripe noni. Thus, it can be concluded that extracts exceeding a dose of 0.81 g/mL seem to be convenient to reduce the proliferation rate of human liposarcoma SW872 cells.
In addition, M. citrifolia fruit extracts significantly restored cell growth of the adipocytes challenged with 1 mM of H2O2 by modulating H2O2-induced oxidative stress in human liposarcoma SW872 cells. Likewise, the levels of ROS in SW872 cells pretreated with noni fruit extracts were observed to decline linearly in a dose–response manner, clearly demonstrating a strong free-radical scavenging activity with unripe noni extracts. The latter was found to exert a slightly stronger antioxidant potential in this SW872 adipocyte cells. It was reported that M. citrifolia fruit extracts at a concentration of 1.5 mg/mL inhibited the proliferation of the breast cancer (MCF7) and neuroblastoma (LAN5) cells by 50%.43 Moreover, damnacanthal, from noni fruit was indicated to reduce the proliferation of human colorectal carcinomas via cell growth arrest and induction of apoptosis.44 Whilst the antiproliferative potential of noni extracts has been reported against a wide array of cancer cell lines, this study also demonstrated the protective effects of the extracts against oxidative stress induced cell death. The findings shed light on the extracts ability to restore cell growth at a concentration of ≥0.102 g/mL. However, in depth mechanistic studies are warranted to explain this dual functional role.
5. Conclusions
The data in this study collectively showed that M. citrifolia L. ripe and unripe fruits, besides having in vitro antioxidant capacities were characterized by strong antiproliferative effects against human liposarcoma SW872 cells, in addition to modulating oxidative stress in the latter. Both extracts were able to restore cell growth by reducing the intracellular ROS level. However, a concentration range of 0.034 g/mL–0.813 g/mL showed dual properties: reducing proliferation of the SW872 cells as well as restoring growth when the latter was challenged with oxidative stress. These properties of the ripe and unripe fruits may be ascribed to the host of polyphenolic antioxidants which needs to be comprehensively characterized in the locally available noni. Thus, M. citrifolia fruits can be encouraged for consumption as fresh produce or processed functional products for management of health in view of their therapeutic benefits in the management of health and disease.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We acknowledge the support of the University of Mauritius for funding. We thank the staff of the Department of Health Sciences laboratory and ANDI Centre of Excellence for Biomedical and Biomaterials Research, CBBR, Building, MSIRI, University of Mauritius for their help and support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
T. Bahorun, Email: tbahorun@uom.ac.mu.
V.S. Neergheen-Bhujun, Email: v.neergheen@uom.ac.mu.
References
- 1.Hybertson B.M., Bifeng Gao B., Bose S.K., Mccord J.M. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Manolagas S.C. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervellati C., Bonaccorsi G., Cremonini E. Bone mass density selectively correlates with serum markers of oxidative damage in post menopausal women. Clin Chem Lab Med. 2013;51:333–338. doi: 10.1515/cclm-2012-0095. [DOI] [PubMed] [Google Scholar]
- 4.Gironés-Vilaplana A., Baenas N., Villaño D. Evaluation of the latin-Americain fruits rich in phytochemicals with biological effects. J Funct Foods. 2014;7:599–608. [Google Scholar]
- 5.Joshi P.M., Chilkawar, Jadhav B.A. Studies on physicochemical properties of noni fruit (Morinda citrifolia) and preparation of Noni beverages. Int J Food Sci Nutr Diet. 2012;1:3–8. [Google Scholar]
- 6.Deng S., West B.J., Jensen C.J. Simultaneous characterization and quantification of flavonol glycosides and aglycones in noni leaves using a validated HPLC-UV/MS method. Food Chem. 2008;111:526–529. doi: 10.1016/j.foodchem.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Wang M.Y., Su C. Cancer preventive effect of Morinda citrifolia (Noni) Ann N Y Acad Sci. 2001;952:161–168. doi: 10.1111/j.1749-6632.2001.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 8.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–938. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 9.Soobrattee M.A., Neergheen V.S., Luximon-Ramma A. Phenolics as potential antioxidant therapeutic agents: mechanisms and actions. Mutat Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signaling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Jonville M.C., Kodja H., Strasberg D. Antiplasmodial, anti-inflammatory and cytotoxic activites of various plant extracts from the Mascarene Archipelago. J Ethnopharmacol. 2011;136:525–531. doi: 10.1016/j.jep.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Chan-Blanco Y., Vaillant F., Mercedes P.A. The Noni fruit (Morinda citrifolia L.): a Review of agricultural research, nutrional and therapeutic properties. J Food Comp Anal. 2006;19:645–654. [Google Scholar]
- 13.Dussossoy E., Brat P., Bony E. Characterization, antioxidative and anti-inflammatory effects of Costa Rican Noni juice (Morinda citrifolia L.) J Ethnopharmacol. 2011;133:108–115. doi: 10.1016/j.jep.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R.K., Patel A.K. Do the health claims made for Morinda citrifolia (Noni) harmonize with the current scientific knowledge and evaluation of its biological effects. Asian Pac J Cancer Prev. 2013;14:4495–4499. doi: 10.7314/apjcp.2013.14.8.4495. [DOI] [PubMed] [Google Scholar]
- 15.Nayak S.B., Marshall J.R., Isitor G. Hypoglycemic and hepatoprotective activity of fermented fruit juice of Morinda citrifolia (Noni) in diabetic rats. Evid Based Complement Alternat Med. 2011 doi: 10.1155/2011/875293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palu A., Kim A., West B.J. The effects of Morinda citrifolia L. (noni) on the immune sustem: Its molecular mechanisms of action. J Ethnopharmacol. 2008;115:502–506. doi: 10.1016/j.jep.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Wang M.Y., West B.J., Jensen C.J. Morinda citrifolia: a literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002:1127–1141. [PubMed] [Google Scholar]
- 18.Mootoosamy A., Mahomoodally F. Ethno medicinal application of native remedies used against diabetes and related complications in Mauritius. J Ethnopharmacol. 2014;151:413–444. doi: 10.1016/j.jep.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 19.Neergheen V.S., Soobrattee M.A., Bahorun T. Characteristics of the phenolic constituents in Mauritian endemic plants as determinants of their antioxidant activities in vitro. J Plant Physiol. 2006;163:787–799. doi: 10.1016/j.jplph.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Zishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effect on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 21.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. The frap assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Neergheen-Bhujun V.S., Seenauth-Beesso V., Joonas N. Alterations in antioxidant status of patients suffering from diabetes mellitus and cardiovascular complications. Arch Med Biomed Res. 2014;1:35–46. [Google Scholar]
- 23.Halliwel B., Guttridge J.M.C., Aruoma O.I. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 24.Aruoma O.I. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol. 1994;32:671–683. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 25.Mandal M., Misra T.K., Ghosal M. Free-radical scavenging activity and phytochemical analysis in the leaf and stem of Drymaria diandra Blume. Int J Biol. 2009;7:80–84. [Google Scholar]
- 26.Rummun N., Somanah J., Ramsaha S. Bioactivity of nonedible parts of Punica granatum L.: a potential source of functional ingredients. Int J Food Sci. 2013 doi: 10.1155/2013/602312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramesh S., Muthubalaji R., Rajeswari A. Physicohemical, phytochemical and antimicrobial studies on Morinda citrifolia L. fruits at different maturity stages. Int J Pharm Pharm Sci. 2012;4:473–476. [Google Scholar]
- 28.Pawlus A.D., Kinghorn A.D. Review of the ethnobotany, chemistry, biological activity and safety of the botanical dietary supplement Morinda citrifolia(noni) J Pharm Pharmacol. 2007;59:1587–1609. doi: 10.1211/jpp.59.12.0001. [DOI] [PubMed] [Google Scholar]
- 29.Singh D.R. Morinda citrifolia L. (Noni): a review of the scientific validation for its nutritional and therapeutic properties. J Diabetes Endocrinol. 2012;3:77–91. [Google Scholar]
- 30.West B.J., Deng S., Jensen C.J. Nutrient and phytochemical analyses of processed noni puree. Food Res Int. 2011;44:2295–2301. [Google Scholar]
- 31.Rosalizan M.S., Rohani M.Y., Khatijah I. Physicochemical characteristics of Morinda citrifolia fruit during growth and maturation. J Agric Food Sci. 2010;38:21–30. [Google Scholar]
- 32.Shovic A.C., Whistler W.A. Food sources of provitamin A and vitamin C in the American Pacific. Tropic Sci. 2001;41:199–202. [Google Scholar]
- 33.Yang J., Gadi R., Thomson T. Antioxidant capacity, total phenols, and ascorbic acid content of noni (Morinda citrifolia) fruits and leaves at various stages of maturity. Micronesica. 2011;41:pp.167–176. [Google Scholar]
- 34.Naczk M., Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Ramful D., Bahorun T., Bourdon E. Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: potential prophylactic ingredients for functional foods applications. Toxicology. 2010;278:75–87. doi: 10.1016/j.tox.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Gacche R.N., Dhole N.A. Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: an attempt to standardize the botanicals for amelioration of diabetes complications. Food Chem Toxicol. 2011;49:pp.1806–1813. doi: 10.1016/j.fct.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Gulcin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006;78 doi: 10.1016/j.lfs.2005.05.103. 830–811. [DOI] [PubMed] [Google Scholar]
- 38.Serafini M.R., Santos R.C., Guimaraes A.G. Morinda citrifolia Linn leaf extract possesses antioxidant activities and reduces noniceptive behavior and leukocyte migration. J Med Food. 2011;14:1159–1166. doi: 10.1089/jmf.2010.0254. [DOI] [PubMed] [Google Scholar]
- 39.Villano D., Pachon F.M.S., Troncoso A.M. Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro. Anal Chim Acta. 2005;38:391–398. [Google Scholar]
- 40.Jayasri M.A., Mathew L., Radha A. A report on the antioxidant activity of leaves and rhizomes of Costuspictus D. Don. Int J Integr Biol. 2008;5:20–26. [Google Scholar]
- 41.AL-Fartosy A.J.M. Antioxidant properties of methanolic extract from Inulagraveolens L. Turk J Agric For. 2011;35:591–596. [Google Scholar]
- 42.Kumar S.N.K., Suresh M., Kumar S.A. Bioactive compound, radical scavenging, antioxidant properties and FTIR spectroscopy study of Morinda citrifolia fruit extracts. Int J Curr Microbiol App Sci. 2014;3:28–42. [Google Scholar]
- 43.Apornsuwan T., Punjanon T. Tumor cell-selective antiproliferative effect of the extracts from Morinda citrifolia fruits. Phytother Res. 2006;20:515–517. doi: 10.1002/ptr.1902. [DOI] [PubMed] [Google Scholar]
- 44.Nualsanit T., Rojanapanthu P., Gritsanapan W. Damnacanthal, a Noni component, exhibits anti-tumorigenic activity in human colorectal cancer cells. J Nutr Biochem. 2012;23:915–923. doi: 10.1016/j.jnutbio.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]