Abstract
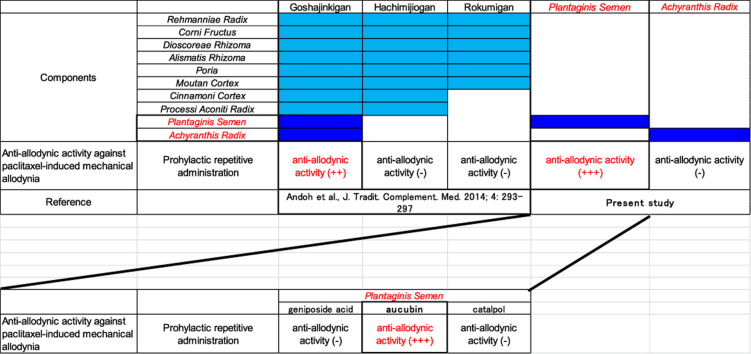
The chemotherapeutic agent paclitaxel (PTX) causes peripheral neuropathy as a major dose-limiting side effect, and this peripheral neuropathy is difficult to control. Our previous report showed that prophylactic repetitive administration of goshajinkigan (牛車腎氣丸 niú chē shèn qì wán), but not hachimijiogan (八味地黃丸 bā wèi dì huáng wán), which lacks two of the constituents of goshajinkigan, inhibited PTX-induced mechanical allodynia in mice. Thus, the herbal medicines Plantaginis Semen (車前子 chē qián zǐ) or Achyranthis Radix (牛膝 niú xī) may contribute to the inhibitory action of goshajinkigan on the exacerbation of PTX-induced mechanical allodynia [Andoh et al, J. Tradit. Complement. Med. 2014; 4: 293–297]. Therefore, in this study, we examined whether an extract of Plantaginis Semen (EPS) or Achyranthis Radix (EAR) would relieve PTX-induced mechanical allodynia in mice. A single intraperitoneal injection of PTX caused mechanical allodynia, which peaked on day 14 after injection. Repetitive oral administration of EPS, but not EAR, starting from the day after PTX injection significantly inhibited the exacerbation of PTX-induced mechanical allodynia. Repetitive intraperitoneal injection of aucubin, one of the main components of EPS, starting from the day after PTX injection also significantly reduced PTX-induced mechanical allodynia. However, repetitive intraperitoneal injection of geniposide acid (a precursor of aucubin) or catalpol (a metabolite of aucubin) did not prevent the exacerbation of mechanical allodynia. These results suggest that prophylactic administration of EPS is effective for preventing the exacerbation of PTX-induced allodynia. Aucubin may contribute to the inhibitory action of EPS on the exacerbation of PTX-induced allodynia.
Keywords: Paclitaxel, Aucubin, Plantaginis Semen, Allodynia
Graphical abstract
1. Introduction
Paclitaxel (PTX) is an anti-microtubule agent that is widely indicated to treat solid neoplasms such as ovarian, breast, and lung cancer.1, 2 Approximately 20% of PTX-treated patients develop sensory neuropathy characterized by mechanical allodynia, cold allodynia, spontaneous pain, tingling, and numbness, with a stocking and glove distribution.3, 4 Since several drugs, such as gabapentin and amifostine, that have been used by PTX-treated patients to relive neuropathy have failed,5, 6, 7 new therapeutic drugs are needed.
Goshajinkigan (牛車腎氣丸 niú chē shèn qì wán) is a traditional herbal formulation that consists of 10 herbal medicines [Rehmanniae Radix (地黃 dì huáng), Achyranthis Radix (牛膝 niú xī), Corni Fructus (山茱萸 shān zhū yú), Dioscoreae Rhizoma (山藥 shān yào), Plantaginis Semen (車前子chē qián zǐ), Alismatis Rhizoma (澤瀉 zé xiè), Poria (茯苓 fú ling), Moutan Cortex (牡丹皮 mǔ dān pí), Cinnamoni Cortex (桂皮 guì pí), and Processi Aconiti Radix (附子 fù zǐ)]. Goshajinkigan has been shown to attenuate the progression of peripheral neuropathy induced by docetaxel and PTX/carboplatin treatment in cancer patients.8, 9 In mice, repetitive administration of goshajinkigan also inhibits the exacerbation of PTX-induced mechanical allodynia.10 Interestingly, hachimijiogan (八味地黃丸 bā wèi dì huáng wán), which consists of the same herbal medicines in goshajinkigan, except Plantaginis Semen and Achyranthis Radix, does not affect the exacerbation of PTX-induced mechanical allodynia.10 This finding suggests that one of the herbal components (Plantaginis Semen or Achyranthis Radix) may contribute to the inhibitory action of goshajinkigan on the exacerbation of PTX-induced mechanical allodynia. Therefore, in the present study, we examined whether extracts of Plantaginis Semen (EPS) or Achyranthis Radix (EAR) could attenuate the exacerbation of PTX-induced mechanical allodynia.
2. Materials and methods
2.1. Animals
Male C57BL/6NCr mice were purchased from Japan SLC Ltd. (Hamamatsu, Japan) and were 6 weeks old at the start of experiments. The mice were housed under controlled temperature (21–23 °C), humidity (45–46%), and light (light from 7:00 AM to 7:00 PM). Food and water were feely available. This study was conducted with the approval of the Committee for Animal Experiments at the University of Toyama and in accordance with the guidelines for investigations of experimental pain in animals published by the International Association for the Study of Pain.
2.2. Drugs
PTX (Sigma, St. Louis, MO, USA) was dissolved in vehicle (physiological saline containing 10% Cremophore EL® [Sigma] and 10% ethanol) and administered intraperitoneally at a dose of 5 mg/kg in a volume of 0.1 mL/10 g of body weight. The dose calculations for PTX were based on the recommended clinical doses.11 Dried water extracts of Plantaginis Semen (EPS: Lot. No. 2111049010) and Achyranthis Radix (EAR: Lot. No. 2101066010) were obtained from Tsumura and Co. Ltd. (Tokyo, Japan) and were dissolved in 5% gum arabic. Aucubin, geniposide acid, and catalpol were purchased from Wako Pure Chemical Industries (Osaka, Japan) and were dissolved in saline. The extracts and agents were administered orally and intraperitoneally, respectively, in a volume of 0.1 mL/10 g of body weight once daily from the day after PTX injection.
2.3. Behavioral experiments
Mechanical allodynia was evaluated using a fine von Frey filament with a bending force of 0.69 mN (North Coast Medical Inc., Morgan Hill, CA, USA).11 The mice were placed individually in an acrylic cage (11 cm × 18 cm × 15 cm) with a wire mesh bottom. After an acclimation period of at least 30 min, the von Frey filament was pressed perpendicularly against the central part of the plantar hind paw of freely moving mice and was held there for 1–3 s by slight buckling. Responses to the stimulus were scored as follows: 0, no reaction; 1, lifting of the hind paw; and 2, licking and flinching of the hind paw. A stimulation of same intensity was applied three times to each hind paw at intervals of several seconds, and the average value of six trials was used as the response score (the maximum score was 2).
2.4. Statistical analysis
All data are presented as the mean and standard error of the mean. Statistical significance was analyzed using Mann–Whitney rank sum test or Kruskal–Wallis one way analysis of variance on ranks followed by Dunn's multiple comparisons (comparisons with a control), and a p value less than 0.05 was considered statistically significant.
3. Results
3.1. Effects of prophylactic administration of EPS and EAR on PTX-induced mechanical allodynia
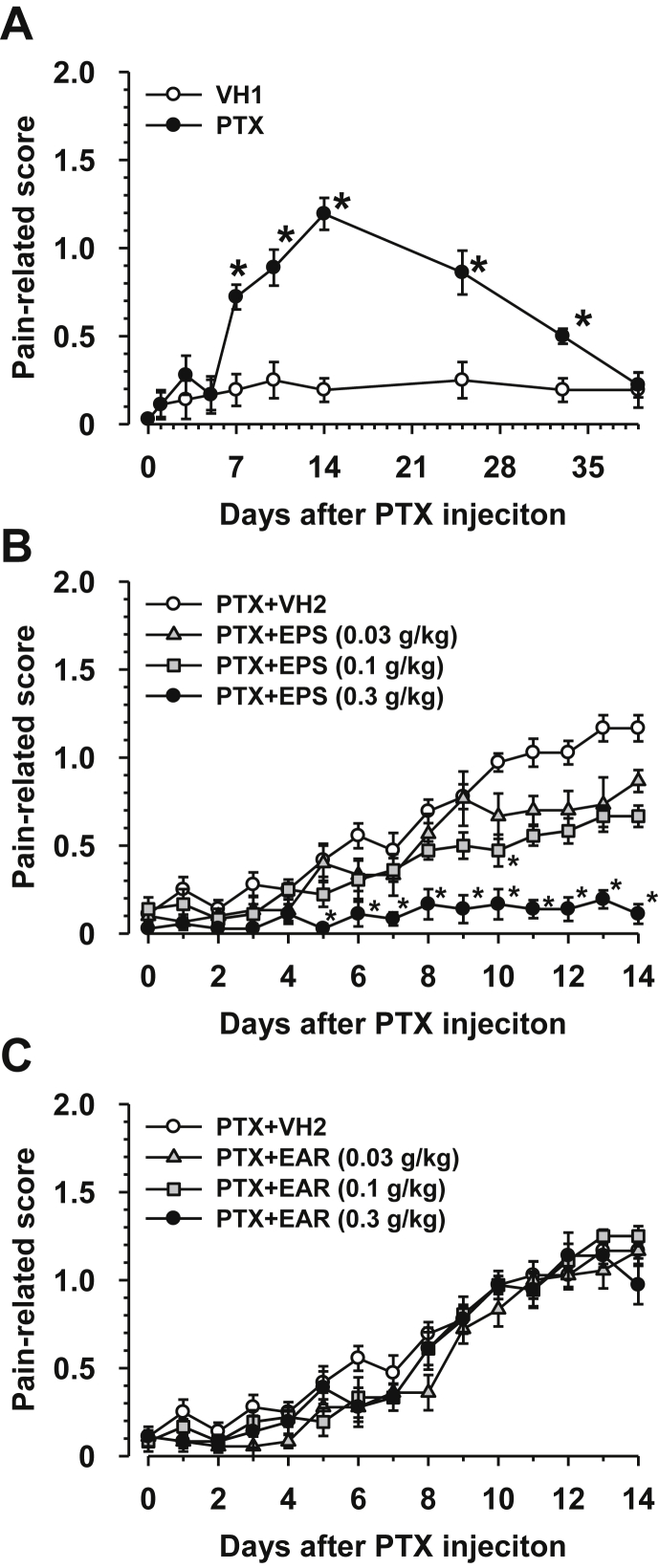
A single intraperitoneal injection of PTX (5 mg/kg) induced mechanical allodynia, which peaked on day 14 and almost subsided by day 39 (Fig. 1A). When administered once daily from the day after PTX injection, EPS (0.3 g/kg, oral) significantly inhibited the exacerbation of allodynia from day 5 (Fig. 1B). A lower dose (0.1 g/kg, oral) of PTX had a tendency to inhibit allodynia from day 9, with a significant inhibition observed on day 10 (Fig, 1B). The lowest dose tested (0.03 g/kg, oral) had a tendency to inhibit allodynia from day 10 (Fig. 1B). On the other hand, daily oral administration of EAR (0.03–0.3 g/kg) did not affect the mechanical allodynia induced by PTX (Fig. 1C). In addition, repetitive administration of EPS and EAR did not cause diarrhea and sedation.
Fig. 1.
Effects of prophylactic administration of extracts of Plantaginis Semen (EPS) and Achyranthis Radix (EAR) on paclitaxel (PTX)-induced mechanical allodynia. (A) Development of mechanical allodynia after a single injection of PTX. (B) Effects of EPS on PTX-induced allodynia. (C) Effects of EAR on PTX-induced allodynia. PTX (5 mg/kg) and its vehicle (VH1) were injected intraperitoneally in mice. EPS, EAR, and their vehicle (VH2) were administered orally once daily from the day after PTX injection. Data are presented as the mean and standard error of the mean (n = 5–6). *p < 0.05 compared to VH1 (Mann–Whitney test) or VH2 (Dunn's multiple comparisons with the control).
3.2. Effects of prophylactic administration of aucubin, geniposide acid, and catalpol on PTX-induced mechanical allodynia
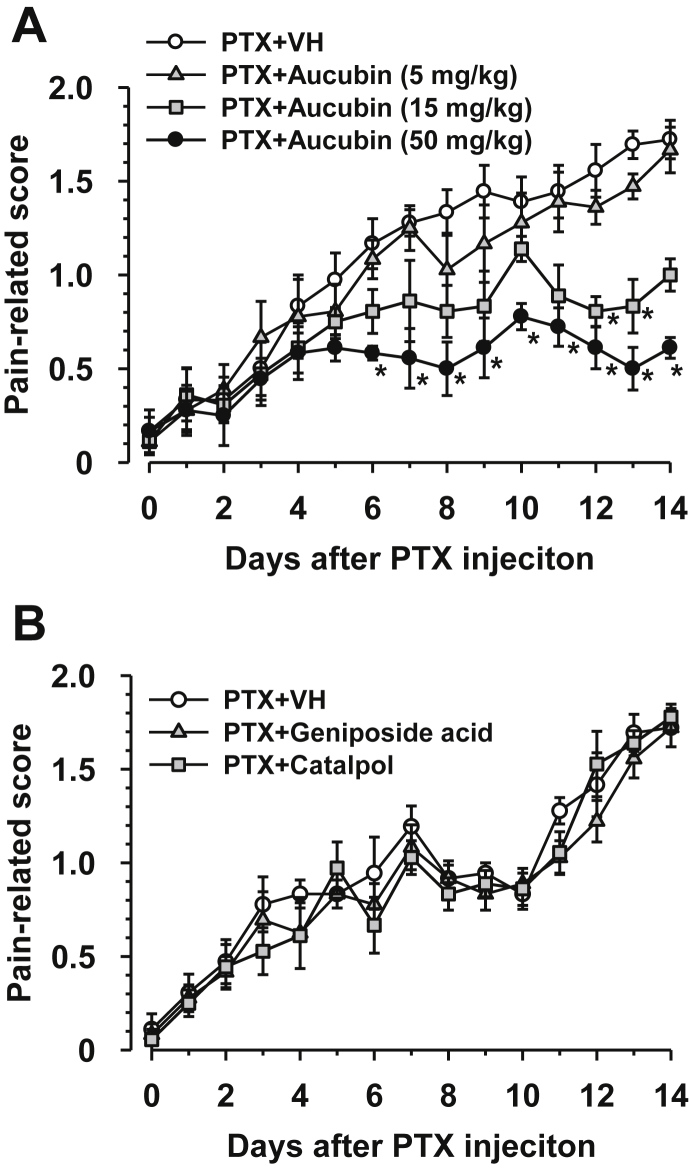
Aucubin is one of the main components of Plantaginis Semen. When administered once daily from the day after PTX injection aucubin (50 mg/kg, intraperitoneal) significantly inhibited the exacerbation of allodynia from day 6 (Fig. 2A). A lower dose (15 mg/kg, intraperitoneal) had a tendency to inhibit allodynia from day 6, with a significant inhibition observed on days 12 and 13 (Fig, 2A). No significant inhibition was observed at the lowest dose tested (5 mg/kg, intraperitoneal) during the experimental period (Fig. 2A). On the other hand, daily intraperitoneal administration of geniposide acid (50 mg/kg, a precursor of aucubin) and catalpol (50 mg/kg, a metabolite of aucubin) did not affect the mechanical allodynia induced by PTX (Fig. 2B). In addition, repetitive administration of aucubin, geniposide acid, and catalpol did not cause diarrhea and sedation.
Fig. 2.
Effects of prophylactic administration of aucubin, geniposide acid, and catalpol on paclitaxel (PTX)-induced mechanical allodynia. PTX (5 mg/kg) was injected intraperitoneally in mice. Aucubin (A: 5, 15, and 50 mg/kg), geniposide acid (B: 50 mg/kg), catalpol (B: 50 mg/kg), and vehicle (VH) were administered intraperitoneally once daily from the day after PTX injection. Data are presented as the mean and standard error of the mean (n = 6). *p < 0.05 compared to VH (Dunn's multiple comparisons with the control).
4. Discussion
Prophylactic repetitive administration of EPS, but not EAR, inhibited the exacerbation of PTX-induced mechanical allodynia, suggesting that EPS is an effective herbal medicine for inhibiting PTX-induced mechanical allodynia. We investigated the antiallodynic effect of the components of EPS. In this study, we examined the effects of a water extract of Plantaginis Semen, and iridoid glycosides, such as aucubin and catalpol, are more efficiently extracted from the plant matrix by the water-based method than by the methanol-based method.12 Aucubin is one of the main components of Plantaginis Semen.13 Thus, the antiallodynic effect of aucubin, its precursor geniposide acid, and its metabolite catalpol were also tested. Prophylactic repetitive administration of aucubin attenuated the exacerbation of PTX-induced mechanical allodynia. However, geniposide acid and catalpol did not attenuate PTX-induced mechanical allodynia. Taken together, these results suggest that aucubin plays an important role in the inhibition of exacerbation of PTX-induced mechanical allodynia.
The mechanisms underlying aucubin- and EPS-mediated inhibition of PTX-induced mechanical allodynia are still unclear. A single oral dose of goshajinkigan has been shown to slightly inhibit established mechanical allodynia after PTX injection.10 In our preliminary experiments, a single intraperitoneal injection of aucubin did not affect the established mechanical allodynia induced by PTX (Andoh, Kato, and Kuraishi, unpublished data). These results suggest that aucubin is the same as goshajinkigan in that it does not have an acute inhibitory effect on PTX-induced mechanical allodynia. In this study, prophylactic repetitive administration of aucubin and EPS prevented the induction of PTX-induced mechanical allodynia. Analysis of the detailed underlying mechanism requires further study. Prophylactic administration of the reactive oxygen species scavenger N-tert-butyl-a-phenylnitrone attenuates PTX-induced mechanical allodynia.14 PTX shows neurotoxicity due to mitochondrial dysfunction leading to oxidative stress in dorsal root ganglion neurons.15 Since aucubin16, 17 and EPS18, 19 have antioxidant activity, this antioxidant activity may be involved in the inhibition of PTX-induced mechanical allodynia. PTX decreases peripheral blood flow, which is related to the exacerbation of mechanical allodynia.11 However, aucubin does not affect the decreased blood flow.20 Therefore, control of peripheral blood flow may not be involved in the inhibitory action of aucubin on the exacerbation of mechanical allodynia.
5. Conclusion
Prophylactic oral administration of EPS prevented the exacerbation of PTX-induced mechanical allodynia, suggesting that Plantaginis Semen is an important herbal component of the clinically used medicine goshajinkigan. In addition, aucubin, one of the major components of Plantaginis Semen, may become a new agent for the prevention of PTX-induced peripheral neuropathy. We need to investigate the mechanisms underlying the preventative effect of EPS on the exacerbation of PTX-induced mechanical allodynia to identify the active components.
Conflict of interest
None of the authors has any conflict of interest to declare.
Acknowledgments
This study was supported by the Adaptable and Seamless Technology Transfer Program through Target-driven R&D, of the Japan Science and Technology Agency (FS-Stage: AS262Z01828Q; to T.A.), the Takeda Science Foundation (2009) (to T.A.), the Foundation of the First Bank of Toyama (H27) (to D.U.), and Japan Society for the promotion of Science KAKENHI (Grant Number: 15H05268; to K.K.).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Chang A.Y., Garrow G.C. Pilot study of vinorelbine (Navelbine) and paclitaxel (Taxol) in patients with refractory breast cancer and lung cancer. Sem Oncol. 1995;22:66–71. [PubMed] [Google Scholar]
- 2.Wani M.C., Taylor H.L., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty P.M., Cata J.P., Cordella J.V., Burton A., Weng H.R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Windebank A.J., Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 5.Carlson K., Ocean A.J. Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin Breast Cancer. 2011;11:73–81. doi: 10.1016/j.clbc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Rao R.D., Michalak J.C., Sloan J.A., North Central Cancer Treatment Group Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110:2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 7.Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Abe H., Mori T., Kawai Y. The Kampo medicine Goshajinkigan prevents docetaxel-related peripheral neuropathy in breast cancer patients. Cancer Res. 2012;72:24. doi: 10.7314/apjcp.2013.14.11.6351. [DOI] [PubMed] [Google Scholar]
- 9.Kaku H., Kumagai S., Onoue H. Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med. 2012;3:60–65. doi: 10.3892/etm.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andoh T., Kitamura R., Fushimi H., Komatsu K., Shibahara N., Kuraishi Y. Effects of goshajinkigan, hachimijiogan, and rokumigan on mechanical allodynia induced by Paclitaxel in mice. J Tradit Complement Med. 2014;4:293–297. doi: 10.4103/2225-4110.128906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauchan P., Andoh T., Kato A., Sasaki A., Kuraishi Y. Effects of the prostaglandin E1 analog limaprost on mechanical allodynia caused by chemotherapeutic agents in mice. J Pharmacol Sci. 2009;109:469–472. doi: 10.1254/jphs.08325sc. [DOI] [PubMed] [Google Scholar]
- 12.Suomi J., Sirén H., Hartonen K., Riekkola M.L. Extraction of iridoid glycosides and their determination by micellar electrokinetic capillary chromatography. J Chromatogr A. 2000;868:73–83. doi: 10.1016/s0021-9673(99)01170-x. [DOI] [PubMed] [Google Scholar]
- 13.Jurisić R., Debeljak Z., Vladimir-Knezević S., Vuković J. Determination of aucubin and catalpol in Plantago species by micellar electrokinetic chromatography. Z Naturforsch C. 2004;59:27–31. doi: 10.1515/znc-2004-1-206. [DOI] [PubMed] [Google Scholar]
- 14.Fidanboylu M., Griffiths L.A., Flatters S.J. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS One. 2011;6:e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melli G., Taiana M., Camozzi F. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008;214:276–284. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Xue H.Y., Jin L., Jin L.J. Aucubin prevents loss of hippocampal neurons and regulates antioxidative activity in diabetic encephalopathy rats. Phytother Res. 2009;23:980–986. doi: 10.1002/ptr.2734. [DOI] [PubMed] [Google Scholar]
- 17.Xue H.Y., Gao G.Z., Lin Q.Y., Jin L.J., Xu Y.P. Protective effects of aucubin on H2O2-induced apoptosis in PC12 cells. Phytother Res. 2012;26:369–374. doi: 10.1002/ptr.3562. [DOI] [PubMed] [Google Scholar]
- 18.Li H., Wang Q. Evaluation of free hydroxyl radical scavenging activities of some Chinese herbs by capillary zone electrophoresis with amperometric detection. Anal Bioanal Chem. 2004;378:1801–1805. doi: 10.1007/s00216-004-2509-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y.Z., Huang S.H., Tan B.K., Sun J., Whiteman M., Zhu Y.C. Antioxidants in Chinese herbal medicines: a biochemical perspective. Nat Prod Rep. 2004;21:478–489. doi: 10.1039/b304821g. [DOI] [PubMed] [Google Scholar]
- 20.Oku H., Ogawa Y., Iwaoka E., Ishiguro K. Allergy-preventive effects of chlorogenic acid and iridoid derivatives from flower buds of Lonicera japonica. Biol Pharm Bull. 2011;34:1330–1333. doi: 10.1248/bpb.34.1330. [DOI] [PubMed] [Google Scholar]