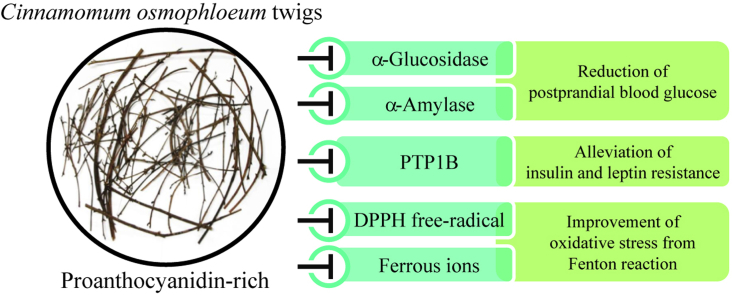
Abstract
This is the first report concerning the α-glucosidase, α-amylase and protein tyrosine phosphatase 1B (PTP1B) inhibitory activities of cinnamon twig extracts. Comparing the antihyperglycemic activity of renewable plant parts, indigenous cinnamon (Cinnamomum osmophloeum; 土肉桂 tǔ ròu guì) twig extracts (CoTE) showed better α-glucosidase and α-amylase activities than leaf, 2-cm branch and 5-cm branch extracts. Chemotype of C. osmophloeum has no influence on the antihyperglycemic activities and proanthocyanidin contents of CoTE. Among four soluble fractions obtained from CoTE by following bioactivity-guided fractionation procedure, the n-butanol soluble fraction (BSF) with abundant proanthocyanidins and condensed tannins, exhibited the best antihyperglycemic and PTP1B inhibitory activities. In addition, the BSF displayed the excellent DPPH free-radical scavenging and ferrous ion-chelating activities. The antihyperglycemic and antioxidant activities of all four soluble fractions from CoTE showed high correlation coefficient with their proanthocyanidin and condensed tannin contents. Furthermore, CoTE had no toxicity on 3T3-L1 preadiocytes. Results obtained demonstrated that CoTE has excellent antihyperglycemic, antioxidant and PTP1B inhibitory activities, and thus has great potential as a source for natural health products.
Keywords: α-Amylase, Cinnamomum osmophloeum, Ferrous ion-chelating, α-Glucosidase, Protein tyrosine phosphatase 1B
Graphical abstract
1. Introduction
The incidence and prevalence of type 2 diabetes have acutely increased since 1990. A dramatic 64% growth between 2010 and 2025 is predicted, influencing 53.1 million people and posing an extremely huge medical and societal cost. The incidence of type 2 diabetes is highly related to age, inheritance, diet, lifestyle and environmental pressure.1 Obesity showed high correlation with risk of type 2 diabetes, and aggravated insulin resistance of type 2 diabetes. Excessive nutrients lead to energy overload, thus affecting the metabolic function of adipocytes. The dysfunction of adipocytes would cause generation of reactive oxygen species (ROS), change secretion of adipokines, increase release of fatty acids and inflammatory factors. Overload of free fatty acids would result in lipotoxicity and dyslipidemia, which in turn affect uptake of glucose and insulin sensitivity.2, 3
Besides injection of insulin, there are three kinds of hypoglycemic drugs, namely insulin secretagogues, insulin sensitizers and α-glucosidase inhibitors for maintaining glucose homeostasis in type 2 diabetes patients.4 These commercial hypoglycemic drugs also have many side effects.5 The antihyperglycemic assays, such as α-glucosidase inhibition, α-amylase inhibition, protein tyrosine phosphatase 1B (PTP1B) inhibition and glucose uptake in cells are commonly used to estimate the beneficial effects on the treatment of type 2 diabetes. Over 400 plant extracts have been estimated for the treatment of diabetes throughout the world. Several phytochemicals in plant extracts were evaluated by antihyperglycemic assays and might have multiple benefits on type 2 diabetes to avoid side effects.6 Aqueous extract from Cinnamomum burmannii bark is rich in proanthocyanidins.7 It was also reported that extracts of C. burmannii contained proanthocyanidins with A-type linkage and had insulin-like biological activity.8 Furthermore, four water extracts of Cinnamomun bark were reported to have significant inhibitory activities of glucose metabolic enzymes, and might be potential substitution of commercial α-glucosidase inhibitory drugs.9
Studies have showed that the coumarin contents in commercial cinnamons, such as Cinnamomum cassia and C. burmannii, are high. The average coumarin content in C. cassia barks and twigs was 5790 mg/kg of sample, and coumarin contents in C. burmannii bark were between 2140 and 9300 mg/kg of sample.10, 11 Coumarin was reported that have hepatotoxic and carcinogenic effects in animals.12, 13 The results of no observed-adverse-effect level for liver toxicity in the most sensitive animal species led the European Food Safety Authority to establish a tolerable daily intake of 0.1 mg of coumarin/kg body weight.
Cinnamomum osmophloeum (Lauraceae) (土肉桂 tǔ ròu guì) is an endemic species in Taiwan and its leaves have high cinnamaldehyde and low coumarin contents.14 Meanwhile, coumarin was not detected in the essential oil of C. osmophloeum twig.15 However, studies on the antihyperglycemic activities of C. osmophloeum twig are lacking, hence its inhibitory activities of glucose metabolic enzymes and active components merit investigation.
With the consideration for sustainability and non-destructive utilization, twigs and branches of C. osmophloeum were used in this study instead of the bark. The antihyperglycemic activities were evaluated by α-glucosidase and α-amylase inhibitory assay. Inhibition of these two glucose metabolic enzymes could decrease the absorption rate of glucose to prevent acute rise of postprandial blood glucose of type 2 diabetes.16 PTP1B inhibitory activities of cinnamon twigs have not been investigated yet. PTP1B has been known to play an important role in inhibiting signaling pathways of insulin and leptin receptors. PTP1B-knockout animals need lower insulin to activate glucose uptake of cells and decreased weight.17 Therefore, benefit effect on insulin and leptin sensitivities were evaluated by PTP1B inhibitory assay. DPPH free-radical scavenging and ferrous ion-chelating activity were utilized to estimate the antioxidant activities. The active components were presumed by the correlation analysis between different phenolic contents and bioactivities. Finally, the viability of 3T3-L1 preadipocytes was examined for the toxicity of extracts. With the above-mentioned assays, the antihyperglycemic potency of C. osmophloeum twigs for nature health products could be elucidated.
2. Materials and methods
2.1. Chemicals
Analytical grade solvents for extraction and chromatography were purchased from Echo Chemical Co. (Taiwan). α-Glucosidase from Bacillus stearothermophilus, α-amylase from porcine pancreas, 3,5-dinitrosalicylic acid (DNS), 4-nitrophenyl phosphate disodium salt hexahydrate (pNPP), 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt (Ferrozine), trizma hydrochloride (Tris–HCl), DL-dithiothreitol (DTT), (+)-catechin hydrate, acarbose and rutin hydrate were purchased from Sigma Chemical Co. (USA). p-Nitrophenyl-α-d-glucopyranoside (pNPG), KNaC4H4O6·4H2O, quercetin dihydrate, ethylenediaminetetraacetic acid disodium salt (EDTA-Na2), FeCl2·4H2O, NH4Fe(SO4)2·12H2O and thiazolyl blue tetrazolium bromide (MTT) were purchased from Acros (Belgium). Ursolic acid, citric acid, vanillin and AlCl3·6H2O were purchased from Merck (Germany). PTP1B from human was purchased from Enzo Life Sciences (Switzerland). Dulbecco's modified Eagle's medium (DMEM) and newborn calf serum (NCS) were purchased from Gibco BRL (USA). Melacacidin was separated and purified from Acacia confusa root according to Lin and Chang.18 All other unlabelled chemicals and reagents were purchased from Sigma Chemical Co. (USA).
2.2. Sampling of plant materials
The leaf, twig (diameter < 0.5 cm), 2-cm and 5-cm branch of cinnamaldehyde type and the other two chemotypes of twig, including mixed and linalool types of C. osmophloeum (土肉桂 tǔ ròu guì) were collected in the July of 2012 from the Hsin-Sheng Nursery (24.841532°N, 121.533524°E) in New Taipei city, and the trees were about 30 years. The species was identified by Mr. Yen-Ray Hsui (Taiwan Forestry Research Institute) and the materials were deposited at the laboratory of Wood chemistry (School of Forestry and Resource Conservation, National Taiwan University).
2.3. Extraction and isolation
Those dried samples were grounded into powder and soaked in 70% acetone at ambient temperature for seven days. The antihyperglycemic twig crude extracts were then extracted successively with n-hexane, ethyl acetate, n-butanol, and water to yield the n-hexane soluble fraction (HSF, 4.0%), ethyl acetate soluble fraction (EASF, 4.7%), n-butanol soluble fraction (BSF, 55.4%), and water soluble fraction (WSF, 35.9%). Each fraction was tested by the various assays including α-glucosidase inhibition, α-amylase inhibition, PTP1B inhibition, DPPH free-radical scavenging activity, and ferrous ion-chelating activity to determine the best active fraction.
2.4. Inhibitory assay for α-glucosidase
The inhibitory activity of α-glucosidase was estimated according to Lin and Lee.19 Briefly, 20 μL of ddH2O, 10 μL of extracts/50% methanol with different concentrations (1–100 μg/mL) and 60 μL of 0.25 unit/mL α-glucosidase/0.1 M phosphate buffer (pH 7.0) were mixed together. The mixture was incubated at 37 °C for 10 min, and then the reaction was initiated by the addition of 10 μL of 20 mM pNPG/0.1 M phosphate buffer for 10 min incubation and the absorbance was measured at 405 nm. Acarbose was used as a positive control.
2.5. Inhibitory assay for α-amylase
The inhibitory activity of α-amylase was estimated according to Apostolidis et al20 with slight modifications. Briefly, 50 μL of extracts/50% methanol with different concentrations (25–1000 μg/mL) were mixed with 50 μL of 0.5 mg/mL α-amylase/20 mM phosphate buffer (pH 6.9). The mixture was incubated at 37 °C for 10 min, and then added 50 μL of 1% starch/20 mM phosphate buffer. After 10 min of incubation at 37 °C, added 100 μL of reagent (1% DNS in 0.4 M NaOH/ddH2O of 12% KNaC4H4O6·4H2O/ddH2O) and incubated at 100 °C for 15 min. Finally, total volume was made up to 1.25 mL with ddH2O and the absorbance was measured at 405 nm. Acarbose was used as a positive control.
2.6. Inhibitory assay for PTP1B
Method of this assay was carried out according to Na et al21 with slight modification. Briefly, 10 μL of extract/10% DMSO with different concentrations (0.1–100 μg/mL), 10 μL of 2 mM pNPP/50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mM EDTA-Na2 and 1 mM DTT) and 100 μL of 1 μg/mL PTP1B/50 mM citrate buffer were mixed together. The mixture was incubated at 37 °C for 30 min, and then added 100 μL of 1 N NaOH/ddH2O to stop the reaction. The absorbance was measured at 405 nm. Ursolic acid was used as a positive control.
2.7. DPPH free-radical scavenging activity assay
The DPPH free-radical scavenging activity was examined according to Chang et al.22 Briefly, 10 μL of the extracts/methanol with different concentrations (1–100 μg/mL), 200 μL of 0.1 mM DPPH/ethanol and 90 μL of 50 mM Tris–HCl buffer (pH 7.4) were mixed together. The mixture stood was incubated at ambient temperature in dark for 30 min. The reduction of DPPH free-radical was measured at 517 nm. Quercetin was used as a positive control.
2.8. Ferrous ion-chelating activity assay
The chelating effect of ferrous ions was evaluated according to Dinis et al23 with slight modifications. Briefly, 100 μL of the extracts solution/DMSO, 370 μL of 50 mM Tris–HCl buffer (pH 7.4) and 10 μL of 2 mM FeCl2/ddH2O were mixed together. The reaction was initiated by adding 20 μL of 5 mM ferrozine/ddH2O with vigorous shake, and incubated at ambient temperature for 10 min, and the absorbance was measured at 562 nm. EDTA-Na2 was used as a positive control.
2.9. Determination of proanthocyanidin contents
A modified vanillin-H2SO4 assay of Hsieh et al24 was adopted for examination of the proanthocyanidin contents. Briefly, 50 μL of extracts/methanol, 125 μL of 1% vanillin/methanol, and 125 μL of 10% H2SO4/methanol were mixed together. After the mixture was incubated at 30 °C for 15 min, the absorbance was measured at 500 nm. The calibration curve was performed with (+)-catechin and expressed as (+)-catechin equivalent (CE) in milligrams per gram sample.
2.10. Determination of condensed tannins contents
A modified acid-butanol assay of Hagerman25 was adopted for examination of the condensed tannins. Briefly, 450 μL of 5% HCl/n-butanol, 75 μL of extracts/DMSO and 15 μL of 2% NH4Fe(SO4)2/ddH2O were mixed together. After the mixture was incubated at 100 °C for 50 min, the absorbance was measured at 550 nm. The calibration curve was performed with melacacidin and expressed as melacacidin equivalent (ME) in milligrams per gram sample.
2.11. Determination of flavonoid contents
The AlCl3 method of Quettier-Deleu et al26 was used for determination of the total flavonoid contents. 150 μL of extracts/methanol and 150 μL of 2% AlCl3/methanol were mixed together. After the mixture was incubated at ambient temperature for 10 min, the absorbance of reaction mixture was read at 450 nm for quercetin and 435 nm for rutin. The calibration curve was performed with quercetin and rutin. The results were expressed as quercetin equivalents (QE) and rutin equivalents (RE) in milligrams per gram sample.
2.12. Cell culture
The 3T3-L1 preadipocytes were purchased from Bioresource Collection and Research Center (BCRC). Cells were cultured in 10-cm plastic dishes with DMEM, containing 10% NCS. Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
2.13. Cell viability assay
Method of this assay was carried out according to Kim et al27 with slight modifications. 3T3-L1 preadipocytes were seeded in 96-well microtiter plates at a density of 2 × 105 cells/100 μL/well and grown for 4 h for adherence. The cells were treated with test samples in fresh DMEM at different concentrations. After 24 h incubation at 37 °C in a 5% CO2 incubator, 0.5 mg/mL MTT/DMEM was added and incubated for 1 h. The supernatant was removed after culture, and the insoluble formazan product was dissolved in 100 μL of DMSO. The absorbance of formazan was measured at 570 nm.
2.14. Statistical analysis
All results are expressed as means ± standard error (SE) (n = 3). The significance of difference was calculated by Scheffe's test, and results with p < 0.05 were considered to be significant. The correlation was calculated by Pearson's test, and results with p < 0.05 were considered to be significant.
3. Results and discussion
3.1. Antihyperglycemic activities and proanthocyanidin contents of extracts from different plant parts of C. osmophloeum (土肉桂 tǔ ròu guì)
The α-glucosidase and α-amylase inhibitory activities of 70% acetone extracts from different parts (twig, 2-cm branch, 5-cm branch, and leaf) of C. osmophloeum were determined and acarbose, a clinical drug, was used as a positive control. As shown in Table 1, the IC50 values of α-glucosidase inhibitory activity in increasing order are as follows: twigs (3.8 μg/mL) < 5-cm branch (6.3 μg/mL) ≤ 2-cm branch (6.7 μg/mL) < leaf (23.2 μg/mL). Moreover, of the four different plant parts studied, the α-amylase and α-glucosidase inhibitory activities showed similar trends, with twigs exhibiting the best α-amylase inhibitory activity (IC50 = 84.5 μg/mL). On the other hand, the proanthocyanidin contents in decreasing order are as follows: 5-cm branch (495.1 mg of CE/g) > 2-cm branch (449.5 mg of CE/g) > twigs (363.8 mg of CE/g) > leaf (trace). These results indicated that leaf extract contained no proanthocyanidins and had weak antihyperglycemic activities. Even with lower proanthocyanidins, twigs showed better antihyperglycemic activity than branches. Therefore, it is rationally presumed that twigs may have other different active components.
Table 1.
α-Glucosidase, α-amylase inhibitory activities and proanthocyanidin contents of 70 % acetone extracts from different chemotypes and plant parts of C. osmophloeum.
| Specimen | Chemotypes | IC50 (μg/mL) |
TPAC |
|
|---|---|---|---|---|
| α-Glucosidase | α-Amylase | CE/extract (mg/g) | ||
| Leaf | Cinnamaldehyde | 23.2 ± 0.1a | > 1000 | – |
| 2-cm Br | Cinnamaldehyde | 6.7 ± 0.0b | 250.1 ± 4.6a | 495.1 ± 4.8a |
| 5-cm Br | Cinnamaldehyde | 6.3 ± 0.1b | 232.5 ± 4.2a | 449.5 ± 5.5a |
| Twig | Cinnamaldehyde | 3.8 ± 0.2c | 84.5 ± 10.0b | 363.8 ± 3.0b |
| Twig | Linalool | 3.6 ± 0.0c | 90.3 ± 1.3b | 370.7 ± 3.7b |
| Twig | Mixed | 3.5 ± 0.1c | 105.6 ± 4.2b | 346.5 ± 3.9b |
Mean ± SE (n = 3). Different letters (a–c) in the table are significantly different at the level of p < 0.05 according to Scheffe's test. – = not detected. Twig = diameter < 0.5 cm, Br = branch. TPAC = total proanthocyanidin contents, CE = (+)-Catechin equivalent. IC50 values of acarbose against α-glucosidase and α-amylase were 0.004 μg/mL and 12.5 μg/mL, respectively.
Salehi et al28 reported that the methanolic extracts of Cinnamomum zeylanicum bark had an excellent α-amylase inhibitory activity (IC50 value was about 38 μg/mL) in the ten plant extracts traditionally used in Iran for diabetes. In another study, Lin and Lee19 reported that aesculitannin B had better α-glucosidase inhibitory activity (IC50 = 3.0 μg/mL) than other compounds from Machilus philippinensis leaf. Compared with the above-mentioned results, the C. osmophloeum twig extracts (CoTE) in this study showed a similar α-amylase inhibitory activity as that of the methanolic extracts of C. zeylanicum bark and a similar α-glucosidase inhibitory activity as that of aesculitannin B. Shihabudeen et al29 reported that the methanolic extracts of C. zeylanicum bark exhibited a strong in vitro α-glucosidase inhibitory activity and inhibited effectively the maltase and sucrase for suppression of postprandial blood glucose spikes in rats. Hence, it is of note that CoTE has excellent α-glucosidase inhibitory activity and the potency of in vivo antihyperglycemic activity.
3.2. Antihyperglycemic activity and proanthocyanidin contents of extracts from different chemotypes of C. osmophloeum twigs
The leaf essential oils of C. osmophloeum could be classified into six chemotypes by GC/MS and cluster analyses.30 Cinnamaldehyde type, linalool type and mixed type were selected and tested using antihyperglycemic assays in order to understand whether chemotypes influence the antihyperglycemic activities of CoTE.
As seen in Table 1, no significant difference in antihyperglycemic activity and proanthocyanidin contents between CoTE of different chemotypes, indicating that chemotypes have no influence on the antihyperglycemic activities and proanthocyanidin contents of CoTE.
Similar to C. cassia bark oil, essential oils from cinnamaldehyde type of C. osmophloeum leaf comprised mainly cinnamaldehyde and rarely coumarin.14, 30 Babu et al31 reported that cinnamaldehyde had antihyperglycemic and antihyperlipidemic activities in streptozotocin-induced diabetic rats. The hot water extracts from C. osmophloeum leaves could enhance secretion of adiponectin, activation of glucose transporter type 4 (GLUT4) translocation and phosphorylation of insulin receptor-β in 3T3-L1 adipocytes.32 Moreover, these hot water extracts also had antihyperlipidemic activities and increased high-density lipoprotein in golden Syrian hamsters fed with high-cholesterol diet.33
According to above-mentioned studies, it is obvious that the essential oils from cinnamaldehyde type of C. osmophloeum leaf have both antihyperglycemic and antihyperlipidemic activities. Furthermore, the hot water extracts from cinnamaldehyde type of C. osmophloeum leaf exhibited similar activities. Twig is a young-woody part and connects to leaf. Rough harvest of leaves from cinnamaldehyde type of C. osmophloeum trees accompanied some twigs. In this study, CoTE exhibited better antihyperglycemic activities than leaf extracts. It is worthy to investigate active components in the CoTE. To explore the further utilization of C. osmophloeum renewable plant parts, twigs of cinnamaldehyde-type C. osmophloeum were chosen for the subsequent tests.
3.3. Various phenolic contents of soluble fractions from CoTE
According to the aforesaid results, CoTE had the best antihyperglycemic activities. To determine the structure of active compounds in the extracts, an in vitro bioactivity-guided fractionation procedure was carried out. The fractionation was done by successive liquid-liquid partition with n-hexane, ethyl acetate, n-butanol, and water to yield four soluble fractions. Then, proanthocyanidin, condensed tannin and flavonoid contents in the crude extract and its four soluble fractions were quantitated.
As shown in Table 2, the proanthocyanidin contents in decreasing order are as follows: BSF (561.1 mg of CE/g) > crude (402.6 mg of CE/g) > WSF (189.0 mg of CE/g) > EASF (88.4 mg of CE/g) > HSF (1.5 mg of CE/g). Moreover, condensed tannin of crude extract and its four soluble fractions showed a similar trend as that of proanthocyanidin contents, with BSF having the highest condensed tannin content (331.5 mg ME/g). In the crude extract and its four soluble fractions, the flavonoid contents calculated by quercetin equivalent were lower than 10 mg of QE/g. However, the flavonoid contents expressed as rutin equivalent in decreasing order are as follows: EASF (52.7 mg of ME/g) > BSF (21.6 mg of ME/g) > crude (10.7 mg of ME/g) > WSF (0.7 mg of ME/g) > HSF (0.5 mg of ME/g). These results indicated that crude extracts of C. osmophloeum had abundant proanthocyanidins and condensed tannins in BSF, while EASF had the highest contents of flavonoid glycosides.
Table 2.
Proanthocyanidin, condensed tannin and flavonoid contents of CoTE and its soluble fractions.
| Specimen | TFC |
TPAC |
CTC |
|
|---|---|---|---|---|
| QE/extract (mg/g) | RE/extract (mg/g) | CE/extract (mg/g) | ME/extract (mg/g) | |
| Crude | 1.4 ± 0.0c | 10.7 ± 0.1c | 402.6 ± 2.9b | 258.2 ± 5.7b |
| HSF | 0.0 ± 0.1d | 0.5 ± 0.2d | 1.5 ± 0.3e | 22.7 ± 0.3e |
| EASF | 6.0 ± 0.1a | 52.7 ± 1.0a | 88.4 ± 1.0d | 90.6 ± 0.7d |
| BSF | 4.1 ± 0.1b | 21.6 ± 0.4b | 561.1 ± 2.4a | 331.5 ± 3.8a |
| WSF | 0.0 ± 0.0d | 0.7 ± 0.1d | 189.0 ± 1.9c | 125.5 ± 3.2c |
Mean ± SE (n = 3). Different letters (a–e) in the table are significantly different at the level of p < 0.05 according to Scheffe's test. HSF = n-hexane soluble fraction, EASF = ethyl acetate soluble fraction, BSF = n-butanol soluble fraction, WSF = water soluble fraction. TFC = total flavonoid contents, TPAC = total proanthocyanidin contents, CTC = condensed tannin contents, CE = (+)-catechin equivalent, ME = melacacidin equivalent, QE = quercetin equivalent, RE = rutin equivalent.
3.4. Antihyperglycemic and PTP1B inhibitory activity of soluble fractions from CoTE
PTP1B plays an important role as a regulator of insulin and leptin signal pathway, and many studies have explored its potential as a validated therapeutic target for the treatment of type 2 diabetes and obesity.22 In this study, not only α-glucosidase and α-amylase assays were employed to determine the antihyperglycemic activity, PTP1B assay was also utilized to determine the improvement of insulin or leptin resistance in the crude extract and its four soluble fractions.
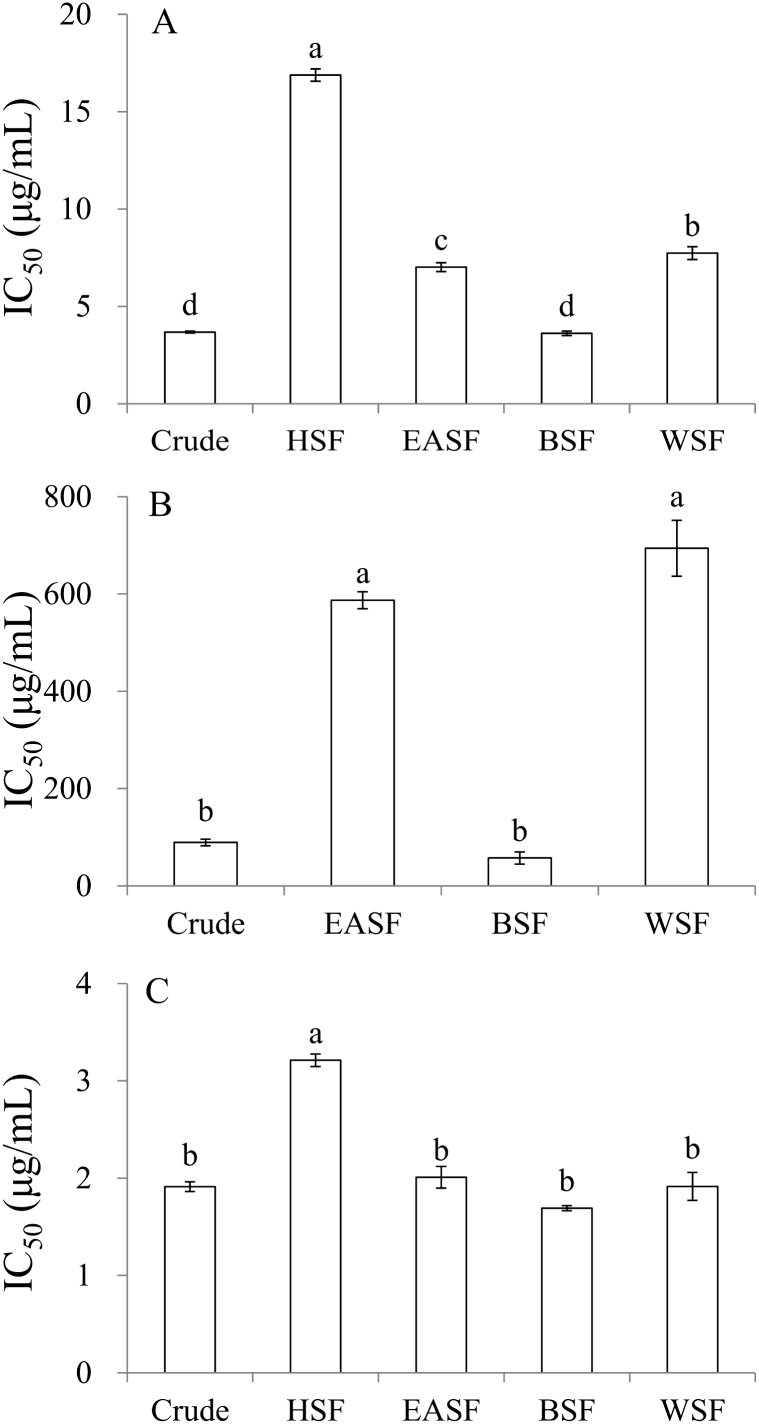
As shown in Fig. 1A, the IC50 values of α-glucosidase inhibitory activity in increasing order are as follows: BSF (3.6 μg/mL) ≤ crude (3.8 μg/mL) < EASF (7.0 μg/mL) < WSF (7.7 μg/mL) < HSF (16.9 μg/mL). Moreover, α-amylase inhibitory activity of the crude extract and its four soluble fractions (Fig. 1B) showed a similar trend as that of α-glucosidase, with BSF exhibiting the best α-amylase inhibitory activity (IC50 = 57.4 μg/mL). Taken together, these results revealed that BSF had the best antihyperglycemic activities. The IC50 values of PTP1B inhibitory activity (Fig. 1C) in increasing order are as follows: BSF (1.7 μg/mL) ≤ crude (1.9 μg/mL) = WSF (1.9 μg/mL) ≤ EASF (2.0 μg/mL) < HSF (3.2 μg/mL). Note that ursolic acid having an IC50 value of 4.6 μg/mL reveals that it is less effective than crude extract and its four soluble fractions. This result demonstrated that CoTE had better PTP1B inhibitory activity than that of the positive control and showed greater potential for improvement of insulin or leptin resistance in type 2 diabetes.
Fig. 1.
IC50 values of (A) α-glucosidase, (B) α-amylase and (C) PTP1B of CoTE and its soluble fractions. Mean ± SE (n = 3). Different letters (a–d) in the figure are significantly different at the level of p < 0.05 according to Scheffe's test. HSF = n-hexane soluble fraction, EASF = ethyl acetate soluble fraction, BSF = n-butanol soluble fraction, WSF = water soluble fraction. IC50 value of HSF against α-amylase was > 1000 μg/mL. IC50 values of acarbose against (A) α-glucosidase and (B) α-amylase were 0.004 μg/mL and 12.5 μg/mL (C) IC50 value of ursolic acid against PTP1B was 4.6 μg/mL.
Yilmazer-Musa et al34 reported that epi-gallocatechin gallate, a proanthocyanidins structure unit, had the best α-glucosidase inhibitory activity. In addition, the grape seed extract owing to their high condensed tannin contents had excellent α-amylase inhibitory activity. The structure and degree of polymerization of condensed tannin were reported to affect the inhibitory activities of glucose and lipid metabolic enzymes.35 Similarly, BSF of CoTE had excellent antihyperglycemic activities because of its highest proanthocyanidin and condensed tannin contents.
In general, it was reported that proanthocyanidin and condensed tannin exhibited strong inhibitory activities against α-glucosidase and α-amylase.36, 37 The proanthocyanidin and condensed tannin contents of WSF (189.0 mg of CE/g and 125.5 mg of ME/g, respectively) were higher than those of EASF (88.4 mg of CE/g and 90.6 mgof ME/g, respectively) (Table 2). It was expected that WSF would have better α-glucosidase and α-amylase inhibitory activities than EASF; however, their α-glucosidase and α-amylase inhibitory activities were in fact similar. On the other hand, EASF has higher contents of flavonoid glycosides (52.7 mg of RE/g) than WSF (0.7 mg of RE/g). Lin and Chang38 reported that 9 kaempferol glycosides were obtained from CoTE, and these kaempferol glycosides contained xylopyranosyl, arabinofuranosyl, rhamnopyranosyl, apiofuranosyl or glucopyranosyl group. It was reported by Pereira et al39 that kaempferitrin significantly inhibited the in vivo activities of maltase and sucrase. Taken together, these results reveal that kaempferol glycosides in CoTE may contribute to extra α-glucosidase and α-amylase inhibitory activities of EASF.
Arya et al40 reported that Centratherum anthelminticum seed extracts in six plants in South India and Southeast Asia had excellent PTP1B inhibitory activity and were the most effective in decreasing blood glucose of diabetic rats. In addition, the total phenolic contents of C. anthelminticum seed extracts were 665.3 mg of GAE/g. In another study, Muthusamy et al41 reported that the condensed tannin from leaf extracts of Cichorium intybus could inhibit PTP1B expression. Therefore, leaf extracts of C. intybus could decrease accumulation of lipid and increase glucose uptake in 3T3-L1 adipocytes. C. anthelminticum seed extracts had a lower PTP1B inhibitory activity than ursolic acid, but CoTE, containing abundant proanthocyanidin and condensed tannin, had higher PTP1B inhibitory activity than ursolic acid. In other words, CoTE has potential for use as an antihyperglycemic and antihyperlipidemic agent, and it merits further investigations using animal tests in the near future.
3.5. Antioxidant activity of soluble fractions from CoTE
Excess nutrients may cause unfolded protein response (UPR) to accumulate reactive oxygen species (ROS) in cells. UPR and ROS would bring about inflammation which led to the symptoms of type 2 diabetes, including insulin resistance, leptin resistance and β-cell dysfunction.42, 43 To evaluate whether the soluble fractions from CoTE can directly decrease ROS or indirectly decrease ROS by inhibiting Fenton reaction. Results from these assays show that extracts with excellent antioxidant abilities had the potential for decreasing oxidative stress. Even more important, they could reduce dysfunction of endocrine cells in type 2 diabetes.
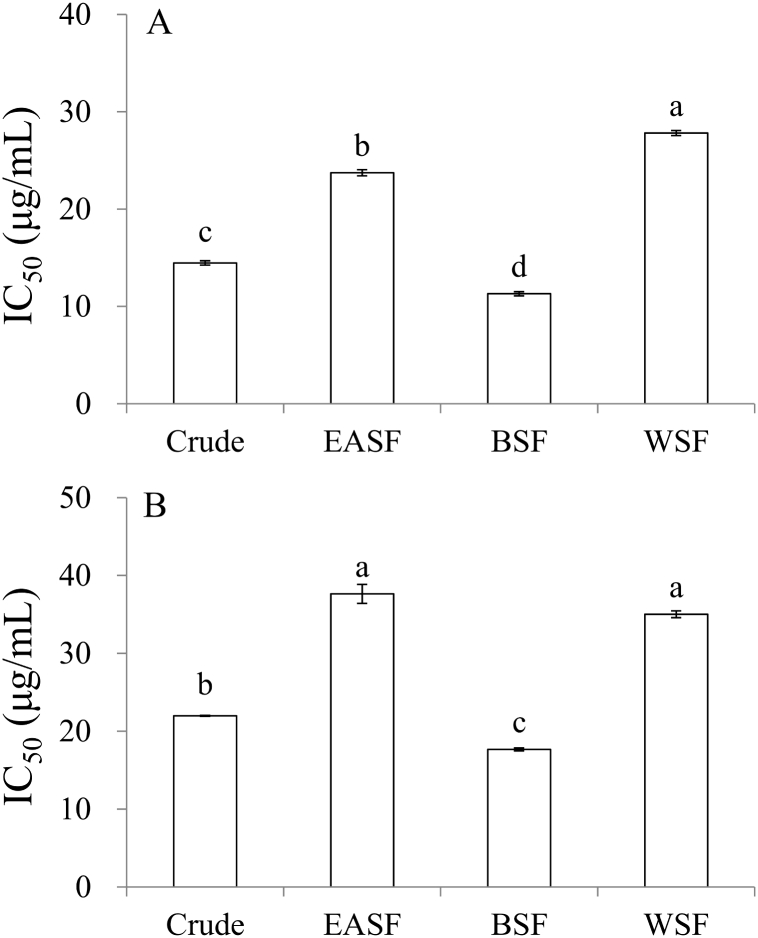
As shown in Fig. 2A, the IC50 values of DPPH free-radical scavenging activity in increasing order are as follows: BSF (11.3 μg/mL) < crude (14.5 μg/mL) < EASF (23.7 μg/mL) < WSF (27.8 μg/mL) < HSF (>100 μg/mL), revealing that BSF possessed the highest antioxidant activity, which might reduce the in vivo oxidative stress in human. As shown in Fig. 2B, the IC50 values of ferrous ion-chelating activity in increasing order are as follows: BSF (17.7 μg/mL) < crude (22.0 μg/mL) < WSF (35.0 μg/mL) < EASF (37.6 μg/mL) < HSF (>100 μg/mL), revealing that BSF possessed the highest ferrous ion-chelating activity, which might reduce generation of hydroxyl radical by Fenton reaction.
Fig. 2.
IC50 values of (A) DPPH free-radical scavenging and (B) ferrous ion-chelating activity of CoTE and its soluble fractions. Mean ± SE (n = 3). Different letters (a–d) in the figure are significantly different at the level of p < 0.05 according to Scheffe's test. EASF = ethyl acetate soluble fraction, BSF = n-butanol soluble fraction, WSF = water soluble fraction. (A) IC50 value of quercetin was 4.0 μg/mL (B) IC50 value of EDTA-Na2 was 9.3 μg/mL.
Chau et al44 reported that the 70% ethanolic extracts of C. osmophloeum twigs had good inhibitory activity on lipid peroxidation, DPPH free radical and superoxide radical. The active compounds were mainly flavonoid glycosides. In contrast, the results obtained in this study showed that CoTE extracted from 70% acetone had good antioxidant activities, and the active compounds are proanthocyanidin and condensed tannin. Taken together, the findings revealed obvious variations in components of C. osmophloeum twig extracts obtained using different solvents. Moreover, CoTE extracted from 70% acetone had better ferrous ion-chelating activity than that of 70% ethanolic extracts. Cooksey et al45 reported that iron chelation therapy significant protected β-cell function and insulin sensitivity from diabetes in leptin-deficient ob/ob mice. Escolar et al46 reported that EDTA-based chelation regimen reduced cardiovascular events in type 2 diabetes patients with myocardial infarction. According to above-mentioned studies, it indicated that CoTE extracted from 70% acetone with great ferrous ion-chelating activity have potential as a substitution for commercial iron chelators to treat type 2 diabetes.
3.6. Correlation between phenolic contents, antioxidant and antihyperglycemic activities
To determine the relationship between phenolic contents and potential for remedying early stages of type 2 diabetes, including antioxidant activities and antihyperglycemic activities, correlation analysis was employed to examine different phenolic contents, inhibitory action of hyperglycemic enzymes such as α-glucosidase, α-amylase and PTP1B and antioxidant activities such as DPPH free-radical scavenging activity and ferrous ion-chelating activity.
As shown in Table 3, the correlation coefficient between proanthocyanidin and condensed tannin contents was 0.997, revealing a highly significant correlation between these two phenolic contents. The correlation coefficients of proanthocyanidin contents with α-glucosidase, α-amylase, and PTP1B inhibitory activities were 0.945, 0.870, and 0.740, respectively, indicating the higher the proanthocyanidin contents, the better the inhibitory activities of enzymes. The correlation coefficients of proanthocyanidin contents with DPPH free-radical scavenging and ferrous ion-chelating activity were 0.959 and 0.974, respectively, revealing the higher the proanthocyanidin contents, the better the antioxidant activities. However, flavonoid glycoside contents showed no correlation with inhibition of enzymes or antioxidant activities. This might be partially due to the glycosylation of flavonoids which weakens the antihyperglycemic or antioxidant activities. In brief, these results demonstrated that both proanthocyanidin and condensed tannin were active compounds in CoTE.
Table 3.
Pearson's correlation for phenolic contents, antihyperglycemic and antioxidant activities of CoTE and its four soluble fractions.
| Assay | TPACa | CTCb | TFCc | Glud | Amyd | PTP1Bd | DPPHe | Chelatinge |
|---|---|---|---|---|---|---|---|---|
| TPAC | 1 | 0.997** | −0.010 | 0.945** | 0.870** | 0.740** | 0.959** | 0.974** |
| CTC | 1 | 0.047 | 0.964** | 0.860** | 0.752** | 0.973** | 0.984** | |
| TFC | 1 | 0.164 | 0.042 | 0.300 | 0.257 | 0.177 | ||
| Glu | 1 | 0.843** | 0.748** | 0.972** | 0.967** | |||
| Amy | 1 | 0.565* | 0.860** | 0.827** | ||||
| PTP1B | 1 | 0.814** | 0.824** | |||||
| DPPH | 1 | 0.991** | ||||||
| Chelating | 1 |
* and ** indicate significant correlation at 5% and 1% level of confidence.
TPAC, total proanthocyanidin contents (mg of catechin equivalent/g extract).
CTC, condensed tannin contents (mg of melacacidin equivalent/g extract).
TFC, total flavonoid contents (mg of rutin equivalent/g extract).
Data from inhibition activity of α-glucosidase, α-amylase and PTP1B expressed as 1/IC50 values.
Data from DPPH free-radical scavenging and ferrous ion-chelating capacity expressed as 1/IC50 values.
Manaharan et al47 reported that selected tropic plant extracts displayed highly significant correlation between total phenolic contents and DPPH free-radical scavenging activity. Moreover, the correlation of antioxidant activity with α-glucosidase or α-amylase inhibitory activity was also high. In another study, Gonçalves et al48 reported that condensed tannin formed complexes with enzymes to quench the activity of enzymes. Our results were consistent with those of the above-mentioned studies, demonstrating that the phenolic structure of proanthocyanidin could scavenge DPPH free radical and condensed tannin when aggregated with enzymes into complexes had inhibitory activities.
The present findings from in vitro assays demonstrated that CoTE has excellent antihyperglycemic and antioxidant effects. Nevertheless, further studies are required to elucidate the in vivo antihyperglycemic and antioxidant activities. Moreover, the relationship between bioactivities and structure of proanthocyanidins, such as monomer, substituted groups, linkages and degree of polymerization, merit further investigations.
3.7. Cell toxicity of soluble fractions from CoTE on 3T3-L1 preadipocyte cells
Under energy overload, adipocytes serve not only as the primary storage site, but also secrete unsaturated or saturated fatty acid to affect the gene expression in muscle or liver, and then regulate fat metabolism and glucose homeostasis. Same as tissues of other endocrine organs, adipose tissue released adipokines such as leptin, adiponectin and resistin, which regulated insulin action and metabolism in other tissues. For this reason, adipocytes played an important role in energy metabolism and might contribute to alternative novel therapies for obesity-related diseases.3
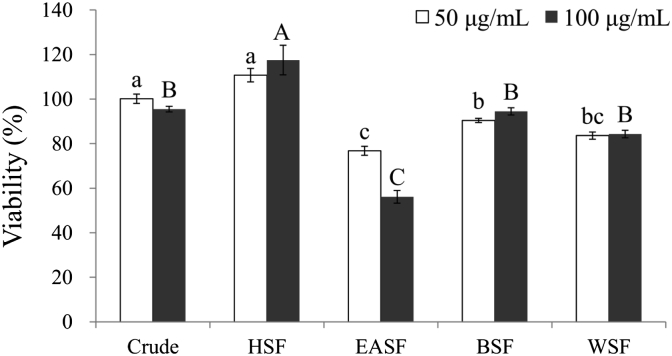
Fig. 3 shows the effects of toxicity of crude extracts and its four soluble fractions on viability of 3T3-L1 preadipocytes. Except for EASF, the viability of crude extracts and other soluble fractions were higher than 80% at 50 μg/mL. At treatment concentration of 100 μg/mL, crude extract and other soluble fractions had no toxicity on viability of 3T3-L1 preadipocytes while EASF decreased cell viability to 56.1%. These results revealed that CoTE had no toxicity on 3T3-L1 preadipocytes at 100 μg/mL. Even though EASF was toxic to 3T3-L1 preadipocytes, crude extract comprised only 4.7% EASF. In other words, the metabolism of adipocytes remained unaffected by extracts at the concentration of 100 μg/mL. In addition, CoTE may still display excellent inhibition on both glucose metabolism enzymes and antioxidant activities, and recover the balance metabolism of adipocytes from energy overload at the dosage of 100 μg/mL.
Fig. 3.
Cell viability of CoTE and its soluble fractions. Mean ± SE (n = 3). Different letters (a-c, A-C) in the figure are significantly different at the level of p < 0.05 according to Scheffe's test. Cell line: 3T3-L1 preadipocytes, HSF = n-hexane soluble fraction, EASF = ethyl acetate soluble fraction, BSF = n-butanol soluble fraction, WSF = water soluble fraction.
4. Conclusions
To the best of our knowledge, this is the first report demonstrating that indigenous cinnamon (C. osmophloeum; 土肉桂 tǔ ròu guì) twig extracts has antihyperglycemic effects as evidenced by both α-glucosidase and α-amylase inhibitory activity assays. Furthermore, it is of note that CoTE has PTP1B inhibitory activity to improve insulin or leptin resistance. In addition, CoTE displays antioxidant activities, and has the potential for decreasing oxidative stress in type 2 diabetes. The above-mentioned activities were attributed to the abundant proanthocyanidins and condensed tannins in CoTE. CoTE has good potential as a renewable source for natural health products due to its antihyperglycemic activity, antioxidant activity and non-toxic properties.
Conflict of interest
All authors declare that there is no conflict of interests.
Acknowledgements
We thank Dr. Shoei-Sheng Lee (School of Pharmacy, College of Medicine, National Taiwan University) for assistance in inhibitory assay of α-glucosidase. We also thank Taiwan Forestry Research Institute for providing specimens.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Rowley W.R., Bezold C. Creating public awareness: state 2025 diabetes forecasts. Popul Health Manag. 2012;15:194–200. doi: 10.1089/pop.2011.0053. [DOI] [PubMed] [Google Scholar]
- 2.Hummasti S., Hotamisligil G.S. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res. 2010;107:579–591. doi: 10.1161/CIRCRESAHA.110.225698. [DOI] [PubMed] [Google Scholar]
- 3.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 4.Bösenberg L.H., van Zyl D.G. The mechanism of action of oral antidiabetic drugs: a review of recent literature. JEMDSA. 2008;13:80–88. [Google Scholar]
- 5.Krentz A.J., Bailey C.J. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 6.Chang C.L.T., Lin Y., Bartolome A.P., Chen Y.C., Chiu S.C., Yang W.C. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid-based Complement Altern Med. 2013;2013:37865733. doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng D.M., Kuhn P., Poulev A., Rojo L.E., Lila M.A., Raskin I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012;135:2994–3002. doi: 10.1016/j.foodchem.2012.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson R.A., Broadhurst C.L., Polansky M.M. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52:65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 9.Adisakwattana S., Lerdsuwankij O., Poputtachai U., Minipun A., Suparpprom C. Inhibitory activity of cinnamon bark species and their combination effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Plant Food Hum Nutr. 2011;66:143–148. doi: 10.1007/s11130-011-0226-4. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y., Wu E.Q., Liang C. Discrimination of cinnamon bark and cinnamon twig samples sourced from various countries using HPLC-based fingerprint analysis. Food Chem. 2011;127:755–760. doi: 10.1016/j.foodchem.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.H., Avula B., Nanayakkara N.P.D., Zhao J.P., Khan I.A. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J Agric Food Chem. 2013;61:4470–4476. doi: 10.1021/jf4005862. [DOI] [PubMed] [Google Scholar]
- 12.Hazleton L.W., Tusing T.W., Zeitlin B.R., Thiessen R., Murer H.K. Toxicity of coumarin. J Pharmacol Exp Ther. 1956;118:348–358. [PubMed] [Google Scholar]
- 13.Lake B.G. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem Toxicol. 1999;37:423–453. doi: 10.1016/s0278-6915(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 14.Yeh T.F., Lin C.Y., Chang S.T. A potential low-coumarin cinnamon substitute: Cinnamomum osmophloeum leaves. J Agric Food Chem. 2014;62:1706–1712. doi: 10.1021/jf405312q. [DOI] [PubMed] [Google Scholar]
- 15.Tung Y.T., Chua M.T., Wang S.Y., Chang S.T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour Technol. 2008;99:3908–3913. doi: 10.1016/j.biortech.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Hanhineva K., Törrönen R., Bondia-Pons I. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip S.C., Saha S., Chernoff J. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem Sci. 2010;35:442–449. doi: 10.1016/j.tibs.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H.Y., Chang S.T. Antioxidant potency of phenolic phytochemicals from the root extract of Acacia confusa. Ind Crops Prod. 2013;49:871–878. [Google Scholar]
- 19.Lin H.C., Lee S.S. Proanthocyanidins from the leaves of Machilus philippinensis. J Nat Prod. 2010;73:1375–1380. doi: 10.1021/np1002274. [DOI] [PubMed] [Google Scholar]
- 20.Apostolidis E., Karayannakidis P.D., Kwon Y.I., Lee C.M., Seeram N.P. Seasonal variation of phenolic antioxidant-mediated α-glucosidase inhibition of Ascophyllum nodosum. Plant Food Hum Nutr. 2011;66:313–319. doi: 10.1007/s11130-011-0250-4. [DOI] [PubMed] [Google Scholar]
- 21.Na M., Kim B.Y., Osada H., Ahn J.S. Inhibition of protein tyrosine phosphatase 1B by lupeol and lupenone isolated from Sorbus commixta. J Enzyme Inhib Med Chem. 2009;24:1056–1059. doi: 10.1080/14756360802693312. [DOI] [PubMed] [Google Scholar]
- 22.Chang S.T., Wu J.H., Wang S.Y., Kang P.L., Yang N.S., Shyur L.F. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001;49:3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- 23.Dinis T.C.P., Madeira V.M.C., Almeida L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh C.Y., Chang S.T. Antioxidant activities and xanthine oxidase inhibitory effects of phenolic phytochemicals from Acacia confusa twigs and branches. J Agric Food Chem. 2010;58:1578–1583. doi: 10.1021/jf903569k. [DOI] [PubMed] [Google Scholar]
- 25.Quettier-Deleu C., Bernard G., Jacques V. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol. 2000;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 26.Hagerman, AE [Internet]. The Tannin Handbook; 2011. Available from: http://www.users.muohio.edu/hagermae/. Accessed on May, 2015.
- 27.Kim M.J., Chang U.J., Lee J.S. Inhibitory effects of fucoidan in 3T3-L1 adipocyte differentiation. Mar Biotechnol. 2009;11:557–562. doi: 10.1007/s10126-008-9170-1. [DOI] [PubMed] [Google Scholar]
- 28.Salehi P., Asghari B., Esmaeili M.A., Dehghan H., Ghazi I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J Med Plants Res. 2013;7:257–266. [Google Scholar]
- 29.Shihabudeen H.M.S., Priscilla D.H., Thirumurugan K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metab. 2011;8:46. doi: 10.1186/1743-7075-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng S.S., Liu J.Y., Hsui Y.R., Chang S.T. Chemical polymorphism and antifungal activity of essential oils from leaves of different provenances of indigenous cinnamon (Cinnamomum osmophloeum) Bioresour Technol. 2006;97:306–312. doi: 10.1016/j.biortech.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Babu P.S., Prabuseenivasan S., Ignacimuthu S. Cinnamaldehyde-A potential antidiabetic agent. Phytomedicine. 2007;14:15–22. doi: 10.1016/j.phymed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Lee M.J., Rao Y.K., Chen K., Lee Y.C., Tzeng Y.M. Effect of flavonol glycosides from Cinnamomum osmophloeum leaves on adiponectin secretion and phosphorylation of insulin receptor-β in 3T3-L1 adipocytes. J Ethnopharmacol. 2009;126:79–85. doi: 10.1016/j.jep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Lin T.Y., Liao J.W., Chang S.T., Wang S.Y. Antidyslipidemic activity of hot-water extracts from leaves of Cinnamomum osmophloeum Kaneh. Phytother Res. 2011;25:1317–1322. doi: 10.1002/ptr.3408. [DOI] [PubMed] [Google Scholar]
- 34.Yilmazer-Musa M., Griffith A.M., Michels A.J., Schneider E., Frei B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J Agric Food Chem. 2012;60:8924–8929. doi: 10.1021/jf301147n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusano R., Ogawa S., Matsuo Y., Tanaka T., Yazaki Y., Kouno I. α-Amylase and lipase inhibitory activity and structural characterization of acacia bark proanthocyanidins. J Nat Prod. 2010;74:119–128. doi: 10.1021/np100372t. [DOI] [PubMed] [Google Scholar]
- 36.Xiao J., Ni X., Kai G., Chen X. A review on structure-activity relationship of dietary polyphenols inhibiting α-amylase. Crit Rev Food Sci Nutr. 2013;53:497–506. doi: 10.1080/10408398.2010.548108. [DOI] [PubMed] [Google Scholar]
- 37.Xiao J., Kai G., Yamamoto K., Chen X. Advance in dietary polyphenols as α-glucosidases inhibitors: a review on structure-activity relationship aspect. Crit Rev Food Sci Nutr. 2013;53:818–836. doi: 10.1080/10408398.2011.561379. [DOI] [PubMed] [Google Scholar]
- 38.Lin H.Y., Chang S.T. Kaempferol glycosides from the twigs of Cinnamomum osmophloeum and their nitric oxide production inhibitory activities. Carbohydr Res. 2012;364:49–53. doi: 10.1016/j.carres.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Pereira D.F., Cazarolli L.H., Lavado C. Effects of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition. 2011;27:1161–1167. doi: 10.1016/j.nut.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Arya A., Looi C.Y., Wong W.F. In vitro antioxidant, PTP-1B inhibitory effects and in vivo hypoglycemic potential of selected medicinal plants. Int J Pharm. 2013;9:50–57. [Google Scholar]
- 41.Muthusamy V.S., Anand S., Sangeetha K.N., Sujatha S., Arun B., Lakshmi B.S. Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chem Biol Interact. 2008;174:69–78. doi: 10.1016/j.cbi.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cnop M., Foufelle F., Velloso L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Chua M.T., Tung Y.T., Chang S.T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour Technol. 2008;99:1918–1925. doi: 10.1016/j.biortech.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Cooksey R.C., Jones D., Gabrielsen S. Dietary iron restriction or iron chelation protects from diabetes and loss of β-cell function in the obese (ob/ob lep−/−) mouse. Am J Physiol Endocrinol Metab. 2010;298:E1236–E1243. doi: 10.1152/ajpendo.00022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escolar E., Lamas D.A., Mark D.B. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the trial to assess chelation therapy (TACT) Circ Cardiovasc Qual Outcomes. 2014;7:15–24. doi: 10.1161/CIRCOUTCOMES.113.000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manaharan T., Teng L.L., Appleton D., Ming C.H., Masilamani T., Palanisamy U.D. Antioxidant and antiglycemic potential of Peltophorum pterocarpum plant parts. Food Chem. 2011;129:1355–1361. [Google Scholar]
- 48.Gonçalves R., Mateus N., de Freitas V. Inhibition of α-amylase activity by condensed tannins. Food Chem. 2011;125:665–672. [Google Scholar]