Abstract
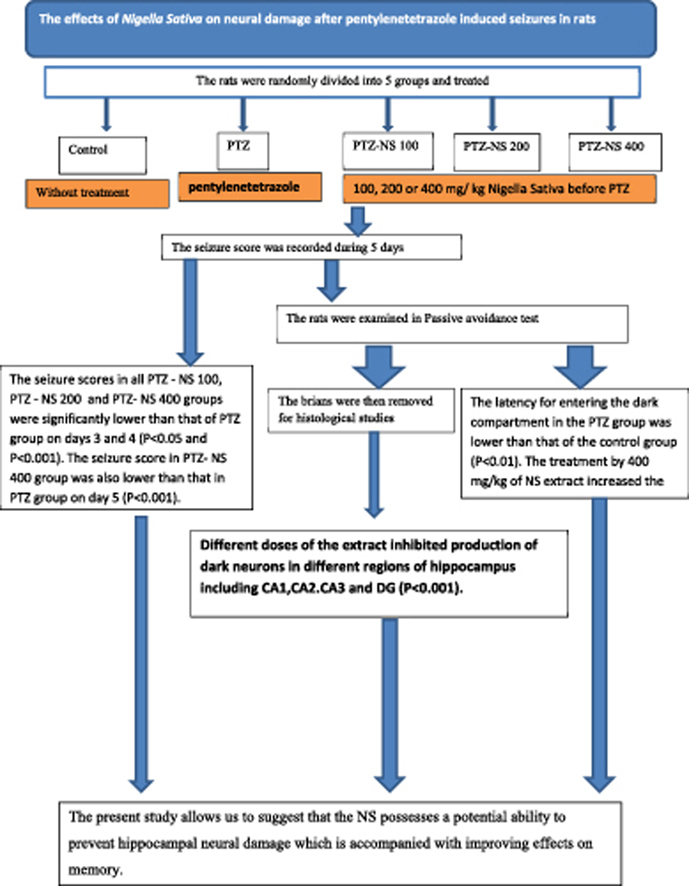
Nigella sativa (NS) has been suggested to have neuroprotective and anti-seizures properties. The aim of current study was to investigate the effects of NS hydro-alcoholic extract on neural damage after pentylenetetrazole (PTZ) – induced repeated seizures. The rats were divided into five groups: (1) control (saline), (2) PTZ (50 mg/kg, i.p.), (3–5) PTZ-NS 100, PTZ-NS 200 and PTZ-NS 400 (100, 200 and 400 mg/kg of NS extract respectively, 30 min prior to each PTZ injection on 5 consecutive days). The passive avoidance (PA) test was done and the brains were then removed for histological measurements. The PTZ-NS 100, PTZ-NS 200 and PTZ-NS 400 groups had lower seizure scores than PTZ group (P < 0.01 and P < 0.001). The latency to enter the dark compartment by the animals of PTZ group was lower than control in PA test (P < 0.01). Pre-treatment by 400 mg/kg of the extract increased the latency to enter the dark compartment (P < 0.05). Meanwhile, different doses of the extract inhibited production of dark neurons in different regions of hippocampus (P < 0.001). The present study allows us to suggest that the NS possesses a potential ability to prevent hippocampal neural damage which is accompanied with improving effects on memory.
Keywords: Nigella sativa, Pentylenetetrazole, Repeated seizures, Rat, Neural damage
Graphical abstract
1. Introduction
Epilepsy is a common and heterogeneous neurological disorder arising from biochemical and molecular events that are incompletely understood. The results of human studies suggest that epilepsy affects cognition, learning and memory.1 Animal studies have also confirmed that prolonged or recurrent seizures cause memory and emotional deficits.2 The rats which had experienced recurrent seizures also showed the damages to the parts of the brain such as the hippocampus and other parts of the limbic system.3 Even brief induced seizures lasting 60–70 s by administering a single convulsive dose of pentylenetetrazole (PTZ, 50 mg/kg, i.p.) has been shown to impair learning and memory.4
Dark neurons are considered as a group of cells undergoing early damage in their cytoskeleton such as microtubules or microfilaments, representing small dense nuclei inside of them.5 Dark neurons appear under specific conditions such as mechanical forces (head injuries or an electric shock), pathological metabolic conditions (hypoglycemia or ischemia) and epilepsy. It has been reported that a percentage of the dark neurons produced under such conditions recover while, the others can no more be alive.6 No specific method is considered for detecting the dark neurons, but they are almost diagnosed by their hyperbasophilia, hyperargyrophilia, and high-electron density properties in histological sections.6 Production of dark neurons has also been reported during epilepsy.7 Dark neurons during seizures are produced due to a cellular stress caused by markedly increased intracellular Ca2+ concentration, which in turn, results in ultrastructure compaction in the neurons.8, 9 Baracskay et al., suggested that generalized seizure produces widespread dark cells throughout the brain especially in the hippocampus and the pontine reticular formation.8, 9 On the other hand, experimental evidence indicates that antioxidant compounds protect against the neuronal damage observed during epilepsy and seizures.10 The protective effects of antioxidant compounds against learning and memory impairments due to epilepsy and seizures has also been well documented.11, 12
Nigella sativa L. (NS) is an annual flowering plant native to different regions of southern Europe and some parts of Asia. The flowers are delicate and are usually colored pale blue and white with small black seeds.13 NS seeds are the source of active components such as 30–40% fixed oil, 0.5–1.5% essential oil, various sugars and proteins and pharmacologically active components containing thymoquinone (TQ), ditimoquinone (DTQ) and nigellin.13, 14, 15 In traditional medicine, this herb was identified to have healing power so that it has been used in the Middle East and Far East for treating diseases such as asthma, headache, dysentery, infections, obesity, back pain, hypertension and gastrointestinal problems. Finally, there is a common Islamic opinion that the NS is useful for all diseases except death.16 The results of previous experimental studies have confirmed the extract of NS seeds and TQ have inhibitory effects on inducible nitric oxide synthase and production of nitric oxide13, 17, 18 as well as anti-inflammatory and anticancer activities.19, 20 Both NS and TQ also showed the beneficial effects in lipopolysaccharide – induced depression like behavior in rats.21 The anti-oxidant effects of NS and TQ in carbon tetrachloride (CCl4)-induced oxidative injury in rat liver,22 isolated rat hepatocytes,23 hypercholesterolemic rats24 and gentamicin and cyclosporine induced kidney injury have been reported.25 The antioxidant effects of NS oil and TQ in hippocampal tissues of the rats subjected to cerebral ischemia-reperfusion has also been reported.26 Neuroprotective effects for NS and TQ has also been suggested.27, 28, 29, 30
Based on the properties of NS which has been reported in traditional medicine and in experimental studies, the present study was designed in order to evaluate possible effects of the plant hydro-alcoholic extract on neural damage after PTZ-induced seizures in rats.
2. Materials and methods
2.1. Preparing the plant extract
Powdered seeds (100 g) of NS were extracted in a Soxhlet extractor with ethanol (70%). The resulting extract (yielded 32%) was concentrated under reduced pressure and kept at −20 °C until being used. The extract was dissolved in saline.30
2.2. Animals and the experimental protocol
Thirty male Wistar rats (8 weeks old and weighted 230 ± 20 g) were kept at 22 ± 2 °C and 12 h light/dark cycle at 7:00 am. They were randomly divided to five groups and treated according to the experimental protocol. Group 1 (control group) received saline instead of NS extract or PTZ. The animals in group 2 (PTZ group) were treated by saline instead of NS extract and were injected PTZ (50 mg/kg, i.p.). Groups 3 (PTZ-NS 100), 4 (PTZ-NS 200) and 5 (PTZ-NS 400) were treated by 100, 200 and 400 mg/kg of NS (i.p.) respectively, before each PTZ injection.
2.3. Behavioral procedures
2.3.1. PTZ-induced repeated seizures
The animals were injected by 50 mg/kg PTZ. Following each injection the rats were placed a Plexiglas cage separately, and observed for 60 min. The resultant seizures were classified according to a modified Racine scale as follows: 1- Mouth and facial movements; 2- Head nodding; 3- Forelimb clonus; 4- Rearing; 5- Rearing and falling. The latencies to the first sign of seizure were also recorded.31, 32
2.3.2. Passive avoidance test
The passive avoidance (PA) learning test based on negative reinforcement was carried out. The apparatus had a grid floor and comprised two compartments: one dark and the other one lighted, with a small gate which connected these two parts. This test is performed with the knowledge that rats have a native preference to the dark environment. Before beginning the training session, the animals were familiarized with the apparatus for two successive days (5 min per day). On the ensuing day, they were placed in the lighted compartment and the time latency for entering the dark compartment was noted down. During the training phase, the animals were located in the lighted compartment while facing toward the walls and away from the gate and received an electric shock (2 mA, 2 s duration) when they were entered the dark part. The animals were then returned to their cages. In retention or test phase which was carried out at 1 h after the training sessions, the rats were placed in the light compartment, and time latency to enter the dark compartment was recorded.33 All behavioral tests were conducted between 16:00 and 18:00 o'clock.
2.4. Histological assessment
One hour after finishing the experimental protocols (see above), the rats were given a high dose of urethane and transcardially perfused with 100 ml of saline followed by 100 ml fixative solution (formaldehyde %4 in 0.2 M buffer phosphate at pH = 7.4). After perfusion, all rats were decapitated and the brains removed. The brains were kept in 4% formaline for at least 72 h and were then processed for histological studies as follows. The paraffin blocks were cut into coronal serial sections of 5 μm thickness. Ten sections including hippocampus tissue from each animal were chosen by systematic randomized method and mounted on poly-l-lysine coated slides for determining of hippocampus dark neurons with Toloidin blue staining.
Since there is no specific immunocytochemical method available for dark neurons demonstration, commonly determination of the dark neurons is based on their increased stainability with cationic dyes (such as Mayer's hematoxylin or toluidine blue), and stainability with anionic dyes (such as acid fuchsin or eosin) also silver staining method that is a selective and sensitive marker of dark neurons in light-microscopic.6
The sections were stained with toluidine blue. The slides were examined with light microscope (BX51, Japan) at magnification of X40 objective lens (UPlan FI, Japan) and digital photographs were taken from hippocampal CA1, CA2, CA3 and dentate gyrus (DG) areas of both hemispheres. For quantitative analysis of dark neurons, the physical dissector method was used.34
2.5. Quantification of TQ in NS seed extract
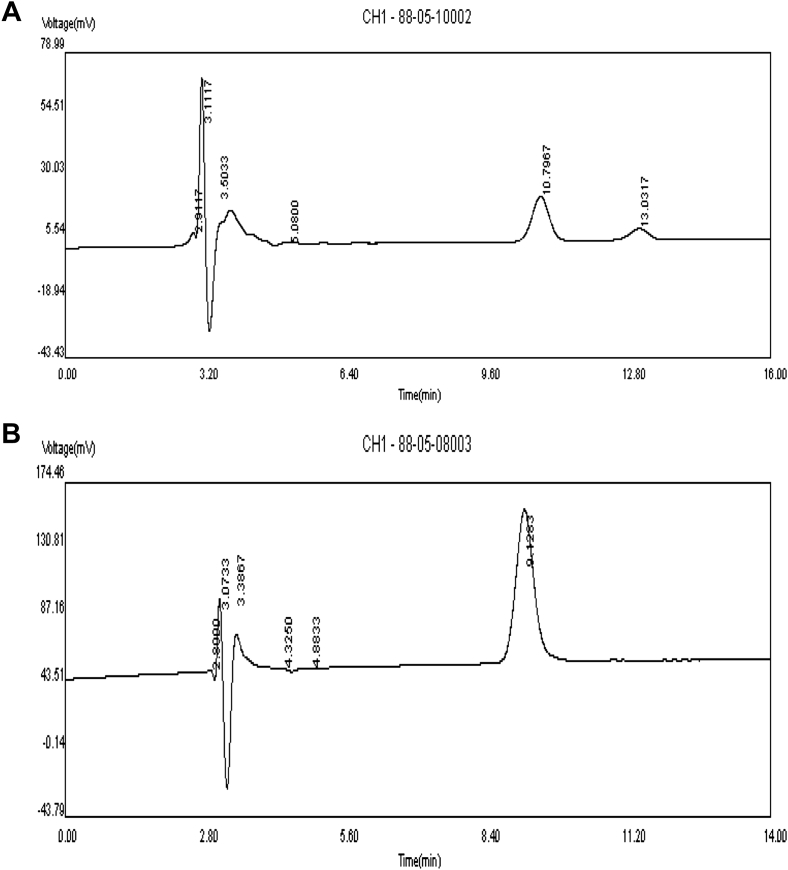
TQ quantification was carried out by HPLC on a reversed-phase C18 analytical column (250 × 4.6 mm, 4.6 mm particle size), using an isocratic mobile phase of water: methanol: 2-propanol (50:45:5% v/v) at a flow rate of 1 ml/min. UV monitoring was carried out at 254 nm. The chromatograms of a sample of NS seed extract and standard TQ were showed in Fig. 6A and B.
Fig. 6.
A chromatogram of purified Nigella sativa seed extract sample (500 μg/ml). (A). HPLC chromatogram of thymoquinone (TQ) dissolved in methanol (5 mg/ml). (B). Detection was carried out at λ 254 nm.
2.6. Statistical analysis
The data were expressed as mean ± SEM. For all data, the one-way ANOVA were run followed by a post hoc comparisons test. The criterion for the statistical significance was P < 0.05.
3. Results
3.1. PTZ-induced repeated seizures
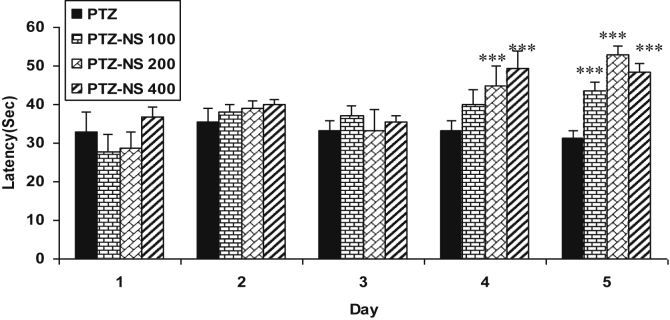
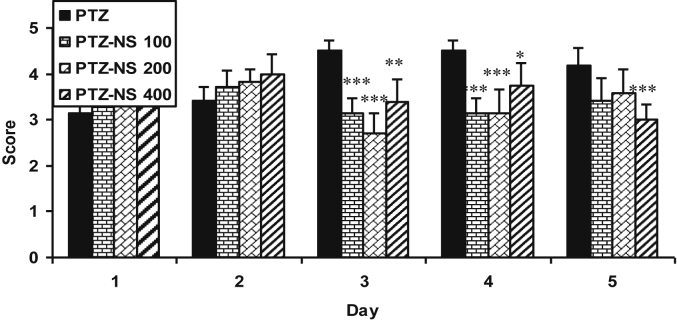
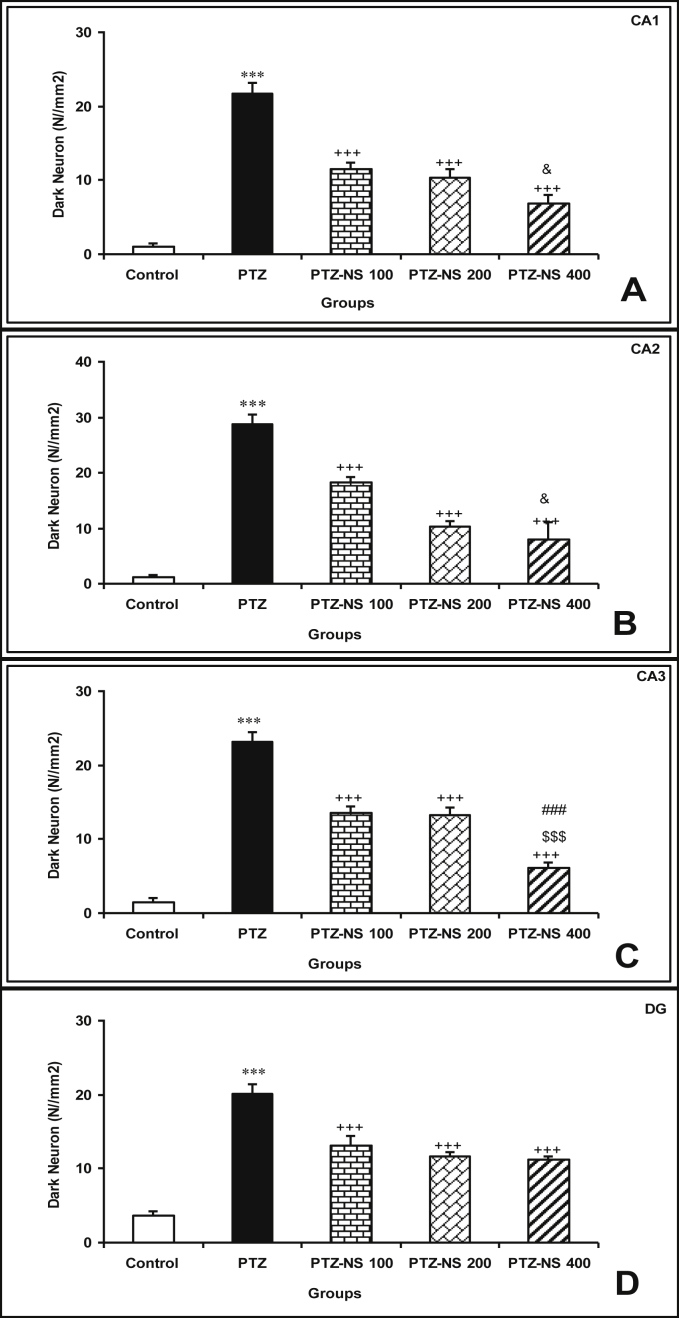
The latency to the onset of seizures of PTZ-NS 200 and PTZ-NS 400 groups on day 4 and of all PTZ-NS 100, PTZ-NS 200 and PTZ-NS 400 groups on day 5 were significantly higher than that of PTZ group (P < 0.001; Fig. 1). The seizure scores in all PTZ – NS 100, PTZ – NS 200 and PTZ-NS 400 groups were significantly lower than that of PTZ group on days 3 and 4 (P < 0.05 and P < 0.001; Fig. 2). The seizure score in PTZ- NS 400 group was also lower than that in PTZ group on day 5 (P < 0.001; Fig. 2).
Fig. 1.
Comparison of latency to the onset of seizures between groups. Data are presented as mean ± SEM (n = 6 in each group). ∗∗∗P < 0.001 in comparison with PTZ group. The animals were injected by PTZ and observed for 60 min. The animals of PTZ- NS 100, PTZ- NS 200 and PTZ-NS 400 groups were treated by 100, 200 and 400 mg/kg of Nigella sativa (NS) extract before PTZ injection. The animals of PTZ group received saline instead of NS extract.
Fig. 2.
Comparison of seizure score between groups. Data are presented as mean ± SEM (n = 6 in each group). ∗P < 0.05 and ∗∗∗P < 0.001 in comparison with PTZ group. The animals were injected by PTZ and observed for 60 min. The animals of PTZ-NS 100, PTZ-NS 200 and PTZ-NS 400 groups were treated by 100, 200 and 400 mg/kg of Nigella sativa (NS) extract before PTZ injection. The animals of PTZ group received saline instead of NS extract.
3.2. Passive avoidance test
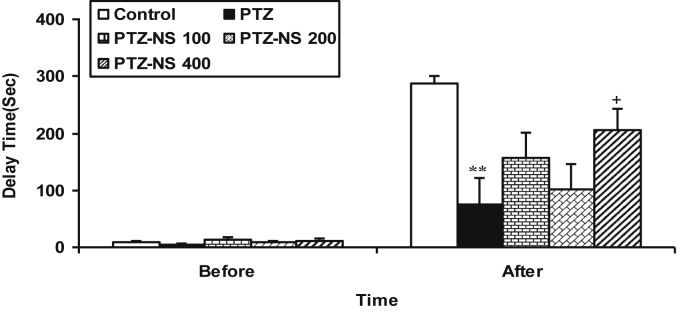
Before receiving the shock, there were no significant differences between the groups. After the shock, the time latency for entering the dark compartment in the PTZ group was lower than that of the control group (Fig. 3, P < 0.01). The treatment of the animals by 400 mg/kg of NS extract significantly increased the time latency for entering the dark compartment after receiving the shock (Fig. 3, P < 0.05). There were no significant differences between PTZ-NS 100, PTZ-NS 200 and PTZ groups (Fig. 3).
Fig. 3.
Comparison of time latency for entering the dark compartment. Data are presented as mean ± SEM (n = 6 in each group). **P < 0.01 in comparison with control group, +P < 0.05 in comparison with PTZ group. The animals were injected by PTZ and observed for 60 min. The animals of PTZ-NS 100, PTZ-NS 200 and PTZ-NS 400 groups were treated by 100, 200 and 400 mg/kg of Nigella sativa extract before PTZ injection. The animals of PTZ group received saline instead of Nigella sativa extract. The animals of control group received saline instead of both the extract and PTZ.
3.3. The effect of NS on production of dark neurons
Dark neurons were identified by the neuronal shrinkage, cytoplasmic esoinophilia, nuclear pyknosis, and surrounding spongiosis in hippocampal formation (Fig. 4). The results showed that the seizures induced by injection of PTZ increased numerical density of dark neurons in CA1, CA2, CA3 and DG areas of hippocampus compared to the control group (P < 0.001; Fig. 5(A–D)). Administration of Nigella sativa at the doses of 100, 200 and 400 mg/kg significantly prevented production of dark neurons due to PTZ – induced seizures in all regions of hippocampus (P < 0.001; Fig. 5 (A–D)). The results also showed that the number of dark neurons in CA1 region of PTZ-NS 400 group were significantly lower than that of PTZ-NS 100 group (P < 0.05) however, there was no significant difference between PTZ-NS 400 and PTZ-NS 200 groups. There was also no significant difference between PTZ-NS 200 and PTZ-NS 100 groups (Fig. 5(A)). Furthermore, the histological results showed that the highest dose (400 mg/kg) of the extract was more effective to prevent the production of dark neurons after repeated seizures in CA1 area compared to the lowest dose (P < 0.05) (Fig. 5(B)). The results showed no significant differences between the groups pre-treated by 400 and 200 mg/kg and also between 100 and 200 mg/kg of the extract (Fig. 5(B)). Similar to the previous areas of hippocampus, the animals of PTZ-NS 400 group had a lowest dark neurons in the CA3 region of their hippocampus compared to both PTZ-NS 100 and PTZ-NS 200 groups (P < 001; Fig. 5(C)) however, there was no significant differences between PTZ-NS 100 and PTZ-NS 200 groups. All doses of the extract prevented production of dark neurons in DG area compared to PTZ group (P < 001) however, there was no significant difference between three doses (Fig. 5(D)).
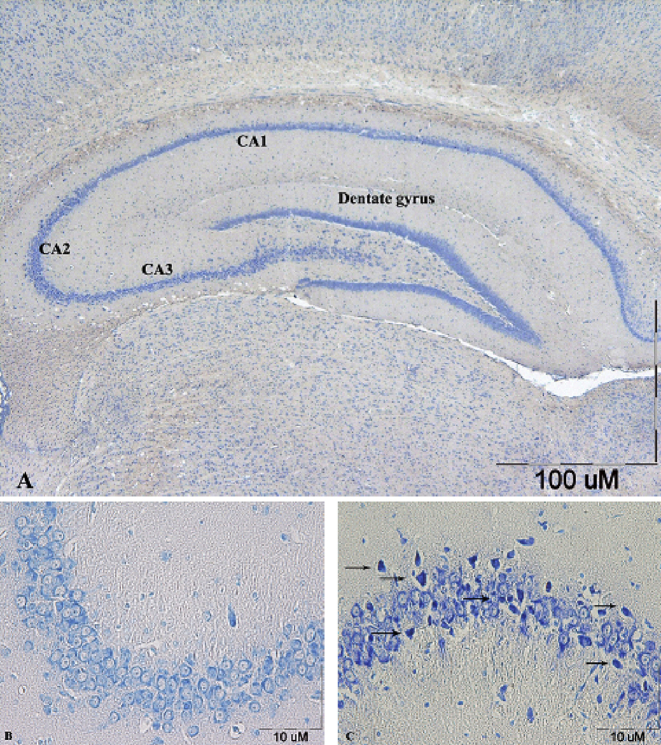
Fig. 4.
Toluidine blue staining of coronal hippocampus sections. A section from control showing the different areas of the hippocampal formation where the hippocampus proper is formed of the Cornu Ammonis (CA) as CA1, CA2 & CA3 regions, and dentate gyrus (DG) is seen surrounding hilar region by its upper & lower limbs (A). A section from control group, showing normal small pyramidal cells in CA2 region (B). A section from PTZ group, showing dark neurons (arrows) among pyramidal cells in CA2 region (C).
Fig. 5.
Comparison of dark neuron numbers per area in CA1(A), CA2(B), CA3(C) and DG(D) areas between groups. Data are presented as mean ± SEM (n = 6 in each group).∗∗∗P < 0.001 in comparison with control group, +++P < 0.001 in comparison with PTZ group, $P < 0.05, $$$P < 0.001 in comparison with PTZ-NS 100 group, ###P < 0.001 in comparison with PTZ-NS 200 group. The animals were injected by PTZ and observed for 60 min. The animals of PTZ-NS 100, PTZ-NS 200 and PTZ-NS 400 groups were treated by 100, 200 and 400 mg/kg of Nigella sativa extract before PTZ injection. The animals of PTZ group received saline instead of Nigella sativa extract. The animals of control group received saline instead of both the extract and PTZ.
The chromatograms of a sample of NS seed extract and standard TQ were showed in Fig. 6A and B.
4. Discussion
The results of the present study showed that PTZ induced repeated seizures damaged to hippocampals neurons of the rats and impaired memory which were prevented by NS extract. Animal and human studies have shown a clear relationship between seizures and pathological conditions in central nervous system (CNS).35, 36, 37 Progressive functional and structural abnormalities in CNS, due to repeated seizures have also been well documented.38, 39, 40 It has been shown that seizures may lead to morphological changes such as production of dark neurons, in brain tissue.10, 32 Dark neurons, previously were considered as histological artifacts in neurosurgical biopsies34, 41 but later, they were seen after brain trauma.34, 42 Recently, it has been well documented that dark neurons are also produced without any trauma or mechanical forces.43 Dark neurons have basophilic appearance and morphological changes and might be seen after hypoglycemia, ischemia, stress and epilepsy.8, 43, 44, 45, 46 Epilepsy has also been introduced as an important cause of dark neuron production.8, 9, 47 The results of present study showed that PTZ-induced repeated seizures were resulted in dark neuron production in the hippocampal regions which were confirmed by our previous studies.10, 32 Several studies have also confirmed hippocampal damages created by seizures.35, 48, 49 The results of present study were consistent with our previous studies in which it was shown that PTZ-induced seizures were followed by production of dark neurons in the brain tissues.10, 32
Furthermore, it has been repeatedly reported that prolonged and recurrent seizures impairs learning and memory.2, 50, 51, 52 Besides of production of dark neurons, our findings using PA test revealed a considerable memory impairment of the rats subjected to the seizure attacks induced by PTZ. Regarding the results of present study the neural damage in hippocampal regions as a link between seizures and memory impairments might be suggested. It has been suggested that release of excitatory neurotransmitters such as glutamate and aspartate as well as neuronal Ca2+ influx have important roles in brain tissue damage and production of dark neurons.43 The brain tissues oxidative damage has also been introduced to have an important role in neural damage, as well as memory impairment after seizure which is preventable by anti-oxidant compounds.11, 12, 53 Regarding the well-known anti-oxidant effects of NS, it is conceivable that the hydroalcoholic extract of the plant have a capability to prevent dark neuron production and attenuates learning and memory impairments due to seizures which was seen in the present study.
The seed extract of NS has been used for many years as a natural remedy for treating of a number of illnesses due to its pharmacological properties and being an immunostimulant, anti-tumor, anti-inflammatory, and respiratory stimulant agent.16, 20, 54, 55, 56 The volatile oil is extracted from the seeds and its main active ingredient TQ, has been shown to exert anti-inflammatory effects in a number of diseases including bronchial asthma.54, 57, 58, 59, 60 TQ supplementation also could alleviate hepatic toxicity induced by lipopolysaccharide as well reduce hepatic malondialdehyde concentrations and apoptosis in rats.61 Mohamed et al. (2005) showed that treatment of rats with TQ prevented the experimental autoimmune encephalomyelitis,62 which was probably due to its antioxidant and anti-inflammatory properties.63 The results of present study also showed that pre-treatment by NS prevented neural damage in the brain regions. Moreover, hippocampal neurodegeneration after chronic tolune exposure in rats has been prevented by NS oil and TQ.64 Hosseinzadeh et al. have also shown that NS and TQ prevented lipid peroxidation increment in hippocampus proteins following global cerebral ischemia-reperfusion injury model in rats.26 Antiepileptic effect of NS and its ability to inhibit excessive reactive oxygen species (ROS) formation in PTZ – induced seizures has been reported.65 Neuroprotective effect of NS in an experimental model of spinal cord injury in rats has also been attributed to its antioxidant capability.66 It has been shown that NS extract and TQ attenuated oxidative stress and neuropathy in diabetic rats.67
Furthermore, It has also been postulated that NS oil and TQ have the antinociception effects which are related to releasing of endogenous opioid peptides in the central nervous system,68 It was also shown that the antinociceptive effect of morphine was reduced in TQ and NS oil-pre-treated mice.68 Considering these effects and the effects of NS on rewarding properties of morphine,69 an interaction between the plant or its ingredients with opioid system might be suggested which may also have a role in attenuating effects of the plant extract on seizures as well as its preventive effects on neural damages and memory impairments which were seen in the present study however, it needs to be more investigated.
On the other hand with keeping in mind that the release of excitatory neurotransmitters such as glutamate and aspartate as well as neuronal transmembrane Ca2+ iflux have important roles in the brain tissue damage and production of dark neurons,43 it might be suggested the effects of the extract which was seen in the present study may at least in part be due to its inhibitory effect on calcium channels70 however, it needs to be more investigated.
As it was mentioned, the beneficial effects of NS has been repeatedly attributed to the main its constituent TQ. Regarding the chromatograms of a sample of NS seed extract and standard TQ (Fig. 6A and B) it is suggested that TQ is probably the main component of responsible for the effect of NS extract which was seen in the present study.
5. Conclusion
The results of the present study showed that the hydro-alcoholic extract of NS prevented neural damage as well as learning and memory impairments after PTZ – induced repeated seizure in rats. These results support the traditional belief about the beneficial effect of NS on the nervous system. Further studies are required for determining (confirming) the protective effect of NS.
Conflicts of interest
The authors do not have any direct financial relationship with the commercial identities mentioned in this article. Therefore, the authors have no conflicts of interest.
Acknowledgments
Authors would like to thank the Vice President of Research in Mashhad University of Medical Sciences for the financial supports.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Banos J.H., LaGory J., Sawrie S. Self-report of cognitive abilities in temporal lobe epilepsy: cognitive, psychosocial, and emotional factors. Epilepsy Behav. 2004;5(4):575–579. doi: 10.1016/j.yebeh.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Mortazavi F., Ericson M., Story D., Hulce V.D., Dunbar G.L. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005;7(4):629–638. doi: 10.1016/j.yebeh.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Ebert U., Brandt C., Loscher W. Delayed sclerosis, neuroprotection, and limbic epileptogenesis after status epilepticus in the rat. Epilepsia. 2002;43(suppl 5):86–95. doi: 10.1046/j.1528-1157.43.s.5.39.x. [DOI] [PubMed] [Google Scholar]
- 4.Mao R.R., Tian M., Yang Y.X., Zhou Q.X., Xu L., Cao J. Effects of pentylenetetrazol-induced brief convulsive seizures on spatial memory and fear memory. Epilepsy Behav. 2009;15(4):441–444. doi: 10.1016/j.yebeh.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Vohra B.P., James T.J., Sharma S.P., Kansal V.K., Chudhary A., Gupta S.K. Dark neurons in the ageing cerebellum: their mode of formation and effect of Maharishi Amrit Kalash. Biogerontology. 2002;3(6):347–354. doi: 10.1023/a:1021303415191. [DOI] [PubMed] [Google Scholar]
- 6.Zsombok A., Toth Z., Gallyas F. Basophilia, acidophilia and argyrophilia of “dark” (compacted) neurons during their formation, recovery or death in an otherwise undamaged environment. J Neurosci Methods. 2005;142(1):145–152. doi: 10.1016/j.jneumeth.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Ingvar M., Morgan P.F., Auer R.N. The nature and timing of excitotoxic neuronal necrosis in the cerebral cortex, hippocampus and thalamus due to flurothyl-induced status epilepticus. Acta Neuropathol. 1988;75(4):362–369. doi: 10.1007/BF00687789. [DOI] [PubMed] [Google Scholar]
- 8.Baracskay P., Szepesi Z., Orban G., Juhasz G., Czurko A. Generalization of seizures parallels the formation of “dark” neurons in the hippocampus and pontine reticular formation after focal-cortical application of 4-aminopyridine (4-AP) in the rat. Brain Res. 2008;1228:217–228. doi: 10.1016/j.brainres.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Gallyas F., Guldner F.H., Zoltay G., Wolff J.R. Golgi-like demonstration of “dark” neurons with an argyrophil III method for experimental neuropathology. Acta Neuropathol. 1990;79(6):620–628. doi: 10.1007/BF00294239. [DOI] [PubMed] [Google Scholar]
- 10.Karimzadeh F., Hosseini M., Mangeng D. Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement Altern Med. 2012;12:76. doi: 10.1186/1472-6882-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao F., Gao Y., Liu Y.F., Wang L., Li Y.J. Berberine exerts an anticonvulsant effect and ameliorates memory impairment and oxidative stress in a pilocarpine-induced epilepsy model in the rat. Neuropsychiatr Dis Treat. 2014;10:2139–2145. doi: 10.2147/NDT.S73210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tome Ada R., Feitosa C.M., Freitas R.M. Neuronal damage and memory deficits after seizures are reversed by ascorbic acid? Arq Neuropsiquiatr. 2010;68(4):579–585. doi: 10.1590/s0004-282x2010000400019. [DOI] [PubMed] [Google Scholar]
- 13.Boskabady M.H., Shirmohammadi B., Jandaghi P., Kiani S. Possible mechanism(s) for relaxant effect of aqueous and macerated extracts from Nigella sativa on tracheal chains of guinea pig. BMC Pharmacol. 2004;4:3. doi: 10.1186/1471-2210-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beheshti F., Hosseini M., Shafei M.N. The effects of Nigella sativa extract on hypothyroidism-associated learning and memory impairment during neonatal and juvenile growth in rats. Nutr Neurosci. 2014 doi: 10.1179/1476830514Y.0000000144. [DOI] [PubMed] [Google Scholar]
- 15.Hosseini M., Mohammadpour T., Karami R., Rajaei Z., Sadeghnia H.R., Soukhtanloo M. Effects of the hydro-alcoholic extract of Nigella sativa on scopolamine-induced spatial memory impairment in rats and its possible mechanism. Chin J Integr Med. 2015;21(6):438–444. doi: 10.1007/s11655-014-1742-5. [DOI] [PubMed] [Google Scholar]
- 16.Ali B.H., Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 17.El-Mahmoudy A., Matsuyama H., Borgan M.A. Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol. 2002;2(11):1603–1611. doi: 10.1016/s1567-5769(02)00139-x. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood M.S., Gilani A.H., Khwaja A., Rashid A., Ashfaq M.K. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res. 2003;17(8):921–924. doi: 10.1002/ptr.1251. [DOI] [PubMed] [Google Scholar]
- 19.Winkler C., Schroecksnadel K., Ledochowski M., Schennach H., Houcher B., Fuchs D. In vitro effects of Nigella sativa seeds extracts on stimulated peripheral blood mononuclear cells. Pteridines. 2008;19:101–106. [Google Scholar]
- 20.Worthen D.R., Ghosheh O.A., Crooks P.A. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18(3A):1527–1532. [PubMed] [Google Scholar]
- 21.Hosseini M., Zakeri S., Khoshdast S. The effects of Nigella sativa hydro-alcoholic extract and thymoquinone on lipopolysaccharide – induced depression like behavior in rats. J Pharm Bioallied Sci. 2012;4(3):219–225. doi: 10.4103/0975-7406.99052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanter M., Coskun O., Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006;80(4):217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 23.Mansour M.A., Ginawi O.T., El-Hadiyah T., El-Khatib A.S., Al-Shabanah O.A., Al-Sawaf H.A. Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol. 2001;110(3–4):239–251. [PubMed] [Google Scholar]
- 24.Ismail M., Al-Naqeep G., Chan K.W. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med. 2010;48(5):664–672. doi: 10.1016/j.freeradbiomed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Yaman I., Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62(2):183–190. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinzadeh H., Parvardeh S., Asl M.N., Sadeghnia H.R., Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14(9):621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Sedaghat R., Roghani M., Khalili M. Neuroprotective effect of thymoquinone, the Nigella sativa bioactive compound, in 6-hydroxydopamine-induced hemi-parkinsonian rat model. Iran J Pharm Res. 2014;13(1):227–234. [PMC free article] [PubMed] [Google Scholar]
- 28.Akhtar M., Imam S.S., Afroz Ahmad M., Najmi A.K., Mujeeb M., Aqil M. Neuroprotective study of Nigella sativa-loaded oral provesicular lipid formulation: in vitro and ex vivo study. Drug Deliv. 2014;21(6):487–494. doi: 10.3109/10717544.2014.886640. [DOI] [PubMed] [Google Scholar]
- 29.Hobbenaghi R., Javanbakht J., Sadeghzadeh S. Neuroprotective effects of Nigella sativa extract on cell death in hippocampal neurons following experimental global cerebral ischemia-reperfusion injury in rats. J Neurol Sci. 2014;337(1–2):74–79. doi: 10.1016/j.jns.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Mousavi S.H., Tayarani-Najaran Z., Asghari M., Sadeghnia H.R. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30(4):591–598. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebrahimzadeh Bideskan A.R., Hosseini M., Mohammadpour T. Effects of soy extract on pentylenetetrazol-induced seizures in ovariectomized rats. Zhong Xi Yi Jie He Xue Bao. 2011;9(6):611–618. doi: 10.3736/jcim20110606. [DOI] [PubMed] [Google Scholar]
- 32.Mansouri S., Ataei M.L., Hosseini M., Bideskan A.R. Tamoxifen mimics the effects of endogenous ovarian hormones on repeated seizures induced by pentylenetetrazole in rats. Exp Neurobiol. 2013;22(2):116–123. doi: 10.5607/en.2013.22.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naghibi S.M., Hosseini M., Khani F. Effect of aqueous extract of Crocus sativus L. on morphine-induced memory impairment. Adv Pharmacol Sci. 2012 doi: 10.1155/2012/494367. 494367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ooigawa H., Nawashiro H., Fukui S. The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate. Acta Neuropathol. 2006;112(4):471–481. doi: 10.1007/s00401-006-0108-2. [DOI] [PubMed] [Google Scholar]
- 35.Toth Z., Yan X.X., Haftoglou S., Ribak C.E., Baram T.Z. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18(11):4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes G.L. Seizure-induced neuronal injury: animal data. Neurology. 2002;59(9 suppl 5):S3–S6. doi: 10.1212/wnl.59.9_suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- 37.Sankar R., Shin D.H., Liu H., Mazarati A., Pereira de Vasconcelos A., Wasterlain C.G. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18(20):8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weniger G., Boucsein K., Irle E. Impaired associative memory in temporal lobe epilepsy subjects after lesions of hippocampus, parahippocampal gyrus, and amygdala. Hippocampus. 2004;14(6):785–796. doi: 10.1002/hipo.10216. [DOI] [PubMed] [Google Scholar]
- 39.Carreno M., Donaire A., Sanchez-Carpintero R. Cognitive disorders associated with epilepsy: diagnosis and treatment. Neurologist. 2008;14(6 suppl 1):S26–S34. doi: 10.1097/01.nrl.0000340789.15295.8f. [DOI] [PubMed] [Google Scholar]
- 40.Zeman A. When a patient with epilepsy complains about poor memory. Pract Neurol. 2009;9(2):85–89. doi: 10.1136/jnnp.2009.172205. [DOI] [PubMed] [Google Scholar]
- 41.Jortner B.S. The return of the dark neuron. A histological artifact complicating contemporary neurotoxicologic evaluation. Neurotoxicology. 2006;27(4):628–634. doi: 10.1016/j.neuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Cammermeyer J. Is the solitary dark neuron a manifestation of postmortem trauma to the brain inadequately fixed by perfusion? Histochemistry. 1978;56(2):97–115. doi: 10.1007/BF00508437. [DOI] [PubMed] [Google Scholar]
- 43.Kherani Z., Auer R. Pharmacologic analysis of the mechanism of dark neuron production in cerebral cortex. Acta Neuropathol. 2008;116(4):447–452. doi: 10.1007/s00401-008-0386-y. [DOI] [PubMed] [Google Scholar]
- 44.Auer R.N., Kalimo H., Olsson Y., Siesjo B.K. The temporal evolution of hypoglycemic brain damage. II. Light- and electron-microscopic findings in the hippocampal gyrus and subiculum of the rat. Acta Neuropathol. 1985;67(1–2):25–36. doi: 10.1007/BF00688121. [DOI] [PubMed] [Google Scholar]
- 45.Czurko A., Nishino H. ‛Collapsed’ (argyrophilic, dark) neurons in rat model of transient focal cerebral ischemia. Neurosci Lett. 1993;162(1–2):71–74. doi: 10.1016/0304-3940(93)90562-y. [DOI] [PubMed] [Google Scholar]
- 46.Ishida K., Shimizu H., Hida H., Urakawa S., Ida K., Nishino H. Argyrophilic dark neurons represent various states of neuronal damage in brain insults: some come to die and others survive. Neuroscience. 2004;125(3):633–644. doi: 10.1016/j.neuroscience.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Soderfeldt B., Kalimo H., Olsson Y., Siesjo B.K. Bicuculline-induced epileptic brain injury. Transient and persistent cell changes in rat cerebral cortex in the early recovery period. Acta Neuropathol. 1983;62(1–2):87–95. doi: 10.1007/BF00684924. [DOI] [PubMed] [Google Scholar]
- 48.Pitkanen A., Tuunanen J., Kalviainen R., Partanen K., Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32(1–2):233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 49.Salmenpera T., Kalviainen R., Partanen K., Pitkanen A. Hippocampal and amygdaloid damage in partial epilepsy: a cross-sectional MRI study of 241 patients. Epilepsy Res. 2001;46(1):69–82. doi: 10.1016/s0920-1211(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 50.Hannesson D.K., Howland J., Pollock M., Mohapel P., Wallace A.E., Corcoran M.E. Dorsal hippocampal kindling produces a selective and enduring disruption of hippocampally mediated behavior. J Neurosci. 2001;21(12):4443–4450. doi: 10.1523/JNEUROSCI.21-12-04443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert T.H., Hannesson D.K., Corcoran M.E. Hippocampal kindled seizures impair spatial cognition in the Morris water maze. Epilepsy Res. 2000;38(2–3):115–125. doi: 10.1016/s0920-1211(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 52.Szyndler J., Rok P., Maciejak P. Effects of pentylenetetrazol-induced kindling of seizures on rat emotional behavior and brain monoaminergic systems. Pharmacol Biochem Behav. 2002;73(4):851–861. doi: 10.1016/s0091-3057(02)00912-7. [DOI] [PubMed] [Google Scholar]
- 53.Hosseini M., Harandizadeh F., Niazamand S., Soukhtanloo M., Mahmoudabady M. Antioxidant effect of Achillea wilhelmsii extract on pentylenetetrazole (seizure model)-induced oxidative brain damage in Wistar rats. Indian J Physiol Pharmacol. 2013;57(4):418–424. [PubMed] [Google Scholar]
- 54.Houghton P.J., Zarka R., de las Heras B., Hoult J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61(1):33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 55.Swamy S.M., Tan B.K. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol. 2000;70(1):1–7. doi: 10.1016/s0378-8741(98)00241-4. [DOI] [PubMed] [Google Scholar]
- 56.el Tahir K.E., Ashour M.M., al-Harbi M.M. The respiratory effects of the volatile oil of the black seed (Nigella sativa) in guinea-pigs: elucidation of the mechanism(s) of action. Gen Pharmacol. 1993;24(5):1115–1122. doi: 10.1016/0306-3623(93)90358-5. [DOI] [PubMed] [Google Scholar]
- 57.Kalus U., Pruss A., Bystron J. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17(10):1209–1214. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 58.Hajhashemi V., Ghannadi A., Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother Res. 2004;18(3):195–199. doi: 10.1002/ptr.1390. Epub 2004/04/23. [DOI] [PubMed] [Google Scholar]
- 59.Mahgoub A.A. Thymoquinone protects against experimental colitis in rats. Toxicol Lett. 2003;143(2):133–143. doi: 10.1016/s0378-4274(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 60.El Gazzar M., El Mezayen R., Nicolls M.R., Marecki J.C., Dreskin S.C. Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochim Biophys Acta. 2006;1760(7):1088–1095. doi: 10.1016/j.bbagen.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Helal G.K. Thymoquinone supplementation ameliorates acute endotoxemia-induced liver dysfunction in rats. Pak J Pharm Sci. 2010;23(2):131–137. [PubMed] [Google Scholar]
- 62.Mohamed A., Afridi D.M., Garani O., Tucci M. Thymoquinone inhibits the activation of NF-kappaB in the brain and spinal cord of experimental autoimmune encephalomyelitis. Biomed Sci Instrum. 2005;41:388. [PubMed] [Google Scholar]
- 63.Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13–14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Kanter M. Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochem Res. 2008;33(3):579–588. doi: 10.1007/s11064-007-9481-z. [DOI] [PubMed] [Google Scholar]
- 65.Ilhan A., Gurel A., Armutcu F., Kamisli S., Iraz M. Antiepileptogenic and antioxidant effects of Nigella sativa oil against pentylenetetrazol-induced kindling in mice. Neuropharmacology. 2005;49(4):456–464. doi: 10.1016/j.neuropharm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Kanter M., Coskun O., Kalayci M., Buyukbas S., Cagavi F. Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum Exp Toxicol. 2006;25(3):127–133. doi: 10.1191/0960327106ht608oa. [DOI] [PubMed] [Google Scholar]
- 67.Hamdy N.M., Taha R.A. Effects of Nigella sativa oil and thymoquinone on oxidative stress and neuropathy in streptozotocin-induced diabetic rats. Pharmacology. 2009;84(3):127–134. doi: 10.1159/000234466. [DOI] [PubMed] [Google Scholar]
- 68.Abdel-Fattah A.M., Matsumoto K., Watanabe H. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol. 2000;400(1):89–97. doi: 10.1016/s0014-2999(00)00340-x. [DOI] [PubMed] [Google Scholar]
- 69.Anvari M., Seddigh A., Shafei M.N. Nigella sativa extract affects conditioned place preference induced by morphine in rats. Anc Sci Life. 2012;32(2):82–88. doi: 10.4103/0257-7941.118537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boskabady M.H., Keyhanmanesh R., Khamneh S., Ebrahimi M.A. The effect of Nigella sativa extract on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs. Clinics (Sao Paulo) 2011;66(5):879–887. doi: 10.1590/S1807-59322011000500027. [DOI] [PMC free article] [PubMed] [Google Scholar]