Abstract
Objectives/Hypothesis
The antitussive properties of (±) baclofen on laryngeal muscle activities have not been determined. The hypothesis of this study was that administration of (±) baclofen would suppress upper airway muscle motor activity in a dose-dependent manner during cough.
Study Design
This is a prospective, preclinical, hypothesis-driven, paired design.
Methods
Electromyograms of the parasternal, rectus abdominis, thyroarytenoid, posterior cricoarytenoid, and thyrohyoid were measured, along with esophageal pressure. Cough was elicited by mechanical stimulation of the lumen of the intrathoracic trachea in spontaneously breathing cats.
Results
Baclofen (±) (3–10 µg kg−1 i.a.) induced decreases in the electromyogram amplitude of the rectus abdominis motor drive during coughing, the inspiratory and active expiratory (E1) phases of cough, and cough number per epoch. There was no effect of (±) baclofen on the EMG amplitudes of any of the laryngeal muscles, the parasternal, or the duration of the passive expiratory (E2) phase.
Conclusions
Results from the present study indicate differential control mechanisms for laryngeal and inspiratory motor drive during cough, providing evidence of a control system regulating laryngeal activity and inspiratory spinal drive that is divergent from the control of expiratory spinal motoneurons.
Keywords: Cough, baclofen, thyrohyoid, parasternal, rectus abdominis, thyroarytenoid, posterior cricoid arytenoid, antitussive, upper airway, larynx
INTRODUCTION
Cough is a coordinated protective airway reflex that generates vigorous and rapid airflow, clearing the airway of foreign bodies from the respiratory tract.1–6 While the cough reflex is usually beneficial, there are scenarios where overexpression of this behavior induces significant morbidity. Chronic cough can result from asthma, upper airway viral infection, chronic bronchitis and other chronic respiratory diseases, and/or gastroesophageal reflux disease.4,7,8 Regardless of origin, chronic cough may result in unpleasant irritation in the throat or chest, which can be painful; chest tightness; hoarse voice; dysphonia and other vocal cord dysfunction symptoms; and acid reflux symptoms.7 In more extreme cases, patients with vigorous chronic cough can develop hiatal or inguinal hernias or fractured ribs.8 Other physical symptoms such as syncope, urinary incontinence, emesis, headaches, and exhaustion can also be caused by chronic cough.7,8 These effects often result in social interference, embarrassment, and withdrawal.9
Antitussive drugs are often prescribed for acute and/or chronic disorders in which cough persists after the underlying condition is resolved. However, clinically available antitussives have not been effective in treating chronic cough.10–20 Centrally acting cough suppressants including opioid and opioid derivatives are considered to be the most effective antitussives; however, there are mixed results with even the most commonly prescribed antitussive codeine and the analgesic/sedative-free dextromethorphan.10–20 Opioids are also associated with side-effects such as physical dependence and respiratory depression.17,21
Baclofen, a centrally acting GABA-Breceptor agonist, has been traditionally used as a treatment for muscle spasticity in patients with spinal cord injuries or degenerative disorders such as multiple sclerosis.22–27 More recently, baclofen has been used to treat cerebral palsy spasticity.27–32 Belvisi et al. (1989) showed that GABA has an effect on neutrally evoked bronchoconstriction in the anesthetized guinea pig via GABA-B receptors.33 Bronchoconstriction is a consistent secondary component and an indication of cough as it increases airflow velocity.4,34 Baclofen has antitussive properties in cat and guinea pig models.35–38 Bolser et al. (1999) found that baclofen decreases primary expiratory motor drive and cough number in a dose-dependent manner without significantly altering primary inspiratory motor drive or total cough cycle time in cats.39 However, the effect of baclofen on laryngeal motor drive, particularly the intrinsic and extrinsic laryngeal muscles, has not been studied. The purpose of this study was to determine the effect of the centrally active antitussive drug baclofen on the cough motor drive and patterns.
MATERIALS AND METHODS
Experiments were performed on five spontaneously breathing male adult cats (mean body weight 4.88 ± 0.71 kg). The animals were anesthetized with sodium pentobarbital by an initial intravenous dose of 35–40 mg kg−1 with supplementary doses of 1–3 mg kg−1 i.v. given as needed. Atropine sulfate (0.1 mg kg−1, i.v.) was administered to reduce airway secretions. The animals were tracheotomized and the right femoral artery and vein were cannulated to monitor arterial blood pressure. End-tidal CO2, body temperature, and blood gas composition were continually monitored and maintained at physiologic levels. Bipolar insulated fine-wire electrodes were inserted into the parasternal, rectus abdominis, posterior cricoarytenoid, thyroarytenoid, and the thyrohyoid muscles to record electromyographic (EMG) activity. Because the anterolateral abdominal muscles are activated together act as a unit during cough,40 only the rectus abdominis was recorded in this study. An esophageal balloon was placed to record esophageal pressure (mm Hg). Correct electrode position was confirmed by visual inspection and EMG activity patterns during breathing and cough. A cough was defined as a parasternal EMG bursting with negative inflection in esophageal pressure, followed by rectus abdominis EMG bursting with a positive inflection in esophageal pressure.
Conditions
There were three conditions for all animals: the first condition was the control condition with no administered (±) baclofen; condition 1 was (±) baclofen 3 µg kg−1 i.a.; and condition 2 was (±) baclofen 10 µg kg−1 i.a. Condition 2 was a cumulative dose with an additional 7 µg kg−1 i.a. to the 3 µg kg−1 i.a. given during condition 1. Baclofen (±) doses were separated by approximately 10 minutes to allow for distribution of the drug throughout all body compartments and completion of all cough trials.
Cough Stimulus and Phases
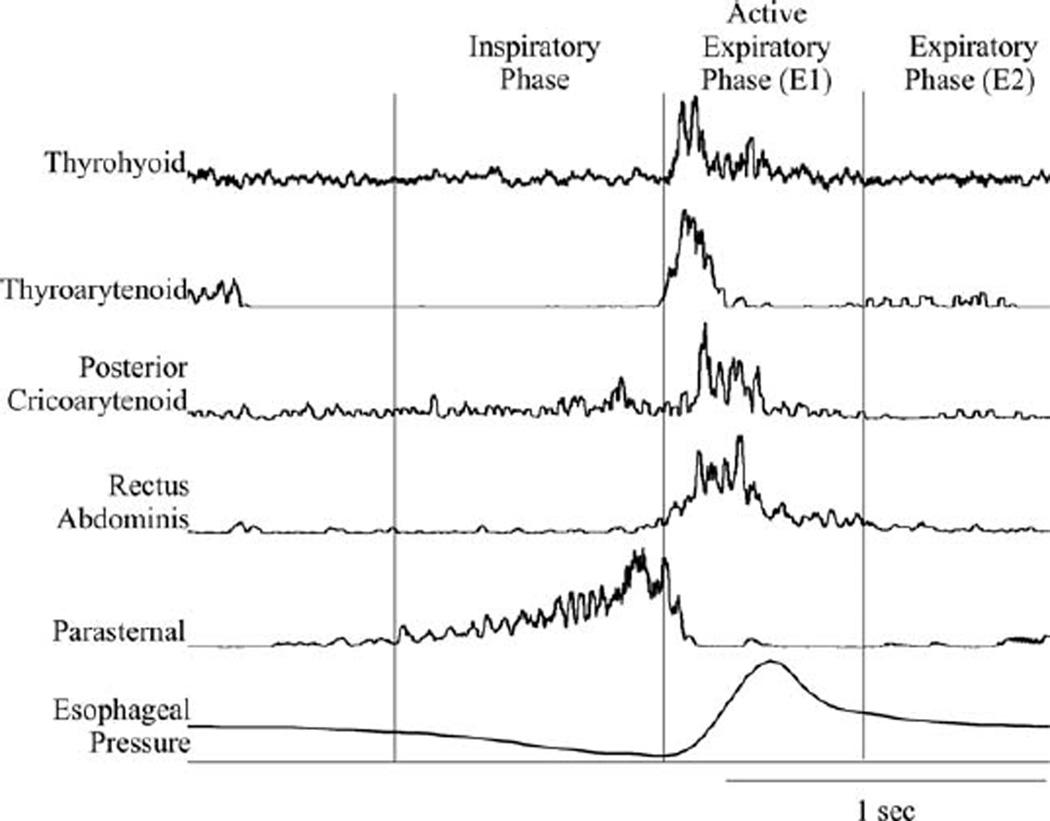
To evoke tracheobronchial cough, the extra- and intrathoracic trachea was mechanically probed with a length of polyethylene tubing repetitively over the stimulus period. Note that due to the tracheostomy there was no laryngeal stimulation during the cough trials. The stimulation consisted of three trials of 20 seconds of mechanical stimulation, with a 1 minute-interstimulus interval. Before the recorded condition trials, approximately 20 consecutive cough trials were conducted to establish a stable baseline response. We used the cough phase duration defined in Wang et al.41 (Fig. 1). The inspiratory phase (CTI), the expiratory phase with active muscle activity (CTE1), the passive expiratory phase (CTE2), and the total cough (CTtot) durations were measured. Duration measures are expressed in milliseconds (ms). CTI was defined as the onset of parasternal activity to the maximum burst of the parasternal EMG; CTE1 was defined as the maximum burst of the parasternal EMG to the end of the rectus abdominis EMG activity; and CTE2 was defined as the end of the rectus abdominis EMG activity to the onset of the parasternal EMG activity for the next cough in the epoch.
Fig. 1.
Example of thyrohyoid activity during a single cough. EMG signals were recorded for the parasternal, rectus abdominis, posterior cricoarytenoid, thyroarytenoid, and thyrohyoid muscles. The inspiratory phase is from the onset of the inspiratory activity to its peak activity. The active expiratory phase (E1) is from the peak of the inspiratory activity to the end of the rectus abdominis activity. The expiratory phase (E2) is from the end of the active expiratory activity to the onset of the next inspiratory burst.
Data Processing and Statistical Analysis
“Spike 2” Version 7 (Cambridge Electronic Design, United Kingdom) was used to automate the analysis process. For duration measures, a mean of the peak noise for 1 second preceding the first cough in the series was set as a threshold marker. The duration measures were marked as onset when the signal was greater than the threshold level and as completion when the signal was again less than or equal to the threshold level. The amplitude measures were marked as the largest amplitude during the EMG burst duration. To enable comparison across animals, EMG data were normalized (% of maximum) in each experiment to the maximum burst during coughing. Differences in dependent variables were analyzed by repeated measures ANOVA with Dunnett’s post hoc test. Data are expressed as mean ± standard error with P <0.05 considered significant.
Baclofen (±) and atropine sulfate were obtained from Sigma-Aldrich (St. Louis, MO) and pentobarbital sodium was obtained from Lundbeck, Inc. (Deerfield, IL). Doses were calculated as their free base.
RESULTS
We conducted 45 cough trials in five animals. Administration of (±) baclofen (3 and 10 µg kg−1, i.a.) resulted in a decrease in the number of coughs and the magnitude of abdominal motor bursts and cough-related esophageal pressures.
Description of Cough Response
Coughing was represented by large ramp-like inspiratory bursts, immediately followed by bursting in the rectus abdominis muscle EMG that was ballistic-like (rate of rise equivalent to rate of decrement). Figure 1 shows the activity of the parasternal, rectus abdominis, thyroartytenoid, and thyrohyoid muscle EMGs during tracheobronchial cough. The thyroarytenoid muscle EMG bursts across the cough inspiratory-expulsive phase transition were consistent with its role in laryngeal adduction for the compression phase (Fig. 1). The thyrohyoid EMG activity pattern was coincident with the thyroarytenoid (laryngeal adductor), and also had a ballistic-like burst. In one animal, thyrohyoid activity began later during the active expiratory phase (E1) than the adductor discharge.
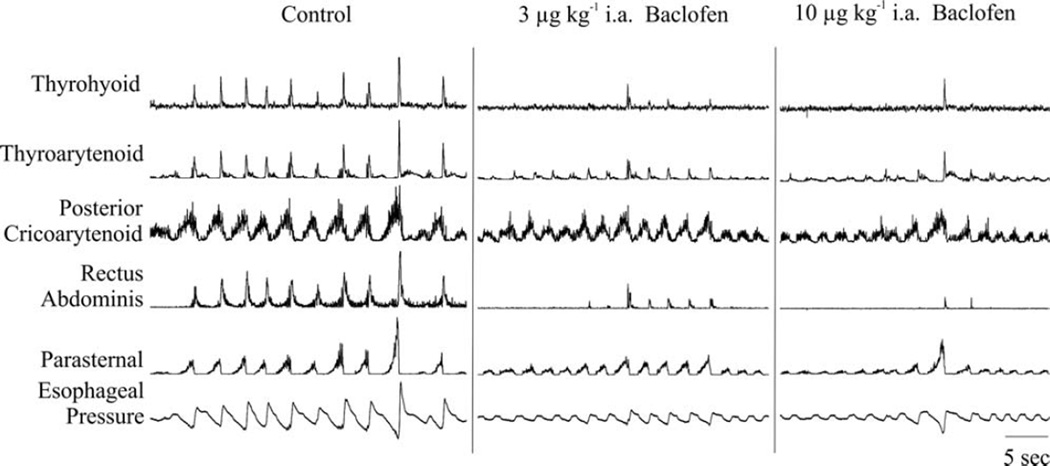
Figure 2 is an example of the effects of increasing doses of intraarterial (±) baclofen on esophageal pressure and respiratory muscle EMG magnitudes. The control record shows respiratory muscle EMG and esophageal pressure responses to repetitive coughing in response to mechanical stimulation of the extra- and intrathoracic trachea. When cough did occur after (±) baclofen administration, the magnitudes of parasternal and posterior cricoarytenoid were relatively unchanged. (Fig. 2). However, thyroarytenoid and thyrohyoid EMG magnitudes appeared to be reduced, and this trend was not statistically significant (Table I).
Fig. 2.
Example of the effects of (±) baclofen on cough. EMG signals were recorded for the parasternal (PS), rectus abdominis (RA), posterior cricoarytenoid (PCA), thyroarytenoid (TA), and thyrohyoid (ThHy) muscles. Esophageal pressure (EP) was also recorded. The control period shows repetitive coughing from stimulation of the trachea. Administration of baclofen significantly affected cough number and expiratory drive. Magnitudes of parasternal and posterior cricoarytenoid were relatively unchanged.
TABLE I.
Effect of (±) Baclofen on the Amplitude of Selected Cough-Related Muscle EMGs.
| Muscle (% of maximum) | Condition | Amplitude (mean ± standard error) |
|---|---|---|
| Parasternal | Control | 48 ± 6 |
| 3 µg kg−1i.a. | 44 ± 9 | |
| 10 µg kg−1i.a. | 33 ± 8 | |
| Rectus abdominis | Control | 52 ± 5 |
| 3 µg kg−1i.a. | 29 ± 7* | |
| 10 µg kg−1i.a. | 18 ± 2† | |
| Posterior cricoarytenoid | Control | 65 ± 5 |
| 3 µg kg−1i.a. | 56 ± 9 | |
| 10 µg kg−1i.a. | 49 ± 8 | |
| Thyroarytenoid | Control | 46 ± 10 |
| 3 µg kg−1i.a. | 36 ± 10 | |
| 10 µg kg−1i.a. | 34 ± 13 | |
| Thyrohyoid | Control | 48 ± 5 |
| 3 µg kg−1i.a. | 42 ± 5 | |
| 10 µg kg−1i.a. | 40 ± 4 |
P <0.05,
P <0.01, relative to control
Effect of Baclofen on Cough Amplitudes
Table I summarizes the magnitudes of the EMG moving averages during the control period and the two doses of (±) baclofen. Baclofen (±) had a significant dose-dependent effect on rectus abdominis (RA) EMG amplitude during cough (P < 0.02). Post-hoc tests revealed differences between control (53 ± 6) and 3 µg/kg (±) baclofen (30 ± 7, P < 0.05) and 10 µg/kg (±) baclofen (18 ±3, P < 0.01). There was no effect on the percent maximum EMG amplitude of the parasternal (P = 0.17), posterior cricoarytenoid (P = 0.11), the thyroarytenoid (P = 0.39), or the thyrohyoid (P = 0.62) during cough.
Effect of Baclofen on Cough Phase Durations
Baclofen (±) administration also significantly prolonged the duration of CTI (P < 0.05). Post hoc tests revealed significant differences between control (785 ± 83 ms) and 10 µg kg−1 (±) baclofen (1169 ± 115 ms, P < 0.05). CTE1 was also affected (P < 0.02), and with control (377 ± 60 ms, P < 0.01) significantly different from the 10 µg kg−1 (±) baclofen (117 ± 25 ms, P < 0.01). CTE2 (P = 0.25) and CTTOT (P = 0.28) did not exhibit a similar effect. Cough number per epoch (P < 0.02) was also affected, and there was a significant difference in cough number between the control condition (10.3 ± 1.2) and 10 µg kg−1 (±) baclofen (3.6 ± 1.495, P < 0.01).
DISCUSSION
This is the first report a) that the extrinsic laryngeal muscle, the thyrohyoid, is active during coughing and b) of the effects of (±) baclofen on the activity of laryngeal muscles (thyrohyoid, posterior cricoarytenoid, and the thyroarytenoid) during this behavior. Baclofen (±) significantly reduced cough number and abdominal expiratory muscle EMG magnitudes, but it had no effect on the amplitudes of laryngeal muscles during coughing. Lastly, (±) baclofen affected specific cough phase durations, lengthening the inspiratory phase and shortening the active expiratory phase (E1), but did not significantly change the total cough phase duration.
The thyrohyoid is an extrinsic laryngeal muscle (innervated by a branch of cervical nerve one; C1) that upon contraction elevates the larynx, which can be used to stiffen the laryngeal complex during dynamic respiratory behaviors.42–45 This muscle was activated in ballistic-like fashion during cough, coincident with the thyroarytenoid (an expiratory phasic laryngeal adductor). We propose that with stronger expiratory drives during coughs, the thyrohyoid acts to proportionally stabilize the larynx to reduce turbulence of the upper airway. Higher turbulence during coughing promotes deposition of particles from the lower airway during cough and adherence to the laryngeal/pharyngeal mucosa.
Baclofen (±) is a specific GABA-B receptor agonist with a central action on the cough reflex in the cat,46–51 suggesting that our results were due to effects of this drug on the brainstem cough control circuit. While acknowledging that GABA-B agonists can inhibit activity of peripheral sensory afferents,52 the work of Bolser et al.46–49,51 established that (±) baclofen given through intraarterial administration at this dosage range has solely central effects. Baclofen (±) administration had no significant effect on laryngeal muscle motor drive during cough, although it decreased the magnitudes of abdominal EMG and esophageal pressure. However, recurrent laryngeal nerve discharge evoked by electrical stimulation of the superior laryngeal nerve (SLN), can be decreased or abolished by administration of antitussive drugs (codeine, dextromethorphan, oxymetebanol, and pentobarbital),53 and NMDA receptor blockade.54 It is probable that the afferent pathways exciting laryngeal motor neurons during SLN stimulation are independent of the cough pattern generator, and that the excitability of these motor neurons during cough is from novel connections within the medullary circuit. Results from the present study indicate differential control mechanisms for laryngeal muscles controlled by the recurrent laryngeal nerve (posterior cricoarytenoid and thyroarytenoid), C1 (thyrohyoid), and inspiratory spinal motor drive versus expiratory spinal motor drive during cough, providing evidence of a control system regulating upper airway activity that is divergent from that controlling drive to thoracic spinal motoneurons.
This study further confirms the work of Bolser et al.46–49,51 demonstrating that 1) (±) baclofen decreased abdominal activity while having little effect on parasternal activity during cough, and 2) there was a significant decrease in cough number. It also extended his work by demonstrating that (±) baclofen did not significantly change total cough time, but did increase inspiratory phase duration and decreased the duration of the active expiratory phase (E1). Wang et al.41 expanded the cough phase definition to examine not only total cough cycle time, but the CTI, CTE1, and CTE2. This work also revealed that the total cough cycle time has a strong linear relationship with CTE2 and appears to be unrelated to CTI and CTE1 durations. This may explain why we found no significant change in CTE2 and total cough cycle time, and confirms Bolser et al.48 finding that there was no significant change in total cough cycle time after administration of (±) baclofen. Bolser et al.55 hypothesized the presence of a gating mechanism that controls the excitability of cough motor pattern. These results also provide evidence to suggest that the control of the amplitude of the muscle activation is separately controlled from its duration. Microinjection of baclofen into the NTS in rabbits did increase the cough phase duration without affecting inspiratory amplitude; and Mutolo et al. hypothesized that this was related to a reduced rate of rise of the inspiratory muscles, and thus a inspiratory depressant effect.50
The caudal nucleus tractus solitarius (NTS) has been hypothesized as a possible action site for the cough gating mechanism.50,56 Furthermore, this gating mechanism may be separated into second-order neuron circuits that control different portions of the cough pattern, for example inspiratory, expiratory, and the compression phase through laryngeal motor control. McGovern et al.57 has shown, through trans-synaptic anatomical tracing in the rat, an average of 3,000 NTS neurons labeled following an injection of herpes simplex virus 1 into the extrathoracic trachea. The transsynaptic labeling progression began in the caudal portion of the NTS, then spread to the medial and dorsolateral subnuclei. The large number of labeled neurons (≈3,000) at the 96-hour time point supports a complex processing network of afferent information in the NTS. This putative complex multisynaptic network differs from current hypotheses of a pauci-synaptic network of second-order interneurons in the NTS.58–61
It is also plausible that many neural structures may respond to i.a. administration of (±) baclofen. These effects may be due to low doses of the drug at many brainstem locations, while a similar effect might be obtained by administration of a higher dose within a small area. The drug effect is highly dependent on the route of administration, and the local concentration within a number of brainstem locations following i.a. administration of antitussive drugs has yet to be verified.
CONCLUSION
Dicpinigaitis62–64 demonstrated the cough suppressive effects of baclofen (decreasing the number of coughs per trial and increasing the cough-response threshold) in healthy subjects, those with cervical spinal cord injury, and those with refractory (i.e., chronic) cough. However, the present study indicates differential control mechanisms for laryngeal muscles, inspiratory muscles, versus expiratory muscle motor drive during cough, providing evidence of a control system regulating upper airway activity which is divergent from that controlling drive to thoracic spinal motoneurons. Future work is needed to further understand the mechanisms that control excitability to the inspiratory muscles, laryngeal muscles, and expiratory muscles in animal and human models. This might elucidate mechanisms for the differential effects of cough suppressants, such as codeine during clinical trials versus preclinical studies. The outcomes of these studies should provide further information about brainstem locations and role of neurotransmitters for the central control of cough.
Acknowledgments
This study was supported by grants HL 89104, HL 103415, and HL 107745 from the National Institutes of Health.
We thank Donald Bolser for his help with article preparation, and Melanie Rose for her expert technical assistance.
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Bucher K. Pathophysiology and pharmacology of cough. Pharmacol Rev. 1958;10:43–58. [PubMed] [Google Scholar]
- 2.Friebel H. Physiologie, Pathologie, und Pharakologie der Bronchialsekretion und des Hustens. Klinische Physiologie. Stuttgart: Thieme. 1963:301–339. [Google Scholar]
- 3.Gunther WV, Krieger E. Husten und Auswurf. Deisenhofen: Dustri Verlag; 1972. [Google Scholar]
- 4.Korpas M, Tomori M. Progress in Respiration Research. Basel, Switzerland: S. Karger; 1979. [Google Scholar]
- 5.Lavietes MH, Smeltzer SC, Cook SD, Modak RM, Smaldone GC. Airway dynamics, oesophageal pressure and cough. Eur Respir J. 1998;11:156–161. doi: 10.1183/09031936.98.11010156. [DOI] [PubMed] [Google Scholar]
- 6.Chung KF, Bolser D, Davenport P, Fontana G, Morice A, Widdicombe J. Semantics and types of cough. Pulm Pharmacol Ther. 2009;22:139–142. doi: 10.1016/j.pupt.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther. 2011;24:267–271. doi: 10.1016/j.pupt.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Young EC, Smith JA. Quality of life in patients with chronic cough. Ther Adv Respir Dis. 2010;4:49–55. doi: 10.1177/1753465809358249. [DOI] [PubMed] [Google Scholar]
- 9.Kuzniar TJ, Morgenthaler TI, Afessa B, Lim KG. Chronic cough from the patient’s perspective. Mayo Clin Proc. 2007;82:56–60. doi: 10.4065/82.1.56. [DOI] [PubMed] [Google Scholar]
- 10.Eddy NB, Friebel H, Hahn KJ, Halbach H. Codeine and its alternates for pain and cough relief. 4. Potential alternates for cough relief. Bull World Health Organ. 1969;40:639–719. [PMC free article] [PubMed] [Google Scholar]
- 11.Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther. 1992;17:175–180. doi: 10.1111/j.1365-2710.1992.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JA, Novack AH, Almquist JR, Rogers JE. Efficacy of cough suppressants in children. J Pediatr. 1993;122:799–802. doi: 10.1016/s0022-3476(06)80031-4. [DOI] [PubMed] [Google Scholar]
- 13.Parvez L, Vaidya M, Sakhardande A, Subburaj S, Rajagopalan TG. Evaluation of antitussive agents in man. Pulm Pharmacol. 1996;9:299–308. doi: 10.1006/pulp.1996.0039. [DOI] [PubMed] [Google Scholar]
- 14.Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol. 1997;49:1045–1049. doi: 10.1111/j.2042-7158.1997.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee PCL, Jawad MS, Eccles R. Antitussive efficacy of dextromethorphan in cough associated with acute upper respiratory tract infection. J Pharm Pharmacol. 2000;52:1137–1142. doi: 10.1211/0022357001774903. [DOI] [PubMed] [Google Scholar]
- 16.Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Curr Opin Allergy Clin Immunol. 2007;7:32–36. doi: 10.1097/ACI.0b013e3280115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung KF. Currently available cough suppressants for chronic cough. Lung. 2008;186(suppl 1):S82–S87. doi: 10.1007/s00408-007-9030-1. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2008:CD001831. doi: 10.1002/14651858.CD001831.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Morice AH, Menon MS, Mulrennan SA, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175:312–315. doi: 10.1164/rccm.200607-892OC. [DOI] [PubMed] [Google Scholar]
- 20.Pavesi L, Subburaj S, Porter-Shaw K. Application and validation of a computerized cough acquisition system for objective monitoring of acute cough: a meta-analysis. Chest. 2001;120:1121–1128. doi: 10.1378/chest.120.4.1121. [DOI] [PubMed] [Google Scholar]
- 21.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 22.Hudgson P, Weightman D. Baclofen in the treatment of spasticity. Br Med J. 1971;4:15–17. doi: 10.1136/bmj.4.5778.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knutsson E, Lindblom U, Martensson A. Differences in effects in gamma and alpha spasticity induced by the GABA derivative baclofen (Lioresal) Brain. 1973;96:29–46. doi: 10.1093/brain/96.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Brogden RN, Speight TM, Avery GS. Baclofen: a preliminary report of its pharmacological properties and therapeutic efficacy in spasticity. Drugs. 1974;8:1–14. doi: 10.2165/00003495-197408010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Penn RD, Kroin JS. Long-term intrathecal baclofen infusion for treatment of spasticity. J Neurosurg. 1987;66:181–185. doi: 10.3171/jns.1987.66.2.0181. [DOI] [PubMed] [Google Scholar]
- 26.Loubser PG, Narayan RK, Sandin KJ, Donovan WH, Russell KD. Continuous infusion of intrathecal baclofen: long-term effects on spasticity in spinal cord injury. Paraplegia. 1991;29:48–64. doi: 10.1038/sc.1991.7. [DOI] [PubMed] [Google Scholar]
- 27.Campbell SK, Almeida GL, Penn RD, Corcos DM. The effects of intrathecally administered baclofen on function in patients with spasticity. Phys Ther. 1995;75:352–362. doi: 10.1093/ptj/75.5.352. [DOI] [PubMed] [Google Scholar]
- 28.Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA. 1991;265:1418–1422. [PubMed] [Google Scholar]
- 29.Albright AL, Barron WB, Fasick MP, Polinko P, Janosky J. Continuous intrathecal baclofen infusion for spasticity of cerebral origin. JAMA. 1993;270:2475–2477. [PubMed] [Google Scholar]
- 30.Albright AL, Gilmartin R, Swift D, Krach LE, Ivanhoe CB, McLaughlin JF. Long-term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg. 2003;98:291–295. doi: 10.3171/jns.2003.98.2.0291. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong RW, Steinbok P, Cochrane DD, Kube SD, Fife SE, Farrell K. Intrathecally administered baclofen for treatment of children with spasticity of cerebral origin. J Neurosurg. 1997;87:409–414. doi: 10.3171/jns.1997.87.3.0409. [DOI] [PubMed] [Google Scholar]
- 32.Butler C, Campbell S. Evidence of the effects of intrathecal baclofen for spastic and dystonic cerebral palsy. AACPDM Treatment Outcomes Committee Review Panel. Dev Med Child Neurol. 2000;42:634–645. doi: 10.1017/s0012162200001183. [DOI] [PubMed] [Google Scholar]
- 33.Belvisi MG, Ichinose M, Barnes PJ. Modulation of non-adrenergic, non-cholinergic neural bronchoconstriction in guinea-pig airways via GABAB-receptors. Br J Pharmacol. 1989;97:1225–1231. doi: 10.1111/j.1476-5381.1989.tb12582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontana GA, Lavorini F. Cough motor mechanisms. Respir Physiol Neurobiol. 2006;152:266–281. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Chapman RW, Danko G, del Prado M, et al. Further evidence for prejunctional GABA-B inhibition of cholinergic and peptidergic bronchoconstriction in guinea pigs: studies with new agonists and antagonists. Pharmacology. 1993;46:315–323. doi: 10.1159/000139062. [DOI] [PubMed] [Google Scholar]
- 36.Bolser DC, Aziz SM, DeGennaro FC, et al. Antitussive effects of GABAB agonists in the cat and guinea-pig. Br J Pharmacol. 1993;110:491–495. doi: 10.1111/j.1476-5381.1993.tb13837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolser DC, DeGennaro FC, O’Reilly S, et al. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol. 1994;113:1344–1348. doi: 10.1111/j.1476-5381.1994.tb17145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hey JA, Mingo G, Bolser DC, Kreutner W, Krobatsch D, Chapman RW. Respiratory effects of baclofen and 3-aminopropylphosphinic acid in guinea-pigs. Br J Pharmacol. 1995;114:735–738. doi: 10.1111/j.1476-5381.1995.tb13265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 40.Bolser DC, Reier PJ, Davenport PW. Responses of the anterolateral abdominal muscles during cough and expiratory threshold loading in the cat. J Appl Physiol. 2000;88:1207–1214. doi: 10.1152/jappl.2000.88.4.1207. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Saha S, Rose MJ, Davenport PW, Bolser DC. Spatiotemporal regulation of the cough motor pattern. Cough. 2009;5:12. doi: 10.1186/1745-9974-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ducharme N, Hackett R, Woodie J, et al. Investigations into the role of the thyrohyoid muscles in the pathogenesis of dorsal displacement of the soft palate in horses. Equine Vet J. 2003;35:258–263. doi: 10.2746/042516403776148200. [DOI] [PubMed] [Google Scholar]
- 43.Mepani R, Antonik S, Massey B, et al. Augmentation of deglutitive thyrohyoid muscle shortening by the Shaker Exercise. Dysphagia. 2009;24:26–31. doi: 10.1007/s00455-008-9167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cook I, Dodds W, Dantas R, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748–G759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 45.Kahrilas PJ. Pharyngeal structure and function. Dysphagia. 1993;8:303–307. doi: 10.1007/BF01321767. [DOI] [PubMed] [Google Scholar]
- 46.Bolser DC. Mechanisms of action of central and peripheral antitussive drugs. Pulmonary Pharmacology. 1996;9:357–364. doi: 10.1006/pulp.1996.0047. [DOI] [PubMed] [Google Scholar]
- 47.Bolser DC, DeGennaro FC. Effect of codeine on the inspiratory and expiratory burst pattern during fictive cough in cats. Brain Res. 1994;662:25–30. doi: 10.1016/0006-8993(94)90792-7. [DOI] [PubMed] [Google Scholar]
- 48.Bolser DC, DeGennaro FC, O’Reilly S, et al. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol. 1994;113:1344–1348. doi: 10.1111/j.1476-5381.1994.tb17145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolser DC, Aziz SM, DeGennaro FC, et al. Antitussive effects of GABAB agonists in the cat and guinea-pig. Br J Pharmacol. 1993;110:491. doi: 10.1111/j.1476-5381.1993.tb13837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol. 2008;295:R243–R251. doi: 10.1152/ajpregu.00184.2008. [DOI] [PubMed] [Google Scholar]
- 51.McLeod RL, Tulshian DB, Bolser DC, et al. Pharmacological profile of the NOP agonist and cough suppressing agent SCH 486757 (8-[Bis(2-Chlorophenyl) Methyl]-3-(2-Pyrimidinyl)-8-Azabicyclo[3.2.1]Octan-3-Ol) in preclinical models. Eur J Pharmacol. 2010;630:112–120. doi: 10.1016/j.ejphar.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green K, Cottrell G. Actions of baclofen on components of the Ca-current in rat and mouse DRG neurones in culture. Br J Pharmacol. 1988;94:235. doi: 10.1111/j.1476-5381.1988.tb11520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mori M, Sakai Y. Effects of antitussive drugs on the activity of the recurrent laryngeal nerve in cats. Jpn J Pharmacol. 1975;25:671. doi: 10.1254/jjp.25.671. [DOI] [PubMed] [Google Scholar]
- 54.Ambalavanar R, Purcell L, Miranda M, Evans F, Ludlow CL. Selective suppression of late laryngeal adductor responses by N-methyl-D-aspartate receptor blockade in the cat. J Neurophysiol. 2002;87:1252–1262. doi: 10.1152/jn.00595.2001. [DOI] [PubMed] [Google Scholar]
- 55.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 56.Bolser D. Central mechanisms II: pharmacology of brainstem pathways. Handb Exp Pharmacol. 2009;187:203–217. doi: 10.1007/978-3-540-79842-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGovern A, Davis-Poynter N, Farrell M, Mazzone S. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience. 2012;207:148–166. doi: 10.1016/j.neuroscience.2012.01.029. Epub 2012. [DOI] [PubMed] [Google Scholar]
- 58.Poliacek I, Morris KF, Lindsey BG, et al. Blood pressure changes alter tracheobronchial cough: computational model of the respiratory-cough network and in vivo experiments in anesthetized cats. J Appl Physiol. 2011;111:861–873. doi: 10.1152/japplphysiol.00458.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther. 2002;15:221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 60.Bonham AC, Sekizawa S, Chen CY, Joad JP. Plasticity of brainstem mechanisms of cough. Respir Physiol Neurobiol. 2006;152:312–319. doi: 10.1016/j.resp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Bonham AC, Chen CY, Sekizawa S, Joad JP. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol. 2006;101:322–327. doi: 10.1152/japplphysiol.00143.2006. [DOI] [PubMed] [Google Scholar]
- 62.Dicpinigaitis PV, Dobkin JB. Antitussive effect of the GABA-agonist baclofen. Chest. 1997;111:996–999. doi: 10.1378/chest.111.4.996. [DOI] [PubMed] [Google Scholar]
- 63.Dicpinigaitis PV, Rauf K. Treatment of chronic, refractory cough with baclofen. Respiration. 1998;65:86–88. doi: 10.1159/000029232. [DOI] [PubMed] [Google Scholar]
- 64.Dicpinigaitis PV, Grimm DR, Lesser M. Baclofen-induced cough suppression in cervical spinal cord injury. Arch Phys Med Rehabil. 2000;81:921–923. doi: 10.1053/apmr.2000.5612. [DOI] [PubMed] [Google Scholar]