Abstract
Background
Applied environmental strategies for asthma control are often expensive, but may save longer-term healthcare costs. Whether these savings outweigh additional costs of implementing these strategies is uncertain.
Methods
We conducted a systematic review to estimate the expenditures and savings of environmental interventions for asthma in the state of Maryland. Direct costs included hospitalizations, emergency room, and clinic visits. Indirect expenditures included costs of lost work productivity and travel incurred during the usage of healthcare services. We used decision analysis, assuming a hypothetical cohort of the approximated 49,290 pediatric individuals in Maryland with persistent asthma, to compare costs and benefits of environmental asthma interventions against the standard of care (no intervention) from the societal perspective.
Results
Three interventions among nine articles met the inclusion criteria for the systematic review: 1) environmental education using medical professionals; 2) education using non-medical personnel; and 3) multi-component strategy involving education with non-medical personnel, allergen-impermeable covers, and pest management. All interventions were found to be cost-saving relative to the standard of care. Home environmental education using non-medical professionals yielded the highest net savings of $14.1 million (95% simulation interval (SI): $−.283 million, $19.4 million), while the multi-component intervention resulted in the lowest net savings of $8.1 million (95% SI: $−4.9 million, $15.9 million). All strategies were most sensitive to the baseline number of hospitalizations in those not receiving targeted interventions for asthma.
Conclusions
Limited environmental reduction strategies for asthma are likely to be cost-saving to the healthcare system in Maryland and should be considered for broader scale-up in other economically similar settings.
Keywords: cost analysis, economics, health outcomes, health policy, health system
Introduction
The prevalence of asthma across the European study centers had increased in the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 1 to Phase 3 from less than 5% to over 20% (1). The point prevalence of asthma in the USA increased from 7.3% in 2001 to 8.4% in 2010, with the highest rates noted among the pediatric population (1). The proportion of these patients with persistent asthma has been difficult to classify for a variety of methodological reasons, but individual studies in a variety of urban settings have reported ranges from 30 to 64% (2–5). At a statewide level, the Maryland Department of Health and Mental Hygiene (DHMH) reported 11.9% of children in 2009 having a history of asthma (6). The economics of the disease is most profoundly felt among lower-income urban families who often reside in homes with higher levels of environmental triggers (7, 8). It has been estimated that environmental determinants may contribute up to 30% of asthma symptoms and may account for $2–4 billion in asthma healthcare expenditures every year in the United States (9, 10).
Interventions to address home environmental asthma triggers span a wide array of complexity and cost, from simple educational programs to complex – and often costly – home remediation strategies. The efficacy of these interventions in improving health is increasingly clear (11, 12), but whether such interventions can also save money is uncertain. The majority of past asthma valuation studies have focused on the pharmacoeconomics of adult medical management and have generally documented the value of rescue and controller medications using a variety of payer perspectives (13, 14). A limited number of studies have conducted cost analyses of multi-component home-based environmental strategies on pediatric asthma outcomes showing a benefit that can match or exceed the program costs (15). However, no study to date has evaluated the effect of environmental strategies on the major drivers of direct and indirect pediatric asthma costs from a population level relevant for statewide decision makers, namely hospitalizations and other clinical encounters. Although high-quality data on the effectiveness of environmental interventions for asthma are sparse, it is nonetheless important to use the available data to inform decision-making, given the rise in pediatric asthma cases in urban locations where increasing numbers of low-income populations are residing in substandard housing, and resources for health are increasingly constrained (10, 15). No study to date has evaluated the effect of environmental strategies on the major drivers of direct and indirect pediatric asthma costs from a population level relevant for statewide decision makers, namely hospitalizations and other clinical encounters. We therefore performed a cost-consequence analysis of environmental strategies for asthma control, using data from the state of Maryland.
Methods
Decision Model
We used decision analysis to estimate all incremental costs and benefits, from a societal perspective, of selected environmental strategies for asthma control (Figure 1). Our study population consisted of a hypothetical cohort of 49,290 children with persistent asthma. We adopted a societal perspective with a time horizon of 1 year.
Figure 1.
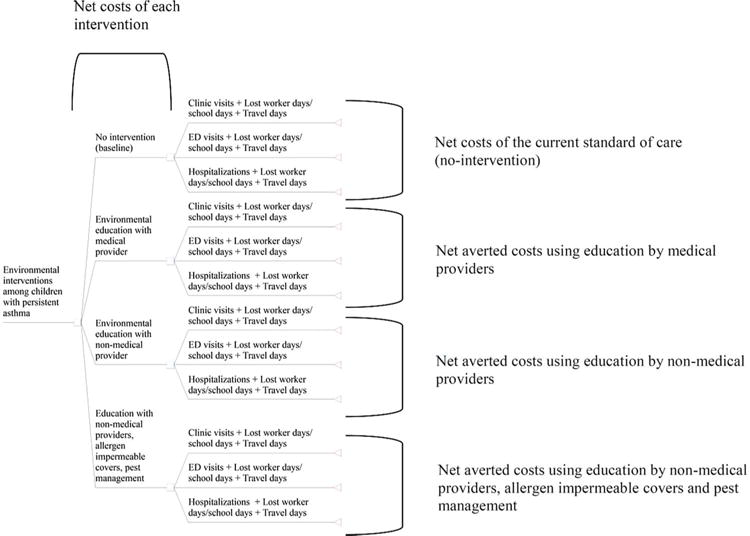
Study analysis figure for direct and indirect asthma outcomes among single and multi-component environmental interventions.
Interventions and Health Resource Utilization: Meta-Analysis of Published Literature
To determine the appropriate study interventions (i.e., those with sufficient data to inform relevant cost modeling), we performed a systematic review and meta-analysis of studies describing environmental strategies for asthma control (e-Appendices 1 and 2 available online at http://informahealthcare.com/doi/suppl/10.3109/02770903.2013.792351). We searched PubMed, Embase, and CINAHL Plus databases from January 1980 through October 2012 using select combinatorial search terms. We included all multimodal interventions that met the following criteria: at least three studies that had the same primary single or multi-component environmental strategies while also reporting the rate of hospitalizations, ER, and urgent asthma-related clinic visits. Analyses were grouped according to the primary environmental strategy employed among the study populations. Environmental education was minimally defined by at least one occasion of evaluation and recommended reduction strategies of potential domestic asthma triggers. Medical professionals were defined as respiratory therapists (RTs) or registered nurses (RNs). Educators not meeting that definition were assumed to be non-medical professionals. Multi-component strategies were assessed for the use of education, allergen-impermeable covers, pest management, air purifiers, mold removal, and air conditioner/heating replacement. Pediatric patients were defined as ≤ 18 years of age. Meta-analysis was performed using version 5.1 Cochrane Review Manager (The Nordic Cochrane Centre, Copenhagen, Denmark). Pooled point probabilities were estimated from dichotomous outcome data. Mantel-Haenszel methods were used to estimate the risk ratios for all strata assuming a fixed effects model. The strength of the evidence for each intervention was rated using a modified Sackett scale containing 5 levels instead of the original 10 subcategories (e-Appendix 3) (16).
Hypothetical Patient Characteristics
We constructed our study population based on health encounters in 2009 within the state of Maryland from the 2011 Maryland Asthma Surveillance Report (MASR) (6). The prevalence of pediatric asthma cases in the state was determined using the CDC Behavioral Risk Factor Surveillance System (BRFSS), Youth Tobacco Surveys (YTS), and Youth Risk Behavior Surveys (YRBS). Data in these reports were weighted to reflect statewide demographics. The frequency of health encounters captured in the MASR were obtained from the Maryland Health Services Cost Review Commission (HSCRC) ambulatory care and hospital discharge profiles using International Classification of Diseases, Ninth Edition (ICD-9) codes 493.0–493.9. The number of persistent asthmatics used to characterize the hypothetical cohort of 49,290 patients in each intervention arm of the decision model was derived from the 31% of current asthmatics (n = 159,000) identified in the MASR who had seen a physician for an urgent outpatient asthma evaluation on at least a single occasion. The total population of persistent asthmatics was then used to calculate hospitalizations, ER, and clinic visits based on MASR percentage data.
Cost Parameters
We considered the following costs of each intervention: costs of implementing the selected interventions, hospitalizations, emergency department visits, urgent care clinic visits, lost time from work, and travel costs. Educational costs provided by medical professionals were averaged from 2009 median salaries of RTs and RNs from the US Department of Labor and then applying a 0.3 full-time equivalent (FTE) commitment (17). The 0.3 FTE component assumed a part-time role for health professionals at approximately 12 hours per week. Costs for non-medical professionals included a salary and time commitment for training by medical professionals. Their salary was based on the 2009 median salary from the US Department of Labor of a medical assistant and applying a 0.3 FTE commitment at 12 hours per week (17). Medical professional training costs used a 0.1 FTE commitment based on the averaged RT and RN median salaries as described above. The expenditures for follow-up visits included those required for training supplies and transportation. Average costs of allergen-impermeable covers were based on pricing from Allergy Control Products (Duluth, Georgia, USA) (18). Pest management expenses were determined based on three treatments sessions (Terminix International Company L.P., Memphis, Tennessee, USA). Clinic costs were based on the 2011 average charge of a moderate complexity follow-up asthma clinic visit (ICD-9 493.xx) by a general pediatrician at the Johns Hopkins Hospital (Baltimore, Maryland, USA). ER and hospitalization charges were based on the 2011 averages at the Johns Hopkins Hospital in which the primary diagnosis was asthma (ICD-9 493.xx) (19). It was assumed that pediatric care centers throughout Maryland had cost-to-charge ratios that approximated that of Johns Hopkins Hospital. Health encounter costs were calculated by the application of the 2009 median national cost-to-charge ratio.
Indirect costs of lost school days were estimated as the forfeiture of a single family member’s income during health encounters. Daily income loss was calculated upon the 2009 average national wage index divided by 250 days of work per year. The model assumed that clinic and ER visits each resulted in a single lost day of work. The average duration of hospitalizations were assumed to be 3 days, resulting in three lost days of work. Base case estimates of travel costs were applied on one occasion for all services. All costs were inflated to 2009 prices using the medical component of the CPI. No costs were discounted, given the 12-month time horizon. We did not incorporate health effects (e.g., quality-adjusted life years) as monetary gains or losses.
Cost-Consequence Analysis and Sensitivity Analyses
The primary outcome of the analysis was the incremental cost of each intervention, relative to the baseline of no asthma-specific interventions. This was calculated as the savings from healthcare visits and savings from caregivers’ lost income from work productivity minus the costs of implementing the intervention. The following equation and variables were used to calculate the incremental averted costs of each intervention:
|
| ||
| Benefits | Costs | |
| (Ccv * Cno) − (Ccv * Ci) + (Cer * ERno) − (Cer * ERi) + (Cho * Hno) − (Cho * Hi) + (Cldw * WDno) − (Cldw * WDi) + (Ctd * TDno) − (Ctd * TDi) |
– |
Hypothetical cohort size * Cint + (Cfu * 3 visits) |
| Variables | ||
| Ccv = Cost of clinic visit | Cno = Clinic visits with no intervention | Ci = Clinic visits with intervention |
| Cer = Cost of emergency room visit | ||
| Cho = Cost of hospitalization | ERno = ER visits with no intervention | ERi = ER visits with intervention |
| Cldw = Cost of loss day of work | Hno = Hospitalizations with no intervention | Hi = Hospitalizations with intervention |
| Ctd = Cost of travel day | ||
| Cint = Cost of the intervention | WDno = Worker days lost with no intervention | WDi = Worker days lost with intervention |
| Cfu = Cost of follow-up visits | TDno = Travel days with no intervention | TDi = Travel days with intervention |
|
| ||
The formula was applied to a situation where every individual in the cohort received the intervention to the situation in which patients received no intervention. We performed one-way sensitivity analyses on all parameters by varying each model parameter by ±50% of its baseline value. Multivariate sensitivity analysis was performed to assess the impact of simultaneous changes in all parameters by varying all estimates over triangular distributions. To account for the possibility that environmental strategies might entail substantially larger costs than our reference estimates, we also created conservative scenario analyses by simultaneously increasing the base case parameter estimates of environmental component costs by 100% and 200% of their baseline values. Expected values were set to base case parameter estimates and maximum/minimum values were based on ±50% of the expected parameter values. The results of 10,000 simulations were presented as 95% simulation intervals (SIs), which correspond to the 2.5% and 97.5% of simulated results. Analyses were performed using TreeAge Pro 2012 (Tree Age Software, Inc., Williamstown, Massachusetts, USA).
Results
Systematic Review and Meta-Analysis
A total of three multimodal interventions met the inclusion criteria, each of which had three supporting studies (Table 1, e-Appendix 4). The three primary environmental components included: 1) environmental education using medically trained professionals; 2) environmental education using non-medical trained individuals; and 3) multi-component approach that included the use of education with non-medical individuals, allergen-impermeable covers and pest management. All interventions were projected to reduce urgent care clinic visits by 36–63% and ER visits by 44–63%. The point estimates used for the multi-component intervention had the least impact on the frequency of urgent care clinic visits (.36, 95% SI: .32, .41) and the highest impact on ER visits (.63, 95% SI: .53, .74). Education by non-medical providers was the sole intervention projected to significantly reduce the hospitalizations (.50, 95% SI: .33, .77). Non-significant point estimates in the model included the frequency of hospitalizations in the interventions using education by medical providers (.71, 95% SI: .42, 1.16) and the multi-component strategies (.83, 95% SI: .64, 1.08). Two randomized control trials qualified as the sole Level 1 evidence studies (26, 27). The remaining seven studies provided Level 2 and 4 evidence according to the modified Sackett scale (16).
Table 1.
Summary of single and multi-component home environmental interventions used in the assessment asthma health outcomes.
| Environmental intervention studiesa | Setting | Sample size | Age (years) | Outcome measurements (Pooled risk ratios attributed to intervention) RR (95% CI) |
||
|---|---|---|---|---|---|---|
| Urgent care clinic visits | ER Visits | Hospitalizations | ||||
| Environmental education using medical providers | ||||||
| Hughes et al. (20) Level 2 Randomized control trial |
Urban (Canada) | 47 | 6–16 | .63 (.41, .95) | .44 (.28, .67) | .71(.42, 1.16) |
| Stout et al. (21) Level 4 Pre-post trial |
Urban (USA) | 23 | 1–9 | |||
| Shelledy et al. (22) Level 4 Pre-post trial |
Urban (USA) | 18 | 3–18 | |||
| Environmental education using non-medical providers | ||||||
| Prinomo et al. (23) Level 49 Pre-post trial |
Urban (USA) | 104 | ≤18 | .66 (.53, .82) | .56 (.44, .73) | .50 (.33,.77) |
| Largo et al. (24) Level 4 Pre-post trial |
Urban (USA) | 243 | 0–17 | |||
| Margellos-Anast et al. (25) Level 4 Pre-post trial |
Urban (USA) | 50 | 2–16 | |||
| Multi-component intervention (education using non-medical providers, allergen-impermeable covers, pest management) | ||||||
| Carter et al. (26) Level 1 Randomized control trial |
Urban (USA) | 30 | 5–16 | .36 (.32, .41) | .63 (.53, .74) | .83 (.64, 1.08) |
| Sullivan et al. (27) Level 1 Randomized control trial |
Urban (USA) | 515 | 5–11 | |||
| Bryant-Stephens and Li (28) Level 2 Randomized control trial |
Urban (USA) | 153 | 2–16 | |||
Evidence level is calculated from a modified Sackett scale containing five levels (e-Appendix 3).
Cost-Consequence Analysis
We estimated that all interventions would be cost-saving relative to the scenario of no intervention (Tables 2 and 3). Health encounters and lost work days were highest in the no-intervention scenario owing to higher healthcare utilization from more frequent exacerbations in the longer term. The cost to institute the intervention was lowest among the cohort using education by non-medical providers ($7.3 million, 95% SI: $4.5, 10.1 million) and highest among those using the multi-component strategy ($14.3 million, 95% SI: $8.8, 20.0 million). Education by non-medical providers resulted in the greatest costs saved from averted urgent care clinic visits ($3.3 million) and ER visits ($2.9 million). The multi-component intervention yielded the highest single health encounter savings from avoided hospitalizations ($10.6 million), but resulted in the lowest averted costs of urgent care clinic visits ($1.8 million). Single component interventions using education with medical and non-medical providers yielded per-cohort annual savings of $13.2 million (95% SI: $.210 million, 21 million) and $14.1 million (95% SI: $−.283 million, 19.4 million), respectively. Because of its greater expense in implementation and less dramatic impact on healthcare utilization, the multi-component intervention generated net savings of $8.1 million (95% SI: $−4.9 million, 15.9 million). Although direct comparisons between interventions could not be made (as each was only compared to a no-intervention scenario in the literature), the non-medical education scenario was estimated to provide the greatest overall savings.
Table 2.
Base case parameter estimates. The frequency of events is based on an estimated cohort size of 49,290 persistent asthmatics in Maryland. All costs are in 2009 US dollars.
| Parameter | Base value | Range for sensitivity analysis (±50%) | Source |
|---|---|---|---|
| Costs per person ($US dollars) | |||
| Environmental strategies | |||
| Education (medical) | $196 | $98–$294 | estimated |
| Education (non-medical) | $148 | $74–$222 | estimated |
| Follow-up education visits | $20 | $10–$30 | estimated |
| Allergen-impermeable covers | $85 | $42–$127 | 18 |
| Pest management | $60 | $30–$90 | estimated |
| Health encounters | |||
| Emergency room visit | $620 | $310–$930 | 19 |
| Hospitalization | $5310 | $2655–$7965 | 19 |
| Urgent care clinic visit | $102 | $51–$153 | 19 |
| Caregiver | |||
| Income lost per day | $172 | $86–$258 | 17 |
| Travel | |||
| Transportation, Parking & Food | $15 | $7–$22 | estimated |
| Frequency of direct and indirect units per hypothetical cohort (N) | |||
| No Intervention | |||
| Urgent care clinic visit | 49,290 | 24,645–73,935 | 6 |
| Emergency room visit | 10,893 | 5446–16,339 | 6 |
| Hospitalization | 2412 | 1206–3618 | 6 |
| Lost worker productivity days | 67,419 | 33,709–10,1128 | – |
| Travel cost days | 62,595 | 31,297–93,892 | – |
| Environmental education by medical providers | |||
| Urgent care clinic visit | 18,237 | 9118–27,355 | 6,20–22 |
| Emergency room visit | 6100 | 3050–9150 | 6,20–22 |
| Hospitalization | 699 | 349–1048 | 6,20–22 |
| Lost worker productivity days | 26,434 | 13,217–39,651 | – |
| Travel cost days | 25,036 | 12,518–37,554 | – |
| Environmental education by non-medical providers | |||
| Urgent care clinic visit | 16,758 | 8379–25,137 | 6,23–25 |
| Emergency room visit | 4793 | 2396–7189 | 6,23–25 |
| Hospitalization | 1206 | 603–1809 | 6,23–25 |
| Lost worker productivity days | 25,169 | 12,584–37,753 | – |
| Travel cost days | 22,757 | 11,378–34,135 | – |
| Environmental education by non-medical providers, allergen-impermeable covers, pest management | |||
| Urgent care clinic visit | 31,546 | 15,773–47,319 | 6,26–28 |
| Emergency room visit | 4030 | 2015–6045 | 6,26–28 |
| Hospitalization | 410 | 205–615 | 6,26–28 |
| Lost worker productivity days | 36,806 | 18,403–55,209 | – |
| Travel cost days | 35,986 | 17,993–53,979 | – |
Table 3.
Base case cost analysis of a hypothetical cohort (n = 49,290) of persistent asthmatics in each environmental strategy. Direct and indirect costs saved by the intervention were calculated from the baseline number of units in the no intervention group. All costs are in 2009 US dollars.
| Parameter | Net intervention Costs (US$) | Urgent care clinic visits (averted)
|
ER visits (averted)
|
Hospitalizations (averted)
|
Lost days of work (averted)
|
Travel days (averted)
|
Total averted costs per cohort-year ($US) | Total net savings per cohort-year (US$) |
|---|---|---|---|---|---|---|---|---|
| Costs (US$ saved) | Costs (US$ saved) | costs) (US$ saved) | Costs (US$ saved) | Costs (US $ saved) | ||||
| No intervention | – | 49290 – |
10893 – |
2412 – |
67419 – |
62595 – |
– | |
| Environmental education by non-medical providers | $9.6 million (95% SI: $5.9, 13.3 million) | 31053 $3.1 million |
4793 $2.9 million |
1713 $9.1 million |
40985 $7.1 million |
37559 $600 thousand |
$22.8 million | $13.2 million (95% SI: $.210, 21.0 million) |
| Environmental education by non-medical providers | $ 7.3 million (95% SI: $4.5, 10.1 million) | 32532 $3.3 million |
6100 $3.8 million |
1206 $6.4 million |
42250 $ 7.3 million |
39838 $600 thousand |
$21.4 million | $14.1 million (95% SI: $ .283, 19.4 million) |
| Environmental education by non-medical providers, allergen impermeable covers, pest management | $14.3 million (95% SI: $8.8, 20.0 million) | 17744 $1.8 million |
6863 $4.4 million |
2002 $10.6 million |
30613 $5.2 million |
26609 $400 thousand |
$22.4 million | $8.1 million (95% SI: $ 4.9, 15.9 million) |
Sensitivity Analyses
One-way sensitivity analyses, ranging all variables by ±50%, showed that single-component interventions generated net savings over a wide range of parameter variation (Figure 2). The estimated net savings were most sensitive to the number of hospitalizations under the current standard of care (no intervention). The multi-component intervention yielded net costs (i.e., negative savings) when varying the number of hospitalizations under the standard of care, lost worker productivity under the standard of care, and the unit cost of hospitalization. Varying all parameters simultaneously along a triangular distribution yielded continued net savings among the education with medical providers, but a net negative saving in 3% of all simulations using education with non-medical professionals (95% SI: $−.283 million, $19.4 million) (e-Figure 1). The multi-component strategy generated net costs relative to single-component interventions in 18% of all simulations. Increasing cost parameters of environmental health strategy components by 100% of the baseline estimates resulted in continued net savings in 35% of the simulations using education with medical providers (95% SI: $−15.2 million, $10.2 million) and 41% of the simulations using education with non-medical providers (95% SI: $−12.2 million, $10.3 million).
Figure 2.
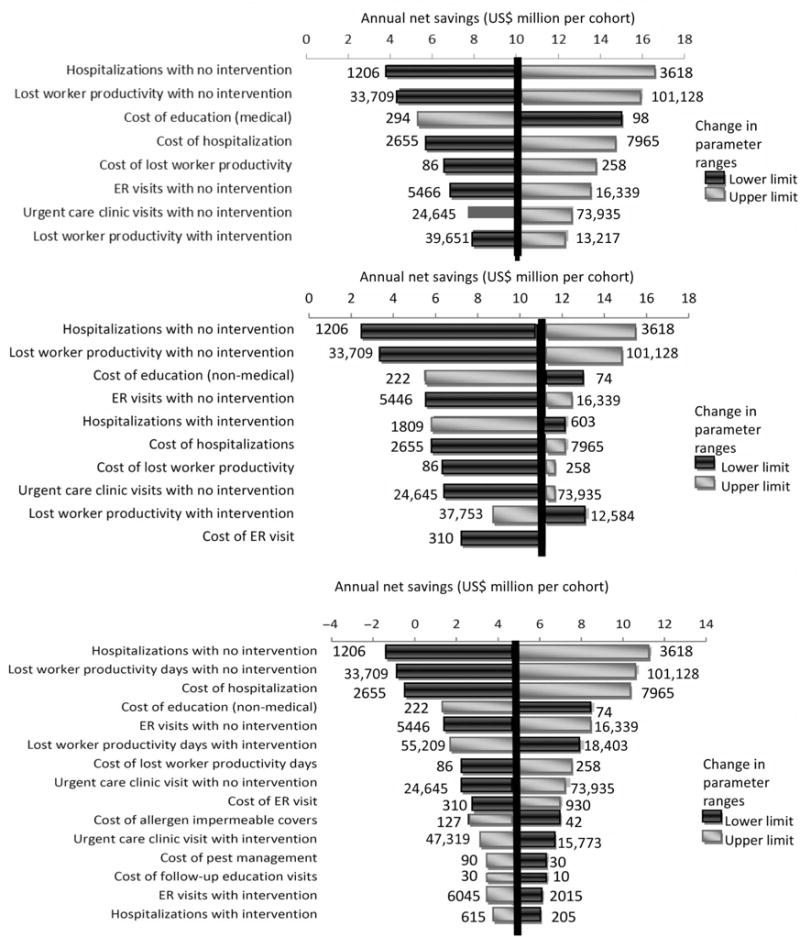
One-way sensitivity analyses. The upper and lower input ranges yielding the changes in annual net savings are represented at the side of each horizontal directional bar. All variables depicted altered the net benefit by at least 20% from base case estimates as noted by the linear vertical line. Net savings are benchmarked against the no intervention approach. The upper, middle and lower panels represent interventions using environmental education by medical providers, environmental education by non-medical providers, and the multi-component intervention, respectively. Costs are in 2009 US dollars.
Discussion
In this model-based analysis, we found that three environmental interventions for the control of persistent asthma are all highly likely to be cost-saving to society in a high-income setting, with projected annual savings of $8.1 million to $13.2 million per cohort. This finding was driven by broad-based savings in clinic costs, emergency department costs, hospitalization costs, and patient costs, and it was robust to wide variation in parameter estimates. These findings lend further support to policy decisions to implement such interventions, as they are likely to be not only beneficial to patient health, but also cost-saving as well. The most cost-saving intervention used environmental education with non-medical personnel. Despite the three elements utilized in the multi-component strategy, the intervention did not appear to be as monetarily beneficial as the single-component methods, nor did it clearly result in better patient outcomes.
Our results are consistent with three previous cost-benefit analyses applying environmental education strategies on the reduction of asthma outcomes (15, 22, 29, 30). These trials had employed either the single or the multi-component strategy with varying levels of net cost savings. Notably, only one of the three studies fulfilled the inclusion criteria of our review (22). Whereas these prior studies aimed for more local relevance, we took an approach (e.g., the use of national salary and statewide health encounter data) that sought to maximize the generalizability of our results. Ultimately, our model extends these prior studies by demonstrating broad-based cost savings for three separate interventions using wide parameter variations.
The goal of the analysis was to evaluate the cost savings that a society may incur with the adoption of home environmental strategies that attenuate asthma triggers. Despite the high costs of the interventions, the savings appeared to outweigh the initial investment using base case estimates among a cohort of persistent asthmatics that characterized Maryland’s pediatric population. The savings were most notable for limited environmental reduction strategies and should be considered for broader scale-up in other economically similar settings. Future research could enhance our findings by employing a randomized controlled trial with micro-costing of home-based environmental trigger reduction strategies and measuring both health encounters and change in quality of life measures, the latter being more likely to reveal subtle differences in health outcomes.
The key limitation of this study, as noted earlier, was its reliance on limited published data to estimate the probabilities of health encounters and efficacy of each intervention in reducing their frequency. Our conservative scenarios that double the estimated environmental strategy costs emphasize the sensitivity of this analysis to these costs. Unfortunately, at this time, high-quality estimates of the cost and effectiveness of multi-component interventions remain elusive; as such, this analysis represents a preliminary attempt, using the best available data, to inform the critical decision of whether to expand such interventions in the face of dramatic rises in pediatric asthma rates. Better data (e.g., from upcoming randomized trials) will provide a more definitive guidance, but until such data are available, the present evaluation provides a much-needed systematic framework for decision making regarding these interventions. Future work will also enhance the translatability of findings across study environments using incremental cost-effectiveness ratios (ICERs) using asthma-specific symptoms and quality-of-life measures as outcomes.
In addition to the primary data limitation on the effectiveness of multi-component interventions, we were also unable to account for the incremental cost of acute and chronic asthma medications due to insufficient data in the peer-reviewed literature. To the extent that environmental interventions would also reduce medication use, our estimates of net savings may be underestimates. Although we used primary data to define the characteristics of our cohort based on the 2009 MASR survey, these data may not generalize to some other settings, especially those with fewer available resources. The number of persistent asthmatics is difficult to estimate given the lack of available statewide data and the large range of prevalence values in the literature (29–31). However, while it may affect the total costs and cost savings, the volume of patients has little impact on the incremental cost per patient. Limiting the population to persistent asthmatics may overestimate the total benefit of asthma control strategies, since people with mild intermittent asthma may consume similar amounts of resources for education or environmental amelioration, with less future savings from averted ED visits or hospitalizations. In addition, the 12-month time horizon used is insufficient to simulate the long-term costs of asthma; however, it may be more realistic of “real-world” gains, as such interventions are implemented with varying levels of adherence and sustainability. Finally, societal costs were limited to lost productive days at work by caregivers and the travel costs they incur from acute exacerbations. We did not include the valuation of lost leisure time, employer friction costs, and poorer quality of life.
Conclusions
Single and multi-component environmental strategies to reduce asthma health encounters are likely to be highly cost-saving (up to $4.9 million or more per year in a cohort of nearly 50,000) when deployed among patients with persistent asthma in high-income settings. This finding is generally robust across a wide array of parameter values. These results lend support for wider deployment of comprehensive management strategies that address the environmental determinants of childhood asthma.
Supplementary Material
Acknowledgments
We are grateful to Kelli Rostkowski, Mary Bonacci, and Barbara Ensor of the Department of Pediatrics (Johns Hopkins School of Medicine) for providing charge data on asthma outcomes at Johns Hopkins Children’s Center.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. No potential conflict of interest exists with any companies or organizations whose products and services may be discussed in this article. This study was not funded by any grant. The study investigators have nothing to disclose.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 2.Bisgaard H, Szefler SJ. Understanding mild persistent asthma in children: The next frontier. J Allergy Clin Immunol. 2005;115:708–713. doi: 10.1016/j.jaci.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Cloutier MM, Wakefield DB, Hall CB, Bailit HL. Childhood asthma in an urban community: Prevalence, care system, and treatment. Chest. 2002;122:1571–1579. doi: 10.1378/chest.122.5.1571. [DOI] [PubMed] [Google Scholar]
- 4.Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, International Study of Asthma and Allergies Phase Three Study Group Global variation in the prevalence and severity of asthma symptoms: Phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2009;64:476–483. doi: 10.1136/thx.2008.106609. [DOI] [PubMed] [Google Scholar]
- 5.Simões SM, Cunha SS, Barreto ML, Cruz AA. Distribution of severity of asthma in childhood. J Pediatr (Rio J) 2010;86:417–423. doi: 10.2223/JPED.2030. [DOI] [PubMed] [Google Scholar]
- 6.Bankowski A, Hess-Mutinda R, McEachern Y, De Pinto C, Maryland Asthma Control Program Maryland Asthma Surveillance Report. Asthma in Maryland 2011. 2011 Available from: fha.dhmh.maryland.gov/mch/documents/Asthma_in_Maryland-2011.pdf.
- 7.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Forno E, Celedon JC. Asthma and ethnic minorities: Socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009;9:154–160. doi: 10.1097/aci.0b013e3283292207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenfeld L, Rudd R, Chew GL, Emmons K, Acevedo-García D. Are neighborhood-level characteristics associated with indoor allergens in the household? J Asthma. 2010;47:66–75. doi: 10.3109/02770900903362676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F, Takaro TK. Childhood asthma and environmental interventions. Environ Health Perspect. 2007;115:971–975. doi: 10.1289/ehp.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisk WJ. Estimates of potential nationwide productivity and health benefits from better indoor environments: An update. In: Spengler J, Samet JM, McCarthy JF, editors. Indoor Air Quality Handbook. 1st. 4. Vol. 2000. New York: McGraw Hill; pp. 1–4.pp. 36 [Google Scholar]
- 12.Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, Hopkins DP, Lawrence BM, Sipe TA. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: A community guide systematic review. Am J Prev Med. 2011;41(2 Suppl 1):S5–S32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Campbell JD, Spackman DE, Sullivan SD. Health economics of asthma: Assessing the value of asthma interventions. Allergy. 2008;63:1581–1592. doi: 10.1111/j.1398-9995.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng JW, Arnold RJ. Pharmacoeconomic review of medical management of persistent asthma. Allergy Asthma Proc. 2008;29:109–122. doi: 10.2500/aap.2008.29.3105. [DOI] [PubMed] [Google Scholar]
- 15.Nurmagambetov TA, Barnett SB, Jacob V, Chattopadhyay SK, Hopkins DP, Crocker DD, Dumitru GG, Kinyota S. Economic value of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity a community guide systematic review. Am J Prev Med. 2011;41(2 Suppl 1):S33–S47. doi: 10.1016/j.amepre.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-Based Medicine: How to practice and teach EBM. Toronto: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- 17.Bureau of Labor Statistics. Labor Force Statistics from the Current Population Survey. Date Last Updated March 11, 2011. Available at: www.bls.gov/bls/blswage.htm.
- 18.Allergy Control Products. Allergy Encasings. Available at: www.allergycontrol.com/c-23-allergy-encasings.aspx.
- 19.Health Services Cost Review Commission. Casemix Information Management. 2012 Available at: http://datamart1.jhmi.edu. Date Last Accessed October 30, 2012.
- 20.Hughes DM, McLeod M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics. 1991;87:54–61. [PubMed] [Google Scholar]
- 21.Stout JW, White LC, Rogers LT, McRorie T, Morray B, Miller-Ratcliffe M, Redding GJ. The Asthma Outreach Project: A promising approach to comprehensive asthma management. J Asthma. 1998;35:119–127. doi: 10.3109/02770909809055413. [DOI] [PubMed] [Google Scholar]
- 22.Shelledy DC, McCormick SR, LeGrand TS, Cardenas J, Peters JI. The effect of a pediatric asthma management program provided by respiratory therapists on patient outcomes and cost. Heart Lung. 2005;34:423–428. doi: 10.1016/j.hrtlng.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Primomo J, Johnston S, DiBiase F, Nodolf J, Noren L. Evaluation of a community-based outreach worker program for children with asthma. Public Health Nurs. 2006;23:234–241. doi: 10.1111/j.1525-1446.2006.230306.x. [DOI] [PubMed] [Google Scholar]
- 24.Largo TW, Borgialli M, Wisinski CL, Wahl RL, Priem WF. Healthy Homes University: A home-based environmental intervention and education program for families with pediatric asthma in Michigan. Public Health Rep. 2011;126(Suppl 1):14–26. doi: 10.1177/00333549111260S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margellos-Anast H, Gutierrez MA, Whitman S. Improving asthma management among African-American children via a community health worker model: Findings from a Chicago-based pilot intervention. J Asthma. 2012;49:380–389. doi: 10.3109/02770903.2012.660295. [DOI] [PubMed] [Google Scholar]
- 26.Carter MC, Perzanowski MS, Raymond A, Platts-Mills TA. Home intervention in the treatment of asthma among inner-city children. J Allergy Clin Immunol. 2001;108:732–737. doi: 10.1067/mai.2001.119155. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan SD, Weiss KB, Lynn H, Mitchell H, Kattan M, Gergen PJ, Evans R, National Cooperative Inner-City Asthma Study (NCICAS) Investigators The cost-effectiveness of an inner-city asthma intervention for children. J Allergy Clin Immunol. 2002;110:576–581. doi: 10.1067/mai.2002.128009. [DOI] [PubMed] [Google Scholar]
- 28.Bryant-Stephens T, Li Y. Outcomes of a home-based environmental remediation for urban children with asthma. J Natl Med Assoc. 2008;100:306–316. doi: 10.1016/s0027-9684(15)31243-8. [DOI] [PubMed] [Google Scholar]
- 29.Jowers JR, Schwartz AL, Tinkelman DG, Reed KE, Corsello PR, Mazzei AA, Bender DR, Lochhead RA. Disease management program improves asthma outcomes. Am J Manag Care. 2000;6(5):585–592. [PubMed] [Google Scholar]
- 30.Oatman L. Reducing Environmental Triggers of Asthma in Homes of Minnesota Children. St. Paul, MN: Minnesota Department of Health; 2007. Available at: http://www.health.state.mn.us/asthma/documents/retafullreport0907.pdf. Date Last Updated September 2007. Date Last Accessed November 15, 2012. [Google Scholar]
- 31.Halterman JS, Yoos HL, Kaczorowski JM, McConnochie K, Holzhauer RJ, Conn KM, Lauver S, Szilagyi PG. Providers underestimate symptom severity among urban children with asthma. Arch Pediatr Adolesc Med. 2002;156:141–146. doi: 10.1001/archpedi.156.2.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


