Abstract
Introduction
The specific goal of this study was to determine whether the inclusion of MRS had a measureable and positive impact on the accuracy of pre-surgical MR examinations of untreated pediatric brain tumors over that of MRI alone in clinical practice.
Methods
Final imaging reports of 120 pediatric patients with newly detected brain tumors who underwent combined MRI/ MRS examinations were retrospectively reviewed. Final pathology was available in all cases. Group A comprised 60 subjects studied between June 2001 and January 2005, when MRS was considered exploratory and radiologists utilized only conventional MRI to arrive at a diagnosis. For group B, comprising 60 subjects studied between January 2005 and March 2008, the radiologists utilized information from both MRI and MRS. Furthermore, radiologists revisited group A (blind review, time lapse >4 years) to determine whether the additional information from MRS would have altered their interpretation.
Results
Sixty-three percent of patients in group A were diagnosed correctly, whereas in 10 % the report was partially correct with the final tumor type mentioned (but not mentioned as most likely tumor), while in 27 % of cases the reports were wrong. For group B, the diagnoses were correct in 87 %, partially correct in 5 %, and incorrect in 8 % of the cases, which is a significant improvement (p<0.005). Re-review of combined MRI and MRS of group A resulted 87 % correct, 7 % partially correct, and 7 % incorrect diagnoses, which is a significant improvement over the original diagnoses (p<0.05).
Conclusion
Adding MRS to conventional MRI significantly improved diagnostic accuracy in preoperative pediatric patients with untreated brain tumors.
Keywords: Pediatric brain tumors, MR imaging, MR spectroscopy, Diagnoses
Introduction
Accurate pre-therapeutic diagnosis is important for clinical management of pediatric brain tumors. Conventional contrast-enhanced MRI is widely employed in brain tumor diagnosis using features such as location, mass effect, signal intensity, contrast enhancement, and diffusion characteristics as well as information such as patient age and onset/duration of symptoms. Prior studies have demonstrated the value of proton 1H-MRS to differentiate between different types of pediatric brain tumors based on the observation of different metabolic properties of various neoplasms [1–11]. To date, only a few studies have examined the benefit of using both MRI with MRS over conventional MRI alone [10, 12–16]. However, most of the patients in these studies were adults and none focused exclusively on a pediatric population. The specific goal of this study was to determine if the combination of conventional MRI with MRS improves diagnostic accuracy of untreated pediatric brain tumors compared with conventional MRI alone in an actual clinical practice setting.
Materials and methods
Patients
The official imaging reports, filed in the hospital medical records, of 120 pediatric patients with newly diagnosed brain tumors examined at our institution between June 2001 and March 2008 were retrospectively reviewed. The first 60 patients (group A) were identical to those included in an earlier publication from our institution [17]. For group B, we then included patients that where examined thereafter that also passed the quality criteria as defined in ref. [17] until an equal number of patients were identified. All patients underwent conventional contrast-enhanced MRI and short echo time 1H-MRS. MR examinations of new tumors, where only MRI was performed, are not included in this report. Following initial MRI/MRS, all tumors were subsequently either biopsied and/or resected and final histopathological diagnoses were available for all patients. The resection/biopsy was generally performed within 3 days of the MRI examination, and specimens were independently reviewed by two neuropathologists (IG-G, FHG). More details on patient demographics and tumor types are provided in Table 1. The Institutional Review Board (IRB) approved this research study. A subgroup of patients in group A was enrolled in a prospective MR spectroscopy research study, and parental/patient consent was obtained. For the remaining subjects, MRS data were obtained as part of the pre-surgical workup and the requirement to obtain parental consent for a retrospective review of already existing data was waived by the IRB.
Table 1. Tumor types and patient demographics.
| Final tumor type | Number (group A) | Number (group B) |
|---|---|---|
| Medulloblastoma/supratentorial PNET | 14 | 18 |
| Anaplastic astrocytoma/GBM | 5 | 7 |
| Astrocytoma (WHO II) | 3 | 4 |
| Pilocytic astrocytoma | 17 | 17 |
| Ependymoma/anaplastic ependymoma | 9 | 6 |
| Choroid plexus papilloma | 3 | 0 |
| Choroid plexus carcinoma | 3 | 2 |
| Germinoma/mixed germ cell tumors | 6 | 6 |
| Total | 60 | 60 |
| Age (years) | 7.2±5.2a | 7.6±5.4 |
| Male/female | 35/25 | 32/28 |
Average±standard deviation
Review of MRI exam reports
Patients were subdivided in two groups. The first group (group A) comprised 60 pediatric patients that were imaged between June 2001 and January 2005. At that time, because the experience with MRS was limited, staff pediatric neuroradiologists relied only on conventional contrast-enhanced MRI to determine an imaging diagnosis. By the end of that period, MRS data were analyzed and findings about the general metabolic features of these tumors were published [17]. Group B comprised 60 pediatric patients that were imaged thereafter between January 2005 and March 2008. For these cases, the staff pediatric neuroradiologists used both conventional MRI as well as MRS when arriving at a diagnosis of tumor type. This was done in conjunction with an MR spectroscopist (SB).
To test whether diagnoses improved significantly from group A to group B, MRI reports as filed in the hospital medical records were compared with the final histopathological reports. Specifically, each imaging diagnosis was determined to be (a) “correct” if the final tumor type was the only mentioned tumor type or was mentioned as most likely diagnosis in the differential diagnosis. The MRI report was rated as (b) “partially correct” if the final tumor type was included elsewhere in the differential diagnosis but not mentioned as the most likely tumor or (c) “incorrect” if the final tumor type was not mentioned in the report.
To exclude the possibility that any improvement was merely due to a learning effect and increased experience over time, three board-certified pediatric neuroradiologists (MDN, KRM, AP) blindly re-reviewed all MR imaging studies in group A. If a differential diagnosis was given, reviewers were asked to limit the studies to three possibilities, from most to the least likely tumor type. Two of these pediatric neuroradiologists (MDN, AP) were involved in the original reporting of these cases. The time lapse between the original reading and the re-review was at least 4 years. The third pediatric neuroradiologist (KRM) was not involved in the original interpretation.
Finally, to test whether additional information from MRS might have improved initial diagnoses of group A, printouts of the MR spectra for each case were prepared. The neuroradiologists were then asked to again name the three tumor types, most likely to least likely, now considering MRI and MRS.
Statistical analyses
We tested whether the number of “correct” versus “partially correct” or “incorrect” MR reports was significantly higher in group B than in group A using a one-tailed Fisher's exact test. In addition, we determined whether the number of “correct” or “partially correct” (i.e., correct answer anywhere in differential diagnosis) versus “incorrect” MR reports was significantly higher in group B than in group A. The Fisher exact test was also used to determine whether there was a significant learning effect between the original interpretations of MRI studies of group A, and the re-review more than 4 years later. Finally, we used this test to determine whether the re-review of group A significantly improved when MR spectroscopy information was added.
MRI and MRS acquisition and analyses
All studies were performed on a 1.5-T clinical scanner (Signa LX, GE Healthcare, Milwaukee, WI). Conventional axial and sagittal T1-weighted, axial T2-weighted, axial T2-weighted FLAIR, axial diffusion-weighted, and axial and coronal/ sagittal contrast-enhanced T1-weighted MR images were obtained. Single-voxel 1H MRS spectra of the tumors were acquired before administration of contrast agent by using a point-resolved spectroscopy sequence (PRESS) with a short TE of 35 ms, a TR of 1500 ms, and 128 signal intensity averages. When the size of the lesion permitted, a second spectrum from a slightly different region of interest was acquired using the same parameters. Approximately 5 min was required for each spectral acquisition. The sizes of the regions of interest ranged from 5 to 10 cm3. Fully automated LCModel software (Stephen Provencher Inc., Oakville, Ontario, Canada) was used for processing and to generate printouts provided to physicians for review.
Results
In the original MRI reports for group A, the correct diagnosis was given as the only or most likely possibility in 38/60 patients (63 %). In 6/60 patients (10 %), the correct diagnosis was included elsewhere in the differential diagnosis whereas in 16/60 patients (27 %) the diagnosis was incorrect (Table 2). For the later group B (MRI and MRS), the correct diagnosis was given as the first or only possibility in 52/60 patients (87 %). In 3/60 patients (5 %), the correct diagnosis was included elsewhere in the differential diagnosis whereas in 5/60 patients (8 %) the diagnosis was incorrect (Table 2). Using Fisher's exact test, the number of “correct” versus “partially correct” or “incorrect” was significantly higher in group B (p<0.005). Similarly, the number of “correct” or “partially correct” (i.e., correct answer anywhere in differential diagnosis) versus “incorrect” was significantly higher in group B (p<0.05).
Table 2. Accuracy of diagnoses.
| Correct | Partially correct | Incorrect | ||
|---|---|---|---|---|
| Medical records | Group A | 63 % | 10 % | 27 % |
| Group B** | 87 % | 5% | 8% | |
| Re-review group Aa | MRI alone† | 71±2 % | 14±3 % | 15±3 % |
| MRI+MRS+ | 87 %±6 % | 7±6 % | 7±6 % |
p<0.005 significantly improved diagnoses groups B over group A
p<0.05 significantly improved diagnoses MRI+MRS over MRI alone of the re-reviewed group A cases
p not significantly improved diagnoses when MRI alone of group A was re-reviewed and compared with original diagnoses
Average±standard deviation of three board-certified neuroradiologists
When a blind re-review of the group A patients was undertaken by the 3 neuroradiologists based on MRI alone, on average, they were correct 71 %, partially correct 14 %, and wrong 15 % of the time. These numbers represent a slight improvement over the original reports filed in the medical records but the difference showed no statistically significant difference. When these reviewers also considered information provided by MRS, 87 % of the diagnoses were correct, 7 % partially correct, and 7 % incorrect, which is a significant improvement from when the re-review only considered MRI alone (p<0.05).
An example of a case where MRI alone did not point conclusively to any particular tumor type is shown in Fig. 1. MRS, on the other hand, was typical for a pilocytic astrocytoma, which was subsequently confirmed after resection of the lesion. In the second example (Fig. 2), the neuroradiologist felt that the posterior fossa tumor is most likely a medulloblastoma and included ependymoma as a second option in the differential after reviewing only MRI. The MRS is, however, not typical for medulloblastoma (low or absent taurine (Tau), high myo-inositol (mIns)) but more consistent with an ependymoma, which was confirmed after the resection of the lesion.
Fig. 1.
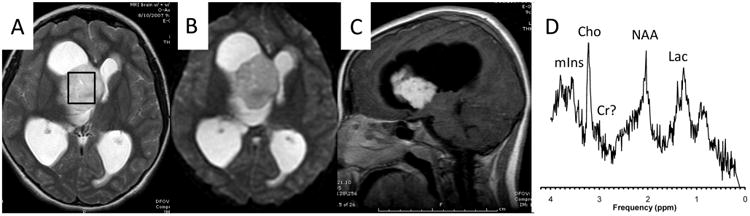
T2-weigthed (a), diffusion-weighted (b), and T1-weighted post-contrast MR images of a 10-year-old female with a newly diagnosed brain lesion. The MRI was interpreted as not specific for a particular tumor type. The MR spectrum, obtained from the region indicated by the box on the T2-weigthed MRI, shows prominent Lac, Cho, essentially depleted Cr, and residual NAA. mIns levels are also low. This pattern is typically observed in pilocytic astrocytoma, which was subsequently confirmed when the lesion was resected. Lac lactate, Cho choline, Cr creatine, NAA N-acetylaspartate, mIns myo-inositol
Fig. 2.
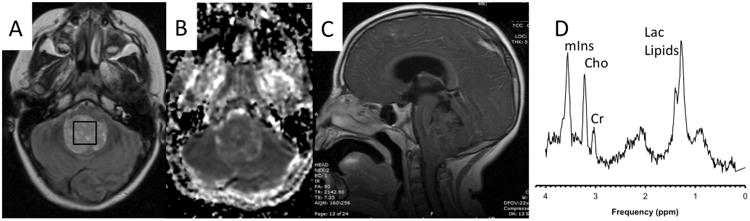
T2-weigthed (a), apparent diffusion coefficient (ADC) map (b), and T1-weighted post-contrast MR images of a 6-year-old male with a newly diagnosed brain lesion in the posterior fossa. Based on MRI alone, a medulloblastoma was felt to be the most likely tumor type with ependymoma included in the differential. The MRS shows prominent Lac and lipids. Cr was below normal tissue levels whereas Cho, albeit elevated relative to Cr, was close to normal tissue levels. NAA was depleted, and mIns was above normal levels. Particularly, the unremarkable Cho levels are not typical for grade IV medulloblastoma. High mIns is also unusual for medulloblastoma. Combining the impression from MRI and MRS, an ependymoma was considered to be the most likely tumor type, which was subsequently confirmed. Lac lactate, Cho choline, Cr creatine, NAA N-acetylaspartate, mIns myo-inositol
Discussion
Surgical resection of brain tumors is a critical step in therapy. In the future, improvement in surgical approaches or considerations of tailored “pretreatments” to, e.g., shrink tumor volumes, will make the accuracy of pre-surgical diagnoses of a brain tumor more crucial. Furthermore, with better understanding of the molecular characterization of brain tumors, understanding the link between neuroimaging phenotypes and molecular correlates may become clinically important in the near future. This approach, called imaging genomics, can utilize information from conventional methods like contrast-enhanced MRI, but may also include functional techniques like MRS [18]. Diagnoses of pediatric brain tumors using conventional imaging is based on factors such as location, size, and extent; enhancement and diffusion characteristics; and T1 and T2 relaxation properties while also considering the age of patient, the length of symptoms, etc. [1]. However, compared with adult brain tumors, pediatric brain tumors are histologically more diverse and include tumor types such as embryonal tumors, germ cell tumors, pilocytic astrocytoma, ependymomas, and others that are not or rarely observed in the adult population. As the imaging features of theses tumors may overlap, definitive diagnoses based on MRI alone are often difficult. On the other hand, several groups have investigated pediatric brain tumors with in vivo MRS and have reported significant biochemical differences between various tumor types [1, 2, 11, 17, 19]. A few studies have examined the diagnostic benefits of combined MRI+MRS over MRI alone; however, these works have focused largely on adult brain tumors [10, 12–16]. Therefore, it remains unclear whether adding MRS to the initial imaging workup of a pediatric patient in actual clinical practice would result in a benefit for patients defined as improved overall accuracy of the diagnoses.
To answer this question, we retrospectively inspected 120 MRI reports that were generated as part of the routine imaging workup of newly diagnosed pediatric brain tumor patients in this institution over a 7-year period. Our results suggest that combined MRI and MRS indeed significantly improved pre-therapeutic diagnoses of pediatric brain tumors over MRI alone. These findings are the result of a comprehensive review of final MRI reports stored in the hospital medical records. A significantly higher accuracy (87 % correct) was noted for a group of patients that were examined at a later time (group B) when MRS findings were routinely reported to the neuroradiologist when compared with a group of generally equivalent tumor types studied at an earlier time (group A, 63 % correct).
To determine to what extent the improved accuracy could have been due to a learning effect or more motivated readers, the group A studies were re-reviewed, at least 4 years later, by three board-certified neuroradiologists. The interpretations using only MRI information improved; however, this was not statistically significant. In contrast, when these neuroradiologists incorporated additional MRS data, the number of correct reports of the re-reviewed group A improved significantly and matched the accuracy of group B. This retrospective approach ensures that our findings are not the result of a transient enthusiasm and increased effort to arrive at the correct diagnoses by the radiologist, which may not be sustainable in routine clinical practice.
To the best of our knowledge, this is the first study to document the ability of combined MRI and MRS to improve diagnostic accuracy of untreated brain tumors in children over MRI alone in a routine clinical setting. Several groups have previously examined whether the addition of MRS has a positive impact on diagnoses and management of patients with brain tumors [10, 12–16]. However, few studies have specifically examined the difference in diagnostic accuracy of using both MRI with MRS over conventional MRI alone in brain tumor patients, and none exclusively in pediatric brain tumor patients in particular.
Methodological approach
For essentially all patients in this study, the MRS protocol consisted of the acquisition of only one single-voxel spectrum, typically not adding more than 5 min to the total examination time. In some cases, a second spectrum was obtained when there was the suspicion of patient movement during a scan, which could have degraded spectral quality or might have shifted the region of interest away from the lesion. It is thus conceivable that more sophisticated MRS methods with longer acquisition times, such as multi-voxel MR spectroscopy, may improve diagnoses above what we have reported here.
There are, however, additional challenges when more complex MRS examinations are considered. Most importantly, processing and documentation of the information obtained by MRS requires additional effort. At our institution, a spectroscopist reviewed all spectra for quality control, processed and quantified all spectra, and provided a preliminary interpretation. The demand for these services increases with more complex MRS studies and may render MRS impractical in institutions with limited resources. Another concern is the timely interpretation of MRS studies. Pediatric brain tumors often present with acute symptoms requiring surgery within 24–48 h after admission. Whereas the infrastructure to report MRI within hours exists in most hospitals, reporting of MRS may be delayed when complex methods such as multi-voxel MRS are being used. We therefore believe our approach of using a robust single-voxel methodology is a sustainable approach that can be adopted by institutions that do not have the support of an MR spectroscopist.
Conclusions
Single-voxel MRS is an FDA-approved modality available on all modern MR scanners. Adding this form of spectroscopy to the initial MR imaging workup of pediatric patients with suspected brain tumors significantly improved the accuracy of diagnoses at our institution.
Acknowledgments
We acknowledge grant support: 5R33CA096032 (National Cancer Institute), U01 CA97452-02 (National Childhood Cancer Foundation), Ian's Friends Foundation and *SC CTSI (NIH/NCRR/NCATS) Grant # KL2TR000131.
Footnotes
Ethical standards and patient consent: We declare that all human and animal studies have been approved by the Institutional Review Board (IRB) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that a subgroup of patients in group A was enrolled in a prospective MR spectroscopy research study and parental/ patient consent was obtained. For the remaining subjects, MRS data were obtained as part of the pre-surgical workup and the IRB waived the requirement to obtain parental consent for a retrospective review of existing data.
Conflict of interest: We declare that we have no conflict of interest.
References
- 1.Wang Z, Sutton LN, Cnaan A, Haselgrove JC, Rorke LB, Zhao H, et al. Proton MR spectroscopy of pediatric cerebellar tumors. AJNR Am J Neuroradiol. 1995;16(9):1821–1833. [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton LN, Wang Z, Gusnard D, Lange B, Perilongo G, Bogdan AR, et al. Proton magnetic resonance spectroscopy of pediatric brain tumors. Neurosurgery. 1992;31(2):195–202. doi: 10.1227/00006123-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lazareff JA, Bockhorst KH, Curran J, Olmstead C, Alger JR. Pediatric low-grade gliomas: prognosis with proton magnetic resonance spectroscopic imaging. Neurosurgery. 1998;43(4):809–817. doi: 10.1097/00006123-199810000-00053. [DOI] [PubMed] [Google Scholar]
- 4.Horska A, Ulug AM, Melhem ER, Filippi CG, Burger PC, Edgar MA, et al. Proton magnetic resonance spectroscopy of choroid plexus tumors in children. J Magn Reson Imaging. 2001;14(1):78–82. doi: 10.1002/jmri.1154. [DOI] [PubMed] [Google Scholar]
- 5.Tzika AA, Vigneron DB, Dunn RS, Nelson SJ, Ball WS., Jr Intracranial tumors in children: small single-voxel proton MR spectroscopy using short- and long-echo sequences. Neuroradiology. 1996;38(3):254–263. doi: 10.1007/BF00596542. [DOI] [PubMed] [Google Scholar]
- 6.Hwang JH, Egnaczyk GF, Ballard E, Dunn RS, Holland SK, Ball WS., Jr Proton MR spectroscopic characteristics of pediatric pilocytic astrocytomas. AJNR Am J Neuroradiol. 1998;19(3):535–540. [PMC free article] [PubMed] [Google Scholar]
- 7.Girard N, Wang ZJ, Erbetta A, Sutton LN, Phillips PC, Rorke LB, et al. Prognostic value of proton MR spectroscopy of cerebral hemisphere tumors in children. Neuroradiology. 1998;40(2):121–125. doi: 10.1007/s002340050551. [DOI] [PubMed] [Google Scholar]
- 8.Arle JE, Morriss C, Wang ZJ, Zimmerman RA, Phillips PG, Sutton LN. Prediction of posterior fossa tumor type in children by means of magnetic resonance image properties, spectroscopy, and neural networks. J Neurosurg. 1997;86(5):755–761. doi: 10.3171/jns.1997.86.5.0755. [DOI] [PubMed] [Google Scholar]
- 9.Dezortova M, Hajek M, Cap F, Babis M, Tichy M, Vymazal J. Comparison of MR spectroscopy and MR imaging with contrast agent in children with cerebral astrocytomas. Childs Nerv Syst. 1999;15(8):408–412. doi: 10.1007/s003810050426. [DOI] [PubMed] [Google Scholar]
- 10.Murphy M, Loosemore A, Clifton AG, Howe FA, Tate AR, Cudlip SA, et al. The contribution of proton magnetic resonance spectroscopy (1HMRS) to clinical brain tumour diagnosis. Br J Neurosurg. 2002;16(4):329–334. doi: 10.1080/0268869021000007687. [DOI] [PubMed] [Google Scholar]
- 11.Davies NP, Wilson M, Harris LM, Natarajan K, Lateef S, Macpherson L, et al. Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS. NMR Biomed. 2008;21(8):908–918. doi: 10.1002/nbm.1283. [DOI] [PubMed] [Google Scholar]
- 12.Moller-Hartmann W, Herminghaus S, Krings T, Marquardt G, Lanfermann H, Pilatus U, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44(5):371–381. doi: 10.1007/s00234-001-0760-0. [DOI] [PubMed] [Google Scholar]
- 13.Galanaud D, Nicoli F, Chinot O, Confort-Gouny S, Figarella-Branger D, Roche P, et al. Noninvasive diagnostic assessment of brain tumors using combined in vivo MR imaging and spectroscopy. Magn Reson Med. 2006;55(6):1236–1245. doi: 10.1002/mrm.20886. [DOI] [PubMed] [Google Scholar]
- 14.Lin A, Bluml S, Mamelak AN. Efficacy of proton magnetic resonance spectroscopy in clinical decision making for patients with suspected malignant brain tumors. J Neurooncol. 1999;45(1):69–81. doi: 10.1023/a:1006387703127. [DOI] [PubMed] [Google Scholar]
- 15.Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658–679. doi: 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julia-Sape M, Coronel I, Majos C, Candiota AP, Serrallonga M, Cos M, et al. Prospective diagnostic performance evaluation of single-voxel 1H MRS for typing and grading of brain tumours. NMR Biomed. 2012;25(4):661–673. doi: 10.1002/nbm.1782. [DOI] [PubMed] [Google Scholar]
- 17.Panigrahy A, Krieger MD, Gonzalez-Gomez I, Liu X, McComb JG, Finlay JL, et al. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. AJNR Am J Neuroradiol. 2006;27(3):560–572. [PMC free article] [PubMed] [Google Scholar]
- 18.Pope WB. Genomics of brain tumor imaging. Neuroimaging Clin N Am. 2015;25(1):105–119. doi: 10.1016/j.nic.2014.09.006. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Kovanlikaya A, Panigrahy A, Krieger MD, Gonzalez-Gomez I, Ghugre N, McComb JG, et al. Untreated pediatric primitive neuroectodermal tumor in vivo: quantitation of taurine with MR spectroscopy. Radiology. 2005;236(3):1020–1025. doi: 10.1148/radiol.2363040856. [DOI] [PubMed] [Google Scholar]


