Abstract
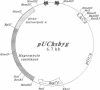
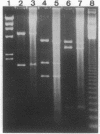
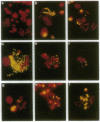
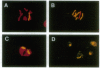
Stable incorporation of high copy numbers (greater than 10,000 per cell) of a plasmid vector containing a gene conferring resistance to the antibiotic hygromycin was achieved in a cell line derived from the Aedes albopictus mosquito. Plasmid sequences were readily observed by ethidium bromide staining of cellular DNA after restriction endonuclease digestion and agarose gel electrophoresis. The plasmid was demonstrated by in situ hybridization to be present in large arrays integrated in metaphase chromosomes and in minute and double-minute replicating elements. In one subclone, approximately 60,000 copies of the plasmid were organized in a large array that resembles a chromosome, morphologically and in the segregation of its chromatids during anaphase. The original as well as modified versions of the plasmid were rescued by transformation of Escherichia coli using total cellular DNA. Southern blot analyses of recovered plasmids indicate the presence of mosquito-derived sequences.
Full text
PDF
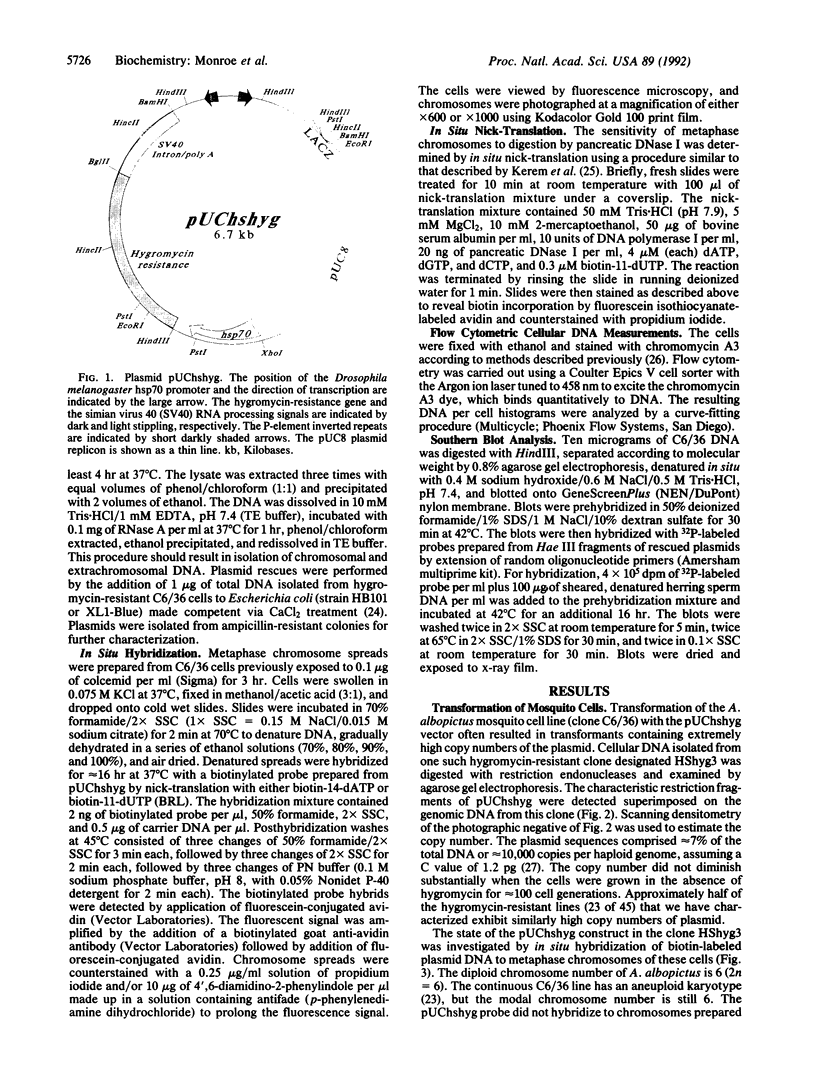
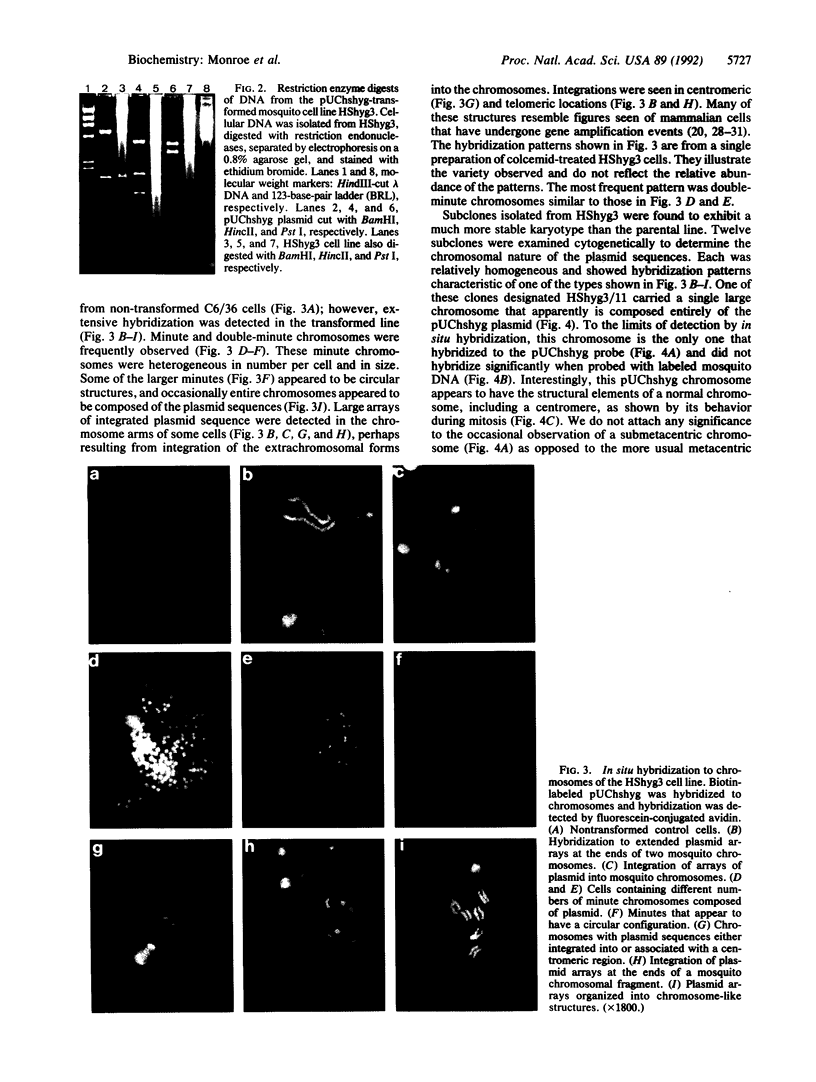
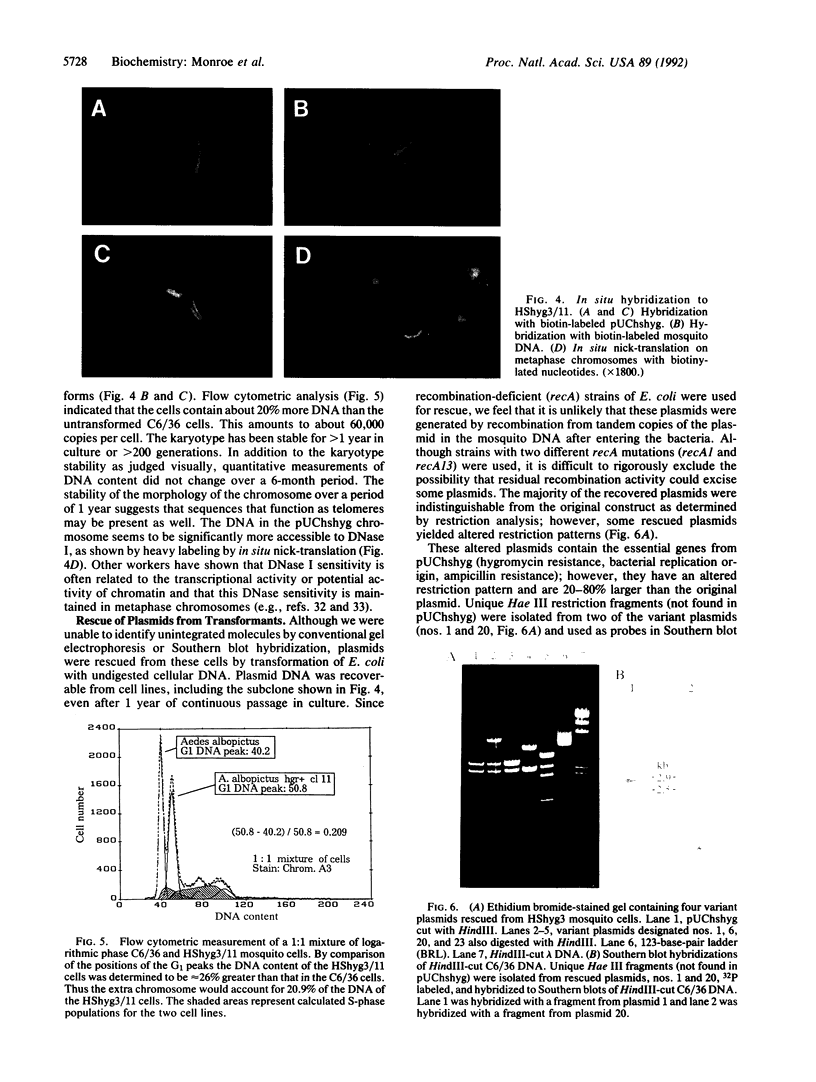

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Bargiello T. A., Jackson F. R., Young M. W. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984 Dec 20;312(5996):752–754. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- Bourouis M., Jarry B. Vectors containing a prokaryotic dihydrofolate reductase gene transform Drosophila cells to methotrexate-resistance. EMBO J. 1983;2(7):1099–1104. doi: 10.1002/j.1460-2075.1983.tb01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., DeRose M. L., Gaudray P., Moore C. M., Needham-Vandevanter D. R., Von Hoff D. D., Wahl G. M. Double minute chromosomes can be produced from precursors derived from a chromosomal deletion. Mol Cell Biol. 1988 Apr;8(4):1525–1533. doi: 10.1128/mcb.8.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Gaudray P., De Rose M. L., Emery J. F., Meinkoth J. L., Nakkim E., Subler M., Von Hoff D. D., Wahl G. M. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency: functional evidence for a mammalian replication origin. Mol Cell Biol. 1987 May;7(5):1740–1750. doi: 10.1128/mcb.7.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988 Mar 4;239(4844):1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Fox M. H., Read R. A., Bedford J. S. Comparison of synchronized Chinese hamster ovary cells obtained by mitotic shake-off, hydroxyurea, aphidicolin, or methotrexate. Cytometry. 1987 May;8(3):315–320. doi: 10.1002/cyto.990080312. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Santi D. V. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986 Aug 1;233(4763):535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Gazit B., Cedar H., Lerer I., Voss R. Active genes are sensitive to deoxyribonuclease I during metaphase. Science. 1982 Aug 13;217(4560):648–650. doi: 10.1126/science.6283640. [DOI] [PubMed] [Google Scholar]
- Hadlaczky G., Praznovszky T., Cserpán I., Keresö J., Péterfy M., Kelemen I., Atalay E., Szeles A., Szelei J., Tubak V. Centromere formation in mouse cells cotransformed with human DNA and a dominant marker gene. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8106–8110. doi: 10.1073/pnas.88.18.8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamkalo B. A., Farnham P. J., Johnston R., Schimke R. T. Ultrastructural features of minute chromosomes in a methotrexate-resistant mouse 3T3 cell line. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1126–1130. doi: 10.1073/pnas.82.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Johansen H., van der Straten A., Sweet R., Otto E., Maroni G., Rosenberg M. Regulated expression at high copy number allows production of a growth-inhibitory oncogene product in Drosophila Schneider cells. Genes Dev. 1989 Jun;3(6):882–889. doi: 10.1101/gad.3.6.882. [DOI] [PubMed] [Google Scholar]
- Kerem B. S., Goitein R., Richler C., Marcus M., Cedar H. In situ nick-translation distinguishes between active and inactive X chromosomes. Nature. 1983 Jul 7;304(5921):88–90. doi: 10.1038/304088a0. [DOI] [PubMed] [Google Scholar]
- Lin C. C., Alitalo K., Schwab M., George D., Varmus H. E., Bishop J. M. Evolution of karyotypic abnormalities and C-MYC oncogene amplification in human colonic carcinoma cell lines. Chromosoma. 1985;92(1):11–15. doi: 10.1007/BF00327240. [DOI] [PubMed] [Google Scholar]
- McArthur J. G., Stanners C. P. A genetic element that increases the frequency of gene amplification. J Biol Chem. 1991 Mar 25;266(9):6000–6005. [PubMed] [Google Scholar]
- McGrane V., Carlson J. O., Miller B. R., Beaty B. J. Microinjection of DNA into Aedes triseriatus ova and detection of integration. Am J Trop Med Hyg. 1988 Nov;39(5):502–510. doi: 10.4269/ajtmh.1988.39.502. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Sakai R. K., Romans P., Gwadz R. W., Kantoff P., Coon H. G. Stable integration and expression of a bacterial gene in the mosquito Anopheles gambiae. Science. 1987 Aug 14;237(4816):779–781. doi: 10.1126/science.3039658. [DOI] [PubMed] [Google Scholar]
- Morris A. C., Eggleston P., Crampton J. M. Genetic transformation of the mosquito Aedes aegypti by micro-injection of DNA. Med Vet Entomol. 1989 Jan;3(1):1–7. doi: 10.1111/j.1365-2915.1989.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Mouchès C., Pasteur N., Bergé J. B., Hyrien O., Raymond M., de Saint Vincent B. R., de Silvestri M., Georghiou G. P. Amplification of an esterase gene is responsible for insecticide resistance in a California Culex mosquito. Science. 1986 Aug 15;233(4765):778–780. doi: 10.1126/science.3755546. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- Pirrotta V., Manet E., Hardon E., Bickel S. E., Benson M. Structure and sequence of the Drosophila zeste gene. EMBO J. 1987 Mar;6(3):791–799. doi: 10.1002/j.1460-2075.1987.tb04821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont G., Degroote F., Picard G. Illegitimate recombination in the histone multigenic family generates circular DNAs in Drosophila embryos. Nucleic Acids Res. 1988 Sep 26;16(18):8817–8833. doi: 10.1093/nar/16.18.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Rai K. Inter and intraspecific variation in nuclear DNA content in Aedes mosquitoes. Heredity (Edinb) 1987 Oct;59(Pt 2):253–258. doi: 10.1038/hdy.1987.120. [DOI] [PubMed] [Google Scholar]
- Raymond M., Callaghan A., Fort P., Pasteur N. Worldwide migration of amplified insecticide resistance genes in mosquitoes. Nature. 1991 Mar 14;350(6314):151–153. doi: 10.1038/350151a0. [DOI] [PubMed] [Google Scholar]
- Rio D. C., Laski F. A., Rubin G. M. Identification and immunochemical analysis of biologically active Drosophila P element transposase. Cell. 1986 Jan 17;44(1):21–32. doi: 10.1016/0092-8674(86)90481-2. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Horsch R. B., Klee H. J., Kishore G. M., Winter J. A., Tumer N. E., Hironaka C. M., Sanders P. R., Gasser C. S., Aykent S., Siegel N. R., Rogers S. G., Fraley R. T. Engineering herbicide tolerance in transgenic plants. Science. 1986 Jul 25;233(4762):478–481. doi: 10.1126/science.233.4762.478. [DOI] [PubMed] [Google Scholar]
- Shotkoski F. A., Fallon A. M. Genetic changes in methotrexate-resistant mosquito cells. Arch Insect Biochem Physiol. 1990;15(2):79–92. doi: 10.1002/arch.940150203. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gorman P. A., Stark M. B., Groves R. P., Stark G. R. Distinctive chromosomal structures are formed very early in the amplification of CAD genes in Syrian hamster cells. Cell. 1990 Dec 21;63(6):1219–1227. doi: 10.1016/0092-8674(90)90417-d. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Lengyel J. A. Small circular DNA of Drosophila melanogaster: chromosomal homology and kinetic complexity. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6142–6146. doi: 10.1073/pnas.76.12.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]