Abstract
Background
Sepsis remains a leading cause of death in intensive care units. There is growing evidence that volatile anesthetics have beneficial immunomodulatory effects on complex inflammation-mediated conditions. The authors investigated the effect of volatile anesthetics on the overall survival of mice in a sepsis model of cecal ligation and puncture (CLP).
Methods
Mice (N = 12 per treatment group) were exposed to anesthetic concentrations of desflurane, isoflurane, and sevoflurane either during induction of sepsis or when the mice showed pronounced symptoms of inflammation. Overall survival, as well as organ function and inflammation was compared with the CLP group without intervention.
Results
With desflurane and sevoflurane conditioning (1.2 minimal alveolar concentration for 2 h immediately after induction of CLP) overall survival was improved to 58% and 83%, respectively, compared with 17% in the untreated CLP group. Isoflurane did not significantly affect outcome. Application of sevoflurane 24 h after sepsis induction significantly improved overall survival to 66%.
Conclusions
Administration of the volatile anesthetics desflurane and sevoflurane reduced CLP-induced mortality. Anesthesia may be a critical confounder when comparing study data where different anesthesia protocols were used.
Sepsis and septic shock remain the leading causes of death in intensive care units worldwide.1 Complex pathophysiology together with heterogeneous disease patterns are key features making the treatment of sepsis extremely challenging.2–5 Numerous approaches have been undertaken to attenuate the harmful host response to infection, with a mostly unsuccessful translation into clinical outcome. Despite new pathophysiological insights and major efforts in developing goal-directed therapies, mortality in septic patients remains considerably high, and the treatment of late stage diagnosed patients is generally associated with bad outcome.6
Volatile anesthetics such as desflurane, isoflurane, and sevoflurane have been identified as effective modifiers of the inflammatory response in various states of tissue injury, exerting beneficial effects on organ function and overall outcome in both animals7–12 and patients.13–16 Potential benefits of the application of volatile anesthetics in in vivo models of experimental sepsis have not been systematically explored, and their effect on survival remains unclear. Previous studies elucidating the protective potential of volatile anesthetics have traditionally focused on ischemia–reperfusion injury and not on sepsis. In addition, biomarkers of organ injury were determined, but outcome parameters were not.17
In this study, we investigated in a model of severe murine sepsis with intraabdominal focus (peritonitis) whether the volatile anesthetics desflurane, isoflurane, and sevoflurane impact on overall survival of septic animals. The cecal ligation and puncture (CLP) model is considered the “gold standard” in sepsis research with a disease profile similar to that in human sepsis in that it encompasses more of the clinical features and drug responses of human sepsis, than, for example, the lipopolysaccharide model,18,19 despite the absence of some key features (e.g., kidney and lung injury). In this study, mice were exposed to desflurane, isoflurane, or sevoflurane during induction of sepsis (conditioning). To investigate the effect in a postconditioning setting, desflurane and sevoflurane were applied 24 h after the CLP procedure, when the mice showed pronounced symptoms of inflammation. Survival as well as markers of renal and hepatic organ function was compared with the CLP group without intervention. Being aware of the limitations of animal models of sepsis, the current study illustrates the beneficial effects of the volatile anesthetics desflurane and sevoflurane in states of severe inflammation leading to a remarkably improved 7-day survival and reduced end-organ damage.
Materials and Methods
Animals
The animal protocol was approved by the University of Illinois Animal Care and Use Committee (Chicago, Illinois). All experiments were performed in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines as defined by the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals. Eight- to 12-week-old male C57BL/6 mice (Charles River Laboratories, Chicago, IL) with body weights between 22 and 31 g (25.8 ± 2.7 g) were used for the experiments. The animals were closely monitored before and during the experiments, assessing respiration, health (fur, teeth, and body weight), and signs of pain or distress. All mice received buprenorphine (0.1 mg/kg subcutaneously) immediately after surgery and as needed thereafter for analgesia. Severely moribund mice were euthanized by anesthetic overdose followed by cervical dislocation.
Anesthesia and Sepsis Induction by CLP
CLP Procedure
The CLP procedure was carried out at the same time of the day to account for circadian rhythm effects on the inflammatory response. Mice were anesthetized using a single dose of ketamine (100 mg/kg body weight; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (10 mg/ kg body weight; Ben Venue Lab, Bedford, OH), applied intraperitoneally unless otherwise stated. CLP was carried out as described by Rittirsch et al.18 In brief, once a surgical plane of anesthesia was reached, as determined by the lack of response to paw or tail pinch and eye reflex, the abdomen was shaved, cleaned, and de-germed using a povidone-iodine solution before a 1-cm midline incision was made. The cecum was exposed and the distal 20% (below the ileocecal valve, approximately 1 cm from the tip) was ligated with a 6-0 suture. The cecum was punctured four times with a 20-gauge needle. When returning the cecum into the abdomen, a well-controlled, small amount of feces was extruded from mesenteric and antimesenteric penetration holes. The wound was cleaned with 0.5 ml of sterile saline, and the peritoneal cavity was closed in two layers with 7-0 and 6-0 prolene sutures, respectively. SHAM animals underwent the same procedure without ligation and puncture of the cecum.
Treatment Groups
Desflurane, isoflurane, and sevoflurane conditioning mice (N = 12 for each group) were anesthetized with 1.2 minimal alveolar concentration (MAC) of desflurane (9 vol%)20 (Baxter Healthcare Corporation, Deerfield, IL), isoflurane (1.5 vol%)20 (Piramal Critical Care, Inc., Bethlehem, PA), or sevoflurane (4 vol%)20 (Baxter Healthcare Corporation) via nose-cone, respectively (instead of ketamine/xylazine). After CLP and injection of saline (20 ml·kg−1·h−1) through the jugular vein, mice were allowed to spontaneously breathe desflurane, isoflurane, or sevoflurane (1.2 MAC) for an additional 2 h. In the sevoflurane postconditioning group, CLP-induced injury was performed under ketamine/xylazine anesthesia. Sevoflurane was administered 24 h after CLP (either 1.2 MAC for 2 h, N = 8, or at a reduced dose of 1 MAC for a duration of 0.5 h, N = 12). For desflurane postconditioning, 1 MAC of desflurane was administered 24 h after CLP for 0.5 h. All animals were closely monitored, and the depth and duration of anesthesia was comparable for all groups included in the experiment.
Survival Studies
For survival studies, mice were returned to their cages after CLP induction and closely monitored for up to 7 days. Mice were given ad libitum access to food and water at all times.
Analysis of Blood and Tissue Samples 24 h after Sepsis Induction
In the next series of experiments, samples were harvested 24 h after CLP induction and animals were euthanized immediately after (N = 6). Peritoneal lavage and blood samples were collected to quantitatively assess markers of disease severity, end-organ damage, and bacterial load. Twenty-four hours after induction of CLP, mice were anesthetized with ketamine/ xylazine and 2 ml of sterile 0.9% NaCl was instilled into the peritoneal cavity. The abdomen was massaged gently for 1 min and then opened with sterile scissors. Recovered peritoneal lavage (1–1.5 ml) was used for cytokine analysis and bacterial count. For detection of cytokines, peritoneal fluid was centrifuged at 2,500 rpm for 10 min at 4°C, and the supernatant was stored at −80°C. For blood sampling after 24 h of injury, 50 μl of heparin (1,000 units/ml) was injected into the inferior vena cava and allowed to circulate for 1 min before euthanizing the animals. Using an angiocath (20-gauge), 500–700 μl of blood was collected. Markers of renal and hepatic organ function (blood urea nitrogen, alkaline phosphatase, alanine transaminase, and aspartate transaminase), total protein, and albumin levels were analyzed in fresh plasma samples with a Hitachi 916 chemistry analyzer (Roche Diagnostics, Laval, Quebec, Canada). For bacterial load determination, peritoneal fluid and blood were serially diluted in sterile saline, and 10 μl of the diluted samples were plated immediately after sample collection on tryptic blood agar plates. After incubation at 37°C for 20 h, bacterial colony forming units were counted. Cytokine concentrations were measured in both plasma and peritoneal fluid using enzyme-linked immunosorbent assay (ELISA) kits for mouse monocyte chemoattractant protein-1 and interleukin-6 (both from BD Biosciences, San Diego, CA). Procedures were carried out according to the protocol provided by the manufacturer.
Statistical Analysis
Sample sizes were determined based on previous studies.9 Survival data were analyzed using Origin (Kaplan–Meier Survival Analysis; OriginLAB, Northampton, MA). Log rank test with Bonferroni correction was used to compare survival distributions. Bar graphs show means ± SD. Markers for organ function and levels of inflammatory mediators for treated and untreated groups were compared using Kruskal–Wallis ANOVA with Mann–Whitney U tests (small sample size, two-tailed) and Bonferroni correction for multiple comparisons. Power analysis was calculated using SPSS version 20.0 (IBM, Zurich, Switzerland). Statistical significance was assigned to a P value of less than 0.05.
Results
Mortality rate in the CLP group was 75% within the first 48 h. One day after induction of CLP, mice showed pronounced symptoms of severe sepsis, including reduced mobility, starry fur, hunched appearance, lethargy, and weight loss (>10%). The overall survival in the CLP group after 7 days was 17%. However, when mice were exposed to 1.2 MAC desflurane during and for additional 2 h after surgery, overall survival increased to 58% (P = 0.049; N = 12; fig. 1A). Similarly, when mice were exposed to 1.2 MAC (4 vol%) sevoflurane, they showed significant improvement in overall survival (83%) compared with the CLP group (P < 0.001; N = 12) (fig. 1A). Symptoms of sepsis were less pronounced during the entire observation period and mice regained full mobility with body weight increasing by day 3 after CLP induction. Exposing mice to equi-MACs of isoflurane (1.2 MAC, 2 h) was less effective and did not significantly improve overall survival compared with the CLP group without conditioning (42% survival; P = 0.12; N = 12; fig. 1A). Only at the 48-h time point was a significant difference observed in the isoflurane group compared with the CLP group (75% survival compared with 50%; P = 0.042).
Fig. 1.
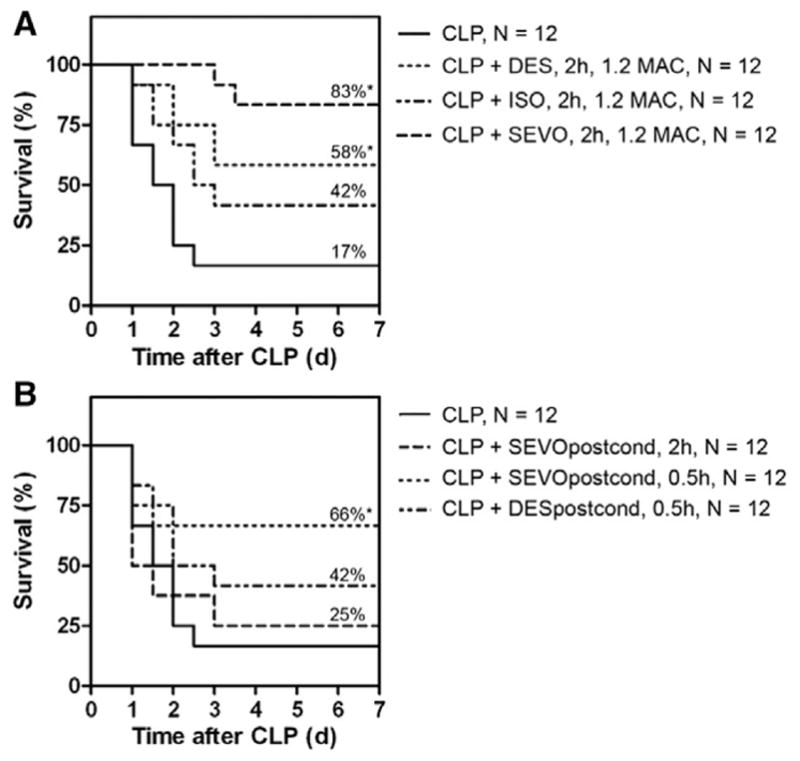
Survival analyses—volatile anesthetics. (A) Conditioning with volatile anesthetics. Survival was assessed over 7 days in a cecal ligation and puncture (CLP) model with a >75% mortality at postoperative day 3 and overall survival of 17% observed in the CLP group. Exposure (1.2 MAC for 2 h) to anesthetic concentrations of desflurane and sevoflurane significantly improved overall survival in CLP mice to 58% (P = 0.049) and 83% (P < 0.001), respectively. Equi-MAC concentrations of isoflurane were less effective (P = 0.12). (B) Postconditioning with volatile anesthetics. Sevoflurane but not desflurane postconditioning (1 MAC for 0.5 h) 24 h after CLP procedure markedly improved overall survival of 66% no mortality after postoperative day 2 (P = 0.045). DES = desflurane; ISO = isoflurane; MAC = minimal alveolar concentration; Postcond = postconditioning; SEVO = sevoflurane. *P < 0.05.
Based on the promising results observed in the desflurane and the sevoflurane conditioning groups, the administration of volatile anesthetics during sepsis was investigated in a postconditioning setting: 24 h after induction of CLP, desflurane or sevoflurane was applied to mice suffering from severe sepsis with pronounced symptoms. When exposing mice to 1.2 MAC sevoflurane for 2 h, however, a large number died either during or within the first few hours after anesthesia. Overall survival was not different from the CLP group without intervention (fig. 1B). Reducing the sevoflurane dose (from 1.2 to 1 MAC) and exposure time (from 2 to 0.5 h) significantly improved overall survival to 66% (P = 0.045). In this group, no deaths were observed after postoperative day 2. Desflurane postconditioning (1 MAC, 0.5 h) did not significantly improve outcome (fig. 1B).
Seven days after CLP, all surviving mice developed abscesses in the region of the cecum. Considering that the primary objective of the host response to bacterial infection is removal of the organisms, bacterial numbers in the peritoneum and blood were counted. No significant differences regarding bacterial load were found either in peritoneal fluid samples or blood samples collected at 24 h after CLP versus CLP + sevoflurane (peritoneal fluid, 2.00 [±1.71] × 107 colony forming units/ml for CLP [N = 6] vs. 2.54 [±0.79] × 107 colony forming units/ml for CLP + sevoflurane (N = 6), no bacteria were detected in samples from SHAM animals [N = 4]).
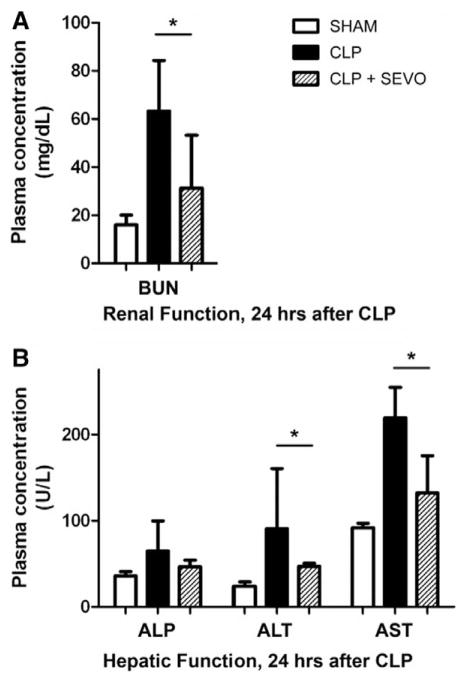
The measurement of biochemical parameters of tissue injury in SHAM, CLP, and CLP + sevoflurane animals revealed that sevoflurane significantly attenuated CLP-induced kidney and liver injury. Assessing markers for renal function 24 h after CLP, increased levels of blood urea nitrogen were found in the CLP group. Conditioning with sevoflurane significantly decreased plasma levels of blood urea nitrogen (P = 0.049; fig. 2A). Increased plasma levels of alanine transaminase and aspartate transaminase were measured for the CLP group compared with the SHAM group. In mice with sevoflurane conditioning, alanine transaminase and aspartate transaminase levels were significantly lower (alkaline phosphatase: P = 0.462; alanine transaminase: P = 0.009; aspartate transaminase: P = 0.002; N = 6; fig. 2B). Total plasma protein and albumin levels were comparable for all groups. Plasma and peritoneal fluid cytokine levels are illustrated in figure 3. CLP induced high levels of cytokines in both plasma and peritoneal fluid with significant increases in CLP compared to SHAM animals in the peritoneal fluid only (interleukin-6: P < 0.05; monocyte chemoattractant protein-1: P < 0.05).
Fig. 2.
Renal (A) and hepatic (B) function in SHAM, cecal ligation and puncture (CLP), and sevoflurane coconditioning groups. Assessing renal injury markers, a CLP-induced increase in blood urea nitrogen (BUN) was found. Hepatic markers (alkaline phosphatase [ALP]; alanine transaminase [ALT]; aspartate transaminase [AST]) were increased in the CLP group (N = 6) compared with the SHAM group (N = 3). Compared with the CLP group, application of sevoflurane (SEVO, 1.2 minimal alveolar concentration, 2 h, N = 6) in CLP animals efficiently reduced markers of organ damage to levels that were comparable with those in SHAM animals. Values are means ± SD. *P < 0.05. SEVO = sevoflurane.
Fig. 3.
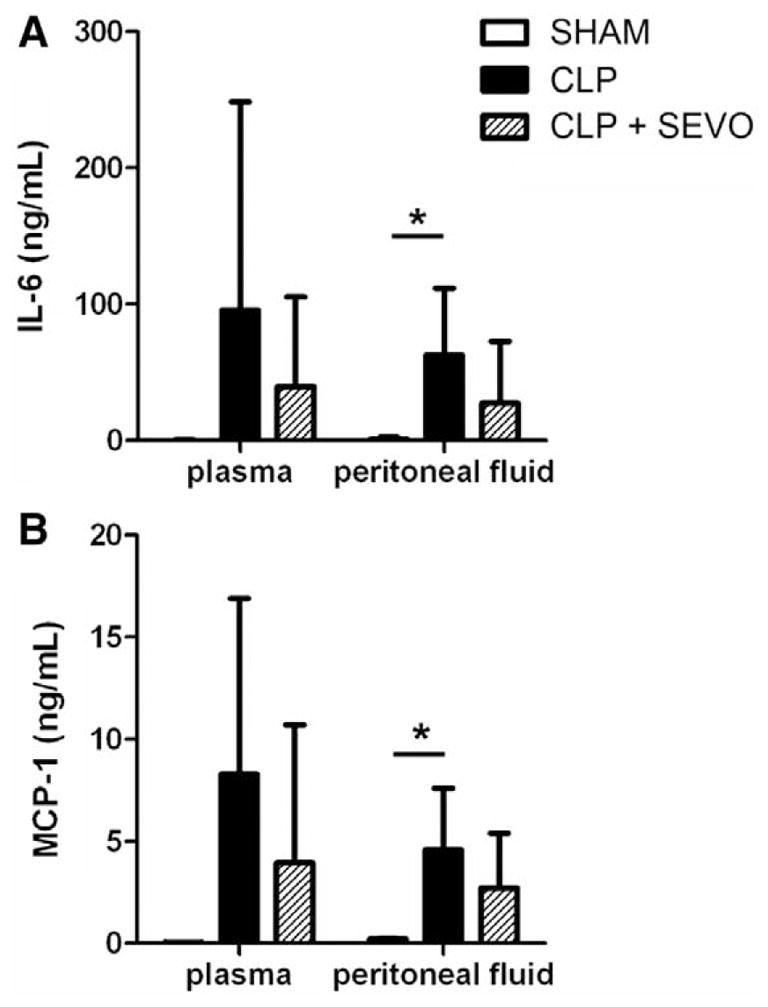
Cytokines—markers of inflammation. Inflammatory mediator levels in blood and peritoneal fluid 24 h after cecal ligation and puncture (CLP) induction. A CLP-induced increase in interleukin-6 (IL-6) (A) and monocyte chemoattractant protein-1 (MCP-1) (B) was found in plasma and peritoneal fluid from CLP mice (N = 6) compared with SHAM animals (N = 3). The inflammatory mediator release was not significantly altered by application of sevoflurane (1.2 minimum alveolar concentration, 2 h, N = 6) in CLP animals. Values are means ± SD. *P < 0.05. SEVO = sevoflurane.
Discussion
The major findings of this study are that application of the volatile anesthetic sevoflurane significantly improved overall survival in a murine model of septic peritonitis when administered during the surgical procedure as well as 24 h after induction of CLP. Similarly, desflurane but not isoflurane positively affected survival in a conditioning setting. In addition, sevoflurane exposure largely prevented CLP-induced renal and hepatic injury.
Volatile Anesthetics in States of Tissue Inflammation
In a number of previous studies, volatile anesthetics have been reported to reduce systemic inflammation and organ damage both in animals7–12 and humans.13–16 However, the underlying mechanisms remain poorly understood, and it has not yet been systematically determined whether these effects could be translated into a positive effect on organ function and outcome in the septic murine peritonitis model.
In the current study, both early and late administration of sevoflurane and exposure to desflurane were shown to be effective at improving the overall survival of CLP mice and in attenuating the expression of tissue damage marker. In contrast, equi-MACs of isoflurane were less effective in improving overall survival. In agreement with our data, a recently published study by Lee et al.9 found early protection using isoflurane, but a 7-day survival of 0% in both the CLP and the CLP + isoflurane group in a comparable animal model. Regarding the effectiveness of volatile anesthetics, studies investigating effects of volatile anesthetics on myocardial reperfusion injury have revealed similar differences among desflurane, isoflurane, and sevoflurane, with isoflurane being least effective.20,21 It has been suggested that different mechanisms of action may be responsible for the observed differences in protective effects.21
Interestingly, desflurane and sevoflurane provided prolonged protection against murine septic peritonitis although both compounds have very low blood and tissue solubility. This indicates that the protective effects may be the result of triggered long-lasting modulatory reactions. Reviewing the literature reveals that protective action appears to be global in nature. Volatile anesthetics have been described to interact with and modulate the immune system,22 coagulation cascade, 23 metabolism24 or adenosine adenosine triphosphate–sensitive potassium channels.25 They attenuate sequestration of organ damage markers and inflammatory mediators including a broad variety of chemokines and cytokines that play a pivotal role in the initial hyperinflammatory phase of sepsis. In the current study, markers of renal and hepatic function 24 h after CLP procedure revealed that sevoflurane provides significant protection and may prevent end-organ damage in murine sepsis. These results are consistent with an earlier study showing that isoflurane protects from CLP-induced hepatic and renal injury.9 Whether prevention of organ damage is a primary mechanism of protection by volatile anesthetics, thereby playing a pivotal role in outcome, rather than a mere consequence of a more controlled immune reaction that prevents host organs from being attacked by leukocytes requires further investigation. The findings are in good agreement with clinical studies reporting that volatile anesthetics reduce ischemia–reperfusion-induced organ damage in patients.16 Regarding inflammation (secondary endpoint), CLP-induced cytokine expression in plasma and peritoneal fluid at 24 h after CLP was not significantly affected by sevoflurane. A priori power analysis based on the measured effect size (inflammatory mediator result from our experiments) reveals that more than 100 animals per group would be required to support the performed statistical tests with sufficient power.
Limitations of the Experimental Model
The current study clearly shows significant beneficial effects, however, requires validation in other animal models and insight into the mechanism of action before first conclusions on the potential benefits of volatile anesthetics in septic peritonitis can be drawn. Particularly, larger animal numbers are required to consolidate the beneficial effects of volatile anesthetics in CLP and to identify their role on the inflammatory response. Regarding the translation of observations made in animal models of sepsis using (antiinflammatory) drugs, clinical trials have failed because most animal models inadequately mirror the immune response in humans.26 Another limitation of the CLP animal model may arise by shifting from a true sepsis model into an intraabdominal abscess model by the formation of a walled-off abscess. Indeed, we observed abscess formation in survivors in the region of the cecum after 7 days; however, bacterial counts in peritoneal fluid and blood were comparable for all groups. Despite promising data from patients undergoing heart, liver, and lung surgery, only clinical studies can ultimately reveal the actual therapeutic benefit of sevoflurane in patients suffering from conditions with complex inflammation-mediated pathophysiology such as sepsis.
Volatile Anesthetics in Animal Models of Sepsis and Inflammation
Importantly, these results reveal that anesthesia has a pivotal effect on the outcome of CLP mice. Thus, special caution is required when using volatile anesthetics during surgical procedures (e.g., CLP induction) as this may significantly affect outcome. Comparison of data among studies using different anesthesia protocols is not recommended. This may not be limited to CLP models in mice, but may also hold for other models investigating inflammation-mediated conditions.
Conclusion and Perspective
This study provides the first experimental demonstration that the application of both desflurane and sevoflurane markedly reduce CLP-induced 7-day mortality in a model of murine septic peritonitis. Improvement in overall survival with volatile anesthetics is accompanied by reduced end-organ damage. Although the mechanism of organ protection by volatile anesthetics remains poorly understood, the action has proven to be robust in nature and therefore holds potential for translation. The use of volatile anesthetics in experimental studies may have a severe consequence on outcome and may be a confounder becoming critical when comparing study data where different anesthesia protocols were used.
What We Already Know about This Topic
Volatile anesthetics have been shown to be antiinflammatory agents in certain conditions
What This Article Tells Us That Is New
Exposing septic mice to volatile anesthetics, particularly sevoflurane, significantly improved survival
Acknowledgments
Support was provided by the National Institutes of Health, Bethesda, Maryland, grant nos. NIH P01 HL60678 and R01 HL71626; Emdo Foundation, Zurich, Switzerland; and Swiss National Science Foundation, Berne, Switzerland, grant no. 320030_141216. Dr. Herrmann and Ms. Castellon contributed equally as first authors. Drs. Minshall and Beck-Schimmer contributed equally as senior authors.
The authors kindly thank David J. Visintine, Technician; Vasily Shinin, M.D., Ph.D., Research Associate, University of Illinois at Chicago, Chicago, Illinois; and Wendelin Stark, Ph.D., Professor, ETH Zurich, Zurich, Switzerland, for their helpful discussions.
Footnotes
Information on purchasing reprints may be found at www.anesthesiology.org or on the masthead page at the beginning of this issue.
References
- 1.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: A critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.Slade E, Tamber PS, Vincent JL. The Surviving Sepsis Campaign: Raising awareness to reduce mortality. Crit Care. 2003;7:1–2. doi: 10.1186/cc1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claessens YE, Dhainaut JF. Diagnosis and treatment of severe sepsis. Crit Care. 2007;11(suppl 5):S2. doi: 10.1186/cc6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Hofstetter C, Boost KA, Flondor M, Basagan-Mogol E, Betz C, Homann M, Muhl H, Pfeilschifter J, Zwissler B. Antiinflammatory effects of sevoflurane and mild hypothermia in endotoxemic rats. Acta Anaesthesiol Scand. 2007;51:893–9. doi: 10.1111/j.1399-6576.2007.01353.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee HT, Emala CW, Joo JD, Kim M. Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis. Shock. 2007;27:373–9. doi: 10.1097/01.shk.0000248595.17130.24. [DOI] [PubMed] [Google Scholar]
- 10.Lee HT, Kim M, Kim M, Kim N, Billings FT, IV, D’Agati VD, Emala CW., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–22. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]
- 11.Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–62. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–7. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Head BP, Patel P. Anesthetics and brain protection. Curr Opin Anaesthesiol. 2007;20:395–9. doi: 10.1097/ACO.0b013e3282efa69d. [DOI] [PubMed] [Google Scholar]
- 14.Julier K, da Silva R, Garcia C, Bestmann L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von Segesser LK, Pasch T, Spahn DR, Zaugg M. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: A double-blinded, placebo-controlled, multicenter study. Anesthesiology. 2003;98:1315–27. doi: 10.1097/00000542-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kato R, Foëx P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: An update for anesthesiologists. Can J Anaesth. 2002;49:777–91. doi: 10.1007/BF03017409. [DOI] [PubMed] [Google Scholar]
- 16.Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, Jochum W, Spahn DR, Graf R, Clavien PA. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg. 2008;248:909–18. doi: 10.1097/SLA.0b013e31818f3dda. [DOI] [PubMed] [Google Scholar]
- 17.De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: Mechanisms and clinical implications. Anesth Analg. 2005;100:1584–93. doi: 10.1213/01.ANE.0000153483.61170.0C. [DOI] [PubMed] [Google Scholar]
- 18.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–6. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 20.Redel A, Stumpner J, Tischer-Zeitz T, Lange M, Smul TM, Lotz C, Roewer N, Kehl F. Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Exp Biol Med (Maywood) 2009;234:1186–91. doi: 10.3181/0902-RM-58. [DOI] [PubMed] [Google Scholar]
- 21.Schlack W, Preckel B, Stunneck D, Thämer V. Effects of halothane, enflurane, isoflurane, sevoflurane and desflurane on myocardial reperfusion injury in the isolated rat heart. Br J Anaesth. 1998;81:913–9. doi: 10.1093/bja/81.6.913. [DOI] [PubMed] [Google Scholar]
- 22.Elena G, Amerio N, Ferrero P, Bay ML, Valenti J, Colucci D, Puig NR. Effects of repetitive sevoflurane anaesthesia on immune response, select biochemical parameters and organ histology in mice. Lab Anim. 2003;37:193–203. doi: 10.1258/002367703766453038. [DOI] [PubMed] [Google Scholar]
- 23.Yuceaktas A, Topal A, Celik JB, Erol A, Otelcioglu S. Effects of desflurane and sevoflurane on thromboelastogram in patients undergoing laparoscopic cholecystectomy: 6AP6-2. Eur J Anaesth. 2011;28:94. [Google Scholar]
- 24.Riess ML, Eells JT, Kevin LG, Camara AK, Henry MM, Stowe DF. Attenuation of mitochondrial respiration by sevoflurane in isolated cardiac mitochondria is mediated in part by reactive oxygen species. Anesthesiology. 2004;100:498–505. doi: 10.1097/00000542-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Schaub MC. Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology. 2002;97:4–14. doi: 10.1097/00000542-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–43. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]