Abstract
Background
Osteopontin (OPN) is a multifunctional glycoprotein with pro-inflammatory properties. In severe sepsis, levels of plasma OPN are significantly higher in non-survivors than in survivors. We hypothesized that OPN results in greater inflammation and worse outcome through modulation of endogenous glucocorticoid production in sepsis.
Methods and Results
Sepsis was induced by cecal ligation and puncture (CLP) in wild type (WT) and OPN gene knockout (OPN−/−) mice. In response to sepsis, the OPN−/− mice had lower levels of plasma cytokines and chemokines than the WT mice. The levels of corticosterone in plasma were similar between WT and OPN−/− sham animals but they increased 24 h after CLP induction in the WT mice, but not in the OPN−/− mice. The mortality rate was lower in the OPN−/− mice than in the WT mice.
Conclusion
OPN is associated with greater inflammatory response and increased mortality, despite the higher corticosterone levels in plasma. Corticosterone production is not impaired in the absence of OPN.
Sepsis is a leading cause of mortality in critically ill patients and is characterized by acute excessive inflammatory responses.1–3 The levels of osteopontin (OPN) in plasma are elevated in patients with sepsis as compared with patients without sepsis in the ICU.4 In septic patients, the non-survivors had higher OPN levels than the survivors.4
OPN is a multifunctional glycoprotein that is considered as both an extracellular matrix protein and a cytokine.5 OPN activates downstream signal transduction pathways via interaction with cell surface integrins and CD44 receptor.6 OPN also acts as a chemotactic factor for neutrophils, T cells and macrophages, and shares the properties with Th-1 cytokines.6 The inflammatory responses mediated by OPN are thought to be associated with its modulatory effects on endogenous glucocorticoid production.7,8
We hypothesized that the inhibition of OPN protects against sepsis by enhancing endogenous corticosteroid production to reduce inflammation.
To test the hypothesis, we employed a clinically relevant model of polymicrobial sepsis induced by cecal ligation and puncture (CLP) in wild type (WT) and in OPN gene knockout mice.
Material and methods
Animals
The experimental protocol was approved by the Animal Care Committee of St Michael’s Hospital. C57BL/6 male (WT, n = 24) mice and OPN gene knockout (OPN−/−, n = 24) mice (6–8 weeks, Charles River, Montreal, Quebec, Canada) were anesthetized by ketamine 150 mg/kg and xylazine 10 mg/kg intraperitoneally. Through a 2-cm ventral midline incision, the cecum was isolated and legated 1.5 cm distally to ileocecal valve with a 4-0 silk ligature. Subsequently, the ligated cecum was punctured with a 22-gauge needle making two punctures. After returning the cecum, abdomen was closed. One milliliter of Normal Saline was injected in all the animals to prevent from dehydration and hypovolemia induced by the surgery. Sham animals underwent the similar surgical procedure without receiving CLP. The animals were then housed and observed for 24 h followed by sacrifice by using overdose anesthesia. Blood samples were collected for measurements of cytokines and corticosterone, and adrenal glands were harvested for measurement of tissue corticosterone.
With two groups of strains of mice that were subjected to CLP and assuming that the mortality is normally distributed, a CLP group size of 12 is required to have an 80% chance to detect a difference, based on a previous study from our group.9
Cytokines assay
Cytokine profiles, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-12 (IL-12), tumor necrosis factoralpha (TNF-α), interferon-gamma, granulocytemacrophage colony-stimulating factor (GM-CSF), keratinocyte growth factor, monocyte chemotactic protein-1, and RANTE (CCLS) were analyzed in plasma by using a Luminex multiplex assay (Bio-Rad, Hercules, CA, USA).
Adrenocorticotropic hormone (ACTH) stimulation
In additional animals, ACTH (α-1-24 corticotropin, Cortrosyn, Organon Canada Ltd., ON, Canada) was injected intramuscularly at 0.8 μg/g body weight at 200 μl 1 h prior to the 24 h of observation.10
Corticosterone assay
Adrenal glands were homogenized and centrifuged at 1300g for 20 min at 4°C. The supernatant was diluted 1 : 16 and used for corticosterone and protein measurements by a commercially available ELISA kit (IBL America, Minneapolis, MN, USA). Corticosterone concentration in adrenal homogenate was expressed per tissue protein.
Statistical analysis
Data are expressed as mean ± standard deviation. Kaplan–Meier survival plot was performed for survival analysis and the survival curves were compared using the Log-rank (Mantel–Cox) test. Comparison between the groups was performed using analysis of variance followed by Holm-Sidak test. A P value <0.05 was considered as significant.
Results
Survival rate in response to CLP
The survival rate was 54% in the WT mice 24 h after CLP induction, and was 83% in the OPN−/− mice (Fig. 1).
Figure 1.
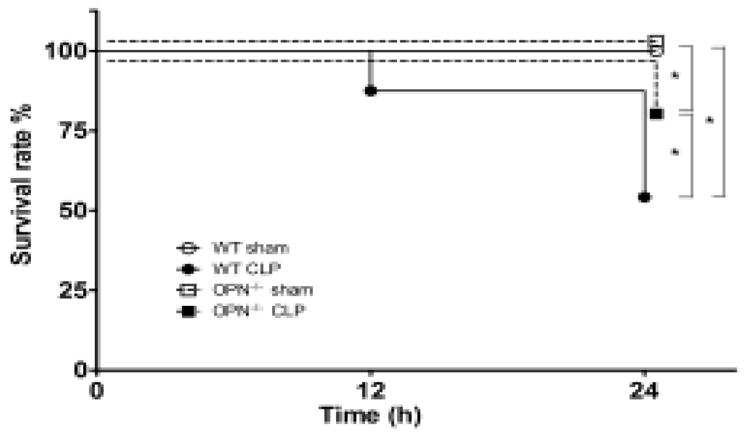
Kaplan–Meier survival curve after cecal ligation and puncture (CLP) in wild type (WT) and osteopontin knockout (OPN−/−) mice. n = 24 each. *P < 0.05.
Inflammatory responses after CLP
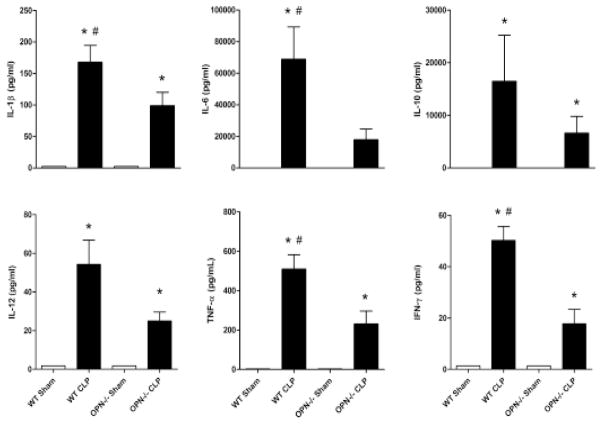
The levels of the 10 cytokines, chemokines and GM-CSF measured in the plasma increased dramatically in the WT mice after 24 h after CLP induction, and the increase was attenuated in the OPN−/− mice (Figs 2 and 3).
Figure 2.
Cytokine profiles in plasma. Plasma concentration of interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-12 (IL-12), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ) in wild type (WT), and osteopontin gene knockout (OPN−/−) mice 24 h after sham or cecal ligation and puncture operation (CLP). n = 6 each. *P < 0.05 vs. sham; #P < 0.05 vs. OPN−/−.
Figure 3.
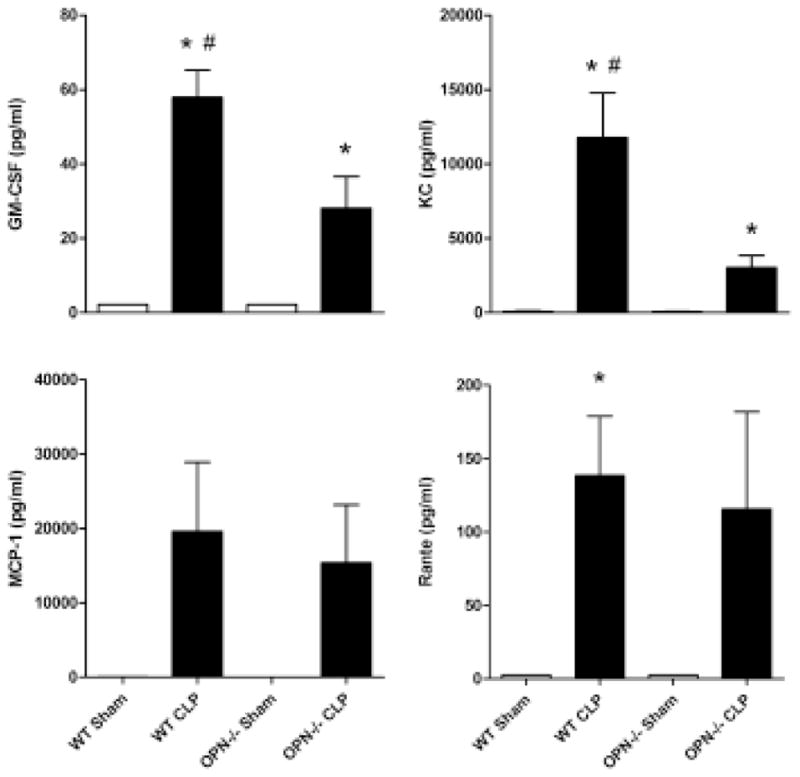
Chemokine profiles and granulocyte-macrophage colony-stimulating factor (GM-CSF) level in plasma. Plasma concentration of GM-CSF, keratinocyte growth factor (KC), monocyte chemotactic protein-1 (MCP-1), RANTE in wild type (WT), and osteopontin knockout (OPN−/−) mice 24 h after sham or cecal ligation and puncture operation (CLP). n = 6 each. *P < 0.05 vs. sham; #P < 0.05 vs. OPN−/−.
Systemic and adrenal corticosterone responses after CLP
The level of corticosterone in plasma increased in 24 h after CLP induction in the WT mice, but not in the OPN−/− mice (Fig. 4A). The level of corticosterone further increased after receiving ACTH in the WT mice and remained similar in the OPN−/− mice (Fig. 4B). A similar pattern of changes was observed when corticosterone levels were measured in the adrenal gland (Fig. 4C).
Figure 4.
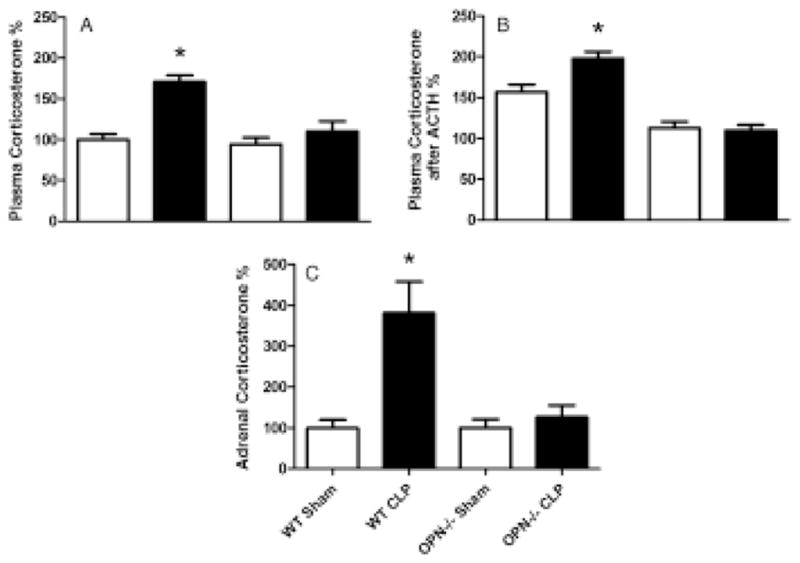
Plasma and adrenal corticosterone levels. (A) Plasma corticosterone levels were measured 24 h after cecal ligation and puncture operation (CLP) in wild type (WT) and osteopontin knockout (OPN−/−) mice. (B) Plasma corticosterone levels were measured 1 h after stimulation with ACTH that was injected at 23 h after CLP. (C) The levels of corticosterone measured in adrenal gland 24 h after CLP. n = 6 each. *P < 0.05 vs. any of other group.
Discussion
The main finding of our study is that the presence of OPN is associated with greater inflammatory responses and an increased mortality rate in the mouse model of CLP-induced sepsis despite the higher corticosterone levels in plasma. Moreover, corticosterone production in OPN−/− sham animals is similar with that of WT sham mice.
OPN is a multifunctional glycoprotein expressed in a variety of cell types including immune and epithelial cells.5,6 In normal conditions, OPN plays an important role in the host against infections caused by virus,11 bacteria,12 and parasite.13 However, the protective effects of OPN may be overweighed by its proinflammatory property.14,15 Our results suggest that the absence of OPN prevents the host from an excessive inflammatory response while reduce the cytokine response to a level that is sufficient to kill the bacteria causing the sepsis.
We observed that the glucocorticoid production is similar between WT sham and OPN−/− sham animals which means that corticosterone production is not impaired in the absence of OPN. Corticosterone did not increase in the OPN−/− mice following CLP. Since the endogenous glucocorticoids are considered protective15 and the OPN knockout mice have lower mortality despite the lower levels of corticosterone, we assumed that this occurs because of the attenuated inflammatory response. This finding is accordance with those reported by other investigators demonstrating that the levels of corticosterone, a major glucocorticoid in rodents, were not altered in response to hindlimb unloading-induced stress in OPN−/− mice.7 It is known that pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, are able to induce glucocorticoid production by activation of hypothalamic–pituitary–adrenal axis.16 This mechanism provides an alternative explanation for the reduced levels of corticosterone seen in the OPN−/− mice.
Another possible explanation for the reduced corticosterone in septic knockout mice is a defect in 11β-hydroxysteroid dehydrogenases (11β-HSDs). There are two types of 11β-HSDs: type I or 11β-HSD1 which catalyzes the reduction of inactive 11-dehydrocorticosterone into active corticosterone and type II or 11β-HSD2 which catalyzes the reverse reaction in the target tissues.17 It is possible that impaired 11β-HSD2 function may have resulted in an increased glucocorticoid negative feedback.18,19
The higher survival rate observed in the OPN−/− mice may be associated with the attenuated inflammatory responses as well as the lower corticosterone production compared with the WT mice. In patients with sepsis, a higher level of plasma OPN has been reportedly associated with worse outcomes.4 Glucocorticoids are usually considered protective as they inhibit the excessive pro-inflammatory response in conditions like sepsis.20 However, high levels of endogenous corticosteroids are not necessarily associated with better outcomes.21,22 In conditions with increased inflammation, the production of glucocorticoids is frequently high, but it is still unable to counterbalance the exaggerated inflammatory response.20
There are few limitations in the present study, including the short period of time (24 h) of the sepsis model and lack of antibiotic treatment as seen in patients. We did not also measure the levels of OPN in plasma or tissue. However, the level of OPN would expect to be undetectable in the OPN−/− mice while the WT mice expressed OPN.23 Nevertheless, our data support that plasma levels of OPN may be a useful biomarker, a predictor for outcome, and possibly a therapeutic target in sepsis.
Acknowledgments
Funding
The study was supported by Canadian Institutes of Health Research (CIHR) grant to HZ. SF was supported by the Alexander S. Onassis Public Benefit Foundation.
Footnotes
Conflicts of interest
Authors have no conflicts of interest germane to this topic.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med. 2003;168:165–72. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Vaschetto R, Nicola S, Olivieri C, Boggio E, Piccolella F, Mesturini R, Damnotti F, Colombo D, Navalesi P, Della Corte F, Dianzani U, Chiocchetti A. Serum levels of osteopontin are increased in SIRS and sepsis. Intensive Care Med. 2008;34:2176–84. doi: 10.1007/s00134-008-1268-4. [DOI] [PubMed] [Google Scholar]
- 5.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–45. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Wang KX, Shi Y, Denhardt DT. Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production. Proc Natl Acad Sci U S A. 2007;104:14777–82. doi: 10.1073/pnas.0703236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang KX, Shi YF, Ron Y, Kazanecki CC, Denhardt DT. Plasma osteopontin modulates chronic restraint stress-induced thymus atrophy by regulating stress hormones: inhibition by an anti-osteopontin monoclonal antibody. J Immunol. 2009;182:2485–91. doi: 10.4049/jimmunol.0803023. [DOI] [PubMed] [Google Scholar]
- 9.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–57. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Brochu M, Wang SP, Rochdi L, Cote M, Mitchell G, Gallo-Payet N. Hormone-sensitive lipase deficiency in mice causes lipid storage in the adrenal cortex and impaired corticosterone. doi: 10.1210/en.2002-220341. [DOI] [PubMed] [Google Scholar]
- 11.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 12.van der Windt GJ, Hoogerwerf JJ, de Vos AF, Florquin S, van der Poll T. Osteopontin promotes host defense during Klebsiella pneumoniae-induced pneumonia. Eur Respir J. 2010;36:1337–45. doi: 10.1183/09031936.00002710. [DOI] [PubMed] [Google Scholar]
- 13.Santamaria MH, Corral RS. Osteopontin-dependent regulation of Th1 and Th17 cytokine responses in Trypanosoma cruzi-infected C57BL/6 mice. Cytokine. 2013;61:491–8. doi: 10.1016/j.cyto.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Van der Windt GJ, Wiersinga WJ, Wieland CW, Tjia IC, Day NP, Peacock SJ, Florquin S, van der Poll T. Osteopontin impairs host defense during established gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis) PLoS Negl Trop Dis. 2010;4:e806. doi: 10.1371/journal.pntd.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Windt GJ, Hoogendijk AJ, Schouten M, Hommes TJ, de Vos AF, Florquin S, van der Poll T. Osteopontin impairs host defense during pneumococcal pneumonia. J Infect Dis. 2011;203:1850–8. doi: 10.1093/infdis/jir185. [DOI] [PubMed] [Google Scholar]
- 16.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 17.Krozowski Z, Li KX, Koyama K, Smith RE, Obeyesekere VR, Stein-Oakley A, Sasano H, Coulter C, Cole T, Sheppard KE. The type I and type II 11beta-hydroxysteroid dehydrogenase enzymes. J Steroid Biochem Mol Biol. 1999;69:391–401. doi: 10.1016/s0960-0760(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 18.Harris HJ, Kotelevtsev Y, Mullins JJ, Seckl JR, Holmes MC. Intracellular regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase (11beta-HSD)-1 plays a key role in regulation of the hypothalamic-pituitary-adrenal axis: analysis of 11beta-HSD-1-deficient mice. Endocrinology. 2001;142:114–20. doi: 10.1210/endo.142.1.7887. [DOI] [PubMed] [Google Scholar]
- 19.Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci U S A. 1997;94:14924–9. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marik PE. Critical illness-related corticosteroid insufficiency. Chest. 2009;135:181–93. doi: 10.1378/chest.08-1149. [DOI] [PubMed] [Google Scholar]
- 21.Schein RM, Sprung CL, Marcial E, Napolitano L, Chernow B. Plasma cortisol levels in patients with septic shock. Crit Care Med. 1990;18:259–63. doi: 10.1097/00003246-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga G, Bokhari F, Vogeser M. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–49. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 23.Guo H, Wai PY, Mi Z, Gao C, Zhang J, Kuo PC. Osteopontin mediates Stat1 degradation to inhibit iNOS transcription in a cecal ligation and puncture model of sepsis. Surgery. 2008;144:182–8. doi: 10.1016/j.surg.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]