Background
Meningiomas arise from the arachnoid meningothelial cells. Intracranially, they are extra-axial masses that are typically T1 isointense to slightly hypointense and T2 hyperintense to cortex, demonstrating avid and often homogeneous post-contrast enhancement. Differentiating meningiomas from other intracranial masses typically involve identifying the extra-axial nature of the mass, with evidence of hyperostosis of the adjacent osseous structures, as well as presence of calcification within the mass helpful for confirming the diagnosis of meningioma. The imaging appearance of meningiomas on MRI is typically characteristic enough that routine imaging sequences of T1-weighted and T2-weighted sequences, as well as postcontrast T1-weighted sequences are sufficient in making the diagnosis.1
Differentiating different subtypes of typical meningiomas and distinguishing typical from atypical/malignant meningiomas may not be possible from the conventional MRI sequences, however. Also, distinguishing meningiomas from other dural-based extra-axial masses, such as in a patient with a known primary malignancy that has a finding of a dural-based extra-axial mass intracranially, would be difficult with conventional T1-weighted and T2-weighted sequences alone. Distinguishing cystic meningiomas from other rim-enhancing/necrotic neoplasms, such as glioblastoma multiforme, also can be difficult, especially when the mass is large and there is difficulty identifying whether or not the mass is intra-axial or extra-axial.1
Diffusion-weighted imaging/diffusion tensor imaging (DWI/DTI), perfusion imaging, and magnetic resonance spectroscopy (MRS) can add additional information that can help refine the diagnostic considerations of a dural-based mass and has the potential to characterize the different subtypes of meningiomas. This article reviews the imaging findings of the techniques as they apply to meningiomas.
Perfusion Imaging
Although routine diagnostic MRI of the brain without and with intravenous contrast is typically able to diagnose a meningioma, it may sometimes be difficult to differentiate a mass as intra-axial or extra-axial in location because of the location or size of the mass. Magnetic resonance perfusion (MR perfusion) can potentially help differentiate a primary glial neoplastic process from an extraaxial mass. MR perfusion can be performed either with a dynamic susceptibility contrast (DSC) technique or a dynamic contrast enhancement (DCE) technique, both of which require the administration of intravenous gadolinium. The two techniques differ in the image acquisition sequence used, with DSC using an echo planar imaging technique and DCE using a gradient echo imaging technique. Both techniques require rapid administration of intravenous gadolinium in a quick bolus, with rapid imaging of the area of interest performed, typically approximately 40 volumes in a 2-minute to 5-min-ute period.2–4
DSC MR perfusion, the more typically used of the MR perfusion techniques, measures relative cerebral blood volume. Neoplastic processes typically have elevated relative cerebral blood volume compared with the contralateral white matter, and MR perfusion can identify masses with elevated relative cerebral blood volume. The time course of DSC MR perfusion also differs for primarily glial neoplastic processes as compared with intracranial metastases/extra-axial masses. The DSC signal time curve of a primary glial neoplastic process typically demonstrates greater than 50% return to baseline, whereas the DSC signal time curve for metastases/extra-axial masses typically demonstrates less than 50% return to base line due to the increased breakdown in blood-brain barrier as well as the presence of dural-based blood supply for metastases/extra-axial masses.5,6
Fig. 1 illustrates the utility of MR perfusion in characterizing meningiomas. A patient from our institution with a history of multiple meningiomas and resection of a large right frontal meningioma in the past presented for follow-up imaging. On the postcontrast axial T1 sequence (see Fig. 1A), there was a left frontal dural-based homogeneously enhancing extra-axial mass compatible with a meningioma, along with a smaller meningioma along the falx. MR perfusion was performed (Fig. 2B), with a region of interest (ROI) placed on the normal, contralateral white matter (ROI #1, green) and another placed on the left frontal meningioma (ROI #2, purple). The contrast enhancement time curve of the left frontal meningioma (curve #2, purple) demonstrates elevated relative cerebral blood volume when compared with the contrast-enhancement time curve of the contralateral white matter (curve #1, green), with the contrast-enhancement time curve of the meningioma demonstrating less than 50% return to baseline, which is the typical perfusion behavior of meningiomas as described in the literature (Fig. 2C).
Fig. 1.
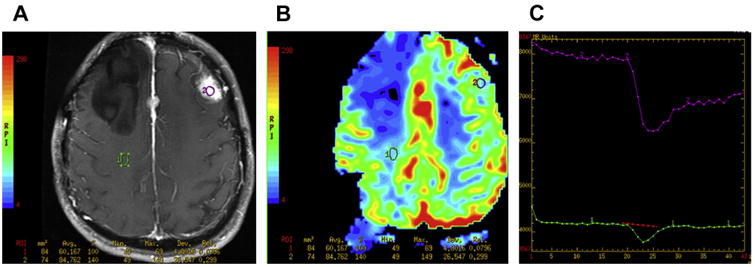
(A) DSC MR perfusion. Postcontrast MR imaging demonstrates an avidly enhancing left frontal dural-based extra-axial mass. The patient had a right frontal meningioma that had been resected in the past, with an additional meningioma seen along the anterior falx. (B) Perfusion analysis is performed by placing an ROI on the enhancing mass in the perfusion sequence (ROI #2, purple) and comparing it with the contralateral white matter (ROI #1, green). (C) The time course of the perfusion curves demonstrates the left frontal mass to have a loss of signal from the susceptibility effect of the gadolinium contrast of a greater magnitude than the contralateral white matter, signifying elevated relative cerebral blood volume, with less than 50% return to baseline for the perfusion curve of the mass, characteristic of extra-axial, nonglial tumors such as meningiomas.
Fig. 2.
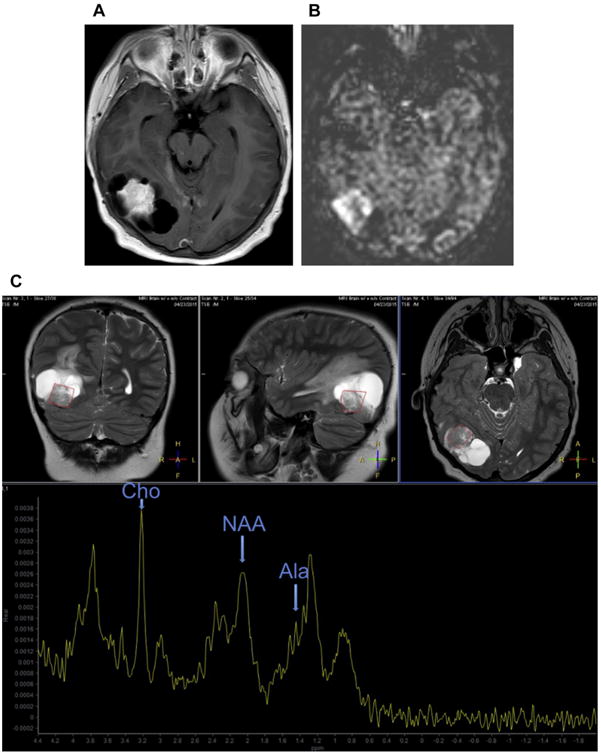
(A) Postcontrast imaging demonstrates a large centrally enhancing right temporo-occipital mass with peripheral cystic components. It is difficult to definitively ascertain if the mass is extra-axial or intra-axial. (B) ASL demonstrates increased signal within the enhancing component of the mass, compatible with elevated cerebral blood flow, which can be seen in meningiomas. (C) MR spectroscopy demonstrates a prominent Ala peak at 1.48 ppm, elevated Cho peak at 3.2 ppm, and decreased NAA peak at 2.0 ppm. These findings are compatible with a meningioma. Elevated spectroscopic peaks are also seen at 3.8 ppm, which has also been reported to be elevated in meningiomas. Prominent lipid/lactate peak also is seen.
MR perfusion also has been used to differentiate subtypes of meningiomas as well as differentiating typical from atypical meningiomas. Angiomatous meningiomas have been shown to have significantly higher tumoral relative cerebral blood volume compared with meningothelial, fibrous, or anaplastic meningiomas, and anaplastic meningiomas have higher peritumoral relative cerebral blood volume compared with the other types of meningiomas.7 Peritumoral edema surrounding malignant meningiomas (World Health Organization [WHO] grade III) also has been shown to have increased relative cerebral blood volume compared with benign meningiomas (WHO grade I) via MR perfusion imaging.8 The volume transfer constant, Ktrans, which is a measurement of vascular permeability, for atypical meningiomas (WHO grade II) has been shown to be higher than that for typical (benign) meningiomas.9
MR perfusion also can be performed to evaluate the contributions of the blood supply to the meningioma. Intra-arterial injection of gadolinium via catheter selection of the internal and external carotid arteries, in combination with intraoperative MR perfusion, can differentiate which portions of the tumors are supplied by which arterial supply. MR perfusion also can be performed after the embolization to assess for residual perfusion to the treated meningioma.10,11
Arterial spin labeling (ASL) is an MRI technique in which a radiofrequency pulse is applied to the arteries proximal to the ROI in such a way that the protons in the inflowing blood have a detectable signal. Cerebral blood flow can therefore be calculated in the ROI as a function of increased amount of “tagged” blood that has flowed into the ROI. ASL does not require the use of intravenous gadolinium, and therefore has the advantage of being usable even in patients with impaired renal function who otherwise may not be able to receive intravenous gadolinium. ASL has been shown to be able to detect increased cerebral blood flow within meningiomas, with the technique also demonstrating statistically significant increased cerebral blood flow in angiomatous meningiomas compared with fibrous and meningothelial meningiomas12 (see Fig. 2B).
Although not typically used to diagnose meningiomas, computed tomography (CT) perfusion, like ASL, can more conspicuously identify intracranial meningiomas due to the increased cerebral blood flow and cerebral blood volume within the meningiomas13 (Fig. 3). Although the meningioma may be less distinct in a noncontrast head CT, blending into the adjacent brain parenchyma (see Fig. 3A), CT perfusion, performed due to suspicion of stroke in this case, demonstrates a left frontal mass with elevated cerebral blood volume (see Fig. 3B) as well as elevated cerebral blood flow (see Fig. 3C) when compared with the adjacent as well as contralateral brain parenchyma. A contrast-enhanced head CT also clearly delineates the left frontal meningioma from the brain parenchyma (see Fig. 3D).
Fig. 3.
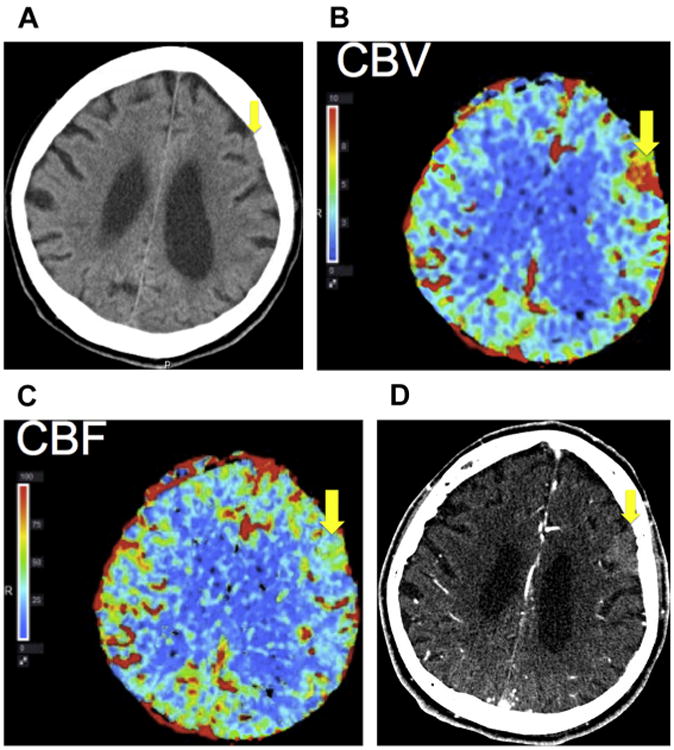
Incidental finding of a meningioma. (A) Noncontrast head CT for a patient with stroke symptoms demonstrates a subtle left frontal extra-axial mass that is isoattenuating to brain parenchyma (yellow arrow). (B) CT perfusion demonstrates elevated cerebral blood volume (CBV) and (C) cerebral blood flow (CBF) within the left frontal mass (yellow arrow), which can be seen in a meningioma. (D) CT angiography of the head demonstrates the left frontal extra-axial mass (yellow arrow) with arterial phase enhancement, compatible with a meningioma.
CT perfusion also has been used to characterize the peritumoral edema around meningiomas, with decreased cerebral blood flow seen in the peritumoral edema, potentially reflective of ischemic tissue that may be salvageable after resection of the tumor.14 The findings are similar to that from MR perfusion, which suggest that the decrease in relative cerebral blood volume may be a consequence of, rather than the cause of, the vasogenic edema, and that ischemic changes may be a secondary facultative phenomenon in the pathogenesis of meningioma-related brain edema.15
CT perfusion also has been used to differentiate meningiomas from hemangiopericytomas, extra-axial masses that are at times difficult to differentiate from meningiomas by conventional imaging features alone and clinically have more aggressive features and higher probability of recurrence and metastasis than meningiomas.16 CT perfusion analysis has demonstrated that hemangiopericytomas have increased cerebral blood volume compared with benign meningiomas, with the permeability surface, a measure of microvascular permeability, also higher in hemangiopericytomas compared with benign meningiomas.17
Perfusion imaging of the brain also can be performed using radionuclides, specifically technetium-99m-d, 1-hexamethyipropyleneamine oxime (99mTc-d, 1-HMPAO). 99mTc-d, 1-HMPAO single-photon emission CT has been used to characterize intracranial tumors, and meningiomas have been shown to have significantly higher HMPAO uptake, corresponding to increased cerebral blood flow, compared with gliomas.18
Diffusion Imaging
Diffusion MRI is an imaging technique that can measure the degree of mobility of water molecules within a biological tissue. By applying gradient fields in a specific way that refocuses the signal of the biological tissue in the MRI scanner except for the signal loss that occurs as a result of the Brownian motion of water, the magnitude of Brownian motion within the biological tissue can be measured, which can be a proxy of cellularity of the tissue, reflect evidence of injury in the context of cytotoxic edema, and elucidate orientation of macromolecular structures, such as white matter fiber tracts.19 Measurements of apparent diffusion coefficients (ADCs) can be made with diffusion MRI, with decreased ADC reflective of decreased Brownian motion of water and therefore suggestive of increased cellularity or cytotoxic edema. Other parameters that can be measured on diffusion MRI include fractional anisotropy (FA), a measure of how directional the water motion in a given area is, with a higher FA indicative of increased directionality of water motion, and a lower FA indicative of more diffuse, random water motion.
The ability for diffusion MRI to characterize cellular tumors has led to research into the application of diffusion MRI to characterize different types of meningiomas. Early research demonstrated that lower ADC values are seen in atypical or malignant meningiomas compared with normal brain parenchyma or benign meningiomas with the exception of densely calcified or psammomatous meningiomas,20 with a subsequent study with more subjects in both the atypical/malignant meningioma as well as benign meningioma groups also demonstrating similar results.21 A study using DTI, which assesses not only for diffusivity of the water molecules but also the directionality of diffusion, also demonstrated that atypical meningiomas have decreased ADC compared with benign meningiomas, and that benign meningiomas have a lower FA, or more spherical diffusivity, compared with atypical meningiomas.22 More recent studies, with a much larger sample size compared with the earlier studies, did not find a statistically significant difference between the ADC values of atypical and benign meningiomas, nor were the ADC values statistically different for the various histologic subtypes of meningiomas.23,24
Spectroscopy
MR spectroscopy has the ability to evaluate for the concentration of metabolites within a given ROI. Rather than detecting the resonance signal of protons (predominantly water) relative to spatial location after the application of a gradient pulse and assessing the morphology of a lesion of interest, the resonance signal of the protons from the different molecular groups within the lesion of interest, such as N-acetylaspartate (NAA), choline (Cho), creatine (Cr), glutamine/glutamate (Glx), alanine (Ala), and lactate (Lac), can be detected. Identifying the chemical composition of the lesion of interest is not only useful for identifying metabolic abnormalities but can be helpful in identifying neoplastic processes as well.
Meningiomas have been shown to have elevated Cho and decreased NAA, which is also seen in many other neoplastic processes. There also is a decrease in Cr.25 Prominent Ala is also seen in meningiomas, much more so than in other neoplastic processes and is considered a spectroscopic signature for meningiomas.26
The utility of MR spectroscopy in characterizing meningiomas is seen in a patient who presents with a large right parietotemporal cystic mass with a large centrally enhancing component (Fig. 2A). Diagnostic considerations based on conventional imaging for this mass include intra-axial masses such as a glioma as well as extra-axial masses such as a meningioma. ASL demonstrates increased signal within the mass, compatible with elevated cerebral blood flow (see Fig. 2B), which can be seen in both meningiomas and gliomas. MR spectroscopy of the mass demonstrates a prominent Ala peak, decreased NAA peak, and elevated Cho peak, however, confirming that the mass is indeed a meningioma (see Fig. 2C).
An elevated metabolite peak at 3.8 parts per million (ppm) also has been described in meningiomas, which is nearly absent in high-grade gliomas and intracranial metastasis in one study.27 Ex vivo MRS evaluation of meningiomas with different genetic mutations also have demonstrated differences in the detected metabolites, with WHO grade I meningiomas having 1p, 14q, and/or 22q genetic mutations, mutations that have been shown to result in rapid recurrence of tumor, demonstrating decreased Ala concentrations compared with WHO grade I meningiomas without those genetic aberrations.28 Ex vivo MRS evaluations have also attempted to characterize the various subtypes of meningiomas, demonstrating differences in macromolecular and lipid peaks in meningothelial, fibrous, and oncocytic subtypes of meningiomas.29 The knowledge that heterogeneity of tumoral tissue may reflect underlying genetic heterogeneity, and that MRS can potentially characterize more clinically aggressive portions of tumors, have led to the use of MRS in neuronavigation for surgical biopsy.30
Nuclear Medicine
Nuclear medicine techniques to evaluate for increased perfusion of meningiomas have already been described in the section on perfusion imaging. Radionuclides also have been made to target somatostatin receptors, which are expressed in meningiomas. Indium-111–labeled octreotide, as well as technetium-99m–labeled depreotide, have been used with single-photon emission CT to image meningiomas,31 with gallium-68–labeled DOTA-D-Phe1-Tyr3-octreotide, which has a high affinity for the somatostatin receptor subtype 2 (SSTR 2), used with PET to image meningiomas. The high tumor-to-background ratio, along with the advent of hybrid PET and MRI systems, allow for highly sensitive and specific diagnosis of intracranial meningiomas.32
Summary
Although the diagnosis of intracranial meningioma often can be made with conventional anatomic imaging, advanced imaging techniques have the potential to not only confirm the presumptive anatomic imaging diagnosis of a meningioma, but they also have the potential to differentiate the histologic subtypes of meningiomas as well as predict the clinical aggressiveness of the tumor. Definitive subtyping of meningiomas is still not possible by imaging alone at this time, but continued advances in imaging technique will give the clinicians more information with regard to the meningioma before surgery.
Key Points.
Conventional anatomic imaging is typically adequate to distinguish intracranial meningiomas from other tumors, but advanced imaging techniques can help confirm the diagnosis in equivocal cases.
Meningiomas demonstrate elevated cerebral blood flow/cerebral blood volume on perfusion imaging, with the perfusion characteristics helpful to distinguish it from other intracranial neoplasms, as well as potentially characterizing the subtype of meningioma.
Different types of meningiomas have been shown to have differences in diffusivity, but more recent larger sample studies have not shown diffusion imaging as a reliable way of differentiating meningioma histopathologic types.
Spectroscopy provides molecular information with regard to meningiomas and can potentially reflect genetic heterogeneity within a tumor and aid biopsy planning.
Radionuclides that bind somatostatin receptors can be a sensitive and specific method of characterizing meningiomas.
Acknowledgments
Funding: M.S. Shiroishi supported in part by SC CTSI (NIH/NCRR/NCATS) Grant KL2TR000131, Toshiba American Medical Systems and is a consultant for Guerbet.
References
- 1.Buetow MP, Buetow PC, Smirniotopoulos JG. Typical, atypical, and misleading features in meningioma. Radiographics. 1991;11(6):1087–106. doi: 10.1148/radiographics.11.6.1749851. [DOI] [PubMed] [Google Scholar]
- 2.Shiroishi MS, Castellazzi G, Boxerman JL, et al. Principles of T2*-weighted dynamic susceptibility contrast MRI techniques in brain tumor imaging. J Magn Reson Imaging. 2015;41:296–313. doi: 10.1002/jmri.24648. [DOI] [PubMed] [Google Scholar]
- 3.Shiroishi MS, Habibi M, Rajderkar D, et al. Perfusion and permeability MR imaging of gliomas. Technol Cancer Res Treat. 2011;10(1):59–71. doi: 10.7785/tcrt.2012.500180. [DOI] [PubMed] [Google Scholar]
- 4.Essig M, Shiroishi MS, Nguyen TB, et al. Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol. 2013;200:24–34. doi: 10.2214/AJR.12.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha S, Knopp EA, Johnson G, et al. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223:11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 6.Hakyemez B, Yildirim N, Erdogan C, et al. Meningiomas with conventional MRI findings resembling intraaxial tumors: can perfusion-weighted MRI be helpful in differentiation? Neuroradiology. 2006;48:695–702. doi: 10.1007/s00234-006-0115-y. [DOI] [PubMed] [Google Scholar]
- 7.Zang H, Rödinger LA, Shen T, et al. Preoperative subtyping of meningiomas by perfusion MR imaging. Neuroradiology. 2008;50:835–40. doi: 10.1007/s00234-008-0417-3. [DOI] [PubMed] [Google Scholar]
- 8.Zang H, Rödinger LA, Shen T, et al. Perfusion MR imaging for differentiation of benign and malignant meningiomas. Neuroradiology. 2008;50:525–30. doi: 10.1007/s00234-008-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S, Law M, Zagzag D, et al. Dynamic contrastenhanced perfusion MR imaging measurements of endothelial permeability: differentiating between atypical and typical meningiomas. AJNR Am J Neuroradiol. 2003;24:1554–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Saloner D, Uzelac A, Hetts S, et al. Modern meningioma imaging techniques. J Neurooncol. 2010;99:333–40. doi: 10.1007/s11060-010-0367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin AJ, Cha S, Higashida RT, et al. Assessment of vasculature of meningiomas and the effects of embolization with intra-arterial MR perfusion imaging: a feasibility study. AJNR Am J Neuroradiol. 2007;28:1771–7. doi: 10.3174/ajnr.A0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura H, Takeuchi H, Koshimoto Y, et al. Perfusion imaging of meningiomas by using continuous arterial spin labeling: comparison with dynamic susceptibility-weighted contrast-enhanced MR images and histopathologic features. AJNR Am J Neuroradiol. 2006;27:85–93. [PMC free article] [PubMed] [Google Scholar]
- 13.Tamrazi B, Yuh E. Stroke mimics imaging atlas: pearls and pitfalls in interpretation of brain CT perfusion studies. Proc Am Soc Neuroradiol. 2013;51:736–7. Available online at: http://www.asnr.org/sites/default/files/proceedings/2013.pdf. [Google Scholar]
- 14.Sergides I, Hussain Z, Naik S, et al. Utilization of dynamic CT perfusion in the study of intracranial meningiomas and their surrounding tissue. Neurol Res. 2009;31:84–9. doi: 10.1179/174313208X331563. [DOI] [PubMed] [Google Scholar]
- 15.Bitzer M, Klose U, Geist-Barth B, et al. Alteration in diffusion and perfusion in the pathogenesis of peritumoral brain edema in meningiomas. Eur Radiol. 2002;12:2062–76. doi: 10.1007/s003300101025. [DOI] [PubMed] [Google Scholar]
- 16.Sibtain NA, Butt S, Connor SEJ. Imaging features of central nervous system haemangiopericytomas. Eur Radiol. 2007;17:1685–93. doi: 10.1007/s00330-006-0471-3. [DOI] [PubMed] [Google Scholar]
- 17.Ren G, Chen S, Wang Y, et al. Quantitative evaluation of benign meningioma and hemangiopericytoma with peritumoral brain edema by 64-slice CT perfusion imaging. Chin Med J. 2010;123(15):2038–44. [PubMed] [Google Scholar]
- 18.Suess E, Malessa S, Ungersböck K, et al. Technetium-99m-d, 1-hexamethylpropyleneamineoxime(HMPAO) uptake and glutathione content in brain tumors. J Nucl Med. 1991;32(9):1675–81. [PubMed] [Google Scholar]
- 19.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217:331–45. doi: 10.1148/radiology.217.2.r00nv24331. [DOI] [PubMed] [Google Scholar]
- 20.Filippi CG, Edgar MA, Uluğ AM, et al. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol. 2001;22:65–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Nagar VA, Ye JR, Ng WH, et al. Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol. 2008;29:1147–52. doi: 10.3174/ajnr.A0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toh CH, Castillo M, Wong AM, et al. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:1630–5. doi: 10.3174/ajnr.A1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santelli L, Ramondo G, Della Puppa A, et al. Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir. 2010;152:1315–9. doi: 10.1007/s00701-010-0657-y. [DOI] [PubMed] [Google Scholar]
- 24.Sanverdi SE, Ozgen B, Oguz KK, et al. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol. 2012;81:2389–95. doi: 10.1016/j.ejrad.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita Y, Kajiwara H, Yokota A, et al. Proton magnetic resonance spectroscopy of brain tumors: an in vitro study. Neurosurgery. 1994;35(4):606–14. doi: 10.1227/00006123-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Demir MK, Iplikcioglu AC, Dincer A, et al. Single voxel proton MR spectroscopy findings of typical and atypical intracranial meningiomas. Eur J Radiol. 2006;60:48–55. doi: 10.1016/j.ejrad.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Kousi E, Tsougos I, Fountas K, et al. Distinct peak at 3.8 ppm observed by 3T MR spectroscopy in meningiomas, while nearly absent in high-grade gliomas and cerebral metastasis. Mol Med Rep. 2012;5:1011–8. doi: 10.3892/mmr.2012.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfisterer WK, Hendricks WP, Scheck AC, et al. Fluorescent in situ hybridization and ex vivo 1H magnetic resonance spectroscopic examinations of meningioma tumor tissue: is it possible to identify a clinically-aggressive subset of benign meningiomas? Neurosurgery. 2007;61(5):1048–61. doi: 10.1227/01.neu.0000303201.62123.5c. [DOI] [PubMed] [Google Scholar]
- 29.Tugnoli V, Schenetti L, Mucci A, et al. Ex vivo HR-MAS MRS of human meningiomas: a comparison with in vivo 1H MR spectra. Int J Mol Med. 2006;18:859–69. [PubMed] [Google Scholar]
- 30.Kanberoglu B, Moore NZ, Frakes D, et al. Neuronavigation using three-dimensional proton magnetic resonance spectroscopy data. Stereotact Funct Neurosurg. 2014;92:306–14. doi: 10.1159/000363751. [DOI] [PubMed] [Google Scholar]
- 31.Valotassiou V, Leondi A, Angelidis G, et al. SPECT and PET imaging of meningiomas. ScientificWorld-Journal. 2012;2012:412580. doi: 10.1100/2012/412580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afshar-Oromieh A, Wolf MB, Kratochwil C, et al. Comparison of 68Ga-DOTATOC-PET/CT and PET/MR hybrid systems in patients with cranial meningiomas: initial results. Neuro Oncol. 2015;17(2):312–9. doi: 10.1093/neuonc/nou131. [DOI] [PMC free article] [PubMed] [Google Scholar]


