Abstract
Objectives
The American Clinical Neurophysiology Society recommends continuous electroencephalographic monitoring after neonatal cardiac surgery because seizures are common, often subclinical, and associated with worse neurocognitive outcomes. We performed a quality improvement project to monitor for postoperative seizures in neonates with congenital heart disease after surgery with cardiopulmonary bypass.
Methods
We implemented routine continuous electroencephalographic monitoring and reviewed the results for an 18-month period. Clinical data were collected by chart review, and continuous electroencephalographic tracings were interpreted using standardized American Clinical Neurophysiology Society terminology. Electrographic seizures were classified as electroencephalogram-only or electroclinical seizures. Multiple logistic regression was used to assess associations between seizures and potential clinical and electroencephalogram predictors.
Results
A total of 161 of 172 eligible neonates (94%) underwent continuous electroencephalographic monitoring. Electrographic seizures occurred in 13 neonates (8%) beginning at a median of 20 hours after return to the intensive care unit after surgery. Neonates with all types of congenital heart disease had seizures. Seizures were electroencephalogram only in 11 neonates (85%). Status epilepticus occurred in 8 neonates (62%). In separate multivariate models, delayed sternal closure or longer deep hypothermic circulatory arrest duration was associated with an increased risk for seizures. Mortality was higher among neonates with than without seizures (38% vs 3%, P<.001).
Conclusions
Continuous electroencephalographic monitoring identified seizures in 8%of neonates after cardiac surgery with cardiopulmonary bypass. The majority of seizures had no clinical correlate and would not have been otherwise identified. Seizure occurrence is a marker of greater illness severity and increased mortality. Further study is needed to determinewhether seizure identification and management lead to improved outcomes.
Keywords: Electroencephalography, EEG, neonatal seizures, congenital heart disease, cardiopulmonary bypass
Graphical Abstract
Neonate on continuous EEG monitoring after surgery.
Neonates undergoing cardiac surgery for repair of congenital heart disease (CHD) are at risk for seizures in the postoperative period.1 Clinically evident seizures have been reported in 5% to 20% of neonates during the postoperative period.2,3 Studies using continuous electroencephalographic (CEEG) monitoring have reported that electroencephalogram (EEG)-only seizures (also termed “nonconvulsive seizures”) may be more common, occurring in 5% to 26% of neonates.3–9 Postoperative seizures are associated with worse neurodevelopmental outcomes, abnormal neurologic examination results, and abnormal neuroimaging study results.10–14 On the basis of these data, the American Clinical Neurophysiology Society’s (ACNS) guideline on neonatal EEG monitoring recommends consideration of CEEG monitoring after neonatal cardiac surgery.15 We implemented this recommendation and performed a single-center quality-improvement project to monitor for postoperative EEG seizures in a contemporary cohort of neonates with CHD after cardiac surgery with cardiopulmonary bypass (CBP). We aimed to determine the incidence of postoperative EEG seizures and to identify risk factors for seizures.
MATERIALS AND METHODS
Patient Population
By using a clinical pathway in which CEEG was recommended for all neonates (≤30 days of age, corrected gestational age ≤44 weeks) after cardiac surgery with CBP, we implemented the ACNS clinical recommendation on June 15, 2012, and reviewed the results for the period ending on December 31, 2013. If a patient underwent multiple surgeries during the neonatal period, only the index surgery was included. The Institutional Review Board of the Children’s Hospital of Philadelphia approved review of the Quality Improvement project.
Surgical Strategy
Operations were performed by 4 cardiac surgeons using a pH-stat blood gas management strategy. Deep hypothermic circulatory arrest (DHCA) was used at the discretion of the surgeon after induction of hypothermia to 18°C. Modified ultrafiltration was performed on all patients. Delayed sternal closure was not routinely used.
Electroencephalogram Monitoring
CEEG was performed by the encephalography service and initiated within 6 hours of returning to the cardiac intensive care unit (CICU) after surgery. The encephalography service includes acquisition and review software, network infrastructure, licensed EEG technologists, and expert encephalographers. CEEG was performed using a Grass-Telefactor video-EEG system (Grass Technologies, West Warwick, RI) with a portable acquisition machine networked to the main EEG server, allowing EEG review at the bedside, from multiple sites in the hospital, and remotely. EEG technologists were present in the hospital 24 hours per day and 7 days per week. CEEG was performed with 12 gold-over-silver scalp electrodes affixed according to the international 10–20 system (modified for neonates), with collodion adhesive (or paste for neonates on extracorporeal membrane oxygenation [ECMO]). Abnormal movements or vital sign fluctuations noted by the clinical team were marked on the recording as push-button events. If an electrographic seizure was identified, the CICU team was alerted by the EEG technologist or encephalographer, and neurologic consultation was obtained. CEEG was continued for 48 hours if no seizures occurred. If seizures were identified, CEEG was continued until 24 hours after the end of the last seizure. The initial antiseizure medications administered were phenobarbital or levetiracetam at the discretion of the on-call neurologist and cardiac inten-sivist. Phenobarbital (20 mg/kg bolus) was administered as a first-line agent in the majority of patients. For patients in whom there were concerns for hemodynamic instability, clinicians sometimes used phenobarbital in divided doses (5 mg/kg × 4 doses over 1 hour) or levetiracetam (20 mg/kg bolus). Patients with seizures usually underwent clinically indicated magnetic resonance imaging (MRI) of the brain or head ultrasound.
Data Collection
Clinical data were obtained from the medical record, including anesthesia and perfusion records. Patients were categorized according to a classification that incorporates cardiac anatomy and perioperative physiology, which has been shown to predict perioperative mortality. Class I is 2 ventricles with no aortic arch obstruction, class II is 2 ventricles with aortic arch obstruction, class III is a single ventricle with no aortic arch obstruction, and class IV is a single ventricle with aortic arch obstruction.16
For purposes of this review, EEG tracings were reinterpreted by a single encephalographer (NSA), blinded to clinical information (except conceptional age), using standardized ACNS neonatal EEG terminology.17 EEG seizures were defined as abnormal, paroxysmal EEG events that were different from the background, lasted more than 10 seconds (or less if associated with a clinical seizure), had a plausible electrographic field, and evolved in frequency, voltage, morphology, and often spatial distribution. EEG seizures were classified as electrographic status epilepticus if any single seizure lasted more than 30 minutes or if recurrent seizures together lasted for more than 30 minutes in any 1-hour epoch (50%seizure burden). EEG seizures were classified as EEG-only seizures (no clinical signs observed by bedside caregivers or on video review) or electroclinical seizures. All available neuroimaging studies were reviewed by a neurologist (DJL) to examine associations between neuroimaging abnormalities and seizure localization.
Data were collected and managed using Research Electronic Data Capture (REDCap), a web-based electronic data application hosted at the Children’s Hospital of Philadelphia Research Institute.18
Statistical Analysis
Summary statistics are reported as medians and interquartile ranges (IQRs) for continuous data and counts and proportions for categoric data. The association of each clinical and interictal EEG variable with seizures was examined using the chi-square test for categoric variables and Wilcoxon’s rank-sum test for continuous variables.
Multiple logistic regression was used for association of seizures with clinical and interictal EEG variables. To avoid collinearity, correlation between predictors was examined by using Pearson correlation coefficients for continuous predictors, and association between continuous and categoric predictors was examined by 2-sample test or analysis of variance. Variables associated with seizures with a P value less than .2 on univariable analysis were included in multivariable analyses. An initial model used only clinical data, whereas a subsequent model also used data obtained from the initial hour of CEEG. All statistics were performed with Stata 10.0 (StataCorp LP, College Station, Tex).
RESULTS
Demographics and Surgical Details
During the 18-month study period, 172 neonates with CHD underwent cardiac surgery with CPB. Postoperative CEEG was obtained in 161 of 172 eligible neonates (94%). The reasons for not undergoing CEEG included unavailability of EEG machine in 1 neonate, CICU team not ordering CEEG (mainly in the initial phase of implementation) in 5 neonates, death in the operating room in 1 neonate, and CICU team deciding not to monitor in 2 neonates (cutis aplasia in 1 and decision that monitoring was not warranted in 1). Pathway adherence improved over time. For the initial period of monitoring June 15 to December 31, 2012, 48 of 55 neonates (87%) who underwent surgery with CPB were monitored. For the middle period from January 1 to June 30, 2013, 54 of 56 neonates (96%) were monitored. For the later period of monitoring from July 1 to December 31, 2013, 57 of 59 neonates (97%) were monitored.
Of the 161 neonates who underwent CEEG, 92 (57%) were male. The median gestational age was 39 weeks (IQR, 38–39), and 26 neonates (16%) were premature (<37 weeks gestational age). The median head circumference at birth was 34 cm (IQR, 32–35). Genetic defects were identified in 21 neonates (13%). The median age at surgery was 5 days (IQR, 3–7). Five neonates had 2 operations with CPB during the neonatal period, and 1 neonate had 3 operations with CPB during the neonatal period. Both were monitored after each surgery.
The cardiac defects were class I in 68 neonates (42%), class II in 35 neonates (22%), class III in 15 neonates (9%), and class IV in 43 neonates (27%). The 5 most common operations were the stage I Norwood operation in 43 neonates (27%), the arterial switch operation in 25 neonates (16%), the systemic to pulmonary artery shunt in 17 neonates (11%), complete repair of tetralogy of Fallot in 14 neonates (9%), and truncus arteriosus repair in 12 neonates (8%). The median duration of CPB was 46 minutes (IQR, 38–62). DHCA was used in 96 neonates (60%), with a median duration of 41 minutes (IQR, 32–50). Twenty-six neonates (16%) had delayed sternal closure. Eleven neonates (7%) required ECMO (2 were placed on ECMO in the operating room before initiation of CEEG, 8 were placed on ECMO during CEEG, and 1 was placed on ECMO while CEEG had been temporarily discontinued during a diagnostic cardiac catheterization). Fifteen neonates (9%) had a cardiac arrest (2 in the operating room before initiation of CEEG, 1 in the CICU before initiation of CEEG, and 12 in the CICU during CEEG). Table 1 summarizes the demographic and clinical characteristics.
TABLE 1.
Demographic and clinical characteristics
| Male, n (%) | 92 (57%) |
| Birth weight (kg), median (IQR) | 3.2 (2.8–3.6) |
| Head circumference (cm), median (IQR) | 34 (32–35) |
| Gestational age (wk), median (IQR) | 39 (38, 39) |
| Premature neonates (<37 wk gestational age), n (%) | 26 (16%) |
| Identified genetic defects, n (%) | 21 (13%) |
| Age at surgery (d) median (IQR) | 5 (3–7) |
| Cardiac defect, n (%) | |
| Class I | 43 (27%) |
| Class II | 15 (9%) |
| Class III | 35 (22%) |
| Class IV | 68 (42%) |
| Duration of CPB (min), median (IQR) | 46 (38–62) |
| DHCA used, n (%) | 96 (60%) |
| DHCA duration (min), median (IQR) | 41 (32–50) |
| Delayed sternal closure, n (%) | 26 (16%) |
| ECMO used, n (%) | 11 (7%) |
| Cardiac arrest, n (%) | 15 (9%) |
Number (%) and median (IQR) are reported as appropriate. IQR, Interquartile range; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; ECMO, extracorporeal membrane oxygenation.
Nonseizure Events
Bedside clinicians identified events concerning for clinically evident seizures in 32 neonates (push-button events). None of these events had an EEG correlate, and they were deemed nonepileptic. Push-button events included episodes of abnormal body movement in 14 neonates, hypertension in 7 neonates, tachycardia in 6 neonates, abnormal face movement in 6 neonates, desaturation in 5 neonates, slow respiratory rate in 2 neonates, bradycardia in 1 neonate, and hypotension in 1 neonate. Events occurred during the initial 12 hours in 22 neonates, later than 12 hours in 10 neonates, and during both time periods in 2 neonates.
Seizures and Electroencephalogram Characteristics
EEG seizures occurred in 13 of 161 neonates (8%). The proportion with seizures has not changed since our previous report4 (14% previously vs 8% currently, P = .10). The median seizure onset was 20 hours (IQR, 15–34) after return to the CICU postoperatively. EEG seizures were EEG only in 11 neonates (85%) and electroclinical in 2 neonates (15%). Electrographic status epilepticus occurred in 8 neonates (62%). Seizures were spatially diffuse in 2 neonates (15%), lateralized in 2 neonates (15%), and focal in 9 neonates (69%). Seizure characteristics are provided in Table 2. The encephalographer performing reinterpretation blinded to clinical information (except conceptional age) was consistent with initial clinical interpretation for seizure occurrence in all patients.
TABLE 2.
Seizure description, treatment, and brain imaging
| Subject | Status epilepticus | Any seizures with clinical correlates | Seizure description | Antiseizure medications administered | Imaging |
|---|---|---|---|---|---|
| 1 | Yes | Yes | 25 focal R central seizures lasting 0.5–3.5 min | PB | Brain MRI Bilateral deep and periventricular foci of PVL |
| 2 | Yes | No | 29 bi-occipital seizures lasting 2–30 min | PB | Brain MRI Bilateral deep and periventricular foci of hemorrhagic PVL |
| 3 | Yes | No | >100 bi-central and vertex seizures lasting 0.5–3 min | LEV, PB | Head US Bilateral IVH, periventricular hemorrhage vs hemorrhagic infarct |
| 4 | No | No | 1 R central and 1 L occipital seizure lasting 1 min | None | Brain MRI Small SDH along flax and tentorium, small intraparenchymal hemorrhage in R cerebellar hemisphere and R posterior parietal lobe |
| 5 | No | No | 5 L central seizures lasting 0.25–1 min | PB | Brain MRI L post frontal and anterior parietal infarction, R frontal lobe infarction |
| 6 | Yes | No | 13 R central seizures lasting 1–3 min | PB, LEV | Head US 2 large intraparenchymal lesions in L hemisphere suggestive of ICH |
| 7 | No | No | 9 L occipital 1-seizures lasting 5 min | PB | Brain MRI Cystic PVL central semiovale and corona radiate bilaterally |
| 8 | Yes | No | 5 bi-occipital seizures lasting 2–13 min | PB | Brain MRI PVL L parietal white matter region, L tentorial SDH, bilateral cerebral microhemorrhages |
| 9 | Yes | No | 34 L occipital seizures lasting 2–4 min | LEV | Brain MRI PVL corpus callosum, periventricular deep white matter, microhemorrhages R frontal and parietal deep white matter, R lateral medulla and cerebellar peduncle, SDH posterior flax and tentorium |
| 10 | Yes | No | >100 R frontal-central-temporal temporal seizures lasting 0.25–1.5 min | PB, LEV | Brain MRI Subacute infarctions in the R frontal lobe and L frontal lobe |
| 11 | No | No | 3 L occipital seizures lasting 1–20 min | PB, LEV | Head US Increased echogenicity bilateral thalami, bilateral SDH |
| 12 | Yes | Yes | ~10 seizures per hour for 3 d from multifocal locations lasting 0.5–5 min | PB, LEV | Head US Large area of increased parenchymal echogenicity in the L parietal-occipital region, compatible with hemorrhagic infarction, IVH left greater than right |
| 13 | No | No | 8 diffuse seizures lasting 0.1–2 min | PB | Head US Evolving left caudothalamic groove germinal matrix hemorrhage with intraventricular synechia |
PB, Phenobarbital; MRI, magnetic resonance imaging; PVL, periventricular leukomalacia; LEV, levetiracetam; US, ultrasound; IVH, intraventricular hemorrhage; SDH, subdural hematoma; R, right; ICH, intracranial hemorrhage.
Univariable and multivariable analyses using clinical variables for seizure prediction are shown in Table 3. On univariable analysis, seizures were more common in younger neonates, with longer CBP and DHCA times, in patients with single-ventricle defects with arch obstruction, in patients returned to the CICU with delayed sternal closure, in patients with postoperative cardiac arrests, and in patients who were placed on ECMO. Variables that were not known on return to the CICU and occurred later would not be useful in identifying patients at higher risk for seizures and in need of CEEG on postoperative CICU admission. Thus, cardiac arrest and ECMO were not included in the multivariable model.
TABLE 3.
Electrographic seizure predictors
| Variable | Univariate analysis
|
Multivariate analysis no. 1*
|
Multivariate analysis no. 2*
|
||||
|---|---|---|---|---|---|---|---|
| No seizures | Seizures | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Gender | .80 | ||||||
| Male | 85 (92%) | 7 (8%) | |||||
| Female | 63 (91%) | 6 (9%) | |||||
| Gestational age (wk) | 39 (38–39) | 37 (37–39) | .42 | ||||
| Identified genetic abnormality | .55 | ||||||
| None | 128 (91%) | 12 (9%) | |||||
| Present | 20 (95%) | 1 (5%) | |||||
| Age at surgery (d) | 5 (3–7) | 3 (2–5) | .05 | 0.91 (0.74–1.11) | .36 | 0.93 (0.77–1.28) | .47 |
| Cardiac defect | .14 | ||||||
| Class I | 65 (96%) | 3 (4%) | 0.46 (0.10–2.18) | .33 | 1.09 (0.18–6.67) | .93 | |
| Class II | 33 (94%) | 2 (6%) | 0.49 (0.08–2.87) | .43 | 0.41 (0.07–2.43) | .33 | |
| Class III | 14 (93%) | 1 (7%) | 0.76 (0.07–7.83) | .82 | 2.28 (0.14–35.06) | .56 | |
| Class IV | 36 (84%) | 7 (16%) | — | — | |||
| Operation | .42 | ||||||
| Stage 1 Norwood Operation | 37 (86%) | 6 (14%) | |||||
| Arterial switch operation | 24 (96%) | 1 (4%) | |||||
| Systemic to pulmonary artery shunt | 16 (94%) | 1 (6%) | |||||
| Complete repair of tetralogy of Fallot | 14 (100%) | 0 (0%) | |||||
| Truncus arteriosus repair | 11 (92%) | 1 (8%) | |||||
| Delayed sternal closure | .002 | 3.99 (1.04–15.29) | .04 | * | * | ||
| No | 128 (95%) | 7 (5%) | |||||
| Yes | 20 (77%) | 6 (23%) | |||||
| Duration of DHCA (min) | 21 (0–42) | 47 (36–49) | .01 | * | * | 1.04 (1.00–1.08) | .04 |
| Duration of CPB (min) | 45 (38–60) | 62 (42–77) | .24 | ||||
| ECMO* | .015 | † | |||||
| No | 140 (93%) | 10 (7%) | |||||
| Yes | 9 (73%) | 3 (27%) | |||||
| Cardiac arrest* | .006 | † | |||||
| No | 137 (94%) | 9 (6%) | |||||
| Yes | 11 (73%) | 4 (27%) | |||||
Number (%) and median (IQR) are reported as appropriate. Boldface indicates statistical significance. OR, Odds ratio; CI, confidence interval; DHCA, deep hypothermic circulatory arrest; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation.
Delayed sternal closure and DHCA duration were highly correlated, so multivariate analysis included only delayed sternal closure (multivariable analysis no. 1) or DHCA duration (multivariable analysis no. 2).
ECMO and cardiac arrest were not included in the multivariable analysis because these variables would not be known at the time of return to the CICU and therefore could not be used to help decide whether EEG monitoring was indicated.
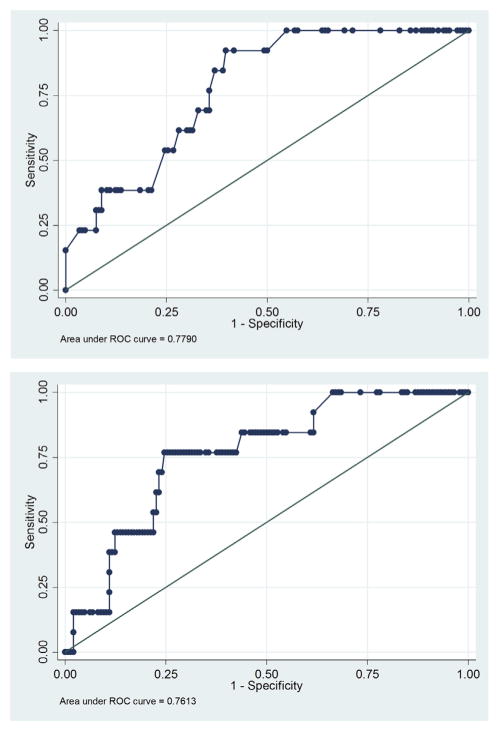
Delayed sternal closure and DHCA duration were strongly associated (P < .0001). Therefore, to avoid collinearity, separate multivariate analyses were performed for delayed sternal closure and DHCA duration to examine their respective associations with seizure occurrence. Both multivariate models are provided in Table 3. Both had similar performance characteristics. The goodness-of-fit for the multivariable logistic regression models indicated that both had a pseudo-R2 of 0.11. Receiver operating characteristic curves (Figure 1) and the c-statistics were not significantly different (P = .8). We performed the leave-one-out cross validation to calculate the predictive probability of seizure occurrence for each individual using the multivariate logistic model and again observed similar results between the 2 models. In their respective models, only delayed sternal closure (odds ratio [OR] 3.99; 95% confidence interval [CI], 1.04–15.29; P = .04) and DHCA duration (OR, 1.04; 95% CI, 1.00–1.08; P = .04) were significantly associated with seizures. Because DHCA was analyzed as a continuous variable, the increase in seizure risk is provided per minute of DHCA. We assessed the potential collinearity of variables. Lower weight was not associated with ECMO (P = .77) or DHCA use (P = .86). Younger age at the time of surgery was not associated with ECMO (P = .41) or DHCA use (P = .23). Arch obstruction was not associated with ECMO (P = .29) but, as expected, was associated with DHCA (P = .0000). When DHCA duration was divided into 3 categories, there was a trend toward higher seizure occurrence with longer DHCA duration (no DHCA 3%; DHCA <40 minutes 7% with OR, 2.48; 95% CI, 0.40–15.56; DHCA ≥40 minutes 15% with OR, 5.36; 95% CI, 1.09–26.42; chi-square P = .07). When DHCA was dichotomized to less than 40 minutes (including no DHCA) or 40 minutes or more, neonates with DHCA 40 minutes or more had a significantly increased seizure risk (15% vs 5%, chi-square P = .03; OR, 3.4; 95% CI, 1.07–11.08; P = .04). When DHCA was changed into a 10-minute scale for subjects with DHCA more than 0 minutes, for every 10-minute increase in DHCA duration time, the OR of developing seizure was 1.47 (95% CI, 0.96–2.15).
FIGURE 1.
Receiver operating characteristic curves for the multivariate model including delayed sternal closure (top) and DHCA duration (bottom). ROC, Receiver operating characteristic.
Several alternative analyses were performed. First, an alternative regression model was explored with the cardiac defect type grouped as 1 versus 2 ventricles, and similar results were observed. Second, an alternative regression model was explored including EEG variables known in the initial hour of EEG recording, including background category, excessive interictal epileptiform discharge presence, and reactivity. The EEG background category was continuous in 30 neonates (19%), appropriately discontinuous in 31 neonates (19%), and excessively discontinuous in 99 neonates (62%). EEG background category did not predict seizure occurrence on univariable analysis (P = .85). Seizures occurred in 5 of 46 neonates (11%) with excessive interictal epileptiform discharges and in 7 of 105 neonates (6%) without excessive epileptiform discharges (P = .06). Among the 112 neonates in whom reactivity could be assessed, seizures occurred in 4 of 96 neonates (4%) with reactivity and 2 of 16 neonates (13%) without reactivity (P = .08). Multivariable analyses including these EEG variables were not substantially different than the model described previously. On multivariable analysis using delayed sternal closure, the only predictor of seizures was delayed sternal closure (OR, 4.09; 95%CI, 1.04–15.99; P = .04). On multivariable analysis using DHCA duration, the only predictor of seizure was DHCA duration (OR, 1.04; 95% CI, 1.00–1.08; P = .04). We compared the receiver operating characteristic curve c-statistics between the model containing only clinical variables and the model containing EEG variables, and they were not significantly different.
Seizure descriptions and management are summarized in Table 2. The most commonly administered antiseizure medication was phenobarbital, followed by a combination of levetiracetam or phenobarbital. The choice of antiseizure medication and bolus parameters were left to the clinician’s discretion. No patients had hemodynamic instability related to medication administration.
Nine neonates (6%) died. Four neonates had multiorgan system dysfunction and withdrawal of technologic support on ECMO, 1 neonate had a large intracranial hemorrhage and withdrawal of technologic support on ECMO, 1 neonate had multiorgan system dysfunction and withdrawal of ventilatory and inotropic support, 1 neonate had a large intracranial hemorrhage and withdrawal of ventilatory and inotropic support, 1 neonate had a cardiac arrest and was deemed to not be an ECMO candidate, and 1 neonate had cardiac arrest and was unable to be cannulated for ECMO. Mortality was higher among neonates with than without seizures (38% vs 3%, P <.01). Neonates with and without seizures did not have significantly different postoperative lengths of stay in the CICU (11 days for both groups, P = .65) or hospital (18 vs 17 days, P = .85). When the 9 neonates who died were excluded, neonates with and without seizures still did not have significantly different lengths of stay in the CICU (11 days for both groups, P = .69) or hospital (17 vs 19 days, P = .40).
Brain Imaging
Brain MRI scans were obtained in 8 of 13 neonates with seizures. Findings included diffuse periventricular leukomalacia in 5 neonates, subdural hematomas in 3 neonates, infarction in 2 neonates, microhemorrhages in 2 neonates, and intraparenchymal hemorrhage in 1 neonate. Five neonates did not undergo MRI (death in 4 and transfer to another hospital in 1) but underwent head ultrasounds that showed intraventricular hemorrhage in 2, infarction in 2, intraparenchymal hemorrhage in 2, and subdural hematoma in 1. Imaging characteristics are provided in Table 2. All neonates with seizures had diffuse or multifocal imaging lesions. Four patients had seizures arising predominantly from one of their injury sites (subjects 4, 5, 10, and 12 in Table 2), but none had a single site of injury with focal seizures arising solely from that region.
DISCUSSION
This is the first report describing the impact of implementation of the ACNS guideline on routine postoperative CEEG among neonates with CHD after surgery with CPB. We identified an EEG seizure incidence of 8% (13/161). The seizure burden was often high (status epilepticus in 62%). Seizures were often EEG only (85%), indicating that CEEG was required for identification because bedside clinical assessment for seizures without CEEG monitoring would be unreliable. The only clinical predictors of seizure occurrence available on return to the CICU postoperatively were delayed sternal closure and longer DHCA duration. Seizures also were more likely in neonates who subsequently required ECMO or experienced cardiac arrest, and seizures were associated with higher mortality.
The 8% incidence of EEG seizures in our current cohort was not significantly different from our previous report of CEEG among neonates who received care from 2001 to 2003 (14%, 15/110).4 However, unlike our previous cohort in which all patients with seizures had EEG-only seizures, 15% of our current cohort of neonates had some clinical correlate to their seizures. These data are similar to the Boston Circulatory Arrest Study of children with transposition of the great arteries in which the incidences of EEG-only and electroclinical seizures were 20% and 6%, respectively.3 Likewise, a study of a heterogeneous CHD cohort of neonates and infants reported perioperative (preoperative, intraoperative, and postoperative) electrographic seizures in 30% of neonates, of which 16% were electroclinical.19 In comparison, Andropoulos and associates20 examined the occurrence of preoperative and postoperative seizures in neonates undergoing surgery with CBP and found that only 1 patient with a single ventricle had an EEG seizure, leading to an overall incidence of 1.5%. The lower incidence of seizures in this last study is likely due to the routine intraoperative and postoperative administration of benzodiazepines, which were not used routinely in the other studies.
Similar to our previous report,4 electrographic seizures were most common in neonates with single-ventricle defects with arch obstruction (16% in current report, 18% in previous report). In our prior study of clinical seizures after infant cardiac surgery, the risk of clinical seizures was also highest in those with single-ventricle defects with arch obstruction.2 This contrasts with other studies that have reported the highest seizure incidence in patients with 2-ventricle defects with aortic arch obstruction.19 In addition, we found that increasing DHCA duration predicted seizure occurrence. This finding is similar to that of the Boston Circulatory Arrest Study, in which identified risk factors for seizures included increasing duration of DHCA, the presence of a ventricular septal defect, and older age at the time of surgery.9 Likewise, we have previously reported risk factors that include coexisting genetic defects, aortic arch obstruction,2 and increasing duration of DHCA.2,4 Since our previous report, the incidence of seizures in patients who had DHCA more than 40 minutes has decreased from 24% to 15%. Of note, avoidance of DHCA does not prevent seizures. In the current study, seizures occurred in 3% of patients in whom DHCA was not used. A recent study by Gunn and associates19 described a high incidence of perioperative seizures (30%). The operative strategy consisted primarily of antegrade cerebral perfusion at one center for all patients; in the second center, DHCAwas used with only brief periods (median duration, 8 minutes [IQR 5–17]) in patients with biventricular circulation during arch reconstruction and during surgery to the atrial septum.19
In this study, on univariable analysis, seizures occurred more often in neonates who were younger at the time of surgery compared with those who were older (aged 3 vs 5 days). This variable was not a seizure predictor in our previous report4 and contradicts the previous finding of older age at the time of surgery in the Boston Circulatory Arrest Study, although it was not possible to separate age and diagnosis of ventricular septal defects as predictors for seizures.3 Seizure onset occurred at median of 20 hours after return to the CICU postoperatively. This is similar to our previous report, in which the median seizure onset time was 21 hours after surgery.5 In the Boston Circulatory Arrest Study, most seizures occurred 13 to 36 hours after surgery.3
Status epilepticus was common in our cohort, occurring in 62% of neonates with seizures. This is consistent with the Boston Circulatory Arrest Study that identified a high occurrence of status epilepticus.3 Patients who had seizures were medically sicker than patients without seizures, as indicated by being more likely to return to the CICU with an open chest, to have longer DHCA durations, to experience a cardiac arrest, and to require ECMO. The occurrence of seizures in our cohort was ominous because 38% (5/13) of neonates with postoperative seizures died. In the Boston Circulatory Arrest Study, 2% (n = 3) of infants died within 1 month of surgery, but no association with postoperative seizures was reported.9 Gunn and colleagues8 reported a 44% early mortality in neonates with hypoplastic left heart syndrome and variants who had seizures after Norwood type operations compared with 13% without postoperative seizures.
Given increasing attention to quality assessment and cost-effective healthcare strategies, physicians must determine whether the seizure incidence and available data regarding the association between seizures and worse neurodevelopmental outcomes justify the routine use of CEEG for all neonates who undergo cardiac surgery with CBP. The ACNS guideline recommends routine monitoring for several neonatal populations who have been identified as having an especially high risk for seizures.15 Neonates at high risk of seizures include 34% to 65% of neonates treated with therapeutic hypothermia for hypoxic ischemic encephalopathy,21,22 90% of neonates with stroke,23 10% to 30% of neonates undergoing ECMO,24–26 and 85% of neonates with meningitis.27 The incidence of seizures in these at-risk populations is higher than has been reported in the population with CHD,3,4,9,19 although in many of those populations the impact of seizures on neurodevelopmental outcomes has not been studied. In adult populations, CEEG has not been shown to significantly increase hospital costs,28,29 but cost-effectiveness analyses have not been performed in neonates with CHD. With implementation of the ACNS guideline at our institution, we were aiming to identify a population of neonates to target for routine postoperative CEEG monitoring. Both delayed sternal closure and longer DHCA durations predicted seizures in our multivariable model, but both ORs included a lower bounds of approximately 1, suggesting the statistically significant findings may not be useful in focusing CEEG implementation on a high-risk group.
Over the last 3 decades, surgical and medical care improvements have increased the survival of neonates, with CHD leading to emphasis on improving functional outcome and quality of life among survivors. Outcome studies have described neurodevelopmental dysfunction in half of all survivors, characterized by mild cognitive impairment, impaired executive function, inattention and impulsive behavior, and impaired language and social skills.30 In the Boston Circulatory Arrest Study, postoperative seizure occurrence was the medical variable most consistently related to worse neuropsychologic outcomes at 16-year follow-up, including lower scores on reading and math composites, general memory index, executive function, and visual special testing.13 In that study, only clinical seizures were treated with antiseizure medications. Most seizures were EEG-only seizures and were untreated, which may have contributed to these unfavorable outcomes. In our previous evaluation of the neurodevelopmental impact of postoperative EEG seizures that were detected and treated with antiseizure medications, seizures were associated with less severe deficits at 4 years of age, including impaired executive function and social interactions,31 compared with the Boston Circulatory Arrest Study. These studies suggest that identifying and treating seizures may reduce secondary brain injury and improve outcomes. Gunn and colleagues8,19 did not find neurodevelopmental impairment at 2-year follow-up in neonates who had perioperative seizures, but testing at this age is limited and does not test higher functions such as memory and executive function. Further, there may have been some misclassification of patients with and without seizures because the study used amplitude-integrated EEG and not conventional full-array EEG.32 High seizure burdens have been associated with worse outcome in older critically ill children.33–35 Although the occurrence of a seizure is a marker of brain injury, there may also be secondary injury if the seizure activity is not terminated. The association between seizures and worse outcomes is consistent with animal models. In baboons with pharmacologically induced seizures and paralysis, thus producing nonconvulsive status epilepticus, severe brain injury occurred.36 Further, seizures in the immature brain have been shown to induce a cascade of events resulting in synaptic changes, altered long-term potentiation, cell injury, and cell death.37–39
Given the association between seizures and worse neurodevelopment outcomes, postoperative CEEG to identify seizures is warranted. Our investigation showed that patients who had delayed sternal closure and longer duration of DHCA had an increased risk of seizures. However, given that neonates with all categories of CHD were at risk of development of seizures and that the majority of seizures in our study were EEG only, widespread monitoring strategies are indicated in the neonatal post-CBP population. Furthermore, push-button events by bedside clinicians, including abnormal movements and hypertensive episodes concerning for possible seizures, did not have any EEG correlate indicating that bedside clinical assessment for seizures without CEEG monitoring is unreliable.
Study Limitations
First, we did not monitor patients preoperatively or intraoperatively, as has been done in previous reports.19,20 Second, we did not evaluate neurodevelopmental outcomes and thus cannot determine whether seizure occurrence was associated with adverse neurodevelopmental outcomes among survivors.
CONCLUSIONS
We implemented routine postoperative CEEG in neonates with CHD who underwent surgery with CPB in accordance with the ACNS guideline and identified an 8% incidence of postoperative EEG seizures. In the majority of neonates, the seizures were EEG only. Bedside clinical assessment for seizures without CEEG monitoring was unreliable. Neonates with all classifications of CHD had postoperative seizures. The only risk factors for electrographic seizures were delayed sternal closure and longer DHCA duration. Seizure occurrence was associated with more severe illness, as indicated by associations with delayed sternal closure, longer DHCA durations, postoperative cardiac arrest, and need for ECMO. Seizures were markers of brain injury, characterized by multifocal neuroimaging lesions and an association with higher mortality. Further study is needed to determine whether identification and management of seizures improve neurodevelopmental outcomes.
Central Message.
Postoperative continuous EEG in neonates after CPB identified subclinical seizures that would not have been identified without monitoring.
Perspective.
Given the known association between seizures and worse neurodevelopmental outcomes among neonates undergoing cardiac surgery with CPB, we implemented routine postoperative continuous EEG monitoring. Seizures were identified in 8% of neonates with a high seizure burden. Most seizures would not have been identified without monitoring.
Acknowledgments
DJL is funded by National Institutes of Health RO1 NS-072338 and the June and Steve Wolfson Family Foundation. NSA is funded by National Institutes of Health K23NS076550.
The authors thank Elizabeth McBride who participated in data collection.
Abbreviations and Acronyms
- ACNS
American Clinical Neurophysiology Society
- CEEG
continuous electroencephalographic
- CHD
congenital heart disease
- CI
confidence interval
- CICU
cardiac intensive care unit
- CPB
cardiopulmonary bypass
- DHCA
deep hypothermic circulatory arrest
- ECMO
extracorporeal membrane oxygenation
- EEG
electroencephalogram
- IQR
interquartile range
- MRI
magnetic resonance imaging
- OR
odds ratio
Footnotes
Read at the 94th Annual Meeting of The American Association for Thoracic Surgery, Toronto, Ontario, Canada, April 26–30, 2014.
You can watch a Webcast of this AATS meeting presentation by going to: http://webcast.aats.org/2014/files/Monday/20140428_435_455pm_Maryam_Naim.mp4
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
References
- 1.Abend NS, Dlugos D, Clancy RR. A review of long term EEG monitoring in critically ill children with hypoxic ischemic brain encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30:134–42. doi: 10.1097/WNP.0b013e3182872af9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clancy RR, McGaurn SA, Wernovsky G, Gaynor JW, Spray TL, Norwood WI, et al. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2003;111:592–601. doi: 10.1542/peds.111.3.592. [DOI] [PubMed] [Google Scholar]
- 3.Helmers SL, Wypij D, Constantinou JE, Newburger JW, Hickey PR, Carrazana EJ, et al. Perioperative electroencephalographic seizures in infants undergoing repair of complex congenital cardiac defects. Electroencephalogr Clin Neurophysiol. 1997;102:27–36. doi: 10.1016/s0013-4694(96)95079-8. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor JW, Nicolson SC, Jarvik GP, Wernovsky G, Montenegro LM, Burnham NB, et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg. 2005;130:1278–86. doi: 10.1016/j.jtcvs.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy RR, Sharif U, Ichord R, Spray TL, Nicolson S, Tabbutt S, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46:84–90. doi: 10.1111/j.0013-9580.2005.22504.x. [DOI] [PubMed] [Google Scholar]
- 6.Chock VY, Reddy VM, Bernstein D, Madan A. Neurologic events in neonates treated surgically for congenital heart disease. J Perinatol. 2006;26:237–42. doi: 10.1038/sj.jp.7211459. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt B, Finckh B, Christen S, Lykkesfeldt J, Schmid ER, Bauersfeld U, et al. Electroencephalographic changes after pediatric cardiac surgery with cardiopulmonary bypass: is slow wave activity unfavorable? Pediatr Res. 2005;58:771–8. doi: 10.1203/01.PDR.0000180554.16652.4E. [DOI] [PubMed] [Google Scholar]
- 8.Gunn JK, Beca J, Penny DJ, Horton SB, d’Udekem YA, Brizard CP, et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann Thorac Surg. 2012;93:170–6. doi: 10.1016/j.athoracsur.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–64. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–55. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 11.Rappaport LA, Wypij D, Bellinger DC, Helmers SL, Holmes GL, Barnes PD, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773–9. doi: 10.1161/01.cir.97.8.773. [DOI] [PubMed] [Google Scholar]
- 12.Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–32. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 13.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–9. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaynor JW, Jarvik GP, Bernbaum J, Gerdes M, Wernovsky G, Burnham NB, et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2006;131:181–9. doi: 10.1016/j.jtcvs.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 16.Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–57. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, et al. American Clinical Neurophysiology Society Standardized EEG Terminology and Categorization for the Description of Continuous EEG Monitoring in Neonates: Report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J Clin Neurophysiol. 2013;30:161–73. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler LR, Tsao JW, Levine SR, Swain-Eng RJ, Adams RJ, Demaerschalk BM, et al. Teleneurology applications: report of the Telemedicine Work Group of the American Academy of Neurology. Neurology. 2013;80:670–6. doi: 10.1212/WNL.0b013e3182823361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn JK, Beca J, Hunt RW, Olischar M, Shekerdemian LS. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. 2012;38:1539–47. doi: 10.1007/s00134-012-2608-y. [DOI] [PubMed] [Google Scholar]
- 20.Andropoulos DB, Mizrahi EM, Hrachovy RA, Stayer SA, Stark AR, Heinle JS, et al. Electroencephalographic seizures after neonatal cardiac surgery with high-flow cardiopulmonary bypass. Anesth Analg. 2010;110:1680–5. doi: 10.1213/ANE.0b013e3181dd5a58. [DOI] [PubMed] [Google Scholar]
- 21.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, Wang A, Cook N, Donnelly M, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol. 2011;26:724–8. doi: 10.1177/0883073810390036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash KB, Bonifacio SL, Glass HC, Sullivan JE, Barkovich AJ, Ferriero DM, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–62. doi: 10.1212/WNL.0b013e31820af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laugesaar R, Kolk A, Tomberg T, Metsvaht T, Lintrop M, Varendi H, et al. Acutely and retrospectively diagnosed perinatal stroke: a population-based study. Stroke. 2007;38:2234–40. doi: 10.1161/STROKEAHA.107.483743. [DOI] [PubMed] [Google Scholar]
- 24.Hahn JS, Vaucher Y, Bejar R, Coen RW. Electroencephalographic and neuroimaging findings in neonates undergoing extracorporeal membrane oxygenation. Neuropediatrics. 1993;24:19–24. doi: 10.1055/s-2008-1071507. [DOI] [PubMed] [Google Scholar]
- 25.Streletz LJ, Bej MD, Graziani LJ, Desai HJ, Beacham SG, Cullen J, et al. Utility of serial EEGs in neonates during extracorporeal membrane oxygenation. Pediatr Neurol. 1992;8:190–6. doi: 10.1016/0887-8994(92)90066-8. [DOI] [PubMed] [Google Scholar]
- 26.Piantino JA, Wainwright MS, Grimason M, Smith CM, Hussain E, Byron D, et al. Nonconvulsive seizures are common in children treated with extracorporeal cardiac life support. Pediatr Crit Care Med. 2013;14:601–9. doi: 10.1097/PCC.0b013e318291755a. [DOI] [PubMed] [Google Scholar]
- 27.ter Horst HJ, van Olffen M, Remmelts HJ, de Vries H, Bos AF. The prognostic value of amplitude integrated EEG in neonatal sepsis and/or meningitis. Acta Paediatr. 2010;99:194–200. doi: 10.1111/j.1651-2227.2009.01567.x. [DOI] [PubMed] [Google Scholar]
- 28.Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999;16:1–13. doi: 10.1097/00004691-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Ney JP, van der Goes DN, Nuwer MR, Nelson L, Eccher MA. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005–2009. Neurology. 2013;81:2002–8. doi: 10.1212/01.wnl.0000436948.93399.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–67. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 31.Gaynor JW, Jarvik GP, Gerdes M, Kim DS, Rajagopalan R, Bernbaum J, et al. Postoperative electroencephalographic seizures are associated with deficits in executive function and social behaviors at 4 years of age following cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2013;146:132–7. doi: 10.1016/j.jtcvs.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- 33.Topjian AA, Gutierrez-Colina AM, Sanchez SM, Berg RA, Friess SH, Dlugos DJ, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;31:215–23. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagenman KL, Blake TP, Sanchez SM, Schultheis MT, Radcliffe J, Berg RA, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne ET, Zhao XY, Frndova H, McBain K, Sharma R, Hutchison JS, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–38. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch Neurol. 1973;29:82–7. doi: 10.1001/archneur.1973.00490260026003. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–63. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- 38.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 39.Zhou C, Lippman JJ, Sun H, Jensen FE. Hypoxia-induced neonatal seizures diminish silent synapses and long-term potentiation in hippocampal CA1 neurons. J Neurosci. 2011;31:18211–22. doi: 10.1523/JNEUROSCI.4838-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]