Abstract
Background
Limited data exist regarding the impact of aldosterone antagonist therapy on cardiac structure and function in heart failure with preserved ejection fraction (HFpEF) and on the prognostic relevance of changes in cardiac structure and function in HFpEF.
Methods and Results
Cardiac structure and function were assessed by quantitative echocardiography at baseline and 12-18 month follow-up in 239 patients with HFpEF (left ventricular [LV] ejection fraction [LVEF] ≥45%) enrolled in the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. The impact of spironolactone therapy on measures of cardiac structure and function was assessed in the study population overall, and change in echocardiographic measures was associated with the subsequent occurrence of the primary composite outcome of cardiovascular (CV) death, heart failure (HF) hospitalization, or aborted cardiac arrest. Spironolactone was not associated with alterations in cardiac structure and function compared to placebo. Decrease in left atrial (LA) volume at follow-up was associated with a lower risk of subsequent occurrence of the primary outcome.
Conclusions
Twelve to 18 months of spironolactone therapy was not associated with alterations in cardiac structure or function in patients with HFpEF. Reduction in LA volume at follow-up was associated with a lower risk of subsequent occurrence of the primary composite outcome.
Clinical Trial Registration
URL: http:///www.clinicaltrials.gov. Unique identifier: NCT00094302.
Keywords: heart failure with preserved ejection fraction, echocardiography, spironolactone, clinical trial
Heart failure with preserved ejection fraction (HFpEF) is common, increasing in prevalence, and is associated with significant morbidity and mortality. Left ventricular (LV) hypertrophy, left atrial (LA) enlargement, elevated LV filling pressure, and pulmonary hypertension have each been associated with worse prognosis in HFpEF.1,2,3,4 Much interest has focused on the potential therapeutic role of aldosterone antagonist therapy in HFpEF. However, limited data exist regarding the impact of aldosterone antagonist therapy on cardiac structure and function in HFpEF and on the prognostic relevance of changes in cardiac structure and function in HFpEF.
In the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial, treatment with spironolactone in HFpEF did not reduce the composite endpoint of cardiovascular (CV) death, aborted sudden death, or heart failure (HF) hospitalization but was associated with a lower incidence of HF hospitalization in the study population overall.5 Cardiac structure and function was assessed by echocardiography at baseline and at 12 to18 months following randomization to either spironolactone or placebo in a subset of patients.6 Among this subset of patients, we determined the impact of randomization to spironolactone versus placebo on measures of cardiac structure and function in HFpEF. In addition, we explored the prognostic relevance of changes cardiac structure and function over 12-18 months on subsequent outcomes.
Methods
Patient population
TOPCAT was a multicenter, international, randomized, double blind placebo-controlled trial of spironolactone compared to placebo to reduce cardiovascular morbidity and mortality in 3,445 adults at least 50 years old with signs and symptoms of HF and a left ventricular ejection fraction (LVEF) ≥45% per local site reading.7 Randomization was stratified by the presence of either one of the following inclusion criteria: at least one hospitalization in the prior 12 months for which HF was a major component or, if no qualifying hospitalization, a B-type natriuretic peptide (BNP) in the prior 60 days ≥100 pg/ml or N-terminal pro-BNP (NT-proBNP) ≥360 pg/ml. All patients provided written informed consent, and the study was approved by the local Institutional Review Board at each site. Baseline demographics and clinical characteristics of the trial population have been previously described in detail.8
The design and baseline findings of the TOPCAT echocardiographic sub-study, including reproducibility metrics for conventional echocardiographic measures, have been previously described in detail.6 At 27 sites, patients consenting to participation in the overall TOPCAT trial were separately consented to participate in the echocardiographic sub-study and underwent echocardiograms by a study-specific protocol at baseline and 12 or 18 months following randomization. Of 935 patients in the TOPCAT echocardiographic study, 305 were enrolled in the dedicated sub-study, in whom follow-up echocardiography was done at 12 months in 213 (70%) and 18 months in 31 (10%). No follow-up echocardiogram was performed in 61 (20%). Of the 244 sub-study participants in whom a follow-up echocardiogram was performed, image quality was adequate for quantitative analysis at baseline and follow-up in 239 (performed at 12 months in 208 participants and at 18 months in 31 participants).
Echocardiographic Methods
Quantitative measurements on all study echocardiograms were performed according to the American Society of Echocardiography recommendations by dedicated analysts at the core laboratory, blinded to clinical information and randomized treatment assignment as previously described.6,9,10 Intra-observer variability in our laboratory for key echocardiographic measures of cardiac structure and function have been previously reported.6
Outcomes
Clinical outcomes included CV death, HF hospitalization, and aborted sudden death during the follow-up period. All events were reported by the primary site investigator and independently adjudicated by the Clinical Endpoints Center. Definitions of these endpoints have been previously published.7
Statistical Analysis
Continuous variables are presented as means and standard deviations or median and interquartile range as specified. Two-sided P-values of less than 0.05 were considered significant. Change in echocardiographic measures from baseline to follow-up was calculated as the follow-up value – baseline value. The relationship between randomization to spironolactone and change in echocardiographic measures was assessed using linear regression, adjusting for the baseline value of the echocardiographic measure of interest. The TOPCAT echocardiography sub-study was initially designed to have 90% power to detect a 1 cm/sec difference in the change in tissue Doppler imaging (TDI) e′ with spironolactone versus placebo, and aimed to enroll 500 patients assuming loss to follow-up rate of 10%, inadequate image quality of 15%, standard deviation of difference 3 cm/sec, and a correlation of baseline with follow-up measure of 0.50. Using the actual study sample size and observed standard deviation of difference and correlation between measures, we had 89% power to detect a difference in the change in e′ of 0.8 cm/s associated with spironolactone. Similarly, we had ≥80% power to detect: a 2.1unit difference in change in E/e′ ratio, a 8 g difference in change in LV mass index, and a 6 ml difference in change in LA volume.
For the assessment of the prognostic implication of changes in cardiac structure and function in HFpEF, the study primary outcome was the composite of first occurrence of HF hospitalization, aborted sudden death, or cardiovascular (CV) death occurring after the date of the follow-up echocardiogram. Unadjusted and multivariable-adjusted Cox proportional hazards analyses were performed to determine the association of changes in cardiac structure and function with adverse outcomes. Due to relatively small numbers of events, all adjusted Cox models were adjusted for the baseline value of the echo parameter and age as continuous covariates. All other categorical variables (gender, race, region, and randomization strata) were used as stratification factors in the Cox model along with Huber-White robust variance estimators. As a sensitivity analysis, all echo parameters variables found to be significant in these adjusted models were further assessed using permutation-based p-values, in which the variable representing change from baseline was randomly permuted 1000 times and the stratified Cox model used to construct the null distribution of parameters used for comparison to the observed parameter. Given the marked regional differences in patient characteristics and treatment effects noted in TOPCAT,11 all analyses were also performed separately by geographic region (Americas vs Russia/Georgia; Supplemental Data).
Results
As compared to the 3,206 patients in TOPCAT without serial echocardiograms, the 239 patients with serial echocardiographic data were older and more frequently enrolled through the BNP stratum. While gender, race, and region of enrollment were similar between groups, patients with serial echocardiographic data had a higher prevalence of prior coronary revascularization and atrial fibrillation and had lower heart rate, blood pressure, and hematocrit at baseline (Supplemental Table 1).
Impact of Spironolactone on Cardiac Structure and Function
Among the 239 patients with echocardiographic data at baseline and follow-up, the 121 patients randomized to spironolactone were well matched to the 118 randomized to placebo, with the exception of a modestly higher prevalence of hypertension among the placebo group (Table 1). No significant difference was noted in the number of interval primary events between the baseline and follow-up studies between treatment arms (11 [9%] versus 4 [3%] in the placebo and spironolactone arms respectively, p=0.07). Randomization to spironolactone compared to placebo was not associated with significant differences in measures of LV structure, LV systolic function, LV diastolic function, LA size, or RV function and pulmonary pressure (Table 2). Similar findings were noted in an on-treatment analysis, excluding 38 patients who permanently discontinued study drug prior to the follow-up echocardiogram (Supplemental Table 2). Similar findings were also noted in analysis stratified by randomization strata (prior HF hospitalization versus elevated natriuretic peptide level; Supplemental Table 3). Supplemental analyses were performed separated by region of enrollment (Supplemental Tables 4-7). Among patients enrolled in the Americas, randomization to spironolactone compared to placebo was associated with modest decrease in LVESV and E/A ratio and increase in LVEF, while randomization to spironolactone was not associated with significant changes in cardiac structure or function among patients enrolled in Russia or Georgia (Supplemental Table 6).
Table 1. Baseline characteristics by randomization treatment arm in the study population overall.
| Overall | ||
|---|---|---|
|
| ||
| Placebo (n=118) | Spirono (n=121) | |
| Age (years) | 70.7 ± 8.4 | 69.7 ± 9.5 |
| Female | 53%) | 51% |
| White | 89% | 84% |
| Enrollment Strata: Prior Hospitalization | 64% | 59% |
| Co-morbidities | ||
| Hypertension | 97% | 89% |
| Myocardial Infarction | 27% | 31% |
| Coronary Revascularization | 30% | 31% |
| Stroke | 7% | 12% |
| Atrial Fibrillation | 44% | 42% |
| Diabetes | 34% | 36% |
| Obesity | 53% | 58% |
| NYHA Functional Class | ||
| 1 | 6% | 5% |
| 2 | 59% | 61% |
| 3 | 36% | 32% |
| 4 | 0% | 2% |
| Physical Characteristics | ||
| BMI (kg/m2) | 32.0 ± 7.4 | 32.8 ± 7.3 |
| Heart rate (bpm) | 68 ± 12 | 65 ± 11 |
| Systolic blood pressure (mmHg) | 126 ± 14 | 127 ± 14 |
| Diastolic blood pressure (mmHg) | 73 ± 11 | 74 ± 10 |
| Laboratory Values | ||
| eGFR (mL/min per 1.73 m2) | 67.5 ± 18.1 | 67.9 ± 19.0 |
| Creatinine (mg/dL) | 1.07 ± 0.29 | 1.10 ± 0.32 |
| Hematocrit (%) | 38.6 ± 4.8 | 39.4 ± 4.6 |
No significant differences for baseline comparisons with the exception of hypertension (p=0.03).; BMI=body mass index; eGFR= estimated glomerular filtration rate
Table 2. Baseline, Follow-up, and Change in echocardiographic measures by treatment arm in the study population overall.
| N | Baseline | Follow-up | Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Spirono | Placebo | Spirono | p | Placebo | Spirono | p | Placebo | Spirono | P* | |
| Overall (n=239) | |||||||||||
| Left Heart Structure | |||||||||||
| LVEDV (ml) | 100 | 101 | 93.0 ± 32.1 | 99.3 ± 31.5 | 0.16 | 96.3 ± 32.3 | 99.7 ± 33.7 | 0.46 | 3.3 ± 14.7 | 0.5 ± 14.0 | 0.23 |
| LVESV (ml) | 100 | 101 | 38.8 ± 21.6 | 40.5 ± 19.3 | 0.55 | 40.9 ± 23.1 | 41.0 ± 21.4 | 0.96 | 2.1 ± 9.2 | 0.5 ± 8.6 | 0.22 |
| Mean wall thickness (cm) | 115 | 117 | 1.16 ± 0.20 | 1.14 ± 0.17 | 0.49 | 1.16 ± 0.20 | 1.14 ± 0.17 | 0.30 | -0.00 ± 0.05 | -0.01 ± 0.08 | 0.27 |
| LV mass index (mg/m2) | 114 | 113 | 106 ± 29 | 106 ± 29 | 0.94 | 107 ± 29 | 105 ± 31 | 0.61 | 0 ± 7 | -1 ± 12 | 0.19 |
| Relative wall thickness | 114 | 113 | 0.49 ± 0.11 | 0.48 ± 0.09 | 0.34 | 0.50 ± 0.12 | 0.48 ± 0.09 | 0.19 | 0.00 ± 0.03 | -0.00 ± 0.04 | 0.22 |
| LAV (ml) | 97 | 93 | 58.1 ± 20.4 | 59.3 ± 22.6 | 0.70 | 60.3 ± 21.1 | 60.3 ± 24.6 | 0.99 | 2.2 ± 12.1 | 1.1 ± 15.3 | 0.61 |
| LA diameter (cm) | 117 | 119 | 4.08 ± 0.56 | 4.10 ± 0.53 | 0.85 | 4.11 ± 0.55 | 4.09 ± 0.50 | 0.77 | 0.02 ± 0.21 | -0.01 ± 0.19 | 0.21 |
| LV Function | |||||||||||
| LVEF (%) | 118 | 121 | 59.2 ± 8.3 | 59.8 ± 7.8 | 0.56 | 58.7 ± 9.4 | 59.9 ± 8.0 | 0.27 | -0.5 ± 6.3 | 0.1 ± 6.1 | 0.33 |
| E/A ratio | 73 | 71 | 0.99 ± 0.43 | 1.04 ± 0.49 | 0.50 | 1.05 ± 0.56 | 0.95 ± 0.41 | 0.25 | 0.05 ± 0.46 | -0.09 ± 0.36 | 0.047 |
| TDI E′ (septal) (cm/sec) | 91 | 95 | 5.76 ± 1.73 | 6.10 ± 2.02 | 0.21 | 5.55 ± 1.87 | 6.00 ± 2.07 | 0.12 | -0.21 ± 1.58 | -0.10 ± 1.79 | 0.31 |
| E/E′ (septal) | 86 | 91 | 15.0± 5.2 | 14.1 ± 5.5 | 0.26 | 15.5 ± 5.6 | 14.4 ± 7.1 | 0.28 | 0.5 ± 4.6 | 0.3 ± 5.2 | 0.67 |
| RV and Pulmonary Vascular | |||||||||||
| TR jet velocity (m/sec) | 36 | 37 | 2.73 ± 0.46 | 2.79 ± 0.39 | 0.53 | 2.66 ± 0.47 | 2.78 ± 0.46 | 0.30 | -0.07 ± 0.55 | -0.02 ± 0.50 | 0.38 |
| RVFAC (%) | 71 | 66 | 0.48 ± 0.07 | 0.49 ± 0.07 | 0.31 | 0.48 ± 0.06 | 0.49 ± 0.07 | 0.32 | 0.00 ± 0.08 | 0.00 ± 0.08 | 0.45 |
P value for between treatment group differences in change is based on linear regression models adjusting for the baseline value
LVEDV – left ventricular end diastolic volume, LVESV – left ventricular end-systolic volume, LVEF – left ventricular ejection fraction; TDI E′ – tissue Doppler early diastolic relaxation velocity; LAV – left atrial volume; TR – tricuspid regurgitation; RVFAC – right ventricular fractional area chance
Prognostic Relevance of Changes in Cardiac Structure and Function in HFpEF
Overall, participants were followed up for a median of 710 days (IQR 361-934 days) after the follow-up echocardiogram, during which time 29 primary outcome events occurred. After adjusting for baseline value, increase in LA volume was associated with a heightened risk of the primary composite endpoint (Table 3; Figure). Associations of marginal statistical significance were also noted between increase in E/A ratio, increase in PASP, and decrease in RVFAC and subsequent occurrence of the primary composite endpoint. The associations between change in LA volume and E/A ratio, and the primary outcome remained statistically significant after further adjustment for patient demographics, region of enrollment, and randomization strata. The significance of change in LAV in adjusted models was further supported by the permutation test, which yielded a p-value of 0.033, as were changes in E/A ratio (permutation test p<0.001). Changes in PASP and RVFAC were no longer associated with subsequent outcomes after adjustment. Changes in E/e′ ratio and measures of LV structure were not associated with subsequent outcomes. Increases in LA volume and PASP, and decrease in LVEF were associated with heightened risk of the primary endpoint among patients enrolled in the Americas, while changes in echocardiographic measures were not associated with subsequent outcomes among patients enrolled in Russia or Georgia (Supplemental Table 7).
Table 3. Change in echocardiographic measures of cardiac structure and function and subsequent outcomes in the study population overall.
| Measure | N | Events | Baseline-adjusted HR | P value | Fully-adjusted HR | P value |
|---|---|---|---|---|---|---|
| ΔLVEDV (per ml) | 186 | 23 | 1.00 (0.97 – 1.03) | 0.83 | ||
| ΔLVESV (per ml) | 186 | 23 | 1.00 (0.96 – 1.04) | 0.96 | ||
| ΔLVMi (per g/m2) | 208 | 29 | 1.00 (0.97 – 1.03) | 0.99 | ||
| ΔLVEF (per 1%) | 219 | 29 | 0.98 (0.92 – 1.04) | 0.52 | ||
| ΔE/A ratio (per 0.1 unit) | 135 | 15 | 1.10 (1.00 – 1.20) | 0.05 | 1.12 (1.04 – 1.19)* | 0.001 |
| Δe′ (per cm/s) | 171 | 22 | 1.07 (0.81 – 1.41) | 0.65 | ||
| ΔE/e′ ratio (per 1 unit) | 163 | 22 | 0.96 (0.88 – 1.04) | 0.29 | ||
| ΔLAV (per ml) | 176 | 24 | 1.03 (1.01 – 1.06) | 0.01 | 1.04 (1.01 – 1.09)§ | 0.019 |
| ΔPASP (per mmHg) | 65 | 8 | 1.06 (1.00 – 1.13) | 0.06 | 1.06 (0.97 – 1.15) | 0.19 |
| ΔRVFAC (per 1%) | 124 | 12 | 0.91 (0.82 – 1.00) | 0.06 | 0.94 (0.84-1.05) | 0.25 |
permutation test p value <0.001;
permutation test p value = 0.033
Figure.
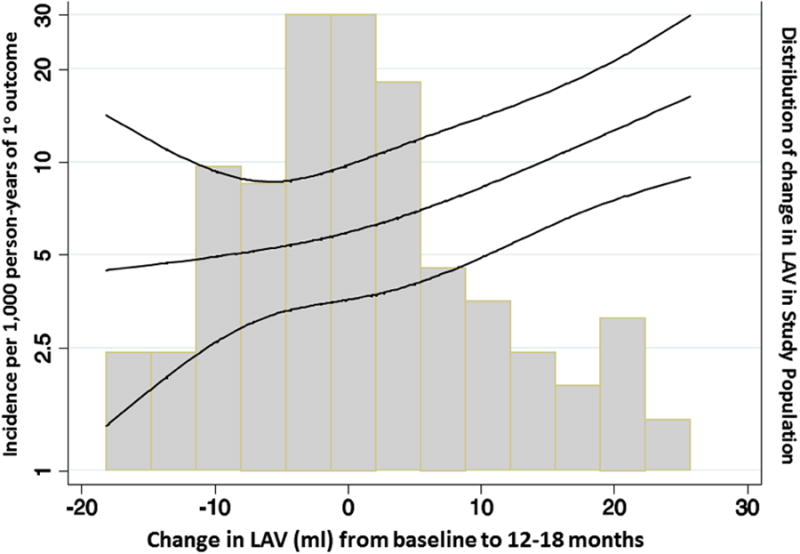
Restricted cubic spline analysis demonstrating the baseline LAV-adjusted incident rate (per 1,000 person-years) for the primary composite endpoint of HF hospitalization, aborted cardiac arrest, or CV death associated with change in LAV. P for linear trend = 0.012.
Discussion
Among 239 HFpEF patients in the TOPCAT trial with echocardiographic data at randomization and 12-18 month follow-up, spironolactone therapy was not associated with major alterations in cardiac structure or function. This was true for the study population overall, and among patients enrolled in the Americas and those enrolled in Russia or Georgia. In the study population overall, improvements in LA volume and E/A ratio from baseline to follow-up were associated with a lower subsequent risk of the primary composite endpoint, of marginal statistical significance.
Previous smaller studies of the impact of aldosterone antagonist therapy on cardiac structure and function in HFpEF have yielded conflicting results (Table 4).12-16 In a study of 44 patients with HFpEF randomized to 12-months of therapy with eplerenone versus placebo, Mak et al observed no significant effect of eplerenone on LV mass index, LA volume index, or multiple measures of LV diastolic function (E/A ratio, e′ velocity, E/e′ ratio; Table 4) although a modest improvement in E wave deceleration time was noted.13 In contrast, Deswal et al observed a significant improvement in the E/e′ ratio among 44 predominantly male HFpEF patients randomized to 6 months of eplerenone compared to placebo, an association that group reproduced in a follow-up study of spironolactone therapy in 48 elderly women with HFpEF.14,15 Notably, no treatment effect was noted on E/A ratio, LA volume, or LV mass in either of those studies. ALDO-DHF, the largest and perhaps the most definitive study to investigate the impact of spironolactone on cardiac structure and function in HFpEF, demonstrated significant improvements in e′, E/e′ ratio, LV mass index, and LV size among 422 HFpEF patients randomized to 12 months of spironolactone versus placebo.16
Table 4. Studies of the impact of aldosterone antagonist therapy on cardiac structure and function in HFpEF.
| Author (year) | N | Intervention | Inclusion | Age (yr) | Female | F/u (mo) | Endpoints |
|---|---|---|---|---|---|---|---|
| Daniel et al 200912 | 11 | Spironolactone 25 mg/day *no placebo arm | Prior HF hosp LVEF >50% | 72±8 | 100% | 4 | ↓ E/e′ No change in E/A ratio, e′, LV dimension or WT |
| Mak et al 200913 | 44 | Epleronone 25-50 mg/daily | Prior HF hosp BNP>100 LVEF >45 % DDfxn on echo | 80±7.8 | 54% | 12 | ↓DT No change in E/A ratio, e′, E/e′, LAVi, LVMi |
| Deswal et al 201114 | 44 | Epleronone 25-50 mg/daily | NYHA Class II/III LVEF >50% BNP>100 | 70±9 | 7% | 6 | ↓E/e′ No change in E/A ratio, e′, LAV, LV dimension, LVMi |
| Kurrelmeyer et al 201415 | 48 | Spironolactone 25 mg/day | NYHA Class II/III LVEF >50% BNP>62 DDfxn on echo | 71±2 | 100% | 6 | ↓E/e′, ↓e′, ↓LV mass No change in E/A ratio, LAV, LV dimension |
| ALDO-DHF 201316 | 422 | Spironolactone 25 mg/day | ≥50 years old NYHA II/III LVEF ≥50% DDfxn on echo Peak VO2 ≤25 | 67±8 | 52% | 12 | ↓E/e′, ↓e′, ↓LVMi, ↓LV dimension, ↑LVEF No change in E/A ratio, LAV |
| TOPCAT Echo | 239 | Spironolactone 15-45 mg/day | ≥50 years old NYHA II/III LVEF ≥45% Prior HF hosp or elevated natriuretic peptide level | 70±9 | 52% | 23±12 | No change in echocardiographic measures* |
Among patients enrolled in the Americas only, spironolactone was associated with modest decrease in LVESV, decrease in E/A ratio, and increase in LVEF (Supplemental Table 8); DT – E wave deceleration time, WT – wall thickness.
The reasons why the TOPCAT findings are partially discordant with those of ALDO-DHF are uncertain. The number of patients with serial studies was smaller in TOPCAT, and limited our power to detect a spironolactone treatment effect. In the overall TOPCAT trial, early study drug discontinuation occurred in over 30% or participants, which would bias analyses of treatment effect towards the null. In addition, differences in inclusion criteria possibly resulted in earlier stage disease in the ALDO-DHF trial compared to TOPCAT. TOPCAT patients included in this analysis tended to be older than ALDO-DHF patients (70 versus 67 years respectively), prior HF hospitalization was present in 62% in TOPCAT versus 37% in ALDO-DHF, and median NT-proBNP level in ALDO-DHF was lower than the TOPCAT inclusion criterion for patients without a prior HF hospitalization. It is possible that impairments in cardiac structure and function in HFpEF are less modifiable by spironolactone in more advanced stages of disease. Concordant with this hypothesis, several studies in patients with co-morbid conditions predisposing to HFpEF – including hypertension with exertional intolerance,17 obesity,18 and metabolic syndrome19 – have demonstrated improvements in LV diastolic function measures with spironolactone.
This is one of the only studies, to our knowledge, to evaluate the prognostic relevance of changes in cardiac structure and function on subsequent outcomes in HFpEF. After adjusting for the baseline value, improvements in measures of LV filling pressure (LA volume, E/A ratio), pulmonary pressure (TR velocity), and RV function (RVFAC) were associated with a lower risk of subsequent clinical events, with most of these associations of borderline statistical significance. These associations are not surprising, as multiple studies have established the prognostic importance of elevated LV filling pressure,4 pulmonary hypertension,2 and RV dysfunction20 in HFpEF. However, the demonstration that improvements in these measures relate to lower subsequent risk is novel, and suggests that these may be relevant surrogates in Phase 2 HFpEF trials.
Each 1 mL reduction in LA volume was associated with a 3% lower risk of the primary endpoint after adjusting for baseline LA volume, and remained significantly associated with similar effect estimate after additional adjustment for patient demographics and randomization strata. Our findings are consistent with prior studies demonstrating the prognostic importance of changes in LA volume in patients with HF or LV systolic dysfunction following myocardial infarction.21 Importantly, whereas we did not observe an effect of spironolactone therapy on LA size, both pharmacologic22 and exercise training23 interventions have been shown to reduce LA size in HFpEF. Ongoing studies will determine whether these intervention-associated benefits in cardiac structure translate into improvements in clinical outcomes.
Several studies have demonstrated the prognostic importance of elevated pulmonary artery pressure,2,4 and more recently associated RV dysfunction,20 in HFpEF. Indeed, there is considerable interest in therapies targeting the pulmonary vasculature in HFpEF patients with pulmonary hypertension,24 although existing data is conflicting.25,26 However, to date there is little data regarding the prognostic implications of reduction in PASP in HF generally, and HFpEF in particular. Therefore, although of marginal statistical significance, our finding of an association between change in PASP – independent of baseline PASP – and subsequent risk in HFpEF is intriguing. We were unable to differentiate changes in PASP related to changes in LA pressure versus changes in pulmonary vascular resistance. Replication of this finding in a larger HFpEF sample, and demonstration that the relationship between change in PASP and outcomes is independent of LA pressure, would further support PASP as a therapeutic target in HFpEF.
Several limitations of this manuscript should be noted. Echocardiographic data at baseline and follow-up were only available in 239 patients enrolled in TOPCAT, limiting our power both to detect treatment effect on echocardiographic measures and associations between changes in echocardiographic measures and subsequent outcomes. In addition, patients with serial echocardiographic data included in this study differed significantly in demographic and clinical comorbidities from the remaining TOPCAT trial population (Supplemental Table 1), possibly limiting the generalizability of our findings. We did not account for multiple testing in our analyses, including the analysis of the relationship between change in echocardiographic measures from baseline to follow-up and subsequent outcomes, which highlights the exploratory nature of these findings. Furthermore, prominent differences in participant characteristics and response to spironolactone therapy were noted by enrollment region (Americas or Russia/Georgia) in TOPCAT11 and may confound our analysis. However, the prognostic relevance of changes in LAV and PASP were also observed in analyses restricted to patients enrolled in the Americas, who demonstrated hospitalization and death rates consistent with prior HFpEF outcomes trials. Survivor bias is an additional potential limitation. However, among patients enrolled in the sub-study, no significant differences in number of deaths prior to the follow-up echocardiogram were noted between the placebo and spironolactone arms in either the Americas (8 versus 6 respectively, p=1.00) or Russia/Georgia (5 versus 3 respectively, p=1.00).
Conclusions
Twelve to 18 months of spironolactone therapy was not associated with improvement in LV structure or function in HFpEF. However, modest sample size and regional differences in TOPCAT limited the power of this analysis. Reduction in LA volume at follow-up was associated with a significantly lower risk of subsequent CV death, aborted cardiac arrest, or HF hospitalization.
Supplementary Material
Clinical Perspective.
Heart failure with preserved ejection fraction (HFpEF) is common, increasing in prevalence, and is associated with significant morbidity and mortality. Limited data exist regarding the impact of aldosterone antagonist therapy on cardiac structure and function in HFpEF and on the prognostic relevance of changes in cardiac structure and function in HFpEF. We assessed cardiac structure and function in 239 patients with HFpEF (left ventricular (LV) ejection fraction ≥45%) enrolled in the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Twelve to 18 months of spironolactone therapy was not associated with alterations measures of LV structure, LV systolic function, LV diastolic function, left atrial (LA) size, or right ventricular (RV) function and pulmonary pressure in patients with HFpEF. However, modest sample size and regional differences in TOPCAT limited the power of this analysis. After adjusting for the baseline value, improvements in measures of LV filling pressure (LA volume, E/A ratio), pulmonary artery systolic pressure, and RV function (RV fractional area change) were associated with a lower risk of subsequent clinical events, with most of these associations of borderline statistical significance. In adjusted analysis, improvements in LA volume and E/A ratio at follow-up remained associated with a lower risk of subsequent occurrence of the primary composite outcome.
Acknowledgments
Sources of Funding: TOPCAT was funded by National Heart, Lung, and Blood Institute, National Institutes of Health (Bethesda, MD), Contract Number HHSN268200425207C. The content of this paper does not necessarily represent the views of the sponsor or of the Department of Health and Human Services. The work for this manuscript was also supported by NHLBI grant 1K08HL116792 (A.M.S.) and AHA grant 14CRP20380422 (A.M.S.).
Footnotes
Disclosures: Dr A. Shah reports receiving research support from Novartis, Gilead, and Actelion. Dr. S. Shah reports receiving research support from Actelion; consulting fees from the American Board of Internal Medicine, AstraZeneca, DC Devices, Novartis, Bayer, and Alnylam; and speaker fees from the Pulmonary Hypertension Association and the American Society of Echocardiography. Dr Deswal reports receiving research support from Novartis. Dr Pitt reports serving as a consultant for Pfizer, Bayer, Elli-Lilly, Novartis, and DaVinci therapeutics, and has a patent pending on site specific delivery of Eplerenone to the myocardium. Dr Pfeffer reports receiving research grants from Amgen, Celladon, Novartis, Sanofi Avantis, and Hamilton Health Sciences, Consulting for Abbot Vascular, Amgen, Bristol-Myers Squibb, Cerenis, Concert, Fibrogen, Genzyme, GlaxoSmithKline, Medtronic, Merck, Novo Nordisk, Roche, Salix, Sanderling, Servier, and University of Oxford. The Brigham and Women's Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of myocardial infarction with Novartis. Dr Pfeffer is a co-inventor. His share of the licensing agreement is irrevocably transferred to charity.
References
- 1.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 2.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta D, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–99. doi: 10.1161/CIRCHEARTFAILURE.113.000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function and Prognosis in Heart Failure With Preserved Ejection Fraction: Findings From the Echocardiographic Study of the Treatment Of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–51. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 6.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function in Heart Failure with Preserved Ejection Fraction: Baseline Findings from the Echocardiographic Study of the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:104–15. doi: 10.1161/CIRCHEARTFAILURE.113.000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–72. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O'Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circ Heart Fail. 20136:184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner J, Leweis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer N, McKinlay S, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 12.Daniel KR, Wells G, Stewart K, Moore B, Kitzman DW. Effect of aldosterone antagonism on exercise tolerance, Doppler diastolic funciton, and quality of life in older women with diastolic heart failure. Congest Heart Fail. 2009;15:68–74. doi: 10.1111/j.1751-7133.2009.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, Patle AK, Baugh JA, McDonald KM. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54:1674–82. doi: 10.1016/j.jacc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in heart failure with Preserved Ejection Fraction trial (RAAM-PEF) J Cardiac Fail. 2011;17:634–42. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Kurrelmeyer KM, Ashton Y, Xu J, Nagueh SF, Torre-Amione G, Deswal A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J Cardiac Fail. 2014;20:560–8. doi: 10.1016/j.cardfail.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The ALDO-DHF randomized controlled trial. JAMA. 2013;209:781–91. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 17.Mottram PM, Haluska B, Leano R, Cowley D, Stowasser M, Marwick TH. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–565. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]
- 18.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomized controlled trial of aldosterone blockade. Heart. 2013;99:320–6. doi: 10.1136/heartjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 19.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, O'Moore-Sullivan T, Marwick TH. A randomized study of the beneficial effects of aldosterone antagonism on LV function, structure, and fibrosis markers in metabolic syndrome. J Am Coll CardiolImg. 2011;4:1239–49. doi: 10.1016/j.jcmg.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–62. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Kober L, Bourgoun M, McMurray JJ, Velazquez EJ, Maggioni AP, Ghali J, Arnold JMO, Zelenkofske S, Pfeffer MA, Solomon SD. Left atrial remodeling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo Study. Eur Heart J. 2009;30:56–65. doi: 10.1093/eurheartj/ehn499. [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJV. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase II randomized-controlled trial. Lancet. 2012;380:1387–95. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 23.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. JACC. 2011;58:1780–91. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CS, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274–85. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–74. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 26.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


