Abstract
Oral vaccines for polio (OPV) and rotavirus are less effective in children in the developing world. The reasons for this are not well understood. We tested for risk factors for poor response to OPV in infants from an urban slum of Dhaka, Bangladesh. Diminished serum neutralizing response to OPV, but not failure of intramuscularly administered vaccines, was associated with malnutrition, diarrhea, and shorter breastfeeding duration. Children with malnutrition (WAZ <−2) had significantly lower OPV 3 titers (p = 0.029). Children who had 2 or more diarrhea episodes during the 1st months of life were more than twice as likely to experience OPV failure as those who had 1 diarrhea episode or no diarrhea (p = 0.0245). In contrast, each additional month in exclusive breastfeeding was associated with an increase in OPV 3 titer by 0.41 (p = 0.0072) and 0.16 (p = 0.0065) at the 25th and 50th percentiles of OPV 3 titers respectively. These data are consistent with a defect in induction of immunity in the gut for OPV but not parenteral vaccines, a defect that may be amenable to intervention in part via promotion of exclusive breastfeeding.
1. Introduction
Oral polio vaccine is less effective in children in the developing world [1]. The per dose efficacy of the trivalent OPV has been estimated to be 50% in the United States but only 21% in India [2]. Similarly, oral rotavirus vaccine was only half as effective at preventing severe rotavirus infection [3,4]. Hypotheses for this lower efficacy of oral vaccination in developing countries include malnutrition, diarrheal disease, and environmental enteropathy [5–12]. Environmental enteropathy is thought to be common in children in the developing world and is pathologically characterized by villous shortening with increased intraepithelial lymphocytes in the small intestine [9–12]. There is little data on the effectiveness of oral vaccines in children with environmental enteropathy, but it has been observed that OPV is less effective if given during episodes of diarrhea [13]. This suggested a potential link of enteric infection and enteropathy with vaccine failure, and led us to test for such an association in infants in Dhaka, Bangladesh.
2. Methods
2.1. Longitudinal birth cohort
The children studied were from an urban slum of the Mirpur Thana of Dhaka, Bangladesh. Subjects were identified by a census for pregnant women in the community, conducted by trained field research assistants. Children were enrolled within the first week of birth starting in January 2008 and followed by twice-weekly household visits until one year of age. A total of 435 children entered the cohort who received OPV (any number of doses). 314 children received at least three doses of OPV by 12 months, of whom 258/314 received 3 doses by 6 months of age. The median number of doses was 3 and the lower and upper quartiles were 3 and 4 respectively. Diarrhea was defined as three loose or unformed stools in 24 h, or by the mother’s report in a breast fed infant under the age of one year. Exclusive breast feeding was defined by the mother’s monthly report of her child’s consumption of human milk without supplementation (including water but excluding medications). The study was approved by the Research and Ethical Review Committees of the International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, and the Institutional Review Board of the University of Virginia. There have been two previous reports from this cohort on enteric infections [14,15].
2.2. Anthropometry
Weight and length of the children were assessed using electronic scales and length boards precise to 10 g and 1 mm respectively (SECA Gmbh & Co, Hamburg, Germany). The mean of two consecutive measurements were recorded. These were converted to weight for age (WAZ) and length for age (LAZ) using the WHO Multicenter Growth Reference Study child growth standards [16]. Underweight was defined as WAZ <−2 and stunting as LAZ <−2.
2.3. Vaccine history and immunogenicity
Immunization histories were obtained from the infants’ mothers, and only children with a minimum of three OPV immunizations were included in the analyses. Serum neutralizing antibodies to the type 1–3 polio strains were measured at the CDC, Atlanta as previously described [17,18]. The log2 based titer was used in all OPV analyses. Tetanus, measles, and diphtheria serum IgG levels were expressed in IU/ml and were measured by ELISA as directed by the manufacturer’s instructions (Virion/Serion GmbH, Germany).
2.4. Statistical analyses
Response to oral poliovirus vaccine was measured by serum neutralizing antibodies in children who had received the recommended minimum of three doses of OPV by age 6 months (for 6 month vaccine response measurements) or 12 months (for 12 month vaccine response measurements). Vaccine failure was defined as a titer of less than 1:8 (log2[titer] < 3). The Kolmogorov–Smirnov test was used to evaluate the association of stunting status with OPV and systemic vaccine responses. Breastfeeding was tested as a potential environmental variable because of its ability to prevent enteric infection and promote development of the infant immune system. Exclusive breastfeeding was defined as the lack of any complementary feeding of any food or liquid except for breast milk. Other independent predictors included nutritional status at month 6 of age (normal weight vs. underweight), number of diarrhea episodes during the first 6 months, as well as family income.
Considering the skewed distribution of OPV titer measure and censoring at low and high ends (2.5 and 10.5 respectively in log2 scale), quantile regression was used to evaluate the predictive effect of independent variables with continuous OPV response at 12 months. In contrast to linear regression, quantile regression has no distribution assumption and is more robust in handling extreme value points and outliers, so the regression coefficients do not vary by the capping on the response variable for most of the percentiles. Because of a concern with the potentially attenuated effect of censored titer measures, the quantile regression was only performed at 25th and 50th percentiles of OPV response. In addition, a generalized linear model with GEE (i.e., longitudinal logistic regression) was used for repeated OPV failures at 6 and 12 months. The repeated analyses for OPV failures at 6 and 12 months models the relationship of covariates with the response over time and accounts for the within-subject correlations, and thus it is potentially more powerful than separate analyses at each time. Statistical significance in this study was set at p < 0.05 and all reported p values were 2-sided. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
3. Results
We tested for diminished immunogenicity of the OPV in a longitudinal prospective cohort of children followed from birth to age one year in an urban slum of Dhaka, Bangladesh. The average family size was 5, monthly family income was 6000 Taka (approximately $80 US), and 40% of mothers lacked any education. The median duration of exclusive breastfeeding was approximately 4 months. Malnutrition was present in 16% of children at birth and one third of children at 12 months of age, as measured by a weight for age (WAZ) score of <−2 (Supplemental Table 1).
OPV type 3 failure (log2[serum neutralizing antibody titer] < 3) was 13.6 and 7.3% for infants who had received at least 3 doses of OPV at 6 or 12 months of age respectively. In contrast, failure rates for type 1 and 2 viruses were lower, as expected from previous experiences with this vaccine (Supplemental Fig. 1). Our analyses therefore focused on the OPV 3 titer.
Malnutrition, measured as weight for age more than 2 standard deviations below the WHO norm (WAZ < −2), was inversely associated with OPV 3 titer at 12 months of age (n = 314; p = 0.0289) (Fig. 1). In contrast, the antibody responses to the intramuscularly administered measles, tetanus, and diphtheria vaccines were not different in malnourished infants (Fig. 1). Premature births were not measured, so the impact of prematurity on OPV response is not known. HAZ ≥−2 at 6 months appeared to be positively associated with OPV 3 titer at 12 months, though it was only marginally significant for the 50th percentile (p = 0.07) and not statistically significant for the 25th percentile (p = 0.45) (data not shown).
Fig. 1.
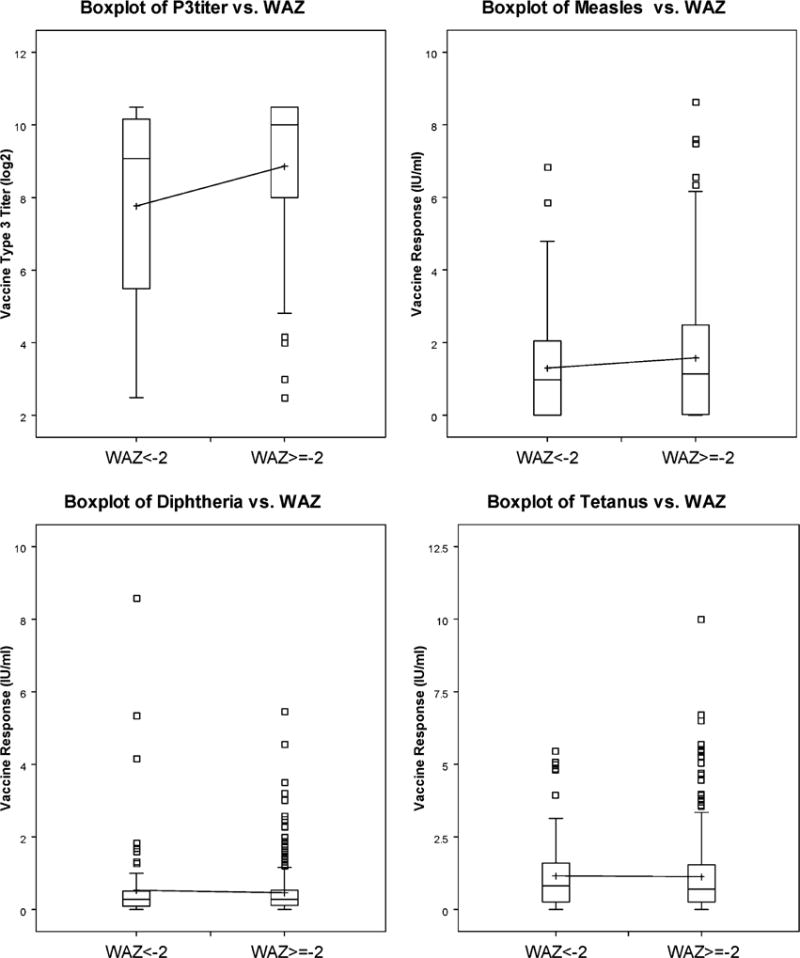
Oral polio vaccine type 3 and parenteral vaccine responses in well and malnourished children. The titers of serum neutralizing antibody responses to OPV type 3 and parenterally-administered vaccines at 12 months of age for children with WAZ scores of <−2 were compared to those with WAZ >−2 at 6 months of age (n = 314; p = 0.0289 for OPV; n = 435 and p = NS for diphtheria, tetanus, and measles vaccine responses; Kolmogorov–Smirnov rank order test).
Exclusive breastfeeding was positively associated with OPV 3 titer at 12 months as determined by quantile regression (Fig. 2). Specifically, at the 25th and 50th percentiles of OPV titer 3, each additional month in exclusive breastfeeding was predicted to increase OPV titer 3 by 0.36 (95% confidence interval (CI): 0.16–0.57, p = 0.0006) and 0.13 (95% CI: 0.02–0.23, p = 0.019) respectively.
Fig. 2.
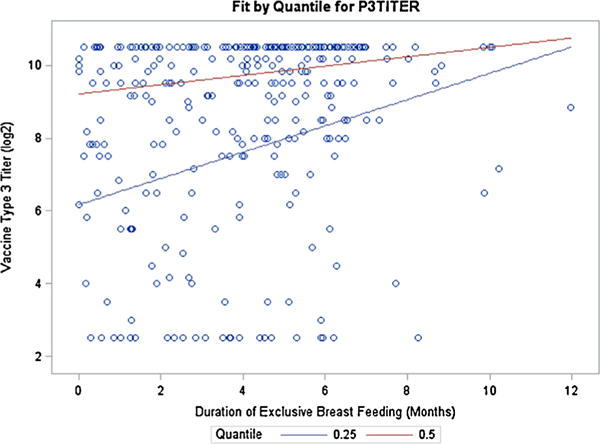
Association of exclusive breastfeeding with oral polio vaccine type 3 response (at 25th and 50th percentiles). Due to the skewed distribution of OPV titer 3 measure (first quartile and median were 7.83 and 9.67 respectively), quantile regression analysis was performed to evaluate the predictive effect of exclusive breastfeeding on OPV titer 3 at 12 months of age (n = 314). The duration of exclusive breastfeeding significantly predicted increased OPV titer measure, with the effect being more pronounced in the first quartile.
The multivariable quantile regression results for continuous OPV type 3 titer are summarized in Table 1. The independent variables in this study included diarrhea episodes, WAZ at 6 months, exclusive breastfeeding duration in first 6 months, and family income. Traditional linear regression for OPV 3 titer models the effects of independent variables on the conditional mean of OPV 3 titer; however, since most children experienced sero-response, and the distribution of OPV 3 titers was skewed with the first quartile and median values of 7.83 and 9.67, the lower conditional quantiles are more critical to answer the questions such as whether these independent variables influence OPV 3 titer differently for children with low titers than for those with average titers where OPV failures occur. Thus, our regression analyses focused on fitting conditional quantiles of OPV 3 titer at 25th and 50th percentiles. The adjusted analysis showed that both malnutrition at 6 months and shorter exclusive breastfeeding within the first 6 months of life were negatively associated with OPV titer, and the effects were greater in the first quartile. Compared with malnourished children, normal children had 2.35 (95% CI: 0.66–4.03, p = 0.0065) and 1.11 (95% CI: 0.31–1.90, p = 0.0063) higher OPV titer 3 measures at the 25th and 50th percentiles of OPV response, respectively. Moreover, each additional month in exclusive breastfeeding was associated with an increase of OPV titer 3 of 0.4 (95% CI: 0.11–0.71, p = 0.0072) and 0.16 (95% CI: 0.05–0.28, p = 0.0065) at the 25th and 50th percentiles, respectively. Neither number of diarrhea episodes nor family income was significantly associated with the continuous OPV type 3 titer in this multivariable analysis. We concluded that a lower immunogenicity, but not failure, of OPV type 3 vaccine was associated with malnutrition and shorter duration of exclusive breastfeeding. OPV type 3 vaccine failure was associated with diarrhea, as discussed below.
Table 1.
Association of independent variables with continuous oral polio vaccine type 3 titers at 12 monthsa.
| Characteristics | 25th quantile regression
|
50th quantile regression
|
||||
|---|---|---|---|---|---|---|
| Effect | 95% CI | p-value | Effect | 95% CI | p-value | |
| Intercept | 3.73 | (1.21, 6.25) | 0.0039 | 8.54 | (7.53, 9.55) | <.0001 |
| ≥2 diarrhea episodes in 1st 6 months | −0.23 | (−1.26, 0. 80) | 0.6599 | −0.17 | (−0.66, 0.33) | 0.5116 |
| WAZ ≥−2 at 6 months | 2.35 | (0.66, 4.03) | 0.0065 | 1.11 | (0.31, 1.90) | 0.0063 |
| Breastfeeding duration in 1st 6 months | 0.41 | (0.11, 0.71) | 0.0072 | 0.16 | (0.05, 0.28) | 0.0065 |
| Family income (1000 Taka)b | 0.05 | (−0.05, 0.15) | 0.3717 | −0.03 | (−0.08, 0.02) | 0.2159 |
Type 3 titer at 12 months of age was analyzed as a continuous outcome in quantile regression (n = 314).
The family income was standardized in 1000 Taka, such that the estimated effect was interpreted as the change in type 3 titers for every 1000 Taka increment (one Taka equals approximately $0.013 US dollars).
We also tested for the predictive effect of environmental variables associated with the probability of repeated failure to seroconvert to type 3 at 6 and 12 months of age, defined as log2[titer] < 3. The multivariable longitudinal logistic regression showed that diarrhea in the first 6 months of age was significantly associated with the risk of OPV 3 failure. Compared with those who had 1 diarrhea episode or no diarrhea, children who had 2 or more diarrhea episodes during the 1st month of age had an odds ratio (OR) = 2.35 for failure (95% CI: 1.12–4.94, p = 0.0245) (Supplemental Table 2). Diarrhea was independent of low birth weight (previously shown to be a risk factor for diarrhea in this cohort (14)) as a predictor of OPV failure. Family income appeared to be marginally associated with OPV failure, in that each 1000-Taka increment reduced the likelihood of OPV failure by 12% (OR = 0.88, 95% CI: 0.77–1.01, p = 0.0599).
4. Discussion
The most important finding of this study is the association of diminished immunogenicity of OPV type 3 virus with undernutrition, shorter duration of exclusive breastfeeding, and diarrhea. In contrast the immunogenicity of intramuscularly administered vaccines was unaffected by these three variables. This study in impoverished infants in Bangladesh therefore points to a specific defect in immune response to the orally administered polio vaccine. Notably, the characteristics associated with OPV underperformance are uniquely common to developing country settings, where up to 40% of children are undernourished, diarrhea is a leading cause of death, and breastfeeding duration is suboptimal [19,20].
It has long been appreciated that OPV was less immunogenic in developing countries. For example, outbreaks of paralytic polio have occurred in developing countries despite the high percentages of children having been immunized, with estimates of the immunogenicity of a single dose of trivalent OPV less than half to that of developed countries [1,2]. This impairment in immune response to OPV in the developing world has been overcome by administering as many as 10 doses to children, although this is burdensome to the public health system. The value of this study is that it points to malnutrition and diarrhea, problems that disproportionately affect children in the developing world, as potential explanations. It also offers potential avenues for improving the efficacy of OPV, which could include extending the duration of exclusive breastfeeding and improving nutrition.
Breastfeeding interaction with OPV has been studied in the past. Work in the developed world demonstrated that breastfed infants had higher serum neutralizing antibody responses to OPV than those formula-fed [21,22]. Withholding of breastfeeding surrounding the time of immunization (to test the contrary hypothesis that maternal breast milk anti-polio IgA could interfere with OPV vaccination) had no effect on OPV titers [23]. Thus, the sum of the data from both the developing (this report) and developed countries is consistent with breastfeeding being advantageous for OPV immunogenicity.
Encouragingly, the study illustrates potential interventions to improve OPV performance. For example, promotion of exclusive breastfeeding may represent a readily implementable intervention. The diminished immunogenicity of OPV, and not measles, diphtheria or tetanus vaccines, also suggests that the apparent intestinal barrier to immunization could be overcome by parenteral vaccination.
Supplementary Material
Acknowledgments
We thank the families of Mirpur for their participation in and support for the study. Roland Sutter, Walter Orenstein, Pearay Ogra, Tom Brewer, Chris Wilson, Nic Grassly, and Jacob John have been invaluable sources of advice. We thank William S. Hendley, Yiting Zhang, Sharla L. McDonald, and Deborah D. Moore for the performance of the serum neutralizing antibody responses. Supported by NIH Grant AI-43596 and by the Bill & Melinda Gates Foundation.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2013.11.056.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- 1.Grassly NC, Jafari H, Bahl S, et al. Asymptomatic wild-type poliovirus infection in India among children with previous oral poliovirus vaccination. J Infect Dis. 2010;201:1535–43. doi: 10.1086/651952. [DOI] [PubMed] [Google Scholar]
- 2.Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150–3. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 3.Zaman K, Anh DD, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 4.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 5.Parashar UD, Glass RI. Rotavirus vaccines—early success remaining questions. N Engl J Med. 2009;360:1063–5. doi: 10.1056/NEJMp0810154. [DOI] [PubMed] [Google Scholar]
- 6.John JT. Oral polio vaccination of children in the tropics. II. Antibody response in relation to vaccine virus infection. Am J Epidemiol. 1975;102:414–21. doi: 10.1093/oxfordjournals.aje.a112180. [DOI] [PubMed] [Google Scholar]
- 7.Zaman K, Sack DA, Yunus M, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27:1333–9. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 8.Panter-Brick C, Lunn PG, Langford RM, et al. Pathways leading to early growth faltering: an investigation into the importance of mucosal damage and immunostimulation in different socio-economic groups in Nepal. Brit J Nutr. 2009;101:558–67. doi: 10.1017/S000711450802744X. [DOI] [PubMed] [Google Scholar]
- 9.Korpe P, Petri WA., Jr Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–36. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veitch AM, Kelly P, Zulu IS, et al. Tropical enteropathy: a T cell-mediated crypt hyperplastic enteropathy. Eur J Gastroenterol Hepatol. 2001;13:1175–81. doi: 10.1097/00042737-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Campbell DI, Murch SH, Elia M, et al. Chronic T cell-mediated enteropathy in rural West African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306–11. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- 12.Papasavvas E, Pistilli M, Reynolds G, et al. Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS. 2009;23:369–75. doi: 10.1097/QAD.0b013e32831e9c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myaux JA, Unicomb L, Besser RE, et al. Effect of diarrhea on the humoral response to oral polio vaccination. Pediatr Infect Dis J. 1996;15:204–9. doi: 10.1097/00006454-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Mondal D, Minak J, Alam M, et al. Contribution of enteric infection, altered intestinal barrier function and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54:185–92. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korpe PS, Liu Y, Siddique A, et al. A prospective cohort study of parasite-specific breast milk antibodies and protection from enteric protozoal infection in breastfed Bangladeshi infants. Clin Infect Dis. 2013;56:988–92. doi: 10.1093/cid/cis1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. The WHO Multicentre Growth Reference Study (MGRS) Internet: http://www.who.int/childgrowth/mgrs/en/.(Accessed 13 January 2013).
- 17.W.H.O. Collaborative study group on oral and inactivated poliovirus vaccines. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. J Infect Dis. 1997;175:S215–27. doi: 10.1093/infdis/175.supplement_1.s215. [DOI] [PubMed] [Google Scholar]
- 18.Report of a WHO informal consultation on polio neutralizing antibody assays, Nashville, 5–6 December 1991. Geneva: World Health Organization; 1991. Expanded Programme on Immunization. [Google Scholar]
- 19.Petri WA, Jr, Miller M, Binder HJ, et al. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–90. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet. 2010;376:1682–8. doi: 10.1016/S0140-6736(10)61230-5. [DOI] [PubMed] [Google Scholar]
- 21.Pickering LK, Granoff DM, Erickson JR, et al. Modulation of the immune system by human milk and infant formula containing nucleotides. Pediatrics. 1998;101:242–9. doi: 10.1542/peds.101.2.242. [DOI] [PubMed] [Google Scholar]
- 22.Hahn-Zoric M, Fulconis F, Minoli I, et al. Antibody responses to parentral and oral vaccines are impaired by conventional and low protein formula as compared to breastfeeding. Acta Paediatr Scand. 1990;79:42. doi: 10.1111/j.1651-2227.1990.tb11401.x. [DOI] [PubMed] [Google Scholar]
- 23.John TJ, Devarajan LV, Luther L, et al. Effect of breast-feeding on seroresponse of infants to oral poliovirus vaccination. Pediatrics. 1976;57:47–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


