Abstract
Currently there are no effective therapies available for the excruciating neuropathic pain that develops after spinal cord injuries (SCI). As such, a great deal of effort is being put into the investigation of novel therapeutic targets that can alleviate this pain. One such target is acrolein, a highly reactive aldehyde produced as a byproduct of oxidative stress and inflammation that is capable of activating the transient receptor potential ankyrin 1 (TRPA1) cation channel, known to be involved in the transmission and propagation of chronic neuropathic pain. One anti-acrolein agent, hydralazine, has already been shown to reduce neuropathic pain behaviors and offer neuroprotection after SCI. This study investigates another acrolein scavenger, phenelzine, for its possible role of alleviating sensory hypersensitivity through acrolein suppression. The results show that phenelzine is indeed capable of attenuating neuropathic pain behaviors in acute, delayed, and chronic administration schedules after injury in a rat model of SCI. Additionally, upon the comparison of hydralazine to phenelzine, both acrolein scavengers displayed a dose-dependent response in the reduction of acrolein in vivo. Finally, phenelzine proved capable of providing locomotor function recovery and neuroprotection of spinal cord tissue when administered immediately after injury for two weeks. These results indicate that phenelzine may be an effective treatment for neuropathic pain after SCI and likely a viable alternative to hydralazine.
Keywords: lipid peroxidation, aldehyde, phenelzine, proalgesic, hyperreflexia
Introduction
Persistent neuropathic pain significantly impairs the quality of life of SCI patients beyond paralysis and is the leading cause for suicide attempts among SCI victims (Hulsebosch et al. 2009). It is well established that inflammation and oxidative stress contribute to the initiation and maintenance of neuropathic pain after SCI (Hulsebosch et al. 2009, Basbaum et al. 2009). However, the exact pathogenic mechanism of this condition remains unclear. Consequently, there are no established analgesic therapies that can effectively alleviate pain among SCI victims. Hence, identifying and establishing an effective pain treatment is of great importance and is highly warranted.
It has recently been reported that acrolein, an α,β-unsaturated aldehyde and a byproduct of oxidative stress and lipid peroxidation, plays a critical role in neuropathic pain-related behavior after SCI (Park et al. 2014b, Due et al. 2014, Park et al. 2014a). Acrolein can be produced from a variety of endogenous sources such as polyamines, lipids, amino acids, and carbohydrates (O’Brien et al. 2005, Stevens & Maier 2008, Esterbauer et al. 1991, Hamann & Shi 2009, Shi et al. 2011a). As a known neurotoxin, acrolein has the ability to trigger neuropathic hyperreflexia by directly activating the electrophile-sensitive transient receptor potential ankyrin 1 (TRPA1) cation channel (Bautista et al. 2006, Barabas et al. 2012, Koivisto et al. 2014). Additionally, as a pro-inflammatory agent, acrolein can stimulate the release of chemokines which likely further intensify pain through known mechanisms (Jung et al. 2008, Beck et al. 2010, Due et al. 2014, Esterbauer et al. 1991, Facchinetti et al. 2007, Facchinetti et al. 1998, Kirkham et al. 2003). It has been shown that acrolein is elevated significantly following SCI which coincides with the emergence of sensory hypersensitivity (Due et al. 2014, Park et al. 2014b, Luo et al. 2005). The microinjection of pathologically relevant concentrations of acrolein directly to the spinal cord of otherwise normal rats produced pain-like behavior and elevated level of TRPA1 mRNA that were similar to that observed after SCI (Due et al. 2014, Park et al. 2014b, Park et al. 2015). Furthermore, lowering endogenous acrolein levels post-SCI with acrolein scavengers has been associated with reducing pain-related behavior and suppressing the post-SCI augmented TRPA1 mRNA levels (Due et al. 2014, Park et al. 2014b, Park et al. 2015). Taken together, it appears that acrolein likely plays a critical role in sensory hypersensitivity following SCI. Likewise, acrolein scavenging may be a novel effective strategy to combat persistent neuropathic pain post-SCI.
It has been shown that a number of hydrazine derivatives, such as hydralazine and phenelzine, have stronger capabilities to scavenge acrolein than other tested compounds, likely utilizing the hydrazine group to trap acrolein (Galvani et al. 2008, Burcham et al. 2000, Kaminskas et al. 2004, Wood et al. 2006). We have shown that hydralazine, an FDA-approved anti-hypertensive medication, can effectively alleviate sensory hypersensitivity, reduce elevated levels of TRPA1 mRNA expression, and offer general neuroprotection while significantly lowering the acrolein levels when administered after SCI in rats (Due et al. 2014, Park et al. 2014b, Liu-Snyder et al. 2006, Park et al. 2015). However, other potential acrolein scavengers, such as phenelzine, also a hydrazine derivative, have not been tested in a similar manner. We reason that if both hydralazine and phenelzine are capable of lowering acrolein, alleviating hyperreflexia, and offering neuroprotection after SCI, then acrolein scavenging may be responsible for the neuronal protection associated with both drugs. Furthermore, due to the potential clinical limitations of hydralazine administration due to its activity as a vasodilator (Pandit 1984, Khan 1953), it is desirable to have an alternative acrolein scavenger particularly in acute neurotrauma situations.
The primary goal of this study was to test the hypothesis that phenelzine could sequester the acrolein and offer the analgesic and neuroprotective effects in rodent SCI. This study was conducted in a well-established rat SCI contusion model where hydralazine was examined to test its neuroprotective effect (Due et al. 2014, Park et al. 2014b). We have found in this study that, similar to hydralazine, phenelzine can reduce acrolein levels and offer significant analgesic and overall neuroprotective effects. This study strengthens the critical pathogenic role of acrolein in post-SCI sensory hypersensitivity and neurodegeneration, and offers further evidence that anti-acrolein treatments could be an effective treatment for SCI victims to relieve pain and enhance overall recovery.
Methods and Materials
Animals
Male Sprague-Dawley rats, weighing 200~250g, were obtained from Harlan Laboratory (Indianapolis, IN, USA) and housed and handled according to the Purdue University Animal Care and Use Committee Guidelines under the Institutional Protocol 111100095. Prior to the surgery, animals were given at least one week for acclimation.
Moderate spinal cord contusion injury model
Intraperitoneal (IP) injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) mixtures were used to anesthetize the rats prior to surgery. Moderate spinal cord contusion injury models were conducted following our previous study (Park et al. 2014b). Briefly, the spinous process and vertebral laminae were removed to expose the dorsal surface of the spinal cord at the T-10 spinal level. Subsequently, the spinal cord was contused using a New York University (NYU) Impactor, with a 10-g rod dropped from 25mm. Sham surgeries were conducted at the same time by removing the vertebral laminae without contusing the spinal cord. After surgery, all rats were placed on a heating pad for recovery, 3 mL of saline was administered subcutaneously to prevent dehydration, and regular manual bladder expression was performed twice daily until the rats regained reflexive control of bladder function.
Application of phenelzine and hydralazine
Phenelzine sulfate salt (Sigma, St. Louis, MO, USA) was dissolved in phosphate buffered saline and sterilized with a 0.45 um filter. Generally, dosages up to 60 mg/kg with IP administration are safe for rodents so 5, 15, and 60 mg/kg of phenelzine were chosen as dosages for this study based on a literature review of the drug’s effectiveness (Musgrave et al. 2011, Paslawski et al. 1996, Baker et al. 1992). In order to evaluate the effectiveness of phenelzine at different SCI stages, phenelzine was administered immediately after injury for 14 days (acute), beginning on the 21st day after surgery for 14 days (delayed), and beginning 2 months after injury for 14 days (chronic). To investigate phenelzine’s effect of suppressing acrolein in both tissue and urine (3-HPMA), phenelzine injections were administered twice: once within 3 min following SCI, and then again 24-h post-SCI. The effect of phenelzine-mediated increase of spinal cord tissue preservation was evaluated 4 weeks post-SCI. Phenelzine was applied IP daily (15 mg/kg) starting immediately following trauma for 2 weeks.
Hydralazine hydrochloride (Sigma, St. Louis, Mo, USA) was dissolved in phosphate buffered saline then sterilized via a filter. Final doses of 5 and 25 mg/kg of hydralazine solution were administrated through IP injection. To investigate hydralazine’s effect of suppressing acrolein, hydralazine injections were administered twice: once within 3 min following SCI, and then again 24-h post-SCI. The level of acrolein in both tissue and urine (3-HPMA) of SCI, or SCI treated with hydralazine or phenelzine were assessed 24 hours post trauma.
Pharmacokinetic study of hydralazine and phenelzine
Male Sprague-Dawley rats were anesthetized using isoflurane; pre-operative analgesia was given subcutaneously. Surgical sites were shaved and scrubbed with betadine and alcohol. Gastric catheters (BASi Culex rat gastric, Bioanalytical System Inc. West Lafayette, IN) were implanted in the peritoneal cavity and the carotid artery (BASi Culex rat carotid, Bioanalytical System Inc. West Lafayette, IN) of each of the rats. Catheters were externalized and secured in the scapular region. The animals were then tethered in a movement-responsive cage and the carotid catheter was connected to a Culex automated pharmacology system. This system periodically flushed the catheters with heparinized saline to maintain patency. Animals were given 24 hr to acclimate to the cage, and recover from surgery prior to dosing.
For hydralazine, the animals were dosed with at 5 mg/kg intraperitoneally via catheter to avoid the stress of handling, and the blood samples (200 μl/sample) were collected at 15, 30, 45 min, 1, 2, 4, 6, 8, 24 hrs post injection. For phenelzine, the animals were dosed at 15 mg/kg intraperitoneally, and the blood samples (200 μl/sample) were collected at 15, 45 min, 2, 4, 6, 8, 9, 16, 24 hrs post injection. Blood samples were deposited into pre-treated heparinized vials and kept at 4°C until processed.
Hydralazine analysis
The sampling vial pretreated with heparin was pre-loaded with 5 μL of 3-cyclohexene-1-carboxaldehyde (derivatization reagent) and 1 μL phenelzine (20 ppm). Phenelzine is capable of reacting with 3-cyclohexene-1-carboxaldehyde so the product serves as the internal standard for analysis. Blood (100 μL) was collected by the Culex sampler and injected into the sampling vial. A volume of 10 μL of blood was then injected into a disposable glass capillary of 0.8 mm i.d. for slug flow microextraction with 10 μL of ethyl acetate. Finally, nanoESI was applied for direct mass spectrometry analysis by a TSQ spectrometer (Thermo Fisher Scientific, Massachusetts, USA). The spray voltage was set at 1500 V, and energy for collision induced dissociation was set at 30 eV. For quantification, ions of m/z 253 → 129 and m/z 229 → 105 were collected for hydralazine and the internal standard.
Phenelzine analysis
The sampling vial pretreated with heparin was pre-loaded with phenelzine-d5 as the internal standard (20 ppm, 1 μL). A volume of 100 μL of blood was collected by the Culex sampler and injected into the sampling vial. A blood sample of 10 μL was injected into a disposable glass capillary of 0.8 mm i.d. for slug flow microextraction with 10 μL ethyl acetate. Finally, nanoESI was applied for direct mass spectrometry analysis by a TSQ spectrometer (Thermo Fisher Scientific, Massachusetts, USA). The spray voltage was set at 1500 V, and energy for collision induced dissociation was set at 30 eV. For quantitation, ions of m/z 137 → 105 and m/z 142 → 110 were collected for phenelzine and the internal standard, respectively.
Behavioral quantification and assessment
Mechanical allodynia
Paw withdrawal thresholds in response to mechanical stimuli were determined using von Frey filaments to quantify mechanical hypersensitivity after SCI and evaluate the effectiveness of phenelzine treatment by someone blind to the treatment. Similar to previous studies, animals were placed inside a transparent plastic box on the top of a metal mesh floor in a quiet area and left alone for at least 10 minutes for acclimation before testing (Due et al. 2014). After that, a series of calibrated Von Frey filaments (range: 0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0 and 15.0 gram, Stoelting, Wood Dale, IL, USA) were applied perpendicular to the plantar surface of the hind-limb with sufficient bending force for three to five seconds. A positive reaction was defined as a brisk movement with or without licking or biting. The level of mechanical withdrawal threshold was calculated according to the up-down method reported by Chaplan (Chaplan et al. 1994). The average of two hind-limb scores was recorded. Finally, to avoid the confounding effect of stress induced by IP injection during behavioral testing, all sensory behavioral tests were conducted 24 hours after the preceding injection.
Behavioral assessment of locomotor function
Locomotor function recovery of rats after SCI was assessed using the Basso, Beattie, and Bresnahan (BBB) Locomotor Rating Scale, a widely used method for assessing SCI rodent models via observing the hind-limbs movement, trunk position and postures of the paws (Basso et al. 1995). Briefly, the scale has 21-points ranging from 0 to 21, of which 0 indicates no movement of the hind limbs and 21 means the rats have completely normal movement. The evaluation was completed in an open field by observation for at least five minutes by an investigator without knowledge of group identity. The left and right hind paws were recorded separately at one day and weekly after SCI until four weeks post-injury. The average of the two hind-paws’ scores was recorded as the final score.
Isolation of the spinal cord
After deep anesthesia, the animals were perfused with oxygenated Kreb’s solution (124 mM NaCl, 2 mM KCl, 1.24 mM KH2PO4, 26 mM NaHCO3, 10 mM ascorbic acid, 1.3 mM MgSO4, 1.2 mM CaCl2, and 10 mM glucose) via the trans-cardiac routine. Subsequently, the whole vertebral column was harvested rapidly and the spinal cord was isolated by removing all the surrounding bone. For the investigation of mRNA expression of TRPA1, the lumbar dorsal root ganglions (DRGs) were also isolated carefully and the skin of hind-paw was acquired at the same time.
Immunoblotting
Immunoblotting was used to detect acrolein-protein adducts at the tissue-specific level. For this purpose, a 1 cm segment of spinal cord, including the damaged site, was incubated with a 1% Triton solution with a Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO, USA), and then was homogenized with a sonicator. The total protein concentration from each sample was measured using a BCA protein assay kit to ensure equal sample loading. Using a Bio-Dot SF Microfiltration Apparatus (Bio-Rad, Hercules, CA, USA), each sample (200 μg) was transferred to the nitrocellulose membrane. Then, the transfer membrane was blocked in 0.2% casein and 0.1% Tween-20 in a PBS blocking buffer and incubated with a monoclonal mouse anti-acrolein antibody (1:1000, ABCAM, Cambridge, MA, USA). After washing the membrane with the blocking buffer, the membrane was transferred to 1:10000 alkaline phosphatase conjugated goat anti-mouse IgG solution (VECTASTAIN ABC-AmP Kit, Burlingame, CA, USA). After final washing with blocking buffer and 0.1% Tween 20 in Tris-buffered saline, the membrane was exposed to the substrate of the ABC-AMP kit and visualized by chemiluminescence. For the band density quantification, Image J (NIH) software was used and an arbitrary unit was used for the expression.
TRPA1 gene expression analysis using real time PCR
To quantify TRPA1 gene expression, DRG cells (L1–L6), dorsal horn of spinal cord (1 cm at T-10 level), and paw skin were collected from sham control, SCI only, and SCI with phenelzine injection groups (Park et al. 2015). All samples were homogenized with a Trizol reagent (Sigma-Aldrich, St. Louis, MO, USA). RNA isolation, followed by chloroform extraction and isopropanol precipitation were carried out. Isolated RNA was measured using NanoDrop 2000c (Thermo Scientific, DE, USA). For cDNA synthesis, the iScript™ cDNA Synthesis kit manual guide (BIO-RAD, 170-8890, USA) was utilized. Primers for TRPA1 channel and 18s were designed based on previous studies (Due et al. 2014). To recognize the TRPA1 channel, 5′-TCCTATACTGGAAGCAGCGA-3′, and 5′-CTCCTGATTGCCATCGACT-3′; 18S primers were used as an internal control against 5′-CGGCTACCACATCCAAGGAA-3′ and 5′-GCTGGAATTACCGCGGCT-3′. The PCR products were detected by the level of iQ™SYBR Green Supermix (BIO-RAD, 170-8880, USA) fluorescence. The target gene expression level was normalized by the expression level of internal control 18s and relative quantification was calculated following previous study (Livak & Schmittgen 2001, Park et al. 2015).
Tissue fixation and histology examination
In brief, the spinal cord tissue was fixed using 4% formaldehyde subsequent to a Kreb’s perfusion and then the fixed tissues were transferred from the formaldehyde solution into 15% and 30% sucrose solution in order for dehydration. The spinal cord tissues were then embedded in Tissue-Tek O.C.T Compound and rapidly frozen. The frozen tissues were transversely sectioned with a cryostat microtome at a thickness of 20 μm focusing on the epicenter lesion. Hematoxylin and eosin staining was performed to observe the spared neural tissue. The area of spared neural tissue was measured and calculated according to a previous report by someone blind to the treatment (Santiago et al. 2009). In brief, all the digital images of the tissues were captured under the same conditions (the same magnification and resolution) using the same microscope. Then, ImageJ was used to measure the total number of pixels of the spared neural tissue (including white and grey matter) and the unit was expressed as “total pixel numbers × 104”.
Urine collection
Standard metabolic cages were used for urine collection. To occasionally induce urination, 0.3 cc of saline was administrated via IP injection. Water and food sources were carefully separated to prevent urine dilution and contamination.
Acrolein metabolite 3-hydroxypropyl mercapturic acid (3-HPMA) test in urine
To prepare the urine samples for solid phase extraction before LC/MS/MS analysis, ENV+ cartridges (Biotage, Charlotte, NC) were used. Each cartridge was conditioned with 1 mL of methanol, followed by 1 mL of water, and then 1 mL of 0.1% formic acid in water. A volume of 500 μL of urine was mixed with 500 μL of 50 mM ammonium formate and 10 μL of undiluted formic acid and 500 μL of urine was spiked with 200 ng of deuterated 3-HPMA (d3-3-HPMA) (Toronto Research Chemicals Inc., New York, Ontario). Then, each cartridge was washed twice using 1 mL of 0.1% formic acid and 1 mL of 10% methanol/90% of 0.1% formic acid in water. All cartridges were completely dried under flowing nitrogen and eluted with 600 μL methanol and 2% formic acid three times. After drying eluates with an evaporation centrifuge, each sample was reconstituted in 100 μL of 0.1% formic acid. For the 3-HPMA analysis, an Agilent 1200 Rapid Resolution liquid chromatography (LC) system coupled to an Agilent 6460 series QQQ mass spectrometer (MS) was used.
Creatinine assay
Creatinine levels were measured using a urine creatinine assay kit (Cayman Chemical Company, MI, USA). Creatinine standards and diluted urine samples (12X and 24X) were incubated with the alkaline picrate solution for 20 min in 96 well plates. To construct a creatinine standard curve, the manufacturer’s manual was followed. The absorbance at 490–500 nm was measured as an initial reading with a standard spectrophotometer. Then, 5 μl of acid solution was added to each solution and incubated for 20 minutes. Again, absorbance at 490–500 nm with a standard spectrophotometry was used to perform the final reading and the difference between the initial and final value was used for quantitative analysis and internal standardization of the 3-HPMA quantification.
Statistical methods
All the averages were expressed as mean ± SEM. Student’s t-test was used to make the comparison when only two groups available. For comparisons involving three or more groups, ANOVA and Tukey’s tests were used to compare the data. p<0.05 was used as statistical significance.
Results
Phenelzine alleviates mechanical hyperreflexia following SCI
Phenelzine (15 mg/kg) was administered immediately after trauma daily for two weeks post-SCI. Figure 1 depicts that SCI resulted in significant tactile hyperreflexia evidenced by the post-SCI day 14 mechanical paw withdraw threshold reduction. The phenelzine treated group, however, exhibited a significant decrease in tactile hyperreflexia when compared with the injury only group at days 15–29 post-injury (P< 0.05) (Fig. 1).
Figure 1.
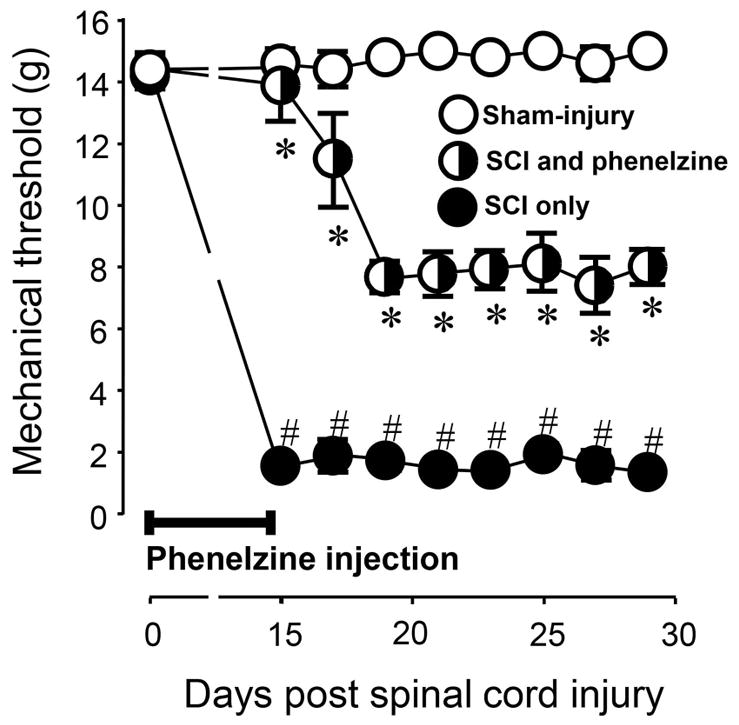
Post-SCI mechanical hyperreflexia and its alleviation by phenelzine applied immediately following trauma for 2 weeks. No difference in mechanical hyperreflexia was found before SCI (at day 0) in sham-injury, SCI only, and SCI treated with phenelzine. On days 15–29, SCI only rats displayed a significantly increased level of mechanical hyperreflexia (# P < 0.05 when compared to sham-injury). This increased display of presumptive pain behavior was significantly attenuated with the application of phenelzine (* P < 0.05 when compared to SCI only). One-way ANOVA and Tukey test were used for statistical analysis. N=5 in each condition. All data were expressed in mean ± SEM.
In a separate experiment, phenelzine was applied with a delay after SCI (Fig. 2). Specifically, the systemic daily treatment of phenelzine began at day 21 post-SCI and concluded at day 35 post SCI: a total of 14 days of phenelzine application. Animal receiving this treatment regimen displayed a significant decrease in paw withdrawal threshold reduction from day 22 to day 36 post-SCI when compared to SCI only group (P < 0.05 or P < 0.01). However, such decrease in tactile hyperreflexia was not significant three days following the termination of phenelzine treatment (day 38 post-SCI) (p > 0.05 between injury and injury treated with phenelzine).
Figure 2.
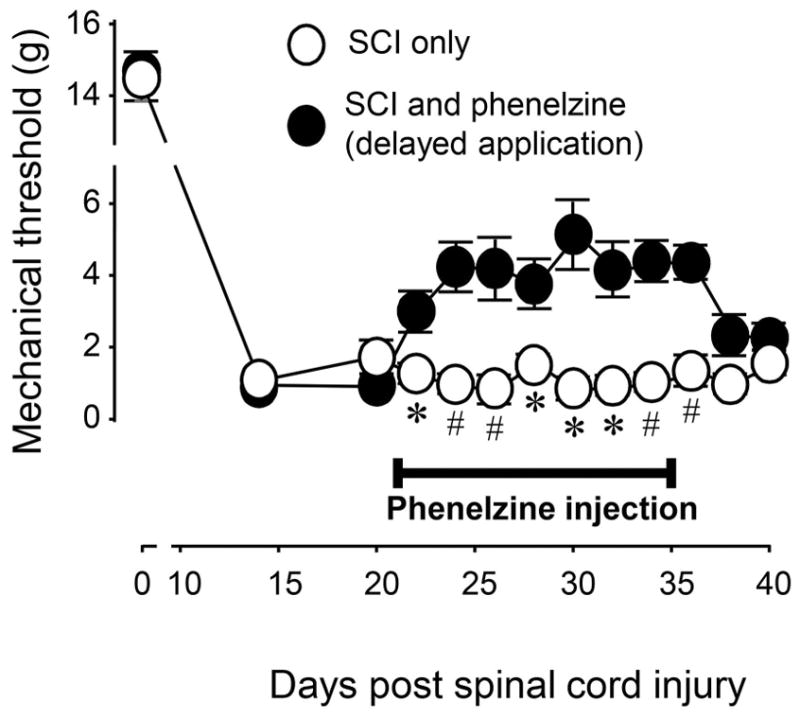
Effective attenuation of post-SCI mechanical hyperreflexia with delayed administration of phenelzine. At day 0 (before SCI) and post-SCI day 14, there was no difference in mechanical hyperreflexia in SCI and SCI + phenelzine groups. However, phenelzine significantly attenuated the mechanical hyperreflexia in SCI starting day 22 until day 36 (* P < 0.05; # P < 0.01 when compared to SCI only group, N = 5 for all groups). Phenelzine was applied at a dosage of 15 mg/kg starting at post-SCI day 21 for a period of two weeks. All data were expressed as mean ± SEM.
Finally, to examine whether phenelzine application was still effective in mitigating mechanical hyperreflexia when applied at chronic stages, phenelzine was administered daily for two weeks beginning at two months post-SCI. Again, as indicated in Figure 3, animals that received this intervention showed reduced tactile hyperreflexia between days 72 to 82 post-SCI when compared to SCI only group (P < 0.05). Similar to the delayed application of phenelzine, the effect seen in treated animals was nonsignificant 3 days following the cessation of systemic phenelzine application (p > 0.05).
Figure 3.
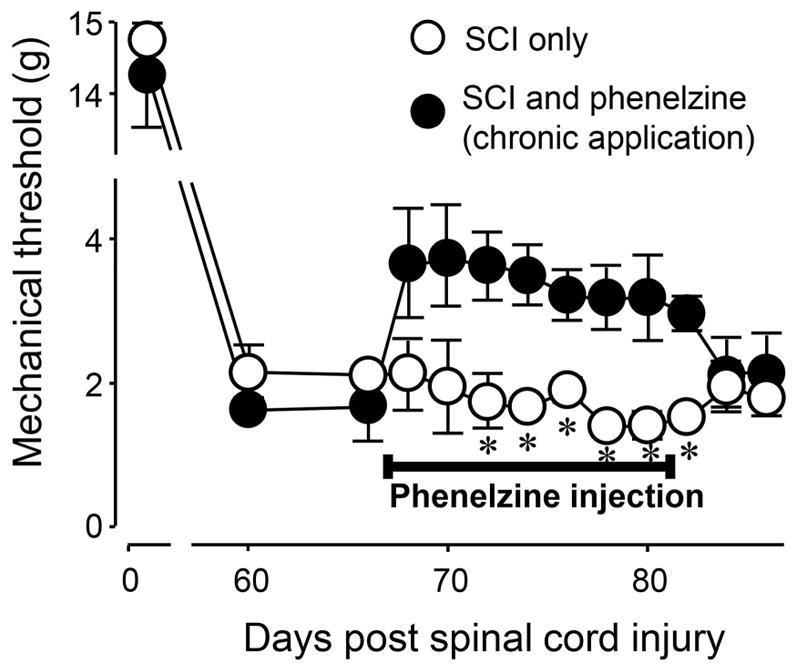
Chronic post-SCI mechanical hyperreflexia and its alleviation by phenelzine. At day 0, before SCI, and post-SCI day 60 and 66, there were no difference in mechanical hyperreflexia in the SCI only and the SCI treated with phenelzine groups. However, phenelzine significantly attenuated the mechanical hyperreflexia in the SCI starting post-SCI day 72 until day 82 (* P < 0.05 when compared to SCI only group, N = 5 for all groups, unpaired student t-test). Phenelzine was applied at a dosage of 15 mg/kg starting at post-SCI day 67 for period of two weeks. All data were expressed as mean ± SEM.
Phenelzine suppresses TRPA1 gene expression levels in various tissues after SCI
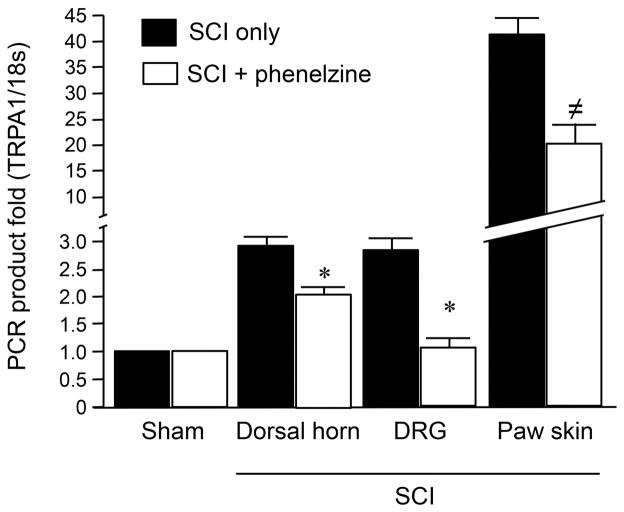
Next, we further tested the possibility that phenelzine treatments could lead to a reduction of TRPA1 gene expression levels after SCI. Specifically, the mRNA gene expression levels of TRPA1 were examined in the spinal dorsal horn, DRG cells, and paw skin one week after SCI with and without phenelzine treatment (Fig. 4). The data indicates that daily phenelzine application for a week immediately following SCI resulted in significant attenuation of TRPA1 mRNA levels elevation in tissues from all three examined sites. Specifically, the change of the value of TRPA1 mRNA in SCI to SCI-treated with phenelzine was: from 2.98 ± 0.15 to 2.02 ± 0.22 (dorsal horn), from 2.86 ± 0.22 to 1.14 ± 0.11 (DRG cells), and from 41.43 ± 2.98 to 20.24 ± 3.65 (paw skin), respectively (P < 0.05 for dorsal horn and DRG, and P < 0.005 for Paw skin).
Figure 4.
Elevation of TRPA1 mRNA level 1 week after spinal cord injury and its suppression by phenelzine. Spinal dorsal horn (1 cm long including T10), dorsal root ganglia (DRG, L1–L6), and paw skin were assessed 7 days after SCI. Specifically, the TRPA1 mRNA levels were significantly increased in dorsal horn, DRG, and paw skin following SCI (P < 0.05 in dorsal horn and DRG, and P < 0.001 in paw skin group when compared to sham). However, this elevated TRPA1 mRNA was significantly attenuated in all three tissue types with the continuous daily IP injection of phenelzine (15 mg/kg) for a week commencing immediately following trauma. (* P<0.05, ≠ P<0.005 when compared to SCI only, One-way ANOVA and Tukey test). N=5–6 in each group. All values were expressed as mean ±SEM.
Phenelzine suppressed acrolein-lysine adduct after SCI
Phenelzine was also examined for its ability to suppress acrolein, which is known to be elevated post-SCI, and compared this effect with hydralazine. Acrolein-lysine adducts were measured in spinal cord tissue one day post-SCI in three groups: SCI only, SCI + phenelzine (15 mg/kg), and SCI + hydralazine (5 mg/kg). As Figure 5 indicates, the acrolein-lysine adduct level in both phenelzine and hydralazine treated groups was significantly lower than that in SCI only group (p<0.001). In addition, the acrolein-lysine level in the phenelzine treated group was statistically lower than that in the hydralazine injection group (p<0.05).
Figure 5.
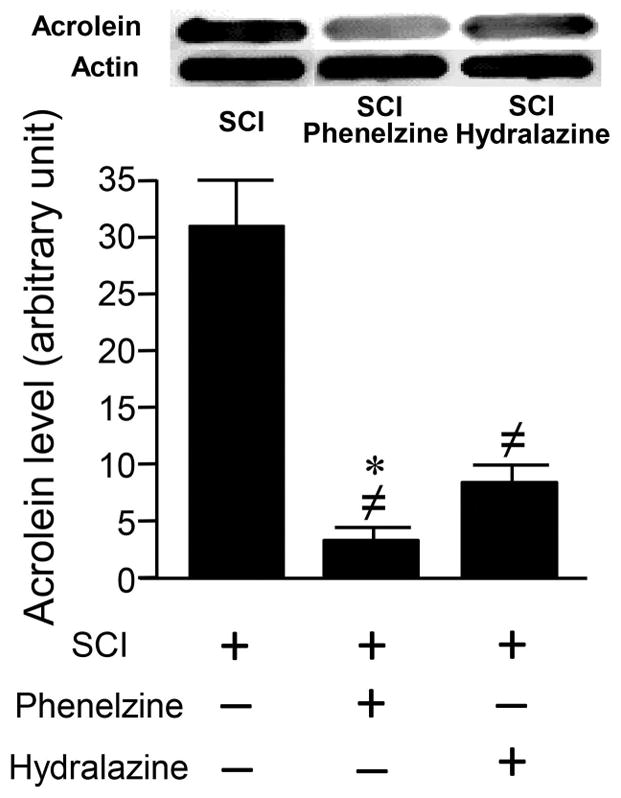
Phenelzine and hydralazine effectively suppressed acrolein-lysine adducts in rat spinal cord injury (SCI). Phenelzine (15 mg/kg) or hydralazine (5 mg/kg) were administered immediately after SCI and again 1 day post-injury. Approximately 2 hrs following the second application, spinal cord tissue was harvested for acrolein determination using dot immunoblotting. Both phenelzine and hydralazine significantly reduced the acrolein-lysine adduct level. Specifically, acrolein-lysine level in SCI only was 31.1 ± 4.2 (au). However, the acrolein-lysine level in SCI treated with phenelzine was 3.4 ± 2.0 (au), and SCI treated with hydralazine was 8.7 ± 1.47 (au). The level of acrolein-lysine in both treated groups are significantly lower than SCI only group. A single blot typical of 6 replicates is depicted. (≠ P < 0.001 when compared to SCI only. * P < 0.05 when compared to hydralazine treated group. One-way ANOVA and Tukey test). N=6 in each group. All values were expressed as mean ±SEM.
Dose-dependent suppression of acrolein metabolite by phenelzine and hydralazine after SCI
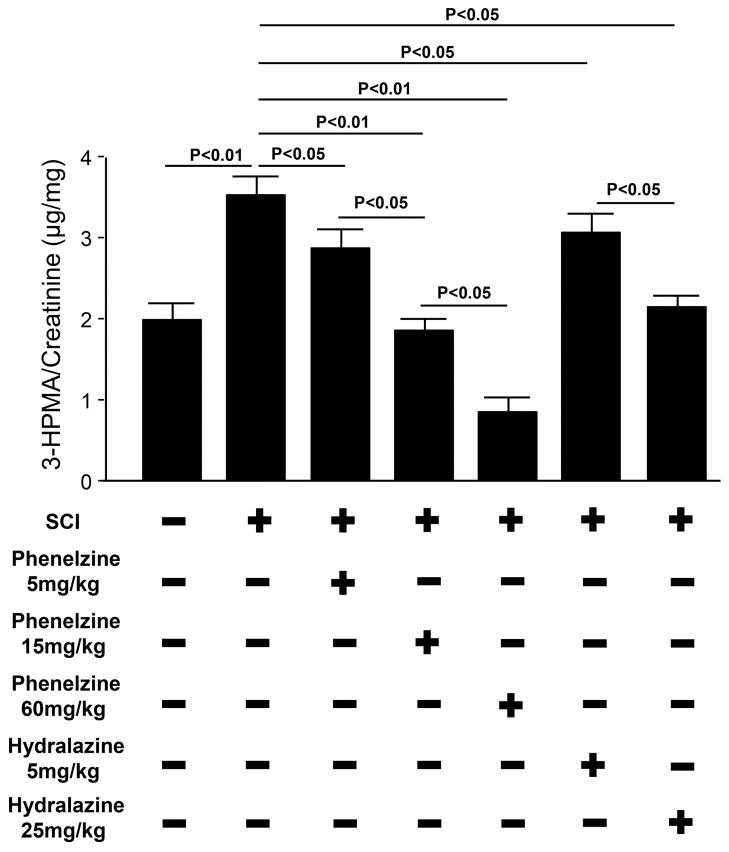
In order to examine if phenelzine application could result in systemic acrolein reduction and its comparison with hydralazine, we measured 3-HPMA, a stable acrolein-glutathione metabolite in urine in SCI rats treated with three safe dosages of phenelzine and two dosages of hydralazine. As indicated in Figure 6, three dosages of phenelzine (5, 15, and 60 mg/kg) produced significant and dose-dependent suppression of 3-HPMA in urine (P < 0.05). In fact, phenelzine at 15 mg/kg could suppress 3-HPMA to a level that was similar to control (uninjured) (P > 0.05), while phenelzine at 60 mg/kg was able to further decrease the 3-HPMA to a level that was lower than control (P < 0.05). Similarly, hydralazine at 5 and 25 mg/kg, also produced significant and dose-dependent suppression of 3-HPMA (P < 0.05, when compared to SCI only). The effectiveness of acrolein-suppression by hydralazine (5 and 25 mg/kg) was comparable to phenelzine at 5 and 15 mg/kg (P > 0.05).
Figure 6.
Post-SCI elevation of urine 3-HPMA and its suppression by phenelzine and hydralazine. 3-HPMA, an acrolein metabolite, was significantly elevated in urine one day after SCI in rats (3.6 ± 0.31 μg/mg), when compared to sham injured group (2.0 ± 0.25 μg/mg). Both hydralazine and phenelzine treatments resulted in dose dependent reduction in 3-HPMA levels after SCI. Rat urine samples were collected one day after SCI to determine 3-HPMA level. On average, the volume of urine could be collected in a period of 3–4 hours is 1–2 mL. N=5~6 in each group. One-way ANOVA and Tukey test were used for statistical analysis. Significance are indicated in the graph. All values were expressed as mean ± SEM.
Pharmacokinetics of phenelzine and hydralazine in plasma following IP injection
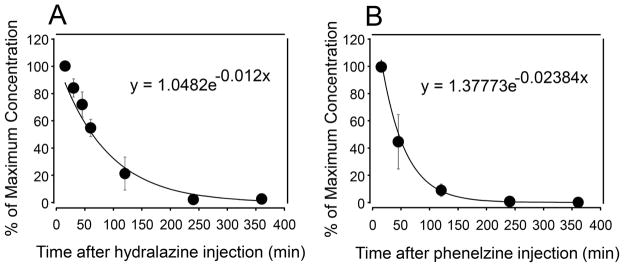
The pharmacokinetics of phenelzine (15 mg/kg) and hydralazine (5 mg/kg) in plasma following IP injection was conducted in uninjured rats. For hydralazine, blood samples were collected at 15, 30, 45 min, 1, 2, 4, 6, 8, 24 hours post injection. In the case of phenelzine, blood samples were collected at 15, 45 min, 2, 4, 6, 8, 9, 16, 24 hours post injection. The samples were analyzed using mass spectroscopy to determine the level of either hydralazine or phenelzine in plasma. As shown in Figure 7, the plasma concentration of both hydralazine and phenelzine were near zero 4 hours post injection. Therefor only the data up to 6 hours post application is displayed. Plasma concentrations of both hydralazine and phenelzine followed an exponential decay consistent with first order kinetics. Using the decay constants displayed in Figure 7, the plasma half-life is estimated to be 59 min. for hydralazine and 29 min. for phenelzine.
Figure 7.
Monitoring of plasma concentration of hydralazine (A) and phenelzine (B) using mass spectrometry following intra-peritoneal injection for 360 min. N = 6 in each data point. All values were expressed as mean ± SD.
Phenelzine improves the motor function recovery in rat SCI
Motor function of SCI rats was evaluated using BBB locomotor score before, 1 day after, and then weekly after SCI for a total of 4 weeks, as depicted in Figure 8. All groups of rats, sham-injury, injury only, and injury and phenelzine, had normal BBB scores (21) before the injury. SCI resulted in the typical reduction of BBB scores which stabilized at around a value of 10. Daily administration of phenelzine for two weeks commenced immediately after SCI resulted in significant improvement of BBB scores at 7, 21, and 28 days post-SCI (P <0.05 for day 7 and P < 0.001 for day 21 and 28, respectively).
Figure 8.
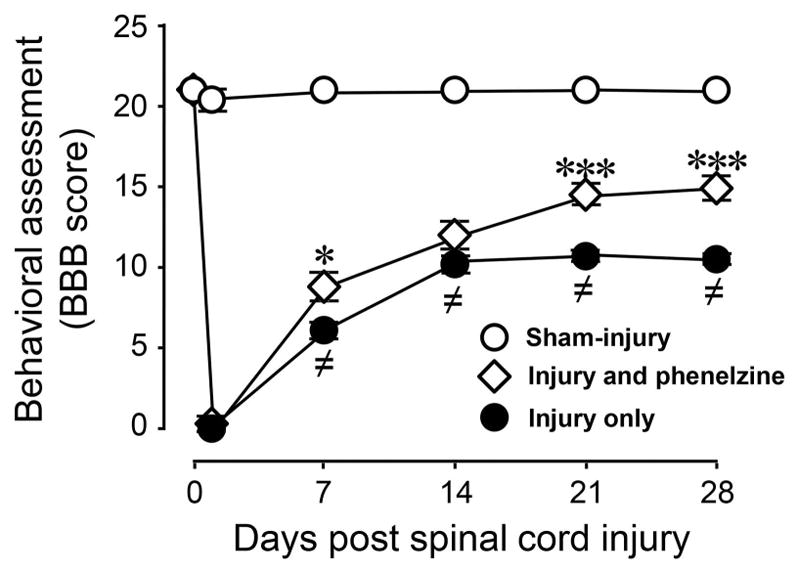
Acute systemic application of phenelzine improved locomotor function recovery after spinal cord injury. Locomotor function was assessed based on Basso, Beattie and Bresnahan (BBB) score in sham-injury, SCI only, and SCI treated with phenelzine. A significant reduction in BBB score was observed in the SCI only group compared to sham injury. Following spinal cord contusion in the SCI + phenelzine group, phenelzine (15 mg/kg) was applied daily through intraperitoneal (IP) injection for 2 weeks immediately following injury. Such treatment significantly improved the BBB score at one, three, and four weeks post-SCI, when compared to the SCI group. One-way ANOVA and Tukey test were used. (≠ P < 0.001 when compared to sham-injured; * P < 0.05 and *** P < 0.001 when compared to injury only. N = 5 in all conditions. All values were expressed as mean ± SEM.
Phenelzine significantly improved tissue preservation after SCI
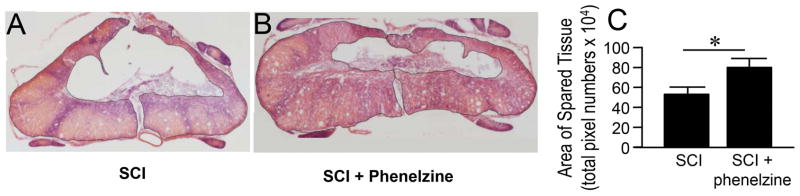
The extent of spinal cord tissue damage and its reduction with the acute application of phenelzine was evaluated 4 weeks post-SCI. Phenelzine was applied IP daily (15 mg/kg) for 2 weeks starting immediately following trauma. As shown in Fig. 9, it can be seen that SCI rats have significant tissue loss. Quantification of spared tissue area in transverse spinal cord sections showed significant tissue preservation in the phenelzine treated group (15 mg/kg) when compared to SCI only (80.96±8.10 vs 54.18±6.42, P < 0.05).
Figure 9.
Phenelzine treatment significantly increased the area of spared tissue after spinal cord injury. (A, B) Conventional hematoxylin and eosin stain of horizontal sections of rat spinal cord reveals the size of the spared tissue at the injury site in SCI only (A), and SCI + Phenelzine (B) groups 4 weeks following injury. Notice the larger area of spared tissue in SCI-phenelzine group compared to SCI only group. (C) Quantitative comparison reveals that the size of the spared area in the SCI + Phenelzine group (80.96±8.10) was significantly higher than that in the SCI only group (54.18±6.42, * p < 0.05, Student’s t-test, n=4~5 in each group).
Discussion
In the current study, we have shown that phenelzine, a hydrazine derivative, has the ability to mitigate post-SCI hyperreflexia (Fig. 1–3), which is also associated with significant improvement of motor behavior, preservation of spinal cord tissue, and suppression of TRPA1 mRNA (Fig. 4, 8, 9). Furthermore, the functional and structural benefits were accompanied by significant suppression of acrolein, known to be elevated post-SCI, both locally at the spinal cord tissue (measured as acrolein-adducts) as well as systemically in urine (measured as 3-HPMA) (Fig. 5, 6). Considering the demonstrated role of acrolein in post-SCI sensory and motor functional deficits and of phenelzine as a known acrolein scavenger (Park et al. 2014a, Park et al. 2014b, Hamann et al. 2008a, Hamann et al. 2008b, Hamann & Shi 2009, Shi et al. 2011a, Due et al. 2014, Singh et al. 2013, Wood et al. 2006, Galvani et al. 2008), we conclude that phenelzine is capable of effectively lowering acrolein in vivo, offering mitigation of sensory and motor deficits and overall improvement of functional recovery follow SCI. As a future study of current investigation, demonstration of the existence of scavenger-acrolein conjugates could confirm the trapping of acrolein by phenelzine.
Phenelzine possesses a hydrazine functional group which is capable of binding and trapping aldehydes such as acrolein (Galvani et al. 2008, Wood et al. 2006). Similar to phenelzine, hydralazine also can offer similar analgesic and neuroprotective effects after SCI (Park et al. 2014b, Hamann & Shi 2009, Park et al. 2015). Although structurally distinct, hydralazine and phenelzine share a common acrolein-scavenging group, hydrazine (Galvani et al. 2008). Since both hydralazine and phenelzine offer acrolein suppressing and related neuronal benefits in SCI, we suggest that acrolein scavenging is more likely to be the common feature that responsible for the observed neuroprotective effects than other effects unique to each drug, such as vasodilation with hydralazine and MAO inhibition with phenelzine a (Khan 1953, Pandit 1984, Cole & Weiner 1960). This is also in agreement with data from other studies with similar conclusions regarding neuroprotective effect of phenelzine. For example, deprenyl, a MAO inhibitor that lacks known aldehyde sequestration capability, was shown to be unable to offer neuro-protection to neuronal cells against aldehyde exposure in vitro (Wood et al. 2006). On the other hand, regarding an analgesic effect, there are recent reports that indicate that MAO inhibitors could provide relieve of pain-related behavior, independent of acrolein scavenging. For example, MAO inhibitors such as moclobemide (Villarinho et al. 2013) and selegiline (Villarinho et al. 2012), both of which lack a hydrazine component, but they are effective in reducing postoperative and sensory hypersensitivity in rodent models. Therefore, while the acrolein-scavenging effect of phenelzine likely plays an important role in reducing pain-related behavior during acute stage (within weeks) post-SCI when acrolein is known to increase significantly, the MAO inhibition effect of phenelzine may be important in reducing hyperalgesia chronically considering that acrolein elevation in chronic SCI is less established (Due et al. 2014). Taken together, these data further strengthen the notion that acrolein is an effective therapeutic target, particularly in acute stage, to mitigate neurological deficit following SCI. In addition, this study also validated the hypothesis that an acrolein-binding group, such as hydrazine, is likely a novel pharmacophore, which could guide the development of future anti-acrolein drugs for treating SCI with higher efficacy and greater safety.
The current in vivo study indicates that phenelzine has comparable efficacy to hydralazine in scavenging acrolein post-SCI (Fig. 5, 6). Despite their acrolein scavenging capability, some possible systemic effects of these two compounds needs to be taken into consideration with in vivo application. Hydralazine is a vasodilator, which could lead to hypotension that could be a concern following SCI (Khan 1953, Pandit 1984). Although no significant blood pressure changes were observed in rats at the dosages of 5 and 25 mg/kg, higher dosages will likely increase the possibility of hypotension (Zheng et al. 2013, Khan 1953). On the other hand, phenelzine is also known for causing severe hypertensive crises in some special circumstances (Yu 1994). Therefore, close monitoring of the blood pressure for SCI animals that receive these two scavengers is warranted due to possible variations in responding to hydralazine or phenelzine treatment.
Another factor to consider for in vivo phenelzine and hydralazine application is the half-life following systemic application. It is reported that the half-life of phenelzine is 11.6 hours (Nardil 2009) which is significantly different than hydralazine’s half-life of 30 minutes to 1 hour in human studies (Reece 1981, Ludden et al. 1980, Shepherd et al. 1980). In the current study (Fig. 7), the plasma half-life of hydralazine is shown to be about 60 minutes and that of phenelzine is around 30 minutes. This indicates that while our hydralazine data in rat is similar to that reported in human, the half-life of phenelzine in rat appears to be shorter than that in humans. However, some variation of phenelzine in human were noted as it has been reported that phenelzine had a half-life of 52–191 min in one study, and 90 to 240 min in another following systemic administration (Robinson et al. 1985, Caddy et al. 1978). The mechanism of these differences remain to be explored. It is known that oxidative stress and acrolein production do not occur transiently, but rather contribute to secondary injury processes in the hours, days, and weeks following the initial trauma (Hall 1989, Luo et al. 2005, Smith et al. 1999, Park et al. 2014b, Due et al. 2014). Therefore, at least in rat studies, or also in humans to a lesser degree, both hydralazine and phenelzine may need to be administered multiple times per day, or in a slow-release fashion, at least in acute stage, in order to achieve optimal effect of acrolein-scavenging and neuroprotective effects in SCI.
One interesting feature of phenelzine-induced analgesic effects post SCI is that it is effective when administered acutely, delayed for three weeks, or two months. It is known that hydralzine can offer similar analgesic effect when applied immediately and delayed for 2 weeks (Due et al. 2014). Therefore, this is the first report that acrolein scavengers are effective in reducing post-SCI sensory hyperreflexia in the chronic stage. Although it is possible that phenelzine has analgesic effect due to MAO inhibition (Villarinho et al. 2013, Villarinho et al. 2012), it also remains a strong possibility that acrolein continues to play a pro-algesic role considerably beyond the period that acrolein has been shown to be significantly elevated (2 weeks post-SCI) (Park et al. 2014b). It is likely that acrolein may still be elevated modestly at this chronic post-SCI stage and continue to activate TRPA1 channels. Another possibility is that elevated expression and sensitivity of TRPA1 following SCI (Due et al. 2014, Park et al. 2015) allows acrolein to contribute to sensory hypersensitivity even at normal concentrations (Fig. 4). The differential role of these possible mechanisms to the underlying post-SCI heightened sensitivity of the sensory system to acrolein remain to be determined.
It is interesting to note that the analgesic effect of phenelzine is long lasting following the termination of treatment in acute administration regimen (lasted at least two weeks), but not when applied with a delay or in chronic post-SCI stage (lasted only days) (Fig. 1, 2, 3). As mentioned above, it is possible that acrolein is an important factor not only for the initiation of the sensory hypersensitivity in the acute stage, likely through directly binding to and activating TRPA1 (Bautista et al. 2006), but also in transitioning the acute hyperreflexia to chronic hypersensitivity stages by acting on mechanisms associated with chronic pain-like behavior. For example, it is known that TRPA1 expression is elevated in SCI examined at 1–2 weeks post trauma and that acrolein has been shown to contribute to such TRPA1 upregulation (Due et al. 2014, Park et al. 2015). It has also been shown in mice that TRPA1 may be a key contributor to the transition from acute to chronic inflammatory pain (Garrison & Stucky 2014), and TRPA1 antagonists have been shown to prevent the transition of acute to chronic inflammation sensory hypersensitivity in mice (Schwartz et al. 2013). Furthermore, acrolein-suppression by hydralazine or phenelzine has also been shown to mitigate the upregulation of TRPA1 mRNA post-SCI (Fig. 4) (Park et al. 2015). It is therefore likely that acrolein acts to up-regulate TRPA1, heighten the sensitivity of the sensory system, and contribute to the transition of acute to chronic post-SCI neuropathic pain (Park et al. 2015). As such, early intervention of acrolein scavenging may not only reduce acute post-SCI hypersensitivity, but more importantly, also mitigate the acrolein-mediated TRPA1 over expression which could delay and even prevent the transition to chronic hyperreflexia.
Conclusion
As an effective acrolein scavenger in both in vitro and in vivo preparations shown in this and other studies (Singh et al. 2013, Wood et al. 2006), phenelzine appears to be a strong option for anti-acrolein treatment in spinal cord injury and possible other neuronal trauma and diseases where acrolein-mediated toxicity is implicated (Leung et al. 2011, Calingasan et al. 1999, Lovell et al. 2001, Pocernich et al. 2001). Furthermore, phenelzine is likely a viable alternative for hydralazine, a proven effective acrolein scavenger (Burcham & Pyke 2006, Burcham et al. 2000, Liu-Snyder et al. 2006, Khan 1953). The availability of multiple acrolein scavengers with demonstrated neuroprotection has greatly strengthened the notion that acrolein scavenging is not only effective, but a feasible strategy for SCI victims. It is predicted that such a study may not only further validate the pathological role of acrolein in neurodegeneration, but also inspire a new class of scavengers to offer more effective neuroprotection with greater safety and efficacy.
Acknowledgments
This work was supported by the Indiana State Department of Health (Grant # 204200 to RS), National Institutes of Health (Grant # NS073636 to RS), Indiana CTSI Collaboration in Biomedical Translational Research (CBR/CTR) Pilot Program Grant (Grant # RR025761 to RS), Project Development Teams pilot grant (Grant #TR000006 to RS), Science and Technology Commission of Shanghai Municipality, Shanghai, China (NO.13430722100 to PC), and grants from the Shanghai Bureau of Health, Shanghai, China (No. XBR2011024 to PC). Riyi Shi is the co-founder of Neuro Vigor, a star-up company with business interests of developing effective therapies for CNS neurodegenerative diseases and trauma.
Abbreviations
- SCI
Spinal cord injury
- TRPA1
transient receptor potential ankyrin 1
- LPO
lipid peroxidation
- DRG
dorsal root ganglia
- IP
Intraperitoneal
- 3-HPMA
3-hydroxypropyl mercapturic acid
- FDA
Food and drug administration
- NanoESI
Nanoelectrospray ionization
- BBB
Basso, Beattie and Bresnahan Locomotor Rating Scale
- O.C.T
optimum cutting temperature
- MS
Mass spectrometry
- LC
Liquid chromatography
- ANOVA
Analysis of variance
Footnotes
ARRIVE guidelines have been followed:
Yes
Conflicts of interest: Riyi Shi is the co-founder of Neuro Vigor, a star-up company with business interests of developing effective therapies for CNS neurodegenerative diseases and trauma.
References
- Baker GB, Coutts RT, McKenna KF, Sherry-McKenna RL. Insights into the mechanisms of action of the MAO inhibitors phenelzine and tranylcypromine: a review. J Psychiatry Neurosci. 1992;17:206–214. [PMC free article] [PubMed] [Google Scholar]
- Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7:e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- Caddy B, Stead AH, Johnstone EC. The urinary excretion of phenelzine. Br J Clin Pharmacol. 1978;6:185–188. doi: 10.1111/j.1365-2125.1978.tb00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. Journal of Neurochemistry. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cole RA, Weiner MF. Clinical and theoretical observations on phenelzine (nardil), an antidepressant agent. Am J Psychiatry. 1960;117:361–362. doi: 10.1176/ajp.117.4.361. [DOI] [PubMed] [Google Scholar]
- Due MR, Park J, Zheng L, Walls M, Allette YM, White FA, Shi R. Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J Neurochem. 2014;128:776–786. doi: 10.1111/jnc.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Amadei F, Geppetti P, et al. Alpha, beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37:617–623. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18:667–682. doi: 10.1023/a:1020685903186. [DOI] [PubMed] [Google Scholar]
- Galvani S, Coatrieux C, Elbaz M, et al. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic Biol Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Garrison SR, Stucky CL. Contribution of transient receptor potential ankyrin 1 to chronic pain in aged mice with complete Freund’s adjuvant-induced arthritis. Arthritis & rheumatology. 2014;66:2380–2390. doi: 10.1002/art.38724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED. Free radicals and CNS injury. Critical Care Clinics. 1989;5:793–805. [PubMed] [Google Scholar]
- Hamann K, Durkes A, Ouyang H, Uchida K, Pond A, Shi R. Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J Neurochem. 2008a;107:712–721. doi: 10.1111/j.1471-4159.2008.05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann K, Nehrt G, Ouyang H, Duerstock B, Shi R. Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J Neurochem. 2008b;104:708–718. doi: 10.1111/j.1471-4159.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- Hamann K, Shi R. Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J Neurochem. 2009;111:1348–1356. doi: 10.1111/j.1471-4159.2009.06395.x. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskas LM, Pyke SM, Burcham PC. Reactivity of hydrazinophthalazine drugs with the lipid peroxidation products acrolein and crotonaldehyde. Org Biomol Chem. 2004;2:2578–2584. doi: 10.1039/B408796H. [DOI] [PubMed] [Google Scholar]
- Khan MA. Effect of hydralazine in hypertension. Br Med J. 1953;1:27–29. doi: 10.1136/bmj.1.4800.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham PA, Spooner G, Ffoulkes-Jones C, Calvez R. Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free Radic Biol Med. 2003;35:697–710. doi: 10.1016/s0891-5849(03)00390-3. [DOI] [PubMed] [Google Scholar]
- Koivisto A, Chapman H, Jalava N, Korjamo T, Saarnilehto M, Lindstedt K, Pertovaara A. TRPA1: a transducer and amplifier of pain and inflammation. Basic & clinical pharmacology & toxicology. 2014;114:50–55. doi: 10.1111/bcpt.12138. [DOI] [PubMed] [Google Scholar]
- Leung G, Sun W, Zheng L, Brookes S, Tully M, Shi R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience. 2011;173:150–155. doi: 10.1016/j.neuroscience.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Snyder P, Borgens RB, Shi R. Hydralazine rescues PC12 cells from acrolein-mediated death. J Neurosci Res. 2006;84:219–227. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiology of Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Ludden TM, Shepherd AM, McNay JL, Lin MS. Hydralazine kinetics in hypertensive patients after intravenous administration. Clin Pharmacol Ther. 1980;28:736–742. doi: 10.1038/clpt.1980.229. [DOI] [PubMed] [Google Scholar]
- Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res. 2005;30:291–295. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- Musgrave T, Benson C, Wong G, Browne I, Tenorio G, Rauw G, Baker GB, Kerr BJ. The MAO inhibitor phenelzine improves functional outcomes in mice with experimental autoimmune encephalomyelitis (EAE) Brain Behav Immun. 2011;25:1677–1688. doi: 10.1016/j.bbi.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Nardil. New York, NY: Pfizer; 2009. [Google Scholar]
- O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- Pandit RB. Long term propranolol and hydralazine in hypertension. J Assoc Physicians India. 1984;32:199–202. [PubMed] [Google Scholar]
- Park J, Muratori B, Shi R. Acrolein as a novel therapeutic target for motor and sensory deficits in spinal cord injury. Neural regeneration research. 2014a;9:677–683. doi: 10.4103/1673-5374.131564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zheng L, Acosta G, Vega-Alvarez S, Chen Z, Muratori B, Cao P, Shi R. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J Neurochem. 2015;135:987–997. doi: 10.1111/jnc.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zheng L, Marquis A, et al. Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J Neurochem. 2014b;129:339–349. doi: 10.1111/jnc.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paslawski T, Treit D, Baker GB, George M, Coutts RT. The antidepressant drug phenelzine produces antianxiety effects in the plus-maze and increases in rat brain GABA. Psychopharmacology. 1996;127:19–24. doi: 10.1007/BF02805970. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochemistry International. 2001;39:141–149. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- Reece PA. Hydralazine and related compounds: chemistry, metabolism, and mode of action. Med Res Rev. 1981;1:73–96. doi: 10.1002/med.2610010105. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Cooper TB, Jindal SP, Corcella J, Lutz T. Metabolism and pharmacokinetics of phenelzine: lack of evidence for acetylation pathway in humans. Journal of clinical psychopharmacology. 1985;5:333–337. [PubMed] [Google Scholar]
- Santiago JM, Rosas O, Torrado AI, Gonzalez MM, Kalyan-Masih PO, Miranda JD. Molecular, anatomical, physiological, and behavioral studies of rats treated with buprenorphine after spinal cord injury. J Neurotrauma. 2009;26:1783–1793. doi: 10.1089/neu.2007.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33:5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Ludden TM, McNay JL, Lin MS. Hydralazine kinetics after single and repeated oral doses. Clin Pharmacol Ther. 1980;28:804–811. doi: 10.1038/clpt.1980.238. [DOI] [PubMed] [Google Scholar]
- Shi R, Luo J, Peasley MA. Acrolein inflicts axonal membrane disruption and conduction loss in isolated guinea pig spinal cord. Neuroscience. 2002;115:337–340. doi: 10.1016/s0306-4522(02)00457-8. [DOI] [PubMed] [Google Scholar]
- Shi R, Luo L. The role of acrolein in spinal cord injury. Applied Neurology. 2006;2:22–27. [Google Scholar]
- Shi R, Page JC, Tully M. Molecular mechanisms of acrolein-mediated myelin destruction in CNS trauma and disease. Free Radic Res. 2015;49:888–895. doi: 10.3109/10715762.2015.1021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Rickett T, Sun W. Acrolein-mediated injury in nervous system trauma and diseases. Mol Nutr Food Res. 2011a;55:1320–1331. doi: 10.1002/mnfr.201100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Sun W, McBride JJ, Cheng JX, Shi R. Acrolein induces myelin damage in mammalian spinal cord. J Neurochem. 2011b;117:554–564. doi: 10.1111/j.1471-4159.2011.07226.x. [DOI] [PubMed] [Google Scholar]
- Singh IN, Gilmer LK, Miller DM, Cebak JE, Wang JA, Hall ED. Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J Cereb Blood Flow Metab. 2013;33:593–599. doi: 10.1038/jcbfm.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarinho JG, Oliveira SM, Silva CR, Cabreira TN, Ferreira J. Involvement of monoamine oxidase B on models of postoperative and neuropathic pain in mice. Eur J Pharmacol. 2012;690:107–114. doi: 10.1016/j.ejphar.2012.06.042. [DOI] [PubMed] [Google Scholar]
- Villarinho JG, de Pinheiro VK, de Pinheiro VF, et al. The antinociceptive effect of reversible monoamine oxidase-A inhibitors in a mouse neuropathic pain model. Progress in neuro-psychopharmacology & biological psychiatry. 2013;44:136–142. doi: 10.1016/j.pnpbp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Wood PL, Khan MA, Moskal JR, Todd KG, Tanay VA, Baker G. Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res. 2006;1122:184–190. doi: 10.1016/j.brainres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Yu PH. Pharmacological and clinical implications of MAO-B inhibitors. Gen Pharmacol. 1994;25:1527–1539. doi: 10.1016/0306-3623(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Zheng L, Park J, Walls M, Tully M, Jannasch A, Cooper B, Shi R. Determination of Urine 3-HPMA, a Stable Acrolein Metabolite in a Rat Model of Spinal Cord Injury. J Neurotrauma. 2013;30:1334–1341. doi: 10.1089/neu.2013.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]