Abstract
Ovarian cancer is currently the most lethal gynecological malignancy with limited treatment options. Improved targeted therapies are needed to combat ovarian cancer. Here, we report the identification of cyclin-dependent kinase 11 (CDK11) as a mediator of tumor cell growth and proliferation in ovarian cancer cells. Although CDK11 has not been implicated previously in this disease, we have found that its expression is upregulated in human ovarian cancer tissues and associated with malignant progression. Metastatic and recurrent tumors have significantly higher CDK11 expression when compared with the matched, original primary tumors. RNAi-mediated CDK11silencing by synthetic siRNA or lentiviral shRNA decreased cell proliferation and induced apoptosis in ovarian cancer cells. Moreover, CDK11 knockdown enhances the cytotoxic effect of paclitaxel to inhibit cell growth in ovarian cancer cells. Systemic in vivo administration of CDK11 siRNA reduced the tumor growth in an ovarian cancer xenograft model. Our findings suggest that CDK11 may be a promising therapeutic target for the treatment of ovarian cancer patients.
Keywords: CDK11, Kinase, Ovarian cancer, Apoptosis
Introduction
Ovarian cancer is currently the most lethal gynecological malignancy and the leading cause of death from gynecological cancers in the United States, which is predominantly a disease of elderly, post-menopausal women over 50 years of age (1). There were more than 239,000 newly diagnosed cases of ovarian cancer worldwide in 2012, including about 20,800 cases in the United States and 65,600 in Europe (2, 3). The current standard treatment for ovarian cancer patients consists of radical surgery with paclitaxel and carboplatin-based chemotherapy (4). Although the surgical and chemotherapeutic strategies lead to small improvements in clinical outcome, most ovarian cancer survivors are eventually diagnosed with recurrent disease that will develop resistance to several types of chemotherapeutic reagents (5, 6). Ultimately, the progression of residual disease leads to significant morbidity and finally mortality with current 5-year overall survival rates being stagnant at approximately 45% (6). Therefore, there is an urgent need to develop novel targeted therapies to improve the treatment of ovarian cancer.
Cyclin-dependent kinases (CDKs) are members of the serine/threonine protein kinase family and play crucial roles in tumor cell proliferation and growth by controlling cell cycle, transcription, and RNA splicing (7). Recently studies have shown that overexpression and activation of CDKs are common features for most human cancers, and targeting CDKs in tumor cells has become a promising therapeutic strategy (7–10). More recently, the Food and Drug Administration (FDA) has approved the CDK4/6 inhibitor palbociclib for treating metastatic breast cancer (11–13). Palbociclib has also shown promising preclinical activity in glioblastoma, renal, and ovarian cancer models that may provide direction for their future clinical development. Other CDK inhibitors, including abemaciclib and ribociclib, have demonstrated very promising clinical activity in breast cancer, melanoma, liposarcoma, and mantel cell lymphoma (8, 14). Among other members of the CDK family, more recent studies have shown that CDK11 (formerly known as PITSLREA or CDC2L1) also plays critical roles in cancer cell growth and proliferation (15). CDK11 is usually overexpressed and/or overactivated in human malignancies, and this overexpression is highly associated with poor outcomes in cancer patients (13, 16, 17). Inhibition of CDK11 has been shown to lead to cancer cell death and apoptosis in difference tumor cells (18–21). Significant evidence suggests that CDK11 may be a novel and promising therapeutic target for the treatment of cancers.
High expression levels and activities of different CDKs, as well as aberrant expression of the CDK inhibitors (such as p16, p21, and p27) have been frequently characterized among women with ovarian cancer (8, 22–25). Although previous studies have found that knockdown of CDK11 decreased cell viability and increased apoptosis in many type of cancers, the significance of CDK11 expression in ovarian cancer is unknown. Therefore, in this study, we first comparatively examined the expression of CDK11 in an ovarian cancer tissue microarray (TMA) by immunohistochemical analysis. Subsequently, we investigated the functional role of CDK11 in the growth and proliferation of ovarian cancer in in vitro cultures and in mouse xenografts model of ovarian cancer in vivo. Our study indicates that CDK11 represents a novel promising drug target for further study in ovarian cancer.
Materials and Methods
Ovarian cancer tissue microarray (TMA) and immunohistochemistry
The TMA utilized in our current study was generated from samples of ovarian cancer patients with long-term follow-up as previously reported (26, 27). The unique features of this ovarian cancer TMA are that tissues are collected from matched primary, metastatic, and recurrent tumor tissues each from 26 individual ovarian cancer patients at the time of surgery. In total, the TMA slide contained 78 epithelial ovarian cancer tissue samples. For immunohistochemistry of CDK11, the slide was baked at 62°C for 1 hour, deparaffinized in xylene for 15 minutes, washed with 100% ethanol for 12 minutes, and then rehydrated with 95%, 70% and 50% ethanol respectively. Antigen retrieval was processed with Target Retrieval Solution from Vector Laboratories (Burlingame, CA) following the manufacturer’s instructions. After antigen retrieval, the slide was washed with PBS three times for 5 minutes and covered with peroxidase blocking reagent for 5 minutes at room temperature. The slide was then incubated with serum blocking reagent, avidin blocking reagent, and then biotin blocking reagent for 15 minutes. Primary CDK11 antibody from Santa Cruz Biotechnology, Inc. (Dallas, Texas, 1:50 dilution) was applied at 4°C overnight in a humidified chamber. The next day, the biotinylated secondary antibody (1:200 dilution) diluted with 1% bovine serum albumin was added to the slide and incubated for one hour. The slide was then incubated with HSS-HRP for 30 minutes. Bound antibody was detected and visualized with the Vectastain ABC kit and 3,3′-diaminobenzidine high-sensitivity substrate from Vector Laboratories. Finally, the slide was counterstained with hematoxylin QS (Vector Laboratories) and mounted with VectaMount AQ (Vector Laboratories).
Evaluation of immunohistochemical staining
Immunostained tissue microarray slides were evaluated under the microscope. The percentage of positive nuclear staining for CDK11 was calculated by reviewing the entire slide. The staining patterns were graded into six groups: 0, no nuclear staining; 1+, <10% of cells stained positive; 2+, 10% to 25% positive cells; 3+, 26% to 50% positive cells; 4+, 51% to 75% positive cells; and 5+, >75% positive cells. Light microscopic images were captured using a Nikon Eclipse Ti-U fluorescence microscope (Nikon Corp.) with an attached SPOT RT digital camera from Diagnostic Instruments, Inc. (Sterling Heights, MI).
Cell lines and cell culture
The human ovarian cancer cell line SKOV-3 was purchased from the American Type Culture Collection (Rockville, MD) in 2014 with certificate of analysis. . Dr. Patricia Donahoe (Massachusetts General Hospital, Boston, MA) kindly provided the human ovarian cancer cell line OVCAR-8 (28). Authentication of the OVCAR-8 cell line was not done. All cell lines were cultured in RPMI 1640 from Invitrogen (Carlsbad, CA) supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Paclitaxel was obtained as unused residual clinical material at the Massachusetts General Hospital. Cells were maintained in a humidified incubator in an atmosphere of 5% CO2-95% air at 37°C. Light microscopic images were documented using Zeiss microscope from Carl Zeiss, Inc. (Oberkochen, Germany) with an attached Nikon D40 digital camera from Nikon Corp. (New York, NY).
Synthetic CDK11 siRNA and transfection
CDK11 knockdown in ovarian cells was performed by transfection of synthetic human CDK11 siRNA (ID: s2734), which was purchased from Ambion at Applied Biosystems (Foster City, CA). The siRNA sequence targeting CDK11 corresponded to coding regions (5′-AGAUCUACAUCGUGAUGAAtt-3′, antisense 5′-UUCAUCACGAUGUAGAUCUtg-3′) of the CDK11 gene. Non-specific siRNA oligonucleotides from Applied Biosystems (Foster City, CA) were used as negative control. The siRNA oligonucleotides were dissolved in nuclease-free water at a concentration of 100 μmol/L and kept at −20°C until the following transfection experiment. Ovarian cancer SKOV-3 or OVCAR-8 cells were either plated on 96-well plates for cell proliferation assays or plated on dishes for Western blot protein isolation. Transfections were performed with Lipofectamine™ RNAiMAX (Invitrogen) according to the manufacturer’s instructions. Media were replaced with RPMI 1640 supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin 24 hours after transfection. Effects of CDK11 siRNA on cellular growth and proliferation were assessed in vitro using the Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, MO) as previously described (29, 30). Experiments were performed in triplicate. All data were processed with the use of GraphPad Prism 4 software from GraphPad Software, Inc. (San Diego, CA). Total protein was isolated with RIPA Lysis Buffer (Upstate Biotechnology) 48 hours after siRNA transfection.
Immunofluorescence
For the immunofluorescence assay, 4×103 SKOV-3 or OVCAR-8 cells were seeded into each well of 8-well glass chamber slides. After 24 hours of culturing, cells were transfected with CDK11 siRNA or non-specific siRNA and continued to incubate for 72 hours. SKOV-3 or OVCAR-8 cells were then briefly rinsed with PBS 3 times and then fixed in 2% paraformaldehyde for 15 minutes at room temperature, followed by ice-cold absolute methanol permeabilization for 10 minutes at −20°C. Permeabilized cells were blocked with blocking buffer (5% goat serum, 0.3% Triton X-100 in PBS) for 1 hour at room temperature. Cells were then incubated with CDK11 and β-actin primary antibodies at 1:200 and 1:1000 dilution in antibody dilution buffer (1% BSA, 0.3% Triton X-100 in PBS) overnight at 4°C. The cells were washed with PBS 3 times at room temperature and incubated with Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 594 goat anti-mouse IgG at 4°C overnight. Cells were then visualized on a Nikon Eclipse Ti-U fluorescence microscope equipped with a SPOT RT digital camera from Diagnostic Instruments, Inc. (Sterling Heights, MI).
Lentiviral CDK11 shRNA transduction
Knockdown of CDK11 induced phenotype changes in ovarian cancer cells, which were further confirmed by transduction of lentiviral CDK11 shRNA. In brief, on the first day, ovarian cancer cell line SKOV-3 or OVCAR-8 cells were diluted to 2×104 cells/ml with complete culture media and added to each well of 96-well plates. On the second day, Hexadimethrine bromide was added to a final concentration of 8 μg/ml and gently mixed well. Lentiviral particles encoding shRNA against CDK11 were added to appropriate wells and incubated for another 24 hours. On the third day, the media was replaced with fresh puromycin (1 μg/ml) containing media to each well for selection of transduced cells. From the third to the sixth day, fresh culture media were replaced as necessary, and cell proliferation in each well was evaluated under the light microscope. On the sixth day, the number of viable cells was determined by using the CellTiter 96® AQueous One Solution Cell Cytotoxicity Assay (Promega, Madison, WI).
Cytotoxicity assay
The in vitro cytotoxicity assays were performed by MTT assay as previously described (29, 30). In brief, 2×103 cells per well were plated in 96-well plates and treated with only CDK11 siRNA or combined with paclitaxel. After the cells were cultured in paclitaxel with or without CDK11 siRNA for 5 days, 10 μL of MTT (5 mg/ml in PBS) was added to each well and the plates were incubated for another 3 hours. The formazan products of MTT were dissolved with acid isopropanol and the absorbance was read at a wavelength of 490nm on a SPECTRAmax Microplate Spectrophtometer from Molecular Devices (Sunnyvale, CA). The relative absorbance values were obtained by assigning the absorbance value of cells without administration of any reagent to 1. Experiments were performed in duplicate. All MTT data were processed with the use of GraphPad Prism 4 software from GraphPad Software, Inc. (San Diego, CA).
Western blotting
Protein lysates were harvested from ovarian cancer cells with 1× RIPA Lysis Buffer from Millipore Corporation (Billerica, MA) and the protein concentrations were determined by Protein Assay Reagents (Bio-Rad) and spectrophotometer quantification from Beckman DU-640, Beckman Instruments, Inc. (Columbia, MD). Twenty-five micrograms of total protein was added on Nu-Page 4–12% Bis-Tris Gel (Invitrogen) and transferred to a pure nitrocellulose membrane from Whatman International Limited (Banbury OX, UK). The rabbit polyclonal antibodies to human Poly (ADP-ribose) polymerase (PARP) were purchased from Cell Signaling Technologies (Cambridge, MA). Antibody against β-actin was bought from Sigma-Aldrich. Primary antibodies were diluted to 1:1000 and incubated in Tris-buffered saline (pH 7.4) with 0.1% Tween 20 overnight at 4°C. Signal was generated through incubation with HRP-conjugated secondary antibodies from Bio-Rad (Hercules, CA) in Tris-buffered saline (pH 7.4) with 5% nonfat milk and 0.1% Tween 20 at 1:2000 dilution for 1 h at room temperature. Positive immunoreactions were detected by SuperSignal West Pico Chemiluminescent Substrate from Pierce (Rockford, IL).
Apoptosis assay
Whole-cell lysates were immunoblotted with specific antibodies to PARP (Cell Signaling Technologies) and its cleavage products. Positive immunoreactions were detected by using Super Signal West Pico Chemiluminescent Substrate. Quantification of apoptosis was also evaluated using the Apo-ONE Homogeneous Caspase-3/7 Assay kit from Promega (Madison, WI). Briefly, 4×103 SKOV-3 or OVCAR-8 cells were seeded per well in a white 96-well plate and transfected with CDK11 siRNA. Seventy-two hours after transfection, the fluorescence of each well was measured at an emission wavelength of 521 nm and an excitation wavelength of 499 nm according to the manufacturer’s instructions on a SPECTRAmax GeminiXS Microplate Spectrophtometer from Molecular Devices (Sunnyvale, CA).
Animal Studies
The protocol for animal use in this project has been approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (SRAC) under the protocol number 2009N000229. In Vivo Ready CDK11 siRNA and non-specific siRNA were purchased from Applied Biosystems. These In Vivo Ready validated CDK11 siRNA were designed using specific algorithms and incorporated with additional chemical modifications for superior serum stability with in vivo applications (17, 19, 31, 32). The Crl:SHO-PrkdcSCIDHrhr female nude mice at 3 to 4 weeks of age were purchased from Charles River Laboratories. To determine the effect of CDK11 siRNA on ovarian cancer growth in a xenograft model, 1×106 SKOV-3 cells were inoculated subcutaneously with matrigel from BD Biosciences (San Jose, CA) into the left and right flank of the mice. Ten days after injection, the mice were randomized into three groups (8 mice/group). Group 1 received injection with sterile saline (0.9% NaCl), group 2 with In Vivo Ready non-specific siRNA, and group 3 with In Vivo Ready CDK11 siRNA. For intratumoral injections, each animal was injected with 30 μL of PBS containing 10 nmol/L of siRNA. All 3 groups were treated twice a week for two times. The health of the mice and evidence of tumor growth were examined daily. Tumor volumes were measured at a regular interval of for up to four weeks with digital calipers. Tumor volume (mm3) was calculated as (W2 × L)/2 (W as width and L as length). Data are presented as mean ± SD. Tumor tissues from the above treated animals were collected and placed in 10% formalin and embedded in paraffin for histology analysis. The silence efficiency of CDK11 In Vivo Ready siRNA on CDK 11 proteins were determined by immunohistochemical staining as described above.
Statistical analysis
GraphPad PRISM® 4 software from GraphPad Software (San Diego, CA) was used to statistically analyze the data. Values shown are representative of triplicate determinations in two or more experiments. Treatment effects were evaluated by a two-sided Student’s t-test. Unpaired t-test was used to compare the CDK11 intensity scores among primary, metastatic and recurrent tumors. Errors are SD of averaged results, and P < 0.05 values were considered as a significant difference between means.
Results
Increased expression of CDK11 in metastatic and recurrent ovarian cancer patient tissues
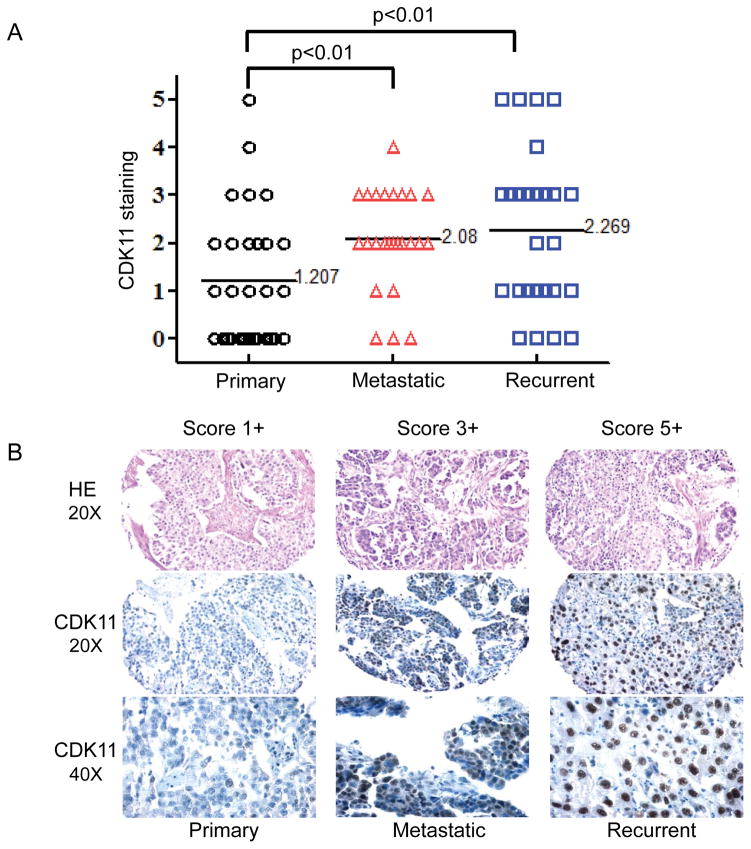
Overexpression of CDK11 has been found in different human cancers. Patients with high CDK11 levels have poor prognosis when compared with patients with low CDK11 levels. In this study, we examined CDK11 expression in primary, metastatic, and recurrent ovarian cancer tissues. This analysis was done in a unique tumor tissue microarray that included the primary tumor, a synchronous metastasis, and a metachronous metastasis from the same patient with the metachronous metastasis being collected at the time of tumor recurrence after treatment with platinum- and taxane-based chemotherapy. The relative levels of CDK11 nuclear staining in tumor samples were scored according to the criteria described in Materials and Methods. Of the 26 ovarian cancer patients with 78 tissue samples, 34 (42.5%) were classified as score 0 to 1+, 38 (47.5%) as score 2+ to 3+, and 8 (10%) as score 4+ to 5+. The results showed that protein expression level of CDK11 was significantly higher in metastatic samples (average 2.08, P < 0.01) and in recurrent samples (average 2.269, P < 0.01) than in primary samples (average 1.207) (Figure 1). There were trends toward greater IL-6 expression in the recurrent tumors as compared with the matched primary tumors (Figure 1A). The results also showed that the CDK11 protein was mainly localized in the nucleus of ovarian cancer cells (Figure 1B), which is consistent with prior publications in other types of human cancer cells (19, 23, 33).
Figure 1. Comparison of CDK11 expression in primary, metastatic, and recurrent ovarian cancer.
A: Distribution of CDK11 immunohistochemical staining scores in primary, metastatic, and recurrent ovarian cancer. Statistical analysis was performed using a Student’s t-test (between two groups) to compare the CDK11 staining intensity scores among primary tumors, recurrent tumors, and tumors with metastasis, and P < 0.01 was accepted as a significant difference between means. B: Representative images of HE and CDK11 immunohistochemical staining in matched primary, metastatic, and recurrent ovarian cancers.
CDK11 knockdown decreases CDK11 expression at the protein level in ovarian cancer cells
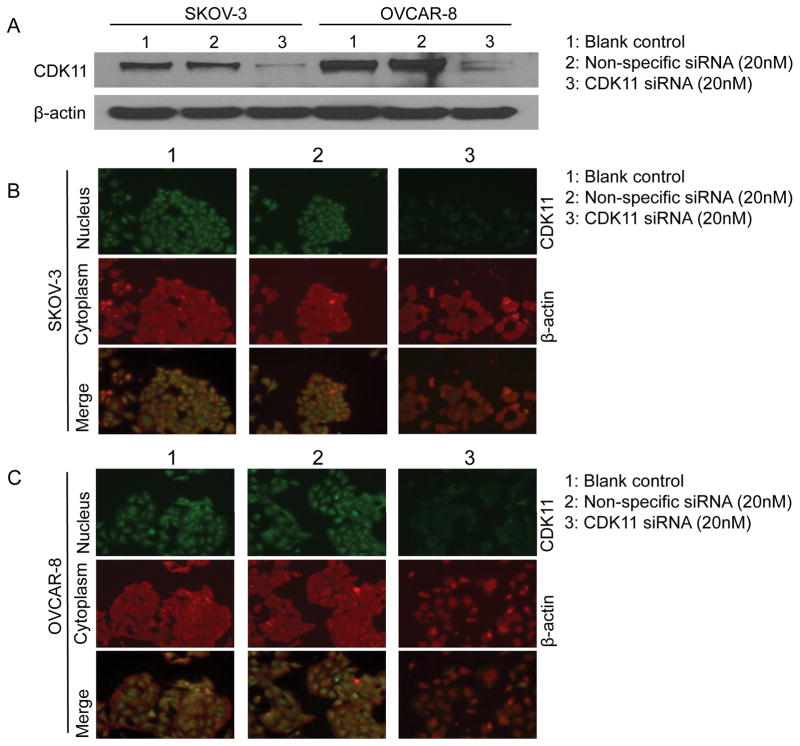
The transfection efficacy of CDK11 siRNA in ovarian cancer cell lines SKOV-3 and OVCAR-8 were accessed by Western blotting and immunofluorescence analysis. Western blotting showed that the CDK11 expression at the protein level was significantly reduced after SKOV-3 or OVCAR-8 cells were transfected with CDK11 siRNA (Figure 2A). To further confirm the expression of CDK11 and determine CDK11 protein subcellular localization in ovarian cancer cell lines, immunofluorescence assay was used in SKOV-3 and OVCAR-8 cell lines. The results further confirmed that the CDK11 expression levels were much lower in SKOV-3 cells (Figure 2B) and in OVCAR-8 cells (Figure 2C) transfected with CDK11 siRNA than in cells of the control group or cells transfected with non-specific siRNA (Figure 2B and Figure 2C). Immunofluorescence assay also showed that the CDK11 protein is mainly localized in the nucleus of ovarian cancer cells.
Figure 2. CDK11 siRNA decreases CDK11 expression in ovarian cancer cells.
A: Evaluation of CDK11 protein knockdown by siRNA with Western blotting analysis. For Western blot analysis, 25 mg of total cellular proteins SKOV-3 or OVCAR-8 cells were subjected to immunoblotting with specific antibody to CDK11, and β-actin. B: Confirmation of CDK11 protein knockdown by siRNA with immunofluorescence analysis. Expression of CDK11 in SKOV-3 or OVCAR-8 cells was assessed by immunofluorescence with antibodies to CDK11 and β-actin. Cells were visualized under a fluorescence microscope after incubation with secondary fluorescent conjugated antibodies Alexa Fluor 488 goat anti-rabbit IgG (green) and Alexa Fluor 594 goat anti-mouse IgG (red) as described in the Materials and Methods.
CDK11 knockdown decreases cell proliferation, viability in ovarian cancer cells
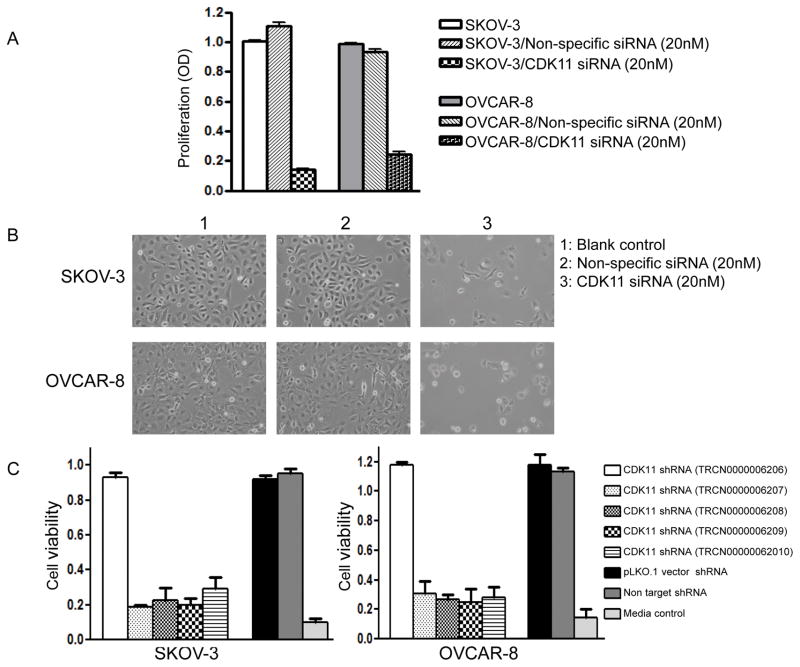
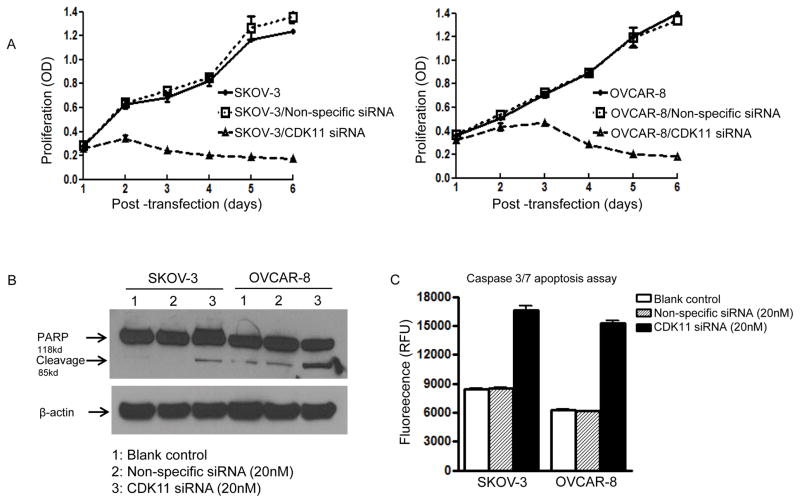
Expression of CDK11 has been shown to be critical for the growth and proliferation of different cancer cells in previous studies. However, information regarding the relationship between expression of CDK11 and ovarian cancer cell growth and survival is lacking. We next determined the effects of CDK11 on cell proliferation and growth in ovarian cancer SKOV-3 and OVCAR-8 cell lines. We observed that when siRNA targeting CDK11 was transfected into ovarian cancer cell lines SKOV-3 or OVCAR-8, it led to significantly reduced cell growth and eventual cell death as shown by the cellular proliferation assay (Figure 3A and Figure 3B). To further characterize the functional role of CDK11 in ovarian cancer cells, we thoroughly validated the CDK11 siRNA results using multiple independent experiments, including multiple shRNA per CDK11 gene and extensively tested with control non-specific shRNA (the sequences of five different shRNA target sites of CDK11 have been reported previously). The results revealed that four out of five CDK11 shRNAs inhibited ovarian cancer cell growth (Figure 3C). Furthermore, there was a time-dependent decreased in cell proliferation after CDK11 knockdown by siRNA in SKOV-3 and OVCAR-8 ovarian cancer cell lines (Figure 4A). These phenotypes were not observed with the non-specific siRNA transfection (Figure 4A).
Figure 3. Effects of CDK11 inhibition by siRNA and shRNA in ovarian cancer cell lines.
A: Effects of siRNA targeting CDK11 in SKOV-3 and OVCAR-8 cell lines. Proliferation was assessed by MTT as described in the Materials and Methods. The data represent the mean ± SD of two experiments carried out in triplicate. B: Phase-contrast photomicrographs of cells were taken after transfection of non-specific siRNA and CDK11 siRNA. CDK11 knockdown induced significantly cell death in ovarian cancer cell lines, but not in non-specific siRNA transfected cells. C: Effects of lentiviral shRNA targeting CDK11 in SKOV-3 and OVCAR-8 cell lines. Proliferation was determined by the CellTiter 96 Aqueous One Solution Cell Assay. The data represent experiments of the 96-well plate of MISSION LentiExpress Human kinases lentiviral shRNA as described. The data represent the mean ± SD of two experiments carried out in triplicate.
Figure 4. Transfection of CDK11 siRNA into ovarian cancer cells induces apoptosis.
A: Transfection of CDK11 siRNA into ovarian cancer cells decreased cell proliferation and inhibited cell growth in a time-dependent manner. SKOV-3 and OVCAR-8 cells were transfected with CDK11 siRNA (20 nM). Proliferation of cancer cells were assessed at different time points post transfection by MTT as described in the Materials and Methods. B: Knockdown CDK11 induced apoptosis in ovarian cancer cells. SKOV-3 and OVCAR-8 cells were transfected with CDK11 siRNA (20 nM). Total cellular proteins were extracted 48 hours post transfection and were subjected to immunoblotting with specific antibodies to PARP and β-actin as described in Materials and Methods. Knockdown of CDK11 led to increased expression of cleaved PARP. C: Apoptosis was also evaluated by the Apo-ONE Homogeneous Caspase-3/7 Assay kit as described in Materials and Methods.
CDK11 knockdown induces apoptosis in ovarian cancer cells
To investigate how CDK11 sustains tumor cell growth and survival, we investigated cellular potential apoptosis events during cell death caused by CDK11 knockdown in ovarian cancer cell lines SKOV-3 or OVCAR-8. We investigated the potential for the induction of apoptosis after CDK11 knockdown by Western blotting for the cleavage of PARP, and by using the Apo-ONE Homogeneous Caspase-3/7 Assay. Significant PARP cleavage was detected 48 hours following transfection with CDK11 siRNA, but not with non-specific siRNA in both SKOV-3 and OVCAR-8 cells (Figure 4B). Consistent with these results, there were greater levels of apoptosis in both ovarian cancer cell lines SKOV-3 and OVCAR-8 after transfection with CDK11 siRNA as determined by Caspase-3/7 Assay (Figure 4C).
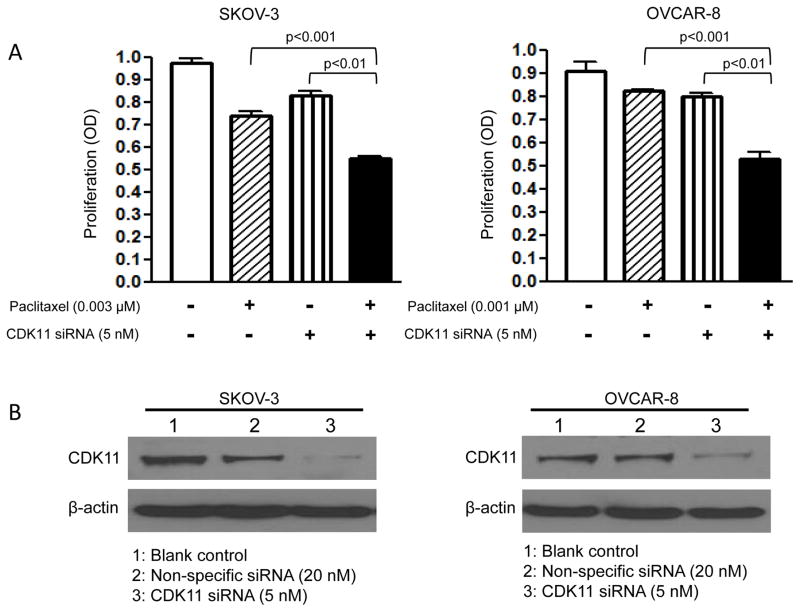
CDK11 knockdown enhances the cytotoxic effect of chemotherapeutic agent paclitaxel in ovarian cancer cell lines
High level expression of CDK11 may contribute to the survival advantage of ovarian cancer cells. We hypothesized that inhibition of the CDK11 pathway in ovarian cancer cells may lower the apoptotic threshold and increase chemotherapy sensitivity. To determine whether CDK11 downregulation influenced the effect of chemotherapeutic agents on ovarian cancer cells, SKOV-3 or OVCAR-8 cells were transfected with a 5 nM non-lethal dose of CDK11 siRNA and then incubated with paclitaxel, a commonly used first-line chemotherapy drug in the treatment of ovarian cancer. The combination of low concentrations of both CDK11 siRNA and paclitaxel significantly inhibited cell growth and survival as compared with those from each treatment alone in SKOV-3 and OVCAR-8 cell lines (Figure 5A). Western blotting confirmed decreased expression of CDK11 in ovarian cancer cells at these concentrations of CDK11 siRNA (Figure 5B). Thus, our results show that CDK11 knockdown enhanced the cytotoxic effect of paclitaxel to inhibit cell growth in ovarian cancer cell lines.
Figure 5. CDK11 knockdown enhances the cytotoxic effect of paclitaxel in SKOV-3 and OVCAR-8 cell lines.
A: The 5 nM non-lethal dose of CDK11 siRNA was chosen for SKOV-3 and OVCAR-8, and 3 nM and 1 nM doses of paclitaxel for SKOV-3 and OVCAR-8, respectively. Proliferation was assessed by MTT as described in the Materials and Methods. The data represent the mean ± SD of two experiments carried out in triplicate. B: The CDK11 expression SKOV-3 and OVCAR-8 was analyzed by Western blotting with specific antibody to CDK11 and β-actin after transfection with CDK11 siRNA or non-specific siRNA.
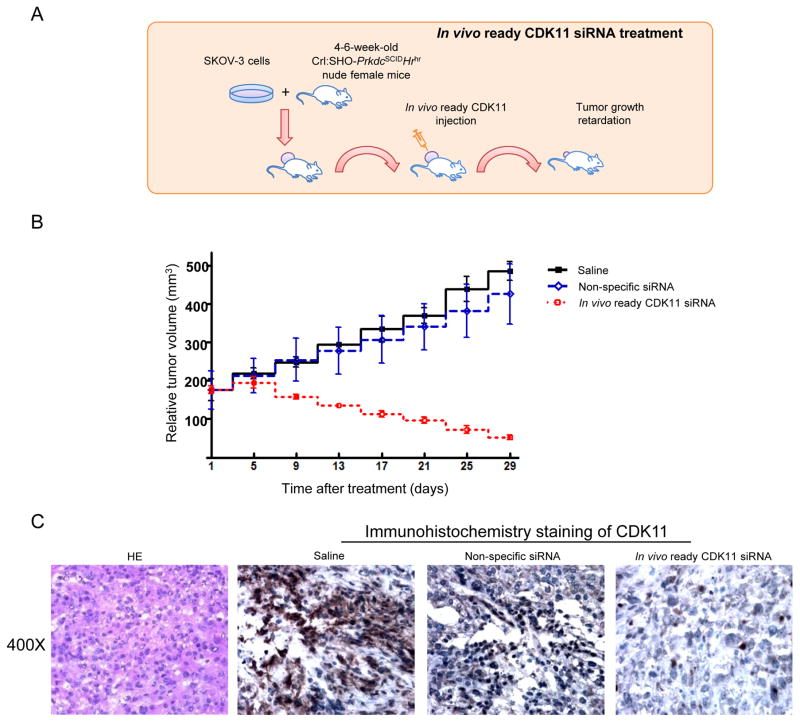
CDK11 siRNA inhibits ovarian cancer growth in vivo
To further support the biological significance of the CDK11 findings in ovarian cancer in vitro, we subsequently investigated the role of CDK11 in ovarian cancer growth in vivo. The experimental scheme is shown in Figure 6A. SKOV-3 ovarian cancer cells were injected subcutaneously into the flank of nude mice. By two weeks, visible tumors had developed at injection sites. CDK11 siRNA was then intratumorally injected twice a week two times. The results revealed that treatment with saline or non-specific siRNA had no obvious effects on ovarian cancer tumor growth suppression in xenograft models. In contrast, treatment with the combination of CDK11 In Vivo Ready siRNA produced a significant inhibitory effect on the growth of the ovarian cancer tumors as compared with control groups (Figure 6B). Immunohistochemical staining indicated a significant decrease of CDK11 expression in tumors treated with CDK11 In Vivo Ready siRNA (Figure 6C). Additionally, on the basis of animal weight and mortality, no considerable toxicity was observed and the animals appeared to have tolerated all the treatment regimens well.
Figure 6. Inhibition of tumor growth in an ovarian cancer xenograft mouse model.
A: A suspension of SKOV-3 cells was injected subcutaneously into the flank of a nude mouse. Two weeks after injection, when the average tumor volume reached ~100 mm3, CDK11 In Vivo Ready siRNA, vehicle (saline) control, and non-specific siRNA were injected into the tumor region twice per week two times. B: Effects on tumor growth rate were assessed in each mouse by determining the tumor volume on the day of treatment relative to the tumor volume on Day 0. Tumors treated with saline are shown in black, tumors with continuous growth with nonspecific siRNA treated alone in blue, and tumors responsive to treatment of CDK11 In Vivo Ready siRNA in red. Curves for representative tumors volume per group and tumor volume were presented as mean ± SD. C: Histological analysis of the effect of CDK11 In Vivo Ready siRNA on CDK11 staining in ovarian cancer tissues showed downregulation of CDK11 expression compared with saline or treatment with non-specific siRNA.
Discussion
Significant evidence has shown the overexpression of CDKs in cancers, and targeting CDK has emerged as a highly promising therapeutic strategy. Specifically, among the members of the CDK family, recent studies have shown that expression of CDK11 is critical for cancer cell growth and proliferation (19, 22, 23, 34). In this study, we found that CDK11 protein is highly expressed in human ovarian cancer cell lines and tumor tissues. Metastatic and recurrent tumors have significantly higher CDK11 expression when compared with the matched, original primary tumors. A wider analysis of CDK11 protein expression in a large cohort of patients with ovarian cancer will be needed to firmly establish if the expression of CDK11 is correlated to progression of ovarian cancer, as it appears to be for breast cancer and sarcomas (15, 22, 23). Inhibition of CDK11 with specific siRNA significantly decreased the growth and proliferation and induced apoptosis of ovarian cancer cells. Furthermore, CDK11 knockdown enhanced the cytotoxic effect of paclitaxel to inhibit cell growth in ovarian cancer cells. Moreover, in vivo administration of CDK11 siRNA reduced the tumor growth in the in vivo model of an established ovarian cancer cell line (SKOV-3). Therefore, our study suggests that CDK11 is a novel therapeutic target for the treatment of ovarian cancer.
CDK11 is a serine/threonine protein kinase and is encoded by the CDK11 gene on chromosome 1p36.3 (15). Previously, several RNAi lethality screenings of the druggable genome have shown that CDK11 is the survival gene in many different cancers, including in multiple myeloma, breast cancer, and osteosarcoma (14, 15, 18, 21–23, 33). CDK11 has been proposed as a potential therapeutic target in these cancers. However, the functional role of CDK11 has not been studied in ovarian cancer. Our study shows that CDK11 knockdown by siRNA inhibited ovarian cancer cell growth and survival. The important role of CDK11 in ovarian cancer cells was further validated by using CDK11 gene-specific lentiviral shRNA to knockdown endogenous CDK11. Both siRNA and follow-up shRNA validation assay results highlighted the importance of CDK11 in supporting the growth and proliferation of ovarian cancer cells. Our results in ovarian cancer are also congruent with myeloma, breast cancer, and sarcoma cell results, in which CDK11 inhibition led to decreased cell growth and induce apoptosis(15, 18, 21, 24, 35).
To establish the mechanisms of CDK11 knockdown-induced cell growth inhibition and apoptosis in ovarian cancer cells, we examined the cleavage of PARP and the apoptosis associated Caspase-3/7 levels. PARP and Caspase-3/7 play a major role in the transduction of apoptotic signals and the execution of apoptosis in cancer cells (36, 37). Significant PARP cleavage and induction of Caspase-3/7 were detected in CDK11 knockdown ovarian cancer cells. These data suggest that CDK11 may control several aspects of apoptosis signaling. In addition, CDK11 knockdown- resulted apoptosis in ovarian cancer cells may also related to the crucial role of CDK11 involved in the regulation of cellular RNA transcription and processing. CDK11 is part of a large protein complex that contains general pre-mRNA splicing factors, including RNA polymerase II and transcriptional elongation factors (38, 39). CDK11 may therefore couple transcription and pre-mRNA splicing by their effect(s) on certain proteins required for the apoptosis (40). For example, CDK11 interacts with the general pre-mRNA splicing factors including RNA-binding protein (RNPS1), 9G8, and RNA polymerase II (RNAP II)(38). CDK11 also exerts the functions in cell cycle and apoptosis by directly interacting with several protein partners, including, cyclin L, 14-3-3 protein, casein kinase 2 (CK2), checkpoint kinase 2 (CHK2) and heat shock protein 70/90 (Hsp70/90)(40, 41).
CDK11 may also play a role in cancer cell migration and invasion, as the migration and invasion activities were significantly reduced in CRISPR-Cas9 CDK11 knockout cancer cells (42). Knockdown of CDK11 by CRISPR-based RNAi technology also showed growth-inhibiting effects in mammalian tuberous sclerosis complex 2 (TSC2)-deficient cell lines, including in human tumor-derived acute myeloid leukemia (AML) cells (20).
CDK11 knockdown also enhanced the cytotoxic effect of chemotherapeutic agent paclitaxel in ovarian cancer cell lines, suggesting that combination therapies could be explored in ovarian cancer clinical trials. These results are consistent with studies showing that the addition of CDK2 inhibitor NU6140 to paclitaxel-treated cells resulted in markedly increased cytotoxic effects and apoptotic response in HeLa cervical carcinoma cells (43). A similar study has been found in lung cancer, in which CDK4/6 siRNA or inhibitor significantly enhanced cytotoxicity of paclitaxel in treated cells (44). The results of the phase I clinical trial also showed that the CDK9 inhibitor flavopiridol potentiates chemotherapy agent doxorubicin efficacy in advanced sarcomas (45). Interestingly, encouraging clinical effects can be achieved with adjuvant CDK9 inhibitor and docetaxel treatment in pancreatic and breast cancer patients (14). In ovarian cancer, flavopiridol showed enhanced radiosensitivity and association with significant downregulation of CDK9 expression in cancer cells (22). Similarly, combinations of the recently FDA approved CDK4/6 inhibitor palbociclib with endocrine therapy, chemotherapy, and targeted therapy may also have potential in various tumors, and phase III clinical trials are under way (16, 35).
Furthermore, silencing of CDK11 reduced tumor volume in an ovarian cancer xenograft mouse model. These in vivo studies with cell line xenografts, recapitulated our in vitro findings suggesting that knockdown CDK11 leads to inhibition of xenograft tumor volume. While further studies will be required to understand the precise mechanism of action, our work clearly identifies and provides a rationale for future pharmacologic investigation of CDK11 as a novel therapy target.
In conclusion, we showed high expression of CDK11 in metastatic and recurrent ovarian cancer and demonstrated that CDK11 is essential for ovarian cancer cell growth and survival in vitro and in vivo. Future studies to understand the upstream and downstream regulations of CDK11 signaling in human cancer will be required. Elucidating these molecular mechanisms may lead to targeting CDK11 through gene therapy or CDK11-specific inhibitors in the treatment of ovarian cancer.
Acknowledgments
This work was supported in part by grants from the Gattegno and Wechsler funds. Dr. Duan is supported, in part, through a grant from the Sarcoma Foundation of America (SFA) a pilot grant from Sarcoma SPORE/NIH, and a grant from the National Cancer Institute (NCI)/National Institutes of Health (NIH), UO1, CA 151452.
Abbreviation Lists
- CDK11
cyclin-dependent kinase 11
- RNAi
RNA interference
- siRNA
small interfering RNA
- shRNA
short hairpin RNA
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Desai A, Xu J, Aysola K, Qin Y, Okoli C, Hariprasad R, et al. Epithelial ovarian cancer: An overview. World journal of translational medicine. 2014;3:1–8. doi: 10.5528/wjtm.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. European journal of cancer. 2015;51:1164–87. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.See HT, Kavanagh JJ, Hu W, Bast RC. Targeted therapy for epithelial ovarian cancer: current status and future prospects. Int J Gynecol Cancer. 2003;13:701–34. doi: 10.1111/j.1525-1438.2003.13601.x. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets. 2003;3:1–19. doi: 10.2174/1568009033333754. [DOI] [PubMed] [Google Scholar]
- 7.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature reviews Drug discovery. 2015;14:130–46. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konecny GE. Cyclin-dependent kinase pathways as targets for women’s cancer treatment. Current opinion in obstetrics & gynecology. 2016;28:42–8. doi: 10.1097/GCO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 9.Liao Y, Feng Y, Shen J, Hornicek FJ, Duan Z. The roles and therapeutic potential of cyclin-dependent kinases (CDKs) in sarcoma. Cancer metastasis reviews. 2015 doi: 10.1007/s10555-015-9601-1. [DOI] [PubMed] [Google Scholar]
- 10.Bruyere C, Meijer L. Targeting cyclin-dependent kinases in anti-neoplastic therapy. Current opinion in cell biology. 2013;25:772–9. doi: 10.1016/j.ceb.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon S. Palbociclib: first global approval. Drugs. 2015;75:543–51. doi: 10.1007/s40265-015-0379-9. [DOI] [PubMed] [Google Scholar]
- 12.Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:4760–6. doi: 10.1158/1078-0432.CCR-15-1185. [DOI] [PubMed] [Google Scholar]
- 13.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer discovery. 2015 doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fornier MN, Rathkopf D, Shah M, Patil S, O’Reilly E, Tse AN, et al. Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5841–6. doi: 10.1158/1078-0432.CCR-07-1218. [DOI] [PubMed] [Google Scholar]
- 15.Malumbres M. Cyclin-dependent kinases. Genome biology. 2014;15:122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidula N, Rugo HS. Cyclin-Dependent Kinase 4/6 Inhibitors for the Treatment of Breast Cancer: A Review of Preclinical and Clinical Data. Clinical breast cancer. 2015 doi: 10.1016/j.clbc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Fujii T, Saito M, Iwasaki E, Ochiya T, Takei Y, Hayashi S, et al. Intratumor injection of small interfering RNA-targeting human papillomavirus 18 E6 and E7 successfully inhibits the growth of cervical cancer. International journal of oncology. 2006;29:541–8. [PubMed] [Google Scholar]
- 18.Tiedemann RE, Zhu YX, Schmidt J, Shi CX, Sereduk C, Yin H, et al. Identification of molecular vulnerabilities in human multiple myeloma cells by RNA interference lethality screening of the druggable genome. Cancer research. 2012;72:757–68. doi: 10.1158/0008-5472.CAN-11-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan Z, Zhang J, Choy E, Harmon D, Liu X, Nielsen P, et al. Systematic kinome shRNA screening identifies CDK11 (PITSLRE) kinase expression is critical for osteosarcoma cell growth and proliferation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4580–8. doi: 10.1158/1078-0432.CCR-12-1157. [DOI] [PubMed] [Google Scholar]
- 20.Housden BE, Valvezan AJ, Kelley C, Sopko R, Hu Y, Roesel C, et al. Identification of potential drug targets for tuberous sclerosis complex by synthetic screens combining CRISPR-based knockouts with RNAi. Science signaling. 2015;8:rs9. doi: 10.1126/scisignal.aab3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiedemann RE, Zhu YX, Schmidt J, Yin H, Shi CX, Que Q, et al. Kinome-wide RNAi studies in human multiple myeloma identify vulnerable kinase targets, including a lymphoid-restricted kinase, GRK6. Blood. 2010;115:1594–604. doi: 10.1182/blood-2009-09-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raju U, Nakata E, Mason KA, Ang KK, Milas L. Flavopiridol, a cyclin-dependent kinase inhibitor, enhances radiosensitivity of ovarian carcinoma cells. Cancer research. 2003;63:3263–7. [PubMed] [Google Scholar]
- 23.Zhou Q, Yu Y. Upregulated CDK16 Expression in Serous Epithelial Ovarian Cancer Cells. Medical science monitor : international medical journal of experimental and clinical research. 2015;21:3409–14. doi: 10.12659/MSM.894990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milde-Langosch K, Riethdorf S. Role of cell-cycle regulatory proteins in gynecological cancer. Journal of cellular physiology. 2003;196:224–44. doi: 10.1002/jcp.10286. [DOI] [PubMed] [Google Scholar]
- 25.Felix AS, Sherman ME, Hewitt SM, Gunja MZ, Yang HP, Cora RL, et al. Cell-cycle protein expression in a population-based study of ovarian and endometrial cancers. Frontiers in oncology. 2015;5:25. doi: 10.3389/fonc.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, et al. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12:5055–63. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Foster R, Yang X, Feng Y, Shen JK, Mankin HJ, et al. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget. 2015;6:9313–26. doi: 10.18632/oncotarget.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephen AE, Pearsall LA, Christian BP, Donahoe PK, Vacanti JP, MacLaughlin DT. Highly purified mullerian inhibiting substance inhibits human ovarian cancer in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:2640–6. [PubMed] [Google Scholar]
- 29.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer research. 1987;47:936–42. [PubMed] [Google Scholar]
- 30.Ulukaya E, Colakogullari M, Wood EJ. Interference by anti-cancer chemotherapeutic agents in the MTT-tumor chemosensitivity assay. Chemotherapy. 2004;50:43–50. doi: 10.1159/000077285. [DOI] [PubMed] [Google Scholar]
- 31.Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer research. 2004;64:3365–70. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- 32.Dormoy V, Beraud C, Lindner V, Thomas L, Coquard C, Barthelmebs M, et al. LIM-class homeobox gene Lim1, a novel oncogene in human renal cell carcinoma. Oncogene. 2011;30:1753–63. doi: 10.1038/onc.2010.557. [DOI] [PubMed] [Google Scholar]
- 33.Kren BT, Unger GM, Abedin MJ, Vogel RI, Henzler CM, Ahmed K, et al. Preclinical evaluation of cyclin dependent kinase 11 and casein kinase 2 survival kinases as RNA interference targets for triple negative breast cancer therapy. Breast cancer research : BCR. 2015;17:19. doi: 10.1186/s13058-015-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia B, Choy E, Cote G, Harmon D, Ye S, Kan Q, et al. Cyclin-dependent kinase 11 (CDK11) is crucial in the growth of liposarcoma cells. Cancer letters. 2014;342:104–12. doi: 10.1016/j.canlet.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark AS, Karasic TB, DeMichele A, Vaughn DJ, O’Hara M, Perini R, et al. Palbociclib (PD0332991)-a Selective and Potent Cyclin-Dependent Kinase Inhibitor: A Review of Pharmacodynamics and Clinical Development. JAMA oncology. 2015:1–8. doi: 10.1001/jamaoncol.2015.4701. [DOI] [PubMed] [Google Scholar]
- 36.Aredia F, Scovassi AI. Poly(ADP-ribose): a signaling molecule in different paradigms of cell death. Biochemical pharmacology. 2014;92:157–63. doi: 10.1016/j.bcp.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Galluzzi L, Kepp O, Kroemer G. Caspase-3 and prostaglandins signal for tumor regrowth in cancer therapy. Oncogene. 2012;31:2805–8. doi: 10.1038/onc.2011.459. [DOI] [PubMed] [Google Scholar]
- 38.Drogat J, Migeot V, Mommaerts E, Mullier C, Dieu M, van Bakel H, et al. Cdk11-cyclinL controls the assembly of the RNA polymerase II mediator complex. Cell reports. 2012;2:1068–76. doi: 10.1016/j.celrep.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Loyer P, Trembley JH, Grenet JA, Busson A, Corlu A, Zhao W, et al. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: influence of cyclin L isoforms on splice site selection. The Journal of biological chemistry. 2008;283:7721–32. doi: 10.1074/jbc.M708188200. [DOI] [PubMed] [Google Scholar]
- 40.Mikolajczyk M, Nelson MA. Regulation of stability of cyclin-dependent kinase CDK11p110 and a caspase-processed form, CDK11p46, by Hsp90. The Biochemical journal. 2004;384:461–7. doi: 10.1042/BJ20040848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng Y, Qi W, Martinez J, Nelson MA. The cyclin-dependent kinase 11 interacts with 14-3-3 proteins. Biochemical and biophysical research communications. 2005;331:1503–9. doi: 10.1016/j.bbrc.2005.04.078. [DOI] [PubMed] [Google Scholar]
- 42.Feng Y, Sassi S, Shen JK, Yang X, Gao Y, Osaka E, et al. Targeting CDK11 in osteosarcoma cells using the CRISPR-Cas9 system. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2015;33:199–207. doi: 10.1002/jor.22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pennati M, Campbell AJ, Curto M, Binda M, Cheng Y, Wang LZ, et al. Potentiation of paclitaxel-induced apoptosis by the novel cyclin-dependent kinase inhibitor NU6140: a possible role for survivin down-regulation. Molecular cancer therapeutics. 2005;4:1328–37. doi: 10.1158/1535-7163.MCT-05-0022. [DOI] [PubMed] [Google Scholar]
- 44.Zhang XH, Cheng Y, Shin JY, Kim JO, Oh JE, Kang JH. A CDK4/6 inhibitor enhances cytotoxicity of paclitaxel in lung adenocarcinoma cells harboring mutant KRAS as well as wild-type KRAS. Cancer biology & therapy. 2013;14:597–605. doi: 10.4161/cbt.24592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luke JJ, D’Adamo DR, Dickson MA, Keohan ML, Carvajal RD, Maki RG, et al. The cyclin-dependent kinase inhibitor flavopiridol potentiates doxorubicin efficacy in advanced sarcomas: preclinical investigations and results of a phase I dose-escalation clinical trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2638–47. doi: 10.1158/1078-0432.CCR-11-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]