Abstract
Activity monitors such as the Actical accelerometer, the Sensewear armband, and the Intelligent Device for Energy Expenditure and Activity (IDEEA) are commonly validated against gold standards (e.g., doubly labeled water, or DLW) to determine whether they accurately measure total daily energy expenditure (TEE) or activity energy expenditure (AEE). However, little research has assessed whether these parameters or others (e.g., posture allocation) predict body weight change over time. The aims of this study were to (i) test whether estimated energy expenditure or posture allocation from the devices was associated with weight change during and following a low-calorie diet (LCD) and (ii) compare free-living TEE and AEE predictions from the devices against DLW before weight change. Eighty-seven participants from 2 clinical trials wore 2 of the 3 devices simultaneously for 1 week of a 2-week DLW period. Participants then completed an 8-week LCD and were weighed at the start and end of the LCD and 6 and 12 months after the LCD. More time spent walking at baseline, measured by the IDEEA, significantly predicted greater weight loss during the 8-week LCD. Measures of posture allocation demonstrated medium effect sizes in their relationships with weight change. Bland–Altman analyses indicated that the Sensewear and the IDEEA accurately estimated TEE, and the IDEEA accurately measured AEE. The results suggest that the ability of energy expenditure and posture allocation to predict weight change is limited, and the accuracy of TEE and AEE measurements varies across activity monitoring devices, with multi-sensor monitors demonstrating stronger validity.
Keywords: accelerometry, Actical, Sensewear, IDEEA, doubly labeled water, activity energy expenditure, total energy expenditure, posture allocation
Keywords: accélérométrie, Actical, Sensewear, IDEEA, eau doublement marquée, dépense énergétique durant une activité, dépense énergétique totale, assignation postural
Résumé
Les moniteurs d’activité tels que l’accéléromètre Actical, le brassard Sensewear et le dispositif IDEEA (Intelligent Device for Energy Expenditure and Activity) sont généralement validés au moyen de tests de référence (p. ex., eau doublement marquée ou DLW), et ce, pour vérifier s’ils mesurent avec précision la dépense énergétique totale au cours d’une journée (TEE) ou la dépense énergétique durant une activité (AEE). Toutefois, peu d’études traitent de ces paramètres ou autres (p. ex. assignation posturale) pour la prédiction de la variation de la masse corporelle avec le temps. Cette étude a pour objectif : (i) vérifier si la dépense énergétique et l’assignation posturale estimées par ces instruments sont associées à la variation de la masse corporelle durant et à la suite d’un régime hypocalorique (LCD) et (ii) comparer les prédictions de TEE et de AEE d’individus libres de leur mouvement au moyen de ces instruments comparativement à DLW avant la modification de la masse corporelle. Quatre-vingt-sept participants dans deux essais cliniques portent deux de ces trois instruments en même temps durant une semaine sur deux lors de l’utilisation de DLW. Puis les participants entreprennent 8 semaines de LCD et sont pesés au début et à la cessation de LCD, puis 6 et 12 mois plus tard. Plus de temps consacré à la marche durant la période initiale au cours de laquelle IDEEA mesure cette activité est associé à une plus grande perte de poids durant les 8 semaines de LCD. Les mesures de l’assignation posturale révèlent une ampleur de l’effet modérée en ce qui concerne la variation de la masse corporelle. L’analyse de Bland–Altman démontre que Sensewear et IDEEA estiment avec précision la TEE et que IDEEA mesure avec précision AEE. D’après ces résultats, l’utilisation de la dépense énergétique et de l’assignation posturale pour prédire la variation de la masse corporelle est limitée, la précision des mesures de TEE et AEE varie d’un capteur à l’autre et les moniteurs équipés de capteurs multiples présentent une plus grande validité. [Traduit par la Rédaction]
Introduction
Energy expenditure represents the amount of energy required to maintain homeostasis and to complete daily activities. During weight maintenance, energy expenditure equals energy intake, and during weight loss, energy expenditure is greater than energy intake. Many weight loss interventions incorporate dietary components, and as energy intake decreases, maintenance of energy expenditure should mathematically translate into weight loss. However, changes in body weight produce complementary changes in energy expenditure that could result in poor long-term weight loss maintenance (Leibel et al. 1995). Given the importance of energy expenditure to understanding the process of weight change, and given the physiological and behavioral adaptations that accompany changes in energy expenditure such as changes in fat mass and fat-free mass, it is important to develop reliable free-living methods that accurately estimate components of this construct (Hall et al. 2012).
Two common units of measurement for energy expenditure are total daily energy expenditure and activity energy expenditure. Total daily energy expenditure (TEE) is the total amount of energy that an individual expends during a 24-h period. Activity energy expenditure (AEE) is a component of TEE that represents energy expended during physical activity and exercise. The gold standard for calculating TEE and AEE involves metabolic methods such as doubly labeled water (DLW) and indirect calorimetry (Schoeller 1988; Westerterp 2009). However, these methods are labor intensive and can be impractical in many applied clinical settings because of cost and participant burden. Furthermore, these procedures do not provide momentary data on changes in activity (AEE), instead relying on retrospective weekly estimates. Hence, it is impossible to intervene and encourage an increase in physical activity in real time.
Because of the shortcomings of gold standard methods, portable electronic activity monitors have become popular tools for free-living energy expenditure assessment. Many contemporary activity monitors are accelerometry based, meaning that they quantify body acceleration and use algorithms to convert acceleration into estimates of energy expenditure (Chen and Bassett 2005). From 2007 to 2011, 18 different accelerometry-based activity monitors were validated against DLW (Plasqui et al. 2013), which reinforces the growing use of these devices to measure energy expenditure.
Given the emergence of these devices in recent years, a burgeoning area of research involves systematically comparing TEE and AEE estimates from multiple devices against one another and against estimates derived using gold standard methods like DLW and indirect calorimetry (Colbert et al. 2011; Dannecker et al. 2013; Wetten et al. 2014). Another emerging area of research involves comparing stand-alone accelerometers versus multi-sensor accelerometry-based activity monitors (Brazeau et al. 2014; Dannecker et al. 2013; Heil et al. 2009), the latter of which integrate data from physiological and supplementary motion sensors to generate multidimensional estimates of energy expenditure (Chen et al. 2012). Replicating these types of designs with novel devices would allow for evidence-based decision-making on what device best suits a specific research question, since most devices’ algorithms are not directly comparable.
In addition to estimating TEE and AEE, some of these activity monitors also quantify posture allocation, which is defined as the amount of time people spend in specific body positions or activities such as sitting, reclining, walking, or running (Levine et al. 2005). Posture allocation is also important to the understanding of weight change, as overweight and obese adults engage in significantly more sedentary behaviors per day than lean adults (Levine et al. 2005; Johannsen et al. 2008), while normal-weight adults spend more time standing and in activity than obese adults (Johannsen et al. 2008). Posture allocation is a component of non-exercise activity thermogenesis that accounts for 269–477 kilocalories (kcal) of energy expenditure per day among obese individuals (Levine et al. 2005). While these cross-sectional and other longitudinal results (Levine et al. 2008) provide initial support for the relationship between posture allocation and weight change, they also encourage further exploration with more systematic comparisons (Levine et al. 2005; Johannsen et al. 2008).
In line with these recommendations for future research we tested whether parameters related to energy expenditure or posture allocation predicted weight loss among overweight and obese individuals during an 8-week low-calorie diet (LCD) and weight loss maintenance over 1 year following completion of the diet. Measures of TEE, AEE, and posture allocation were obtained from 3 activity monitoring devices: the Actical, the Sensewear armband, and the Intelligent Device for Energy Expenditure and Activity (IDEEA). Baseline AEE (Ekelund et al. 2005) and TEE (Piaggi et al. 2013) have been associated with longitudinal weight change; however, the ability of posture allocation to predict longitudinal weight change remains unclear. Because a more active lifestyle that includes greater time spent walking is associated with successful weight loss maintenance (Elfhag and Rossner 2005), we hypothesized that higher rates of physical activity and more time spent in active postures would be associated with greater weight loss during study enrollment.
A secondary aim was to compare the accuracy of TEE and AEE estimates from these devices with gold standard measures from DLW and indirect calorimetry. All of these devices have been previously evaluated for their ability to assess energy expenditure in free-living conditions (Heil 2006; Johannsen et al. 2010; Whybrow et al. 2013). Biases associated with these devices range from 112 kcal·day−1 for TEE estimates from the Sensewear (Johannsen et al. 2010) to 479 kcal·day−1 for TEE estimates from the IDEEA (Whybrow et al. 2013). However, the IDEEA and the Sensewear are both multi-sensor accelerometry-based activity monitors, which integrate more types of data into their TEE and AEE estimates than accelerometers like the Actical. Therefore, we hypothesized that the Sensewear and the IDEEA would estimate energy expenditure more accurately than the Actical.
Materials and methods
Participants
Data were collected from a sample of 87 participants recruited for 2 clinical trials (Martin et al. 2009, 2012). These trials employed very similar procedures, and samples from these studies were pooled. Exclusion criteria included the following: (i) pregnant or planning to become pregnant during the trial (females only); (ii) previous diagnosis of diabetes, cardiovascular disease, or cancer; (iii) use of medications that influence appetite or body weight during the previous 3 months; and (iv) weight instability, defined as a weight change of >0.5 kg based on regressed daily body weights for 1 week at screening. The first trial enrolled only participants whose BMI was ≥25 and <40, while the second trial enrolled participants whose BMI was ≥18.5 and <40. Both study protocols were approved by the Institutional Review Board at Pennington Biomedical Research Center (PBRC), and all participants provided written informed consent prior to enrollment in their respective study.
Of the 87 participants considered for these secondary analyses, 5 were excluded because they did not finish baseline accelerometry assessment, and an additional 5 were excluded because they did not enter the LCD phase due to BMIs < 25. Finally, 7 more participants from the original sample were excluded because they did not successfully complete all aspects of the DLW dosing period. Thus, for this report, a final sample size of 70 was considered for analysis.
Metabolic measures
In both parent studies, DLW was used to measure TEE, and the dosing procedures were identical between the two trials. Participants in each study received 1.425 g of 10% enriched H2 18O and 0.075 g of 99.9% enriched 2H2O per kilogram body mass. They provided baseline urine samples before receiving a DLW dose and follow-up samples on days 7 and 14 of the 2-week DLW cycle. Each urine sample was analyzed for 18O and 2H abundance using isotope ratio mass spectrometry and automated devices for deuterium (H/Device, Finnigan) and 18O (GasBench, Finnigan). The isotope concentrations in the samples collected after dosing compared with the pre-dose samples were used to calculate elimination rates (kD and kO) using linear regression. The rate of CO2 production (rCO2) was calculated using previously validated equations (Schoeller 1988) that were subsequently modified (Racette et al. 1994). TEE was determined by multiplying rCO2 by the energy equivalent of CO2 based on the estimated food quotient of the diet (0.86). These procedures resulted in 2 weeks of energy expenditure calculations, but for the purposes of this study, only the week that corresponded with wearing the activity monitors was used for analysis. This allowed for direct evaluation of the accuracy of the activity monitors when compared with DLW, as both measures were collected simultaneously.
Resting metabolic rate (RMR) was measured on Day 0 of the DLW phase, and as with the DLW procedures, data collection was done in an identical fashion across both studies. These data were collected over 60 min using a Deltatrac II metabolic cart (Datex-Ohmeda, Helsinki, Finland). The analyzer was calibrated before each participant, using standardized gases containing 5% CO2 and 95% O2. A clear plastic hood was placed over the head of a participant after he or she had rested for 20 min. Consumption of O2 and production of CO2 were calculated based on continuous measurements of CO2 and O2 concentrations under the plastic hood, which fluctuated according to inspired and expired air and was diluted with a constant air flow (40 L·min−1) generated by the analyzer. Participants were asked to remain motionless and awake throughout the entire data collection period, and data from the last 30 min of the measurement period were used to calculate RMR and energy expenditure. These RMR data were used to calculate AEE from DLW and IDEEA TEE using the formula TEE − [RMR + (0.1 × TEE)] (Ravussin and Rising 1992). The term 0.1 × TEE represents a population estimate for the thermic effect of food.
Activity monitors
The Actical Physical Activity Monitor (Philips Respironics, Inc., Bend, Ore., USA) provides estimates of AEE and time spent in sedentary, light, moderate, and vigorous activities. The Actical is small, noninvasive, and easily attached to a belt or clothing because of its small size (28 × 2710 mm3) and mass (17 g). The Model C Actical detects low-frequency accelerations (0.5–3.0 Hz), which are then filtered and digitally sampled at 32 Hz before being summarized into epochs of 15 to 60 s. For this analysis, participants wore 2 Actical monitors, one on the waist and one on the dominant wrist, both of which were programmed to record at sample epochs of 60 s. Previously established thresholds were used to classify activity into different intensities, including sedentary, light, moderate, and vigorous, and parameters were examined at the day level. The Light/Moderate cut point was 0.031 kcal·min−1·kg−1, and the Moderate/Vigorous cut point was 0.083 kcal·min−1·kg−1 (Heil 2006). More information on the technological and computational characteristics of the Actical can be found elsewhere (John and Freedson 2012).
The Sensewear armband (BodyMedia, Inc., Pittsburgh, Pa., USA) is a multi-sensor activity monitor worn on the upper part of the dominant arm. It integrates data from multiple physiological sensors and a biaxial accelerometer to estimate TEE and AEE. The algorithms that calculate these estimates, which are proprietary and unpublished, also incorporate anthropometric data such as sex, age, handedness, height, and body mass. The Sensewear samples data at a rate of 32 Hz and includes memory storage for up to 2 weeks of data. Data were transferred from the monitoring device to a personal computer via a USB cable. More information on the physical and measurement characteristics of the Sensewear armband can be found elsewhere (Andre et al. 2006).
The IDEEA (MiniSun LLC, Fresno, Calif., USA) objectively identifies 32 types of physical activities and postures via algorithms by Zhang et al. (2004), and it presents output in terms of time (minutes) and energy expenditure (kilocalories) for each posture and activity. The IDEEA incorporates data from 5 sensors that are placed on the body: 1 on the chest, 2 on the front of the thighs, and 2 on the feet. The sensors are connected with small flexible wires to a small recorder (59 g), which can be clipped to an article of clothing. The recorder includes a 33 MHz, 32-bit microprocessor for computational analysis. A detailed description of the IDEEA and the algorithms used to calculate TEE and AEE is provided elsewhere (Zhang et al. 2003).
Procedures
A diagram of the procedures followed in each study is presented in Fig. 1. Participants in both studies wore multiple activity monitors simultaneously for 1 week during a 2-week DLW period. In both of the parent studies, the order of device measurement was balanced, such that half the participants wore the activity monitors for the first week of the DLW period and the other half wore them for the second week of the DLW period. Participants in Study 1 wore the two Actical monitors and the IDEEA, while participants in Study 2 wore the two Actical monitors and the Sensewear. Participants were asked not to remove any of the devices unless necessary (e.g., taking a bath) and received detailed instructions on how to reapply the monitors after removal. Participants wearing the IDEEA monitor returned to PBRC every 2 to 3 days during baseline testing for data upload and battery replacement.
Fig. 1.
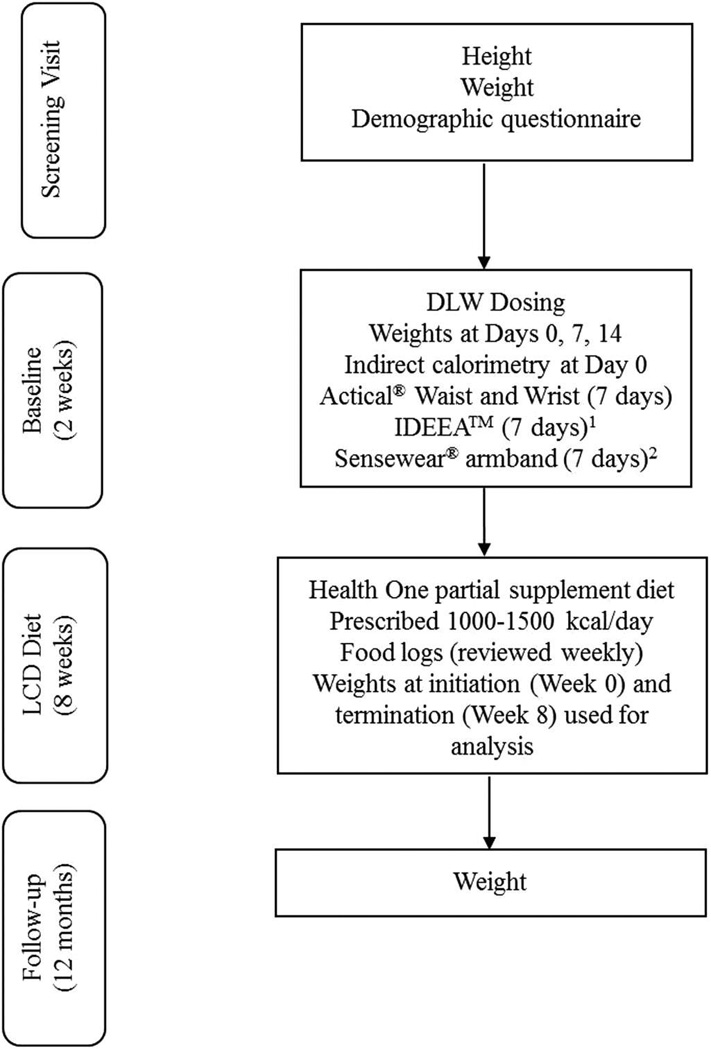
Procedural diagram for Studies 1 and 2. DLW, doubly labeled water; LCD, low-calorie diet. Activity devices in Studies 1 and 2 were worn during the same 7-day period during the DLW dosing. 1Worn in Study 1. 2Worn in Study 2.
After baseline testing, participants in both studies received a partially supplemented LCD for 8 weeks. Only participants whose BMI fell in the overweight or obese range (i.e., 25 ≤ BMI ≤ 40) were enrolled in the diet phase of the parent studies; thus, during Study 2, 5 participants with BMIs < 25 did not participate in the weight loss diet. The LCD meal plan consisted of a 1000– 1500 kcal·day−1 diet composed of Health One shakes (Health and Nutrition Technology, Carmel, Calif., USA) and prepackaged portion-controlled foods or home-cooked meals. Women were prescribed a 1000–1200 kcal·day−1 diet, while men were prescribed a 1200– 1500 kcal·day−1 diet.
Participants were asked to return to PBRC for follow-up evaluations 6 and 12 months after they had completed the LCD. Body mass (to the nearest 0.1 kg) was directly measured at the following time points: screening; Days 0, 7, and 14 of the DLW period; Weeks 0 and 8 of the LCD; and 6 and 12 months after completion of the LCD. For all analyses involving body weight, an intent-to-treat design was utilized; thus, if only a 6 month body weight was available, it was carried forward to the 12 month time point. Height was measured at screening using a stadiometer, and BMI at each time point was calculated by dividing weight by height squared (kg·m−2). Participants received monetary compensation for completing the trials.
Data analysis
All statistical analyses were performed using SPSS Version 20. Differences between subject characteristics across study samples and across completers and non-completers were evaluated using one-way ANOVA. The primary aim of the study was evaluated with linear regression analysis to determine whether TEE, AEE, or measures of posture allocation predicted weight loss during the 8-week LCD and weight maintenance at 12 months post-LCD. Percent weight change from diet initiation to termination was the dependent variable for the weight loss regressions, and percent weight change from diet termination to follow-up at 12 months was the dependent variable for the weight maintenance regressions. Because of the large number of analyses conducted for this study aim, alpha was set at 0.01 to help control alpha inflation from multiple comparisons, and both p values and effect sizes (R2 values with standard errors of the estimates) were reported.
The secondary aim of the study was evaluated using 2 analytic approaches. First, the Bland–Altman technique (Bland and Altman 1986) was used to determine whether TEE and AEE estimates from each device were significantly different from DLW-derived estimates. The Bland–Altman technique is often used to compare new measurement techniques against a gold standard (Bland and Altman 1986) and is frequently used in activity monitor research (St-Onge et al. 2007; Zhang et al. 2004). The Bland–Altman approach used in this analysis involved 2 steps. First, the difference between each device’s estimate and the DLW (gold standard) measure was evaluated using one-sample t tests, which determined whether the device had significant error (bias) compared with the gold standard. Next, linear regression was used to evaluate whether error variance differed across levels of the variable being measured (i.e., TEE or AEE).
In addition to the Bland–Altman approach, percent differences from DLW were calculated for each device and evaluated with one-way ANOVA. Tukey’s HSD post hoc analyses were used to identify pairwise differences in percent deviation among devices for TEE and AEE. This approach allowed for further evaluation of whether either multi-sensor device (i.e., either the Sensewear or the IDEEA) was associated with more accurate estimates than the stand-alone accelerometer (i.e., the Actical). Since these analyses represented planned comparisons, the alpha level was set at 0.05 for both the ANOVA and the pairwise Tukey tests.
Results
Descriptive statistics and attrition
Sample characteristics from each study and weight change during and following the LCD are reported in Table 1. No significant differences emerged between the two original study samples, so participants from both studies were collapsed for all primary and secondary analyses. Four participants were lost to follow-up during the 8-week LCD, and 17 participants were lost to follow-up at 12 months post-LCD. Thus, 94.3% of participants included in these analyses completed the LCD, and 75.7% of participants included in these analyses completed the 12-month follow-up. The only significant demographic difference between completers and non-completers was age, as participants who were lost to follow-up were significantly younger (mean ± SD: 35.6 ± 13.4 years) than those who completed the study (mean ± SD: 44.5 ± 12.8 years) (F1,85 = 8.075, p = 0.006).
Table 1.
Descriptive characteristics for the entire sample and for the individual studies.
| Characteristic | Total sample (n = 87) |
Study 1 (n = 40) |
Study 2 (n = 47) |
p |
|---|---|---|---|---|
| Sex (no. of males, no. of females) | 15 M, 72 F | 9 M, 31 F | 6 M, 41 F | 0.24 |
| Race (% Caucasian) | 66.67 | 75.0 | 59.57 | 0.13 |
| Age (y) | 42 (13) | 43 (14) | 41 (13) | 0.60 |
| Height (cm) | 164.9 (7.6) | 165.3 (6.9) | 164.5 (8.1) | 0.63 |
| Screening body mass (kg) | 85.9 (15.6) | 87.2 (14.1) | 84.9 (16.9) | 0.41 |
| Screening BMI (kg·m−2) | 31.6 (4.5) | 31.9 (3.7) | 31.3 (5.0) | 0.50 |
| Week 8 body mass (kg)a | 81.4 (14.1) | 80.8 (14.1) | 82.0 (14.3) | 0.72 |
| Month 12 body mass (kg)b | 81.4 (15.7) | 82.5 (14.3) | 80.6 (16.9) | 0.65 |
| % Weight loss following 8-week LCDa | −6.2 (2.5) | −6.6 (2.4) | −5.9 (2.6) | 0.29 |
| % Weight change from Week 8 to 12 months post-LCDb | 3.1 (5.6) | 4.1 (6.3) | 2.2 (4.9) | 0.23 |
Note: BMI, body mass index; LCD, low-calorie diet. Continuous variables are reported as the mean (standard deviation).
Study 1, n = 32; Study 2, n = 34.
Study 1, n = 25; Study 2, n = 28.
Prediction of weight change
Results of the linear regression evaluating the ability of these devices to predict weight change are reported in Table 2. No measures of energy expenditure were significant predictors of weight change during or after the 8-week LCD. When posture allocation was considered, the only statistically significant predictor of weight change during the 8-week LCD was raw time spent walking measured by the IDEEA. More time spent walking predicted greater weight loss during the 8-week LCD (p = 0.01). No indices of posture allocation predicted weight change during the 12-month follow-up period after the 8-week LCD.
Table 2.
Linear regression results for device parameters predicting percent weight change during and after an 8-week low-calorie diet.
| Baseline to Week 8 |
Week 8 to Month 12 |
|||
|---|---|---|---|---|
| Device parameter | R2 (SEE) | p | R2 (SEE) | p |
| Actical AEE (waist) | 0.00 (2.54) | 0.75 | 0.00 (5.82) | 0.89 |
| Actical % time sedentary (waist) | 0.02 (2.50) | 0.31 | 0.01 (5.79) | 0.52 |
| Actical % time light activity (waist) | 0.07 (2.44) | 0.05 | 0.00 (5.82) | 0.80 |
| Actical % time moderate activity (waist) | 0.02 (2.50) | 0.35 | 0.03 (5.75) | 0.29 |
| Actical % time vigorous activity (waist) | 0.05 (2.40) | 0.73 | 0.01 (5.79) | 0.50 |
| Actical AEE (wrist) | 0.00 (2.53) | 0.71 | 0.02 (5.68) | 0.40 |
| Actical % sedentary time (wrist) | 0.00 (2.53) | 0.94 | 0.01 (5.69) | 0.45 |
| Actical % time light activity (wrist) | 0.00 (2.53) | 0.67 | 0.01 (5.71) | 0.62 |
| Actical % time moderate activity (wrist) | 0.00 (2.53) | 0.61 | 0.02 (5.66) | 0.32 |
| Actical % time vigorous activity (wrist) | 0.04 (2.64) | 0.80 | 0.01 (5.92) | 0.64 |
| Sensewear armband TEE | 0.00 (2.69) | 0.77 | 0.00 (5.34) | 0.84 |
| Sensewear armband AEE | 0.10 (2.55) | 0.11 | 0.09 (5.11) | 0.19 |
| Sensewear physical activity duration | 0.06 (2.62) | 0.24 | 0.17 (4.88) | 0.07 |
| Sensewear lying down | 0.03 (2.65) | 0.38 | 0.12 (5.02) | 0.13 |
| Sensewear steps | 0.15 (2.49) | 0.05 | 0.01 (5.32) | 0.63 |
| IDEEA TEE | 0.03 (2.59) | 0.34 | 0.05 (6.24) | 0.27 |
| IDEEA % walking time | 0.03 (2.60) | 0.40 | 0.07 (6.19) | 0.22 |
| IDEEA time walking (minutes) | 0.22 (2.33) | 0.01 | 0.13 (5.98) | 0.09 |
| IDEEA % standing time | 0.13 (2.46) | 0.06 | 0.24 (5.59) | 0.02 |
| IDEEA time standing (minutes) | 0.19 (2.37) | 0.02 | 0.21 (5.69) | 0.02 |
Note: SEE, standard error of the estimate; AEE, activity energy expenditure; TEE, total daily energy expenditure. Bolded and italicized entries represent predictors with p values < 0.01.
Validity against DLW
Bland–Altman analysis: TEE
Estimates of TEE obtained from the Sensewear and the IDEEA were compared with estimates of TEE obtained by DLW; all estimates were expressed in kilocalories per day. Bland–Altman plots for these comparisons are presented in Fig. 2, and statistics corresponding to these plots can be found in Table 3.
Fig. 2.
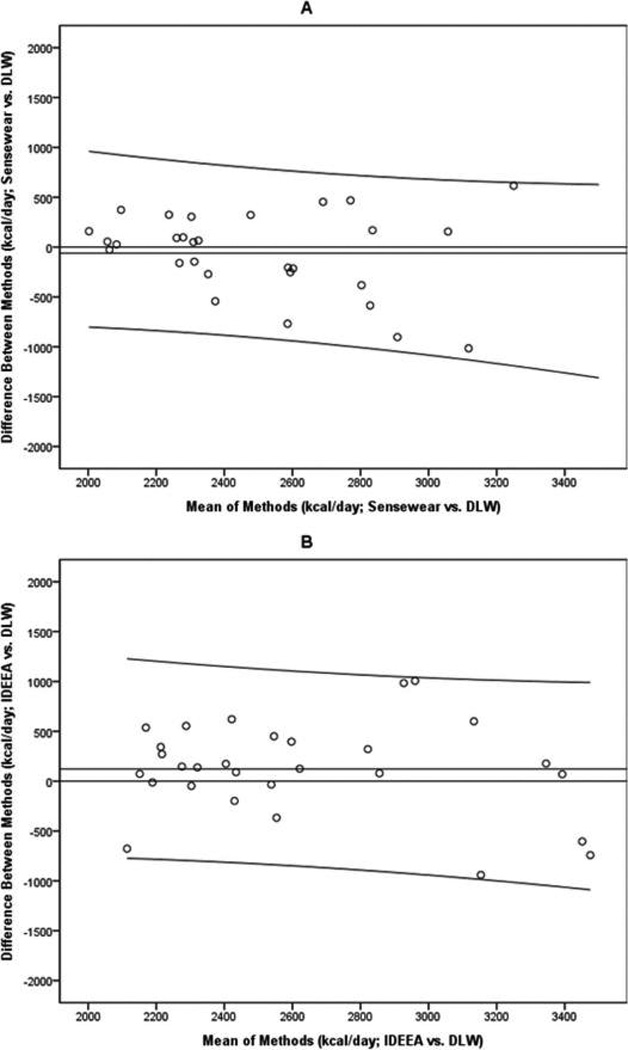
Bland–Altman plots comparing total daily energy expenditure (TEE) estimates from the Sensewear (Panel A) and the IDEEA (Panel B) with those derived by the doubly labeled water method (DLW).
Table 3.
One-sample t test and linear regression results for Bland and Altman comparisons of device measurements against doubly labeled water.
| Device and measurement | Difference between estimates |
p | R2 (SEE) | p |
|---|---|---|---|---|
| Sensewear TEE | −59.98 | 0.44 | 0.054 (407.55) | 0.22 |
| IDEEA TEE | 121.72 | 0.17 | 0.035 (467.98) | 0.33 |
| Actical waist AEE | −111.38 | 0.01 | 0.197 (319.73) | 0.00 |
| Actical wrist AEE | 194.52 | 0.00 | 0.007 (455.73) | 0.49 |
| Sensewear AEE | −416.95 | 0.00 | 0.286 (318.89) | 0.00 |
| IDEEA AEE | 108.77 | 0.21 | 0.080 (427.22) | 0.15 |
Note: SEE, standard error of the estimate; TEE, total daily energy expenditure; AEE, activity energy expenditure.
First, one-sample t tests were used to evaluate differences between the activity monitors and DLW. TEE estimates from the Sensewear (p = 0.44) and the IDEEA (p = 0.17) did not differ significantly from DLW-derived estimates. Linear regressions showed that TEE estimates from the Sensewear and the IDEEA did not vary from DLW measures as a function of kilocalories expended (p values > 0.22). This suggests that as the TEE measured by DLW increased, the difference between measures did not change for either device.
Bland–Altman analysis: AEE
Estimates of AEE obtained from the Actical, Sensewear, and IDEEA were compared with AEE measured by DLW and indirect calorimetry. Bland–Altman plots for these comparisons are presented in Fig. 3, and all statistics used to generate these plots are shown in Table 3, with estimates expressed in kilocalories per day.
Fig. 3.
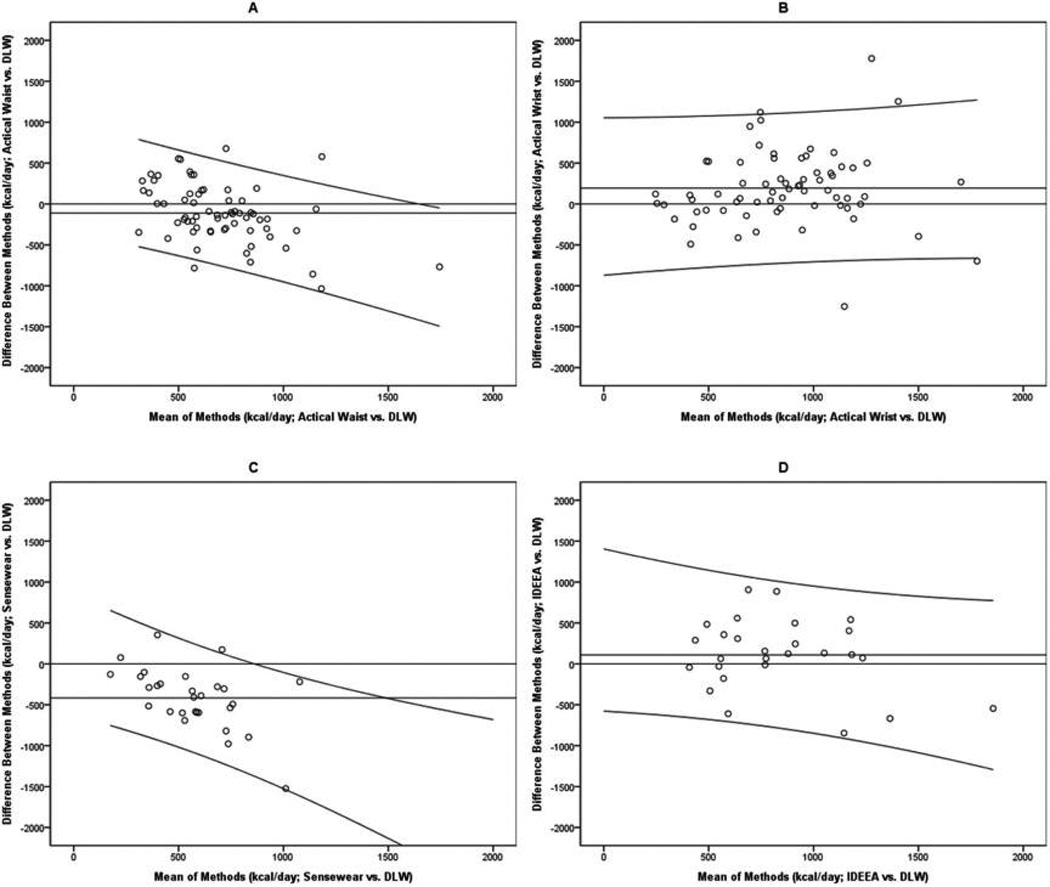
Bland–Altman plots comparing activity energy expenditure (AEE) estimates from activity monitors with those derived by the doubly labeled water method (DLW). Panels A and B report results from the Actical, Panel C reports results from the Sensewear, and Panel D reports results from the IDEEA.
Three of the devices produced mean AEE estimates that differed significantly from AEE measured by DLW (p values ≤ 0.01). The waist-worn Actical and the Sensewear significantly underestimated AEE, and both of these underestimates increased as the number of kilocalories expended per day increased (p values < 0.01). The wristworn Actical significantly overestimated AEE, on average (p < 0.01). However, the degree of overestimation did not change significantly as a function of kilocalories expended per day (p = 0.49). The IDEEA did not produce AEE estimates that differed significantly from DLW-derived estimates, and the difference between these estimates did not change as a function of kilocalories expended per day (p = 0.15).
Comparisons across devices
Percent deviation from DLW was quantified for TEE estimates from the multi-sensor devices and for AEE estimates from all four devices; these deviations were then compared across devices. For TEE estimates, percent deviation from DLW did not significantly differ between the Sensewear (0.84 ± 14.44) and the IDEEA (6.69 + 17.60) (F1,56 = 1.91, p = 0.17). Percent deviation from DLW for AEE estimates did significantly differ across the four devices (F3,187 = 3.02, p = 0.03). Tukey HSD post hoc tests confirmed that mean percent deviation from DLW for the wrist-worn Actical (55.31 ± 123.84) was significantly greater than mean percent deviation from DLW for the waist-worn Actical (7.83 ± 72.60) (p = 0.022).
Discussion
The purpose of this study was to evaluate how well parameters from the Actical, Sensewear, and IDEEA activity monitors predicted weight change during and following an LCD and to determine how accurately these devices estimated energy expenditure. Our primary hypothesis was partially supported, as only measures of posture allocation from the Sensewear and the IDEEA showed modest relationships (Cohen 1988) with weight change during and following an 8-week LCD. Our secondary hypothesis was also somewhat supported, as multi-sensor devices demonstrated modest validity when compared with metabolic measures. The IDEEA accurately estimated AEE when compared with DLW, and both the Sensewear and the IDEEA produced relatively accurate estimates of TEE. However, the Sensewear significantly underestimated AEE, and the estimates became more discrepant as the number of kilocalories expended per day increased.
Our results support other research establishing the poor performance of the Actical at accurately measuring components of energy expenditure (Paul et al. 2007), particularly among obese individuals (Feito et al. 2011). However, our results contrast with previous research suggesting that the Sensewear produces inaccurate TEE estimates (Bäcklund et al. 2010; Johannsen et al. 2010) and the IDEEA produces inaccurate estimates of AEE (Löf et al. 2013) and TEE (Whybrow et al. 2013). Taken together, our Bland– Altman findings provide modest support for the assertion that multi-sensor activity monitors produce more accurate estimates of physical activity and energy expenditure (Van Remoortel et al. 2012). Our data also encourage further parameter development and continuing validation research with these multi-sensor activity monitors, as predicted by Intille et al. (2012).
Our results suggest that parameters of energy expenditure are not strong predictors of weight change either during or following an active weight loss period. This is in contrast to findings from 2 other studies suggesting that baseline AEE (Ekelund et al. 2005) and TEE (Piaggi et al. 2013) are associated with weight change. However, these studies used other objective methods to measure energy expenditure (heart rate monitoring and a 24-h respiratory chamber), and there are several other methodological factors in our study that may explain our contrasting findings (e.g., sample size, sample composition, and follow-up duration).
Our results support other research (Plasqui et al. 2013) showing inconsistencies across devices in accurately assessing AEE and TEE. Our findings expand on this by suggesting that although multi-sensor activity monitors provide relatively accurate assessments of TEE, inconsistencies may exist in their utility for AEE assessment. One factor that could be related to these inconsistencies is reactivity during activity assessment, which is conceptualized as participants modifying their usual rate of engaging in a behavior when they know that the behavior in question is being monitored (Motl et al. 2012). Measurements collected during activity monitoring may not reflect participants’ habitual rates of physical activity, which could threaten the internal validity of the assessment (Dössegger et al. 2014). Thus, reactivity is a possible confound in intervention studies or studies designed to quantify levels of habitual physical activity, though reactivity presents far fewer problems for validity studies such as the present study. Evaluating reactivity to activity monitors is a critical but often overlooked aspect of this research area that requires further exploration.
Much like TEE and AEE, nearly all measures of posture allocation were not significantly associated with longitudinal weight change, with the exception of raw time spent walking measured by the IDEEA, which predicted weight loss during the LCD. Several previous reports describe the relationships between walking and successful weight loss maintenance (Anderson et al. 2001) and between sedentary behavior and weight regain following weight loss (Weiss et al. 2007). Even though elevated physical activity and reduced sedentary behavior can produce several positive health outcomes, the ability of exercise and physical activity to promote weight loss can be modest (Fogelholm and Kukkonen-Harjula 2000), and their association with weight loss maintenance can vary across individuals (Swift et al. 2014).
Thus, our results support both empirical (Lee et al. 2010) and editorial (Westerterp 2010) assertions that physical activity has a minimal role in preventing weight gain, particularly among the overweight and obese population. The relationship between physical activity and body weight remains a controversial topic in the obesity and weight loss research fields (Blair et al. 2013; Luke and Cooper 2013), and there is still much work to be done to elucidate whether physical activity promotes acute weight loss or weight loss maintenance. Our results suggest that there is a modest relationship between a physically active lifestyle and weight loss, and other individualized variables that are physiological (Martin et al. 2011), metabolic (Martin et al. 2007), or behavioral (Church et al. 2009) likely interact with physical activity to influence weight regulation, especially during calorie restriction (Catenacci et al. 2008).
The findings reported here should be considered within the scope of the study’s limitations. The finding that participants lost to follow-up were younger than completers is a trend that is commonly seen in weight loss trials (Fabricatore et al. 2009) and is an important limitation for this particular study, as age is inversely associated with physical activity (Sallis 2000) and longitudinal weight gain (Colditz et al. 1990). TEE, AEE, and posture allocation were not assessed outside of the baseline period because of budgetary considerations, and this limits our understanding of how these constructs may have changed during the LCD or the follow-up period, thereby also limiting our ability to identify potential physiological and behavioral adaptations occurring with changes in energy expenditure or changes in body weight. The algorithms used for some of the devices in this study are proprietary and undocumented. Access to raw accelerometry data and the algorithms used to process the raw data are limited with many accelerometers, and these data would facilitate comparability of accelerometry research and promote replication. Finally, our study did not assess body composition; therefore, changes in fat mass and fat-free mass were not quantified. Nonetheless, both fat mass and fat-free mass decrease during low-calorie diets (Heilbronn et al. 2006) and the same is expected in the present sample.
Nevertheless, these data provide some evidence supporting the validity of multi-sensor activity monitors like the Sensewear and the IDEEA for estimating physical activity and energy expenditure. Our results reflect the inconsistencies seen in the research literature on activity monitors, and they encourage further evaluation of the utility of these devices (especially those that are multi-sensor) in clinical settings. They also encourage further exploration of the many parameters acquired by these devices to determine their roles in evaluating physical activity and serving as intervention components.
Acknowledgments
This work was partially supported by the following funding sources: (i) NIH grant R21 AG032231, entitled “Validation of Innovative Technology to Measure the Energy Intake of Free-Living Humans,” sponsored by the National Heart, Lung, and Blood Institute; (ii) NIH grant K23 DK 068052, entitled “Energy Expenditure: Relation with Body Mass over Time,” sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); (iii) NORC grant 2P30DK072476, entitled “Nutritional Programming: Environmental and Molecular Interactions,” sponsored by NIDDK; and (iv) NIH grant 1 U54 GM104940, entitled “Louisiana Clinical and Translational Science Center,” sponsored by the National Institute of General Medical Sciences. The authors acknowledge Mandy Cowley and Nina Grubisic for their assistance with data management and project development, respectively.
Contributor Information
John B. Correa, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA.
John W. Apolzan, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA
Desti N. Shepard, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA.
Daniel P. Heil, Montana State University, Bozeman, MT 59717, USA
Jennifer C. Rood, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA
Corby K. Martin, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA
References
- Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am. J. Clin. Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. PMID:11684524. [DOI] [PubMed] [Google Scholar]
- Andre D, Pelletier R, Farringdon J, Safier S, Talbott W, Stone R, Vyas N, Trimble J, Wolf D, Vishnubhatla S, et al. The development of the SenseWear® armband, a revolutionary energy assessment device to assess physical activity and lifestyle. [accessed 6 June 2014];2006 Available from http://www.bodymedia.com/site/docs/papers/wp_accuracy_ee.pdf. [Google Scholar]
- Bäcklund C, Sundelin G, Larsson C. Validity of armband measuring energy expenditure in overweight and obese children. Med. Sci. Sports Exerc. 2010;42(6):1154–1161. doi: 10.1249/MSS.0b013e3181c84091. PMID:19997020. [DOI] [PubMed] [Google Scholar]
- Blair SN, Archer E, Hand GA. Commentary: Luke and Cooper are wrong: physical activity has a crucial role in weight management and determinants of obesity. Int. J. of Epidemiol. 2013;42(6):1836–1838. doi: 10.1093/ije/dyt160. PMID:24415617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. PMID:2868172. [PubMed] [Google Scholar]
- Brazeau AS, Suppere C, Strychar I, Belisle V, Demers SP, Rabasa-Lhoret R. Accuracy of energy expenditure estimation by activity monitors differs with ethnicity. Int. J. Sports Med. 2014;35:847–850. doi: 10.1055/s-0034-1371837. PMID:24816887. [DOI] [PubMed] [Google Scholar]
- Catenacci VA, Ogden LG, Stuht J, Phelan S, Wing RR, Hill JO, Wyatt HR. Physical activity patterns in the National Weight Control Registry. Obesity. 2008;16(1):153–161. doi: 10.1038/oby.2007.6. PMID:18223628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KY, Bassett DR., Jr The technology of accelerometry-based activity monitors: current and future. Med. Sci. Sports Exerc. 2005;37(S11):S490–S500. doi: 10.1249/01.mss.0000185571.49104.82. PMID:16294112. [DOI] [PubMed] [Google Scholar]
- Chen KY, Janz KF, Zhu W, Brychta RJ. Redefining the roles of sensors in objective physical activity monitoring. Med. Sci. Sports Exerc. 2012;44(1S):S13–S23. doi: 10.1249/MSS.0b013e3182399bc8. PMID:22157770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, N.J.: Erlbaum; 1988. [Google Scholar]
- Colbert LH, Matthews CE, Havighurst TC, Kim K, Schoeller DA. Comparative validity of physical activity measures in older adults. Med. Sci. Sports Exerc. 2011;43(5):867–876. doi: 10.1249/MSS.0b013e3181fc7162. PMID:20881882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Stampfer MJ, London SJ, Segal MR, Speizer FE. Patterns of weight change and their relation to diet in a cohort of healthy women. Am. J. Clin. Nutr. 1990;51(6):1100–1105. doi: 10.1093/ajcn/51.6.1100. PMID:2349925. [DOI] [PubMed] [Google Scholar]
- Dannecker KL, Sazonova NA, Melanson EL, Sazonov ES, Browning RC. A comparison of energy expenditure estimation of several physical activity monitors. Med. Sci. Sports Exerc. 2013;45(11):2105–2112. doi: 10.1249/MSS.0b013e318299d2eb. PMID:23669877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dössegger A, Ruch N, Jimmy G, Braun-Fahrländer C, Mäder U, Hänggi J, Hofmann H, Puder JJ, Kreimler S, Bringolf-Isler B. Reactivity to accelerometer measurement of children and adolescents. Med. Sci. Sports Exerc. 2014;46(6):1140–1146. doi: 10.1249/MSS.0000000000000215. PMID:24219978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wong MY, Wareham NJ. Physical activity energy expenditure predicts changes in body composition in middle-aged healthy whites: effect modification by age. Am. J. Clin. Nutr. 2005;81(5):964–969. doi: 10.1093/ajcn/81.5.964. PMID:15883416. [DOI] [PubMed] [Google Scholar]
- Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes. Rev. 2005;6(1):67–85. doi: 10.1111/j.1467-789X.2005.00170.x. PMID:15655039. [DOI] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Heymsfield SB, Nguyen AM. Predictors of attrition and weight loss success: results from a randomized controlled trial. Behav. Res. Ther. 2009;47(8):685–691. doi: 10.1016/j.brat.2009.05.004. PMID:19497559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feito Y, Bassett DR, Tyo B, Thompson DL. Effects of body mass index and tilt angle on output of two wearable activity monitors. Med. Sci. Sports Exerc. 2011;43(5):861–866. doi: 10.1249/MSS.0b013e3181fefd40. PMID:20962689. [DOI] [PubMed] [Google Scholar]
- Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain – a systematic review. Obes. Rev. 2000;1(2):95–111. doi: 10.1046/j.1467-789x.2000.00016.x. PMID:12119991. [DOI] [PubMed] [Google Scholar]
- Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. Am. J. Clin. Nutr. 2012;95(4):989–994. doi: 10.3945/ajcn.112.036350. PMID:22434603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res. Q. Exerc. Sport. 2006;77(1):64–80. doi: 10.1080/02701367.2006.10599333. PMID:16646354. [DOI] [PubMed] [Google Scholar]
- Heil DP, Bennett GG, Bond KS, Webster MD, Wolin KY. Influence of activity monitor location and bout duration on free-living physical activity. Res. Quart. Exerc. Sport. 2009;80(3):424–433. doi: 10.1080/02701367.2009.10599580. PMID:19791628. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J Pennington CALERIE Team. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–1548. doi: 10.1001/jama.295.13.1539. PMID:16595757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intille SS, Lester J, Sallis JF, Duncan G. New horizons in sensor development. Med. Sci. Sports Exerc. 2012;44(1 S1):S24–S31. doi: 10.1249/MSS.0b013e3182399c7d. PMID:22157771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen DL, Welk GJ, Sharp RL, Flakoll PJ. Differences in daily energy expenditure in lean and obese women: the role of posture allocation. Obesity. 2008;16(1):34–39. doi: 10.1038/oby.2007.15. PMID:18223609. [DOI] [PubMed] [Google Scholar]
- Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med. Sci. Sports Exerc. 2010;42(11):2134–2140. doi: 10.1249/MSS.0b013e3181e0b3ff. PMID:20386334. [DOI] [PubMed] [Google Scholar]
- John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med. Sci. Sports Exerc. 2012;44(1 S1):S86–S89. doi: 10.1249/MSS.0b013e3182399f5e. PMID:22157779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Djoussé L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA. 2010;303(12):1173–1179. doi: 10.1001/jama.2010.312. PMID:20332403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New Engl. J. Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. PMID:7632212. [DOI] [PubMed] [Google Scholar]
- Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307(5709):584–586. doi: 10.1126/science.1106561. PMID:15681386. [DOI] [PubMed] [Google Scholar]
- Levine JA, McCrady SK, Lanningham-Foster LM, Kane PH, Foster RC, Manohar CU. The role of free-living daily walking in human weight gain and obesity. Diabetes. 2008;57(3):548–554. doi: 10.2337/db07-0815. PMID:18003759. [DOI] [PubMed] [Google Scholar]
- Löf M, Henriksson H, Forsum E. Evaluations of Actiheart, IDEEA® and RT3 monitors for estimating activity energy expenditure in free-living women. J. Nutr. Sci. 2013;2(e31):1–10. doi: 10.1017/jns.2013.18. PMID:25191581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A, Cooper RS. Physical activity does not influence obesity risk: time to clarify the public health message. Int. J. Epidemiol. 2013;42(6):1831–1836. doi: 10.1093/ije/dyt159. PMID:24415616. [DOI] [PubMed] [Google Scholar]
- Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity. 2007;15(12):2964–2973. doi: 10.1038/oby.2007.354. PMID:18198305. [DOI] [PubMed] [Google Scholar]
- Martin CK, Han H, Coulon SM, Allen HR, Champagne CM, Anton SD. A novel method to remotely measure food intake of free-living individuals in real time: the remote food photography method. Br. J. Nutr. 2009;101(3):446–456. doi: 10.1017/S0007114508027438. PMID:18616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Das SK, Lindblad L, Racette SB, McCrory MA, Weiss EP, Delany JP, Kraus WE. Effect of calorie restriction on the free-living physical activity levels of nonobese humans: results of three randomized trials. J. Appl. Physiol. (1985) 2011;110(4):956–963. doi: 10.1152/japplphysiol.00846.2009. PMID:21292847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Correa JB, Han H, Allen HR, Rood JC, Champagne CM, Gunturk BK, Bray GA. Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity. 2012;20(4):891–899. doi: 10.1038/oby.2011.344. PMID:22134199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl RW, McAuley E, Dlugonski D. Reactivity in baseline accelerometer data from a physical activity behavioral intervention. Health Psychol. 2012;31(2):172–175. doi: 10.1037/a0025965. PMID:22023436. [DOI] [PubMed] [Google Scholar]
- Paul DR, Kramer M, Moshfegh AJ, Baer DJ, Rumpler WV. Comparison of two different physical activity monitors. BMC Med. Res. Methodol. 2007;7:26. doi: 10.1186/1471-2288-7-26. PMID:17592631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J. Clin. Endocrinol. Metab. 2013;98(4):E703–E707. doi: 10.1210/jc.2012-3529. PMID:23418317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasqui G, Bonomi AG, Westerterp KR. Daily physical activity assessment with accelerometers: new insights and validation studies. Obes. Rev. 2013;14(6):451–462. doi: 10.1111/obr.12021. PMID:23398786. [DOI] [PubMed] [Google Scholar]
- Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am. J. Physiol. 1994;267(4.1):E585–E590. doi: 10.1152/ajpendo.1994.267.4.E585. PMID:7943308. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Rising R. Daily energy expenditure in humans: Measurements in a respiratory chamber and by doubly labeled water. In: Kinney JM, Tucker HN, editors. Energy metabolism: Tissue determinants and cellular corollaries. New York: Raven Press; 1992. pp. 81–96. [Google Scholar]
- Sallis JF. Age-related decline in physical activity: a synthesis of human and animal studies. Med. Sci. Sports Exerc. 2000;32(9):1598–1600. doi: 10.1097/00005768-200009000-00012. PMID:10994911. [DOI] [PubMed] [Google Scholar]
- Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J. Nutr. 1988;118(11):1278–1289. doi: 10.1093/jn/118.11.1278. PMID:3142975. [DOI] [PubMed] [Google Scholar]
- St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am. J. Clin. Nutr. 2007;85(3):742–749. doi: 10.1093/ajcn/85.3.742. PMID:17344495. [DOI] [PubMed] [Google Scholar]
- Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 2014;56(4):441–447. doi: 10.1016/j.pcad.2013.09.012. PMID:24438736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remoortel H, Giavedoni S, Raste Y, Burtin C, Louvaris Z, Gimeno-Santos E, Langer D, Glendenning A, Hopkinson NS, et al. Validity of activity monitors in health and chronic disease: a systematic review. Int. J. Behav. Nutr. Phys. Act. 2012;9(1):84. doi: 10.1186/1479-5868-9-84. PMID:22776399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am. J. Prev. Med. 2007;33(1):34–40. doi: 10.1016/j.amepre.2007.02.040. PMID:17572309. [DOI] [PubMed] [Google Scholar]
- Westerterp K. Assessment of physical activity: a critical appraisal. Eur. J. Appl. Physiol. 2009;105(6):823–828. doi: 10.1007/s00421-009-1000-2. PMID:19205725. [DOI] [PubMed] [Google Scholar]
- Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr. Rev. 2010;68(3):148–154. doi: 10.1111/j.1753-4887.2010.00270.x. PMID:20384845. [DOI] [PubMed] [Google Scholar]
- Wetten AA, Batterham M, Tan SY, Tapsell L. Relative validity of three accelerometer models for estimating energy expenditure during light activity. J. Phys. Act. Health. 2014;11(3):638–647. doi: 10.1123/jpah.2011-0167. PMID:23417054. [DOI] [PubMed] [Google Scholar]
- Whybrow S, Ritz P, Horgan GW, Stubbs RJ. An evaluation of the IDEEA activity monitor for estimating energy expenditure. Br. J. Nutr. 2013;109(1):173–183. doi: 10.1017/S0007114512000645. PMID:22464547. [DOI] [PubMed] [Google Scholar]
- Zhang K, Werner P, Sun M, Pi-Sunyer FX, Boozer CN. Measurement of human daily physical activity. Obes. Res. 2003;11(1):33–40. doi: 10.1038/oby.2003.7. PMID:12529483. [DOI] [PubMed] [Google Scholar]
- Zhang K, Pi-Sunyer FX, Boozer CN. Improving energy expenditure estimation for physical activity. Med. Sci. Sports Exerc. 2004;36(5):883–889. doi: 10.1249/01.mss.0000126585.40962.22. PMID:15126725. [DOI] [PubMed] [Google Scholar]


