Abstract
Traumatic brain injury (TBI) is associated with adverse effects on brain functions, including sensation, language, emotions and/or cognition. Therapies for improving outcomes following TBI are limited. A better understanding of the pathophysiological mechanisms of TBI may suggest novel treatment strategies to facilitate recovery and improve treatment outcome. Aberrant activation of cyclin-dependent kinase 5 (Cdk5) has been implicated in neuronal injury and neurodegeneration. Cdk5 is a neuronal protein kinase activated via interaction with its cofactor p35 that regulates numerous neuronal functions, including synaptic remodeling and cognition. However, conversion of p35 to p25 via Ca2+-dependent activation of calpain results in an aberrantly active Cdk5/p25 complex that is associated with neuronal damage and cell death. Here we show that mice subjected to controlled cortical impact (CCI), a well-established experimental TBI model, exhibit increased p25 levels and consistently elevated Cdk5-dependent phosphorylation of microtubule-associated protein tau and retinoblastoma (Rb) protein in hippocampal lysates. Moreover, CCI induced neuroinflammation as indicated by increased astrocytic activation and number of reactive microglia. Brain-wide conditional Cdk5 knockout mice (Cdk5 cKO) subjected to CCI exhibited significantly reduced edema, ventricular dilation and injury area. Finally, neurophysiological recordings revealed that CCI attenuated excitatory post-synaptic potential field responses (fEPSP) in the hippocampal CA3-CA1 pathway 24 h after injury. This neurophysiological deficit was attenuated in Cdk5 cKO mice. Thus, TBI induces increased levels of p25 generation and aberrant Cdk5 activity, which contributes to pathophysiological processes underlying TBI progression. Hence, selectively preventing aberrant Cdk5 activity may be an effective acute strategy to improve recovery from TBI.
Keywords: traumatic brain injury, cyclin-dependent kinase 5, microtubule-associated protein tau, retinoblastoma protein, neuroinflammation, p25
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability, contributing to approximately 30% of all injury deaths in the United States (Thurman et al. 1999). Each year approximately 2.5 million emergency room visits, hospitalizations or deaths are associated with TBI in the United States (Bruns & Hauser 2003, Faul et al. 2010). Falls (40.5 %), traffic accidents (14.3 %), unintentional blunt trauma (15.5 %) and violence (10.7 %) are the most common causes of TBI amongst the civilian populations (Faul et al. 2010). Open head or penetrating TBI occurs through gun shot or missile wounds and is observed during armed combat, as well as in cases of gun violence (Morales et al. 2005).
Experimental animal models of TBI that replicate the human condition are crucial for understanding the pathophysiological changes following TBI. A number of animal models have been developed to induce brain trauma with clinical accuracy. The controlled cortical impact (CCI) is a widely used penetrative model of TBI which replicates many of the hallmarks of secondary injury identified in human TBI patients such as neuronal cell death and degeneration, astrogliosis, microglia activation and breakdown of blood-brain barrier (Smith et al. 1995), axonal injury (Hall et al. 2008), brain edema (Elliott et al. 2008) and cortical spreading depression (von Baumgarten et al. 2008). The CCI injury model is highly reliable because of its degree of deformation and the reproducibility of the tissue damage, as well as of cytotoxic and vasogenic edema (Elliott et al. 2008, Albert-Weissenberger & Siren 2010). As CCI is a well-established reproducible animal model of TBI with standardized equipment and methodology, it is commonly used to characterize the molecular basis of TBI and to identify novel targets for the development of new pharmacological therapies for brain injuries.
Cdk5 is a proline-directed serine/threonine kinase that is essential for many neuronal functions, such as neuronal development (Nikolic et al. 1998, Cheung et al. 2007), synaptic plasticity, learning and memory (Angelo et al. 2006, Plattner et al. 2014, Hawasli et al. 2007). Interaction with either p35 or p39 is essential for Cdk5 activation (Lew et al. 1994, Tang et al. 1995, Tsai et al. 1994). Several neurotoxic insults such as excitotoxicity and β-amyloid peptide treatment increase intracellular calcium concentration (Mattson et al. 1992). In response to this calcium influx, calpain becomes active and mediates the cleavage of p35 to p25 (Kusakawa et al. 2000, Lee et al. 2000, Nath et al. 2000, Patrick et al. 1999, Meyer et al. 2008). The resulting Cdk5/p25 complex engenders aberrant activity and abnormally phosphorylates substrates that result in neurotoxicity and neuronal death. For example, aberrant Cdk5 is produced in response to ischemia and contributes to stroke injury (Meyer et al. 2014). Furthermore, p25 production, aberrant Cdk5 activation and phosphorylation of abnormal substrates characterizes several neurodegenerative diseases (Lee et al. 1999, Wang et al. 2003, Smith et al. 2003, Nguyen et al. 2001). Interestingly, post-mortem brain tissue from both TBI patients and sufferers of neurodegenerative disorders feature accumulation of similar proteins including the microtubule-associated protein tau, presenilin 1, amyloid precursor protein (APP) and α-synuclein (Chen et al. 2004, Uryu et al. 2007). Moreover, increasing evidence suggests that even a single moderate to severe episodes of head trauma may be already sufficient to facilitate neurodegenerative diseases. However, the molecular mechanisms that contribute to the progression of TBI-related pathophysiology remain largely unknown (Salib & Hillier 1997, Guo et al. 2000, Johnson et al. 2012).
Using the CCI paradigm, we show here, that TBI induces p25 production and aberrant Cdk5/p25 activation which in turn leads to increased phosphorylation of microtubule-associated protein tau and retinoblastoma (Rb) protein. Attenuation of Cdk5 activity resulted in reduced lesion volume and maintenance of neurophysiological responses in conditional Cdk5 knockout mice (Cdk5 cKO). Taken together, we demonstrate that Cdk5 can contribute to TBI progression and that aberrant Cdk5 activity may be a suitable target for the development of acute treatment strategies for TBI.
Methods
Antibodies and Materials
The p35 (C-19) sc-820 antibody was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The phospho-Rb (Ser807/811) (#9308) and Rb (D20) (#9313) antibodies were from Cell Signaling Technology, tau antibody (A0024) was from Dako, GFAP antibody (PA3-16727) was from Pierce, and β-actin (AC-15) (ab6276) antibody from Abcam. Goat anti-mouse IgG and goat anti-rabbit IgG peroxidase conjugated secondary antibodies were from Thermo Fisher Scientific (Rockford, IL, USA). The phospho-tau (S202) CP13 antibody was kindly received from Dr. Peter Davis. All materials were obtained from Sigma-Aldrich unless stated otherwise.
Animals
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center or the CDC-NIOSH. All animal experiments were performed in adult, 3–4 month-old male mice in a C57BL/6 background. Adult, male Cdk5 cKO mice and littermate controls were generated as described previously (Hawasli et al. 2007). Breeding mice in a C57BL/6 background were obtained from the Jackson Laboratory and all mouse strains were maintained in a C57BL/6 background. Mice were housed 2–4 per cage in a colony maintained at 23° C with a 12 h light/dark cycle and access to food and water ad libitum.
Controlled Cortical Impact (CCI)
A controlled cortical impact device was used to cause brain injury as previously described (Kernie et al. 2001). Briefly, mice were anesthetized with 0.15 ml mixture of ketamine (100 mg/ml) and xylazine (20 mg/ml) at a ration of 10:1 and placed in an injury device stereotactic frame. The skull was exposed after a midline incision and reflection of underlying soft tissues. A circular craniectomy, 5 mm by 5 mm, was made between bregma and lambda, 1 mm lateral from midline. Mice were then subjected to CCI with a 3 mm stainless steel tipped impact device at a velocity of 4.4 m/sec and at a depth of 1 mm as measured directly with a digital motion detector (CDT-2000, EPD Technologies). After injury, the bone flap was replaced and sealed with liquid adhesive (Loctite 454), the scalp was closed with suture, and the animals were allowed to recover. Sham injured animals were anesthetized and received a craniectomy, but were not subjected to CCI. Core body temperature was monitored throughout the surgery via rectal probe and temperature was maintained at 37 ± 1°C with a T/Pump heating system (Gaymar Industries).
Histopathology
The histopathologic analysis was performed 24 h after injury to evaluate effect of CCI-TBI on neuroanatomy and neuroinflammation. Animals were euthanized by CO2 asphyxiation and perfused transcardially with ice-cold PBS with protease and phosphatase inhibitors, then brains were removed and placed in 4% PFA for 24 hours. Brains were cut coronally at the center of the cortical lesion, then block-sectioned into 4 coronal slabs, paraffin-embedded, and serially sectioned at 5 μm. Standard protocols were utilized for staining with hematoxylin and eosin (H&E) (Fischer et al. 2008), and Fluoro-Jade B (FJB, Millipore Corporation) (Schmued & Hopkins 2000). For the immunohistochemical analysis, paraffin-embedded sections were labeled with glial fibrillary acidic protein (GFAP) to detect astrocyte activation (1:1200; Chemicon) and ionized Ca2+-binding adapter molecule (Iba1) to detect microglial activation (1:1000; Wako) and visualized by immunoperoxidase method (Sinclair et al. 1981).
Quantitative Immunoblot analysis
The hippocampi were quickly dissected on an ice-cold steel plate and snap-frozen on dry ice. Frozen tissue was immediately sonicated in boiling lysis buffer (10 mM NaF in 1% sodium dodecyl sulfate), and boiled for 5 min to denature proteins and protect samples from dephosphorylation and protein degradation. Protein concentrations were determined by bicinchoninic acid protein assay (Thermo Fisher Scientific). Equal amounts of total protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by overnight transfer to nitrocellulose membranes. Membranes were blocked in 5% powdered skim milk dissolved in Tris-buffered saline-Tween 20, then probed with primary antibodies raised against p35 (C-19) or β-actin or GFAP or phospho-Rb protein or total Rb protein, phospho-tau Ser202 (CP13 (Herskovits & Davies 2006)) or total tau. Blots were washed three times for 30 min and then incubated with HRP-conjugated secondary antibodies for 1–2 h. Antibodies were detected using enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA). Multiple exposure times were evaluated for each blot to ensure linearity.
Magnetic Resonance Image
All MRI were conducted using a 7-Tesla small animal MRI system (Varian Inc., Palo Alto, CA) with a 40 mm (i.d.) Millipede coil and a 400 mT/m gradient coil set. Under anesthesia by inhalation of 1.5 – 2% isoflurane mixed in medical-grade air via nose cone, the animals were placed supine with the respiratory sensor, head first with the head centered with respect to the center of a RF coil. Through the imaging session, animals were monitored for respiration rate with an MRI compatible monitoring system from SA Instruments (Stony Brook NY) to adjust anesthesia depth.
Two-dimensional (2D) fast spin-echo (FSE) images on three orthogonal planes (transverse, coronal and sagittal) were firstly acquired to ensure the orientation of the head as a localizer imaging. For volume measurements of the brain injury, high-resolution T2-weighted FSE axial image was acquired on the entire brain. The imaging parameters were: TR/TE = 2000/60 msec, FOV = 25.6×25.6 mm, matrix size = 256×256, slice thickness = 1 mm, no gap, 8 averages, affording 100 μm in-plane resolution and a total scan time of 8 min and 32 sec. For the quantification of the volume of abnormalities, three sequential axial images (slice no. 7, 8 & 9) were selected. Hyper-intense areas, reflecting high content of water, such as edema and dilated/expanded ventricles were calculated on the selected T2-weighted images by manual segmentation using NIH Image J (v1.42q). The calculated hyper-intense areas were multiplied by slice thickness (1 mm), for a total of three slices, to obtain total volume of injury. Then, the volume of abnormality was divided by the total brain volume (of all three slices) and compared among the groups.
Electrophysiology
Transverse slices (300 μm thick) were prepared in artificial cerebrospinal fluid (ACSF) with a vibratome (3000 sectioning system, the Vibratome Company) from the ipsilateral hippocampus 24 hours after CCI injury or sham procedure. The ACSF was composed of (in mM) 120 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 25.5 NaHCO3, and 25 glucose, bubbled with 95% O2/5% CO2. Slices were incubated in ACSF at room temperature for 3 h before recordings. During recordings, the slices were perfused with oxygenated ACSF at a flow rate of 2–3 ml/min at 25°C. The Schaffer collateral afferent pathway was stimulated by a concentric bipolar electrode connected to a computer-controlled biphasic stimulus isolator (BSI-950, Dagan) and field excitatory postsynaptic potentials (fEPSPs) were recorded in stratum radiatum of hippocampal area CA1 with glass electrodes filled with ACSF (~ 3 MΩ resistance). Signals were recorded with a bridge and voltage clamp amplifier (BVC 700A, Dagan), digitized with an analog to digital converter (ITC-18, Instrutech Corp.) and stored in a computer using Igor Pro software (Wavemetrics, Inc.). The fEPSP slope was compared between CCI and sham controls, as well as the CCI/sham fEPSP slope ratio between Cdk5 cKO and WT mice.
Data analysis
For quantitative immunoblot analysis, immunoreactivity signals were captured by autoradiography, scanned and quantified with Image J software (NIH). Data are presented as mean ± SEM. Student’s t-test or one-way ANOVA was performed to compare data sets using GRAPHPAD Prism (GraphPad Software Inc., San Diego, CA, USA). A p-value < 0.05 was considered statistically significant; p* < 0.05, p** < 0.01 and P*** < 0.001.
Results
CCI induced neuroinflammation and neuropathology in hippocampus
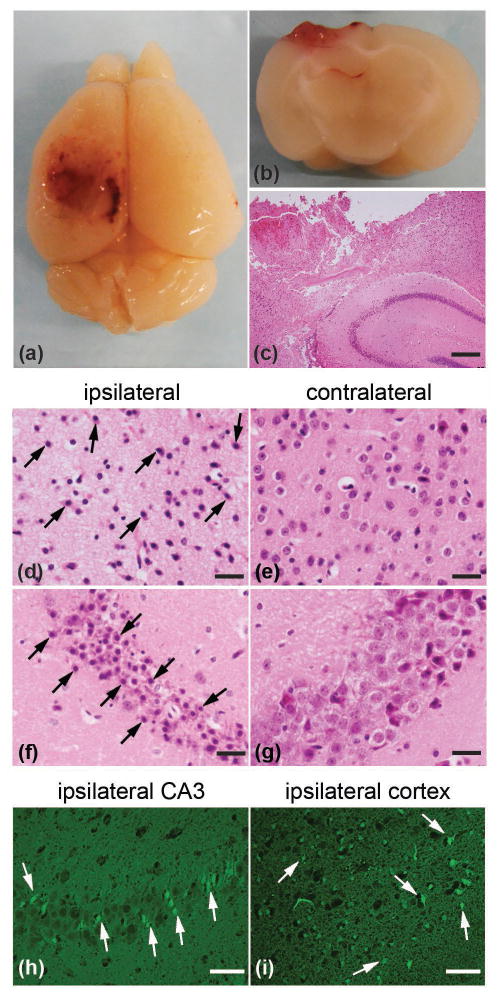
The effects of CCI on neuroanatomy and neuroinflammation were evaluated using standard histopathological and immunohistochemical techniques. Consistent with previous CCI studies (e.g., Smith et al. 1995), gross examination of brains harvested 24 h post-CCI revealed extensive cortical lesions in the left ipsilateral hemisphere, where the pneumatic impactor engaged the cortical surface (Fig. 1a). The area surrounding the cortical lesion was characterized by loss of tissue, hemorrhage and necrosis (Fig. 1a and b). Hematoxylin and eosin (H&E) stained coronal sections of brains harvested 24 h post-CCI showed that the CCI led to neuronal and structural damage within the cortical layers, subcortical white matter and in some cases induced some damage in the hippocampus, predominantly in area CA2 and CA3 (Fig. 1c). Cerebral edema, which is characterized by decreased H&E staining and dilation of extracellular and perivascular spaces, was observed in the ipsilateral, injured area of the cortex (Fig. 1c). Shrunken, pyknotic ischemic neurons, which are characterized by eosinophilic and shrunken cytoplasm and hyperchromatic nucleus, were observed in the ipsilateral cortex (Fig. 1d), but not in contralateral cortex (Fig. 1e). Shrunken, pyknotic ischemic neurons were also seen in the ipsilateral hippocampus, particularly in hippocampal regions, CA2 and CA3 (Fig. 1f), but not in the contralateral hippocampus (Fig 1g). Fluoro-Jade B (FJB) staining confirmed the presence of degenerating neurons in the ipsilateral hippocampal region CA3 and cortex (Fig. 1h and i).
Figure 1.
CCI induces neuropathological changes in the mouse brain. (a) Top view of the mouse brain showing extensive cortical lesion in the left, ipsilateral hemisphere one day after injury. (b and c) Coronal section across area of cortical lesion characterized by loss of tissue, hemorrhage and necrosis in the cortex, as well as subcortical white matter and hippocampus. H&E staining was used in (c). (d and e) Cortical edema and shrunken, pyknotic neurons (arrows indicate characteristic examples) were observed in the injured ipsilateral cortex (d), but not contralateral cortex (e) using H&E staining. (f and g) Loss of pyramidal neurons and shrunken, pyknotic neurons (arrows indicate characteristic examples) were found in ipsilateral hippocampal area CA3 and CA2 (f), but not contralateral CA3 and CA2 (g) using H&E staining. Degenerative neurons (arrows indicate characteristic examples) in ipsilateral hippocampal area CA3 and cortex were confirmed by Fluoro-Jade B (FJB) staining (h and i). Scale bars: 200 μm (c); 50 μm (d, e, h and i); 20 μm (f and g).
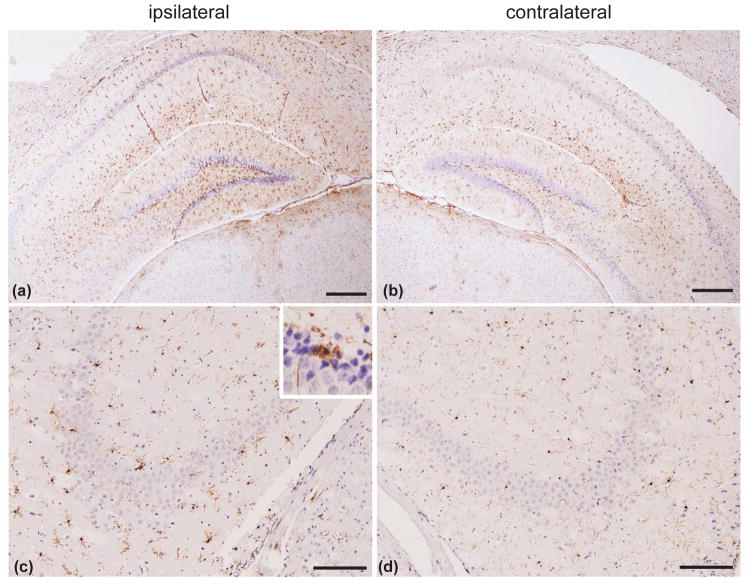
Traumatic brain injury induces neuroinflammation that is characterized by astrogliosis and microglial activation. Consistent with previous CCI studies (e.g., Smith et al. 1995), 24 h after CCI the number of reactive astrocytes was increased in the hippocampus on the ipsilateral, injured side (Fig. 2a) as compared to the contralateral side (Fig. 2b). Levels of activated microglia, which are also known as amoeboid microglia, are elevated in the ipsilateral hippocampus, in particular hippocampal regions, CA2 and CA3 (Fig. 2c, see also insert) as compared to the contralateral side (Fig. 2d). Interestingly, no significant microglial activation was seen in the cortex, neither in the ipsilateral nor in the contralateral side (data not shown). Taken together, these data show that the CCI protocol used here induces brain tissue lesion, hemorrhage and neuronal damage that in turn trigger a neuroinflammatory response. These results are consistent with previous studies on CCI and suggest that our CCI paradigm yields consistent and reproducible TBI and thus can be used to study pathophysiological changes underlying TBI.
Figure 2.
CCI induces neuroinflammation in the mouse brain. (a and b) Neuroinflammation indicated by increased astrocyte activation in the ipsilateral hippocampus (a), as compared to the contralateral hippocampus (b) using GFAP immunostaining. (c and d) Neuroinflammation indicated by increased number of reactive microglia in the ipsilateral hippocampal area CA3 and CA2 (c), as compared to contralateral CA3 and CA2 (d) using Iba1 immunostaining. Insert in panel (c), represents magnification of a region in hippocampal area CA3 with activated microglia. Scale bars: 200 μm (a and b); 100 μm (c and d).
CCI increased levels of the Cdk5 activator, p25, and phosphorylation of aberrant Cdk5 substrates
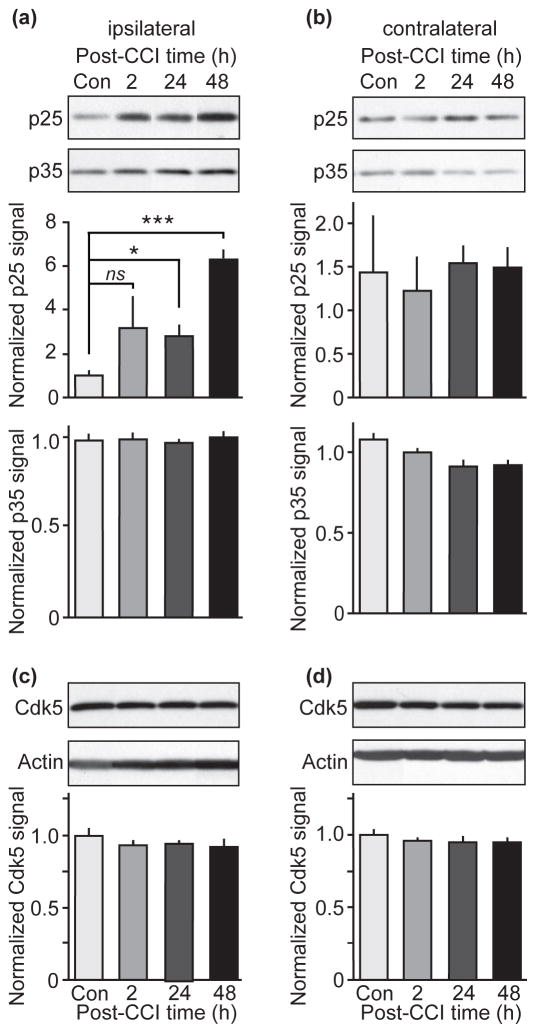
TBI induces excessive release of excitatory neurotransmitters, such as glutamate, and thereby triggers prolonged activation of neuronal receptors and channels. The resulting increase in intracellular Ca2+ leads to overactivation of the Ca2+-dependent protease, calpain (Palmer et al. 1993, Shapira et al. 1989, Fineman et al. 1993, Pike et al. 1998). An important downstream target of calpain that has been linked to neuronal injury and neurodegeneration is the Cdk5 activator, p35. Activated calpain cleaves p35 into p25. Association of p25 with Cdk5 aberrantly activates the kinase and directs it towards a different subset of substrates, a process that has been shown to induce neuronal damage and neurodegeneration (Kusakawa et al. 2000, Lee et al. 2000, Patrick et al. 1999). To evaluate whether Cdk5/p25 plays a role in neuronal injury in TBI, the effects of CCI on the levels of p35 and p25 were examined in the hippocampus by quantitative immunoblotting. The levels of p25 were significantly increased at 24 and 48 hours post-CCI in hippocampal lysates from the ipsilateral (Fig. 3a), but not in the contralateral side (Fig. 3b). At 2h post-CCI the p25 levels showed a trend towards an increase in the ipsilateral hippocampus. In contrast, no significant change in overall p35 expression was observed at 2, 24 or 48 h post-CCI in either the ipsilateral or the contralateral side of the hippocampus (Fig. 3a and b). Furthermore, levels of Cdk5 were not changed at 2, 24 or 48 h post-CCI in either the ipsilateral or the contralateral side of the hippocampus (Fig. 3c and d).
Figure 3.
Quantitative immunoblot analysis of Cdk5 and its activators p35 and p25 in hippocampal lysates at various post-CCI time points. (a and b) Representative immunoblots probed with an anti-p35 carboxy-terminus antibody that detects p35, as well as p25 and corresponding quantification are shown. (c and d) Representative immunoblots probed with anti-Cdk5 antibody and corresponding quantification are shown. Immunoblotts were loaded with hippocampal lysates from ipsilateral (a and c) and contralateral (b and d) side from brains harvested at 2, 24 and 48 hours after CCI injury and control brains. All data are presented as mean ± SEM; n = 4–5; *p < 0.05, ***p < 0.001; ns = non significant; Student’s t-test.
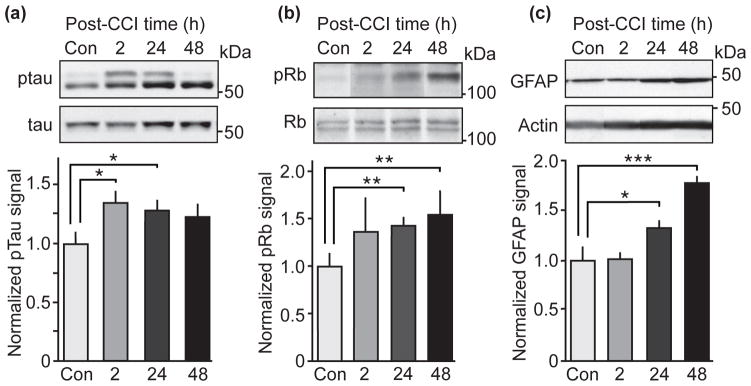
To examine whether the increase in p25 leads to aberrant Cdk5 activation, well-characterized aberrant Cdk5 phosphorylation sites on the microtubule-associated protein tau (Ser202) and retinoblastoma (Rb) protein (Ser807/811) were evaluated (Hashiguchi et al. 2002, Futatsugi et al. 2012, Hamdane et al. 2005, Pozo et al. 2013). Phosphorylation at Ser202 on tau was increased at 2, 24, and 48 h after CCI in lysates from ipsilateral hippocampus (Fig. 4a). The increase in Cdk5-dependent tau phosphorylation are in agreement with previous human postmortem studies showing elevated tau phosphorylation and formation of neurofibrillary tangles after head injury (McKee et al. 2009, Johnson et al. 2012). Consistent with the increase in tau phosphorylation, the levels of Rb protein phosphorylation were significantly elevated at 24 and 48 h and showed a trend towards higher phosphorylation levels at 2 h in lysates from ipsilateral hippocampus (Fig. 4b). No signal for phospho-Rb protein was detected in contralateral hippocampal lysate (data not shown). This effect is consistent with the identification of Rb protein as an aberrant Cdk5 substrate and observations that the invocation of cell cycle signaling characterizes the initiation of neurodegeneration pathology (Hisanaga & Asada 2012). Astrogliosis is a hallmark of CNS injury. The immunohistochemical analysis 24 h post-CCI showed elevated astrogliosis (Fig. 2a), accordingly glial fibrillary acidic protein (GFAP) expression, a marker of activated astrocytes, was significantly increased at 24 and 48 h post-CCI in ipsilateral (Fig. 4c), but not contralateral hippocampal lysate (data not shown).
Figure 4.
Analysis of Cdk5-dependent phosphorylation of tau and Rb protein and GFAP levels in hippocampal lysates at various post-CCI time points. (a, b and c) Quantitative immunoblot analysis from lysates of ipsilateral hippocampus at 2, 24 and 48 hours post-CCI probed for tau phosphorylation at Ser202 (CP13; Fig. a), Rb protein phosphorylation Ser807/811 (b) and GFAP levels (c). Representative immunoblots and corresponding quantifications are shown. Molecular weights are indicated in kDa on the right side of the immunoblots. All data are presented as mean ± SEM; n = 4–5; *p < 0.05, **p < 0.01, ***p < 0.001; Student’s t-test.
These results demonstrate that CCI induces the generation of p25 and aberrant Cdk5 activity as demonstrated by increased phosphorylation of Cdk5 substrates, tau and Rb protein. Altered phosphorylation of tau and Rb protein have been linked to pathophysiological processes.
Loss of Cdk5 attenuates CCI-induced neuronal damage
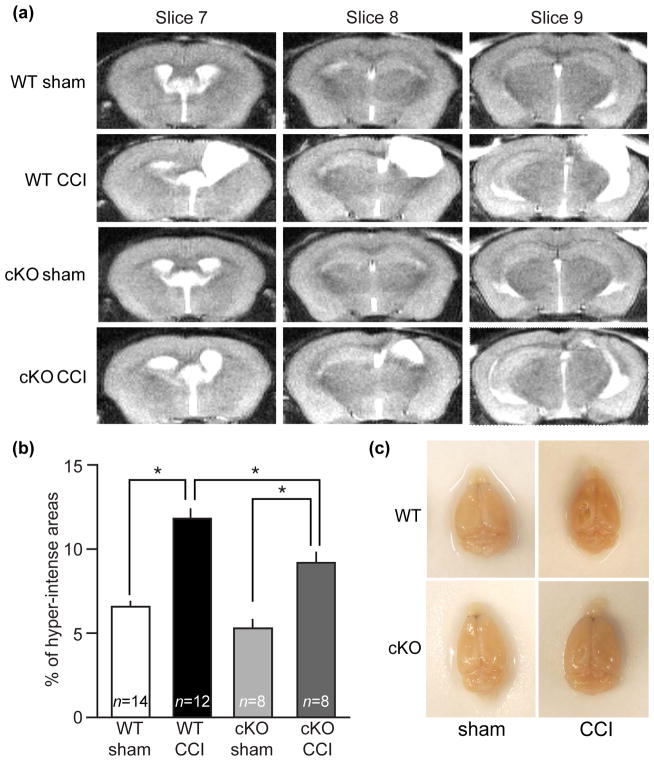
The biochemical analysis indicated that aberrant Cdk5/p25 activity may be involved in the pathogenesis of TBI. Therefore, the effect of Cdk5 loss on injury progression after CCI was investigated using a conditional Cdk5 knockout mouse model (Cdk5 cKO) that shows brain-wide reduction in Cdk5 expression (Hawasli et al. 2007). The size of CCI-induced brain injury was evaluated using high-resolution MRI at 28 days after CCI injury. The areas of brain injury were determined by volume measurement of hyper-intense regions reflective of high water content, which is observed in edema and dilated/expanded ventricles. CCI markedly increased the volume of hyper-intense areas in both Cdk5 cKO and WT mice in comparison to their respective sham procedure controls at 28 days post-CCI (Fig. 5a, b and c). However, the size of hyper-intense injured area was significantly reduced in Cdk5 cKO as compared to WT mice after CCI (Fig. 5a and b). A low level of background hyper-intense signaling was observed in sham animals that did not undergo CCI (Fig. 5a and b). Dissection of brains from Cdk5 cKO mice subjected to CCI confirmed the reduction in lesion size that was detected by MRI (Fig. 5c).
Figure 5.
Loss of Cdk5 attenuates CCI-induced lesion volume. (a) Representative MRI images of axial slices from conditional Cdk5 knockout mice (Cdk5 cKO) and wildtype littermates (WT), acquired at 28 days post-CCI. (b) Hyper-intense areas were expressed as percentage of total brain volume. (c) Top view of dissected brains from Cdk5 cKO and WT showing reduced lesion in Cdk5 cKO. All data are presented as mean ± SEM; WT sham n = 14, WT CCI n = 12, cKO sham n = 8 and cKO CCI n = 8; *p < 0.05, One-way ANOVA with Turkey’s multiple comparison test.
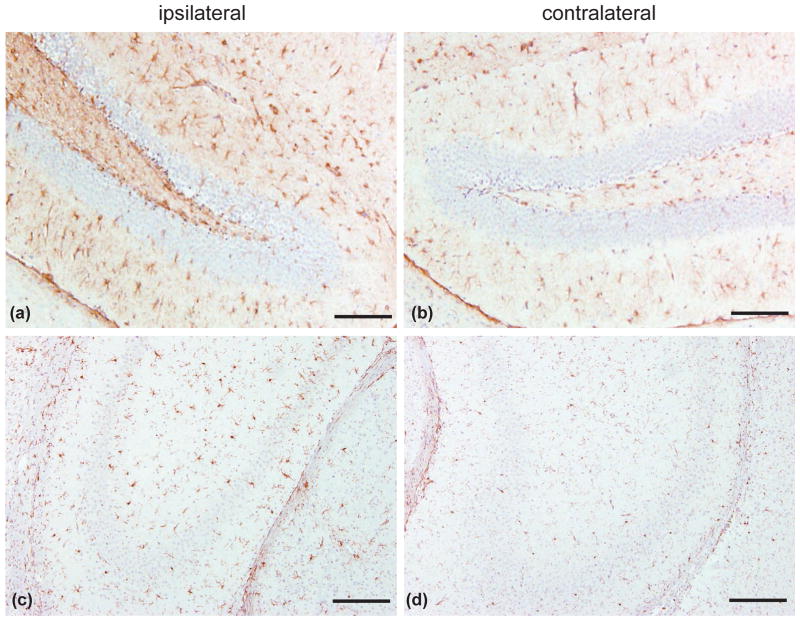
As observed with the WT controls (Fig. 2) the Cdk5 cKO mice showed increased neuroinflammation (Fig. 6). Twenty-four hours after CCI the Cdk5 cKO mice exhibited an increased number of reactive astrocytes in the ipsilateral hippocampus (Fig. 6a) as compare to the contralateral side (Fig. 6b). Moreover, Cdk5 cKO mice showed elevated levels of activated microglia in the ipsilateral hippocampus (Fig. 6c) as compared to the contralateral side (Fig. 6d).
Figure 6.
CCI induces neuroinflammation in Cdk5 cKO mice. (a and b) Neuroinflammation indicated by increased astrocyte activation in the ipsilateral hippocampal area of the dentate gyrus (DG) (a), as compared to the contralateral DG (b) using GFAP immunostaining. (c and d) Increased number of reactive microglia in the ipsilateral hippocampal CA3 region (c), as compared to contralateral CA3 (d) using Iba1 immunostaining. Scale bars: 100 μm.
Taken together these results show that loss of Cdk5 attenuates CCI injury suggesting that aberrant Cdk5 activity may contribute to pathophysiological processes underlying TBI. Interestingly, the level of neuroinflammation was not significantly affected by loss of Cdk5.
Loss of Cdk5 reduces CCI-induced deficits in synaptic responses
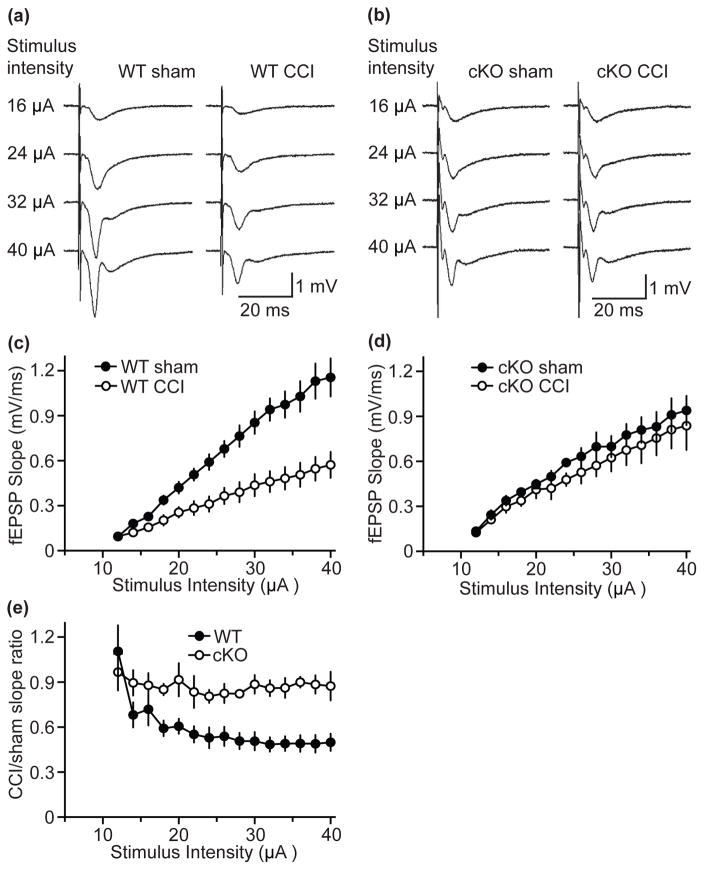
As loss of Cdk5 attenuated CCI-induced increase in brain injury volume, the impact of CCI on neurophysiology was assessed in Cdk5 cKO and WT mice using extra-cellular field recordings in hippocampal brain slices. WT animals subjected to CCI exhibited a significant reduction in maximal field excitatory postsynaptic potential (fEPSP) response as compared to sham treated WT mice 24 h after CCI (Fig. 7a). In contrast, no difference in fEPSP was seen between sham and CCI treated Cdk5 cKO (Fig. 7b). The results of the recordings were plotted as input–output curves and synaptic responses were measured by the rise slope of the fEPSP waveform (i.e., fEPSP slope) at the tested stimulus intensities. These results revealed a significant decrease in the fEPSP slope in WT mice subjected to CCI as compared to sham treated WT mice (Fig. 7c). In contrast, Cdk5 cKO mice exhibited no significant impairment in synaptic transmission after CCI, as the fEPSP did not fluctuate for KO TBI mice compared to KO sham (Fig. 7d). Further analysis showed that the ratio of the fEPSP slope from CCI-treated and sham WT mice was reduced by approximately 50% as compared to the ratio of the fEPSP slope from CCI-treated and sham Cdk5 cKO mice (Fig. 7e). These results suggest that hippocampal circuitry functioning was impaired as a result of CCI, and this impairment was in part mediated by aberrant Cdk5 function. Taken together, these findings are consistent with the role of Cdk5 in hippocampal synaptic function (Hawasli et al. 2007) and suggest a mechanistic and physiological basis for memory impairments associated with TBI.
Figure 7.
Brain injury diminishes synaptic responses in hippocampal CA3-CA1 pathway. (a and b) Traces of field excitatory postsynaptic potential (fEPSP) from hippocampal slices in response to graded stimulation in WT mice (a) and Cdk5 cKO (b). Mice have been subjected to CCI or sham procedure 24 hours prior to slice preparation. (c and d) Stimulus intensity/fEPSP slope relationship for WT subjected to CCI and WT sham (c) as well as for cKO CCI and cKO sham (d). (e) CCI/sham field EPSP slope ratio for Cdk5 cKO and WT mice. All data are presented as mean ± SEM; n = 5.
Discussion
In the present study, we report increased neuroinflammation, as well as formation of p25 in hippocampus following CCI injury. The increase in p25 induced aberrant Cdk5 activation as indicated by elevated Cdk5-dependent phosphorylation of microtubule-associated protein tau and Rb protein. The deleterious effects of CCI, including lesion formation and deficit in synaptic transmission were attenuated in mice with brain-wide Cdk5 reduction (Cdk5 cKO) as compared to WT controls. Taken together, these results suggest an important role for Cdk5 in the pathophysiological processes underlying TBI.
The CCI model of brain injury is a commonly used animal model of TBI that shows widespread damage, including acute cortical, hippocampal and thalamic degeneration (Hall et al. 2005). Astrocytes and microglia are considered the key players in initiating an inflammatory response after neuronal injury. Several studies conducted in CCI model reported the elevated expression and activation of astrocytes and microglia in both hippocampus and cortex (Villapol et al. 2014, Sandhir et al. 2008, Chen et al. 2003). Likewise here we have shown that CCI results in early ischemic neurons in both hippocampus and cortex, while the number of astrocytes and microglia differs markedly with higher GFAP and Iba1 staining in hippocampus at the site of injury.
Excitotoxicity is a common mechanism of secondary brain injury after TBI (Palmer et al. 1993). Elevated intracellular Ca2+-levels and activation of calpain have been reported in the affected brain region after TBI (Shapira et al. 1989, Fineman et al. 1993, Pike et al. 1998). Moreover, activated calpain causes conversion of p35 to p25, which in turn leads to mislocalization and deregulation of Cdk5 kinase activity and redirection toward aberrant substrates, initiating morphological degeneration and profound apoptotic cell death of primary neurons (Kusakawa et al. 2000, Lee et al. 2000, Nath et al. 2000, Patrick et al. 1999). Deregulation of Cdk5 by p25 is also thought to contribute to the etiology of several neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and stroke (Lee et al. 1999, Smith et al. 2003, Nguyen et al. 2001, Meyer et al. 2014). Cdk5/p25 has been reported to phosphorylate Rb protein at Ser807/811 both in vivo and in vitro (Lee et al. 1997, Hamdane et al. 2005, Pozo et al. 2013). In addition, it has been suggested that phosphorylation of Rb protein is an early event in p25/Cdk5-induced neurotoxicity (Hamdane et al. 2005). Another well-characterized Cdk5/p25 substrate is tau that can be phosphorylated at Ser202 by Cdk5/p25 (Hashiguchi et al. 2002). TBI is associated with tau hyperphosphorylation and neurofibrillary tangles accumulation (Johnson et al. 2012, McKee et al. 2009). Consequently, in our present study, CCI induces the formation of p25, as well as phosphorylation of the aberrant Cdk5 substrates, Rb protein and tau, thereby indicating the association of Cdk5/p25 hyper activation in CCI induced neurotoxic insult.
TBI leads to increased cell membrane permeability, ionic pump failure and cellular reabsorption of osmotically active solutes. These events consecutively activate intracellular water accumulation of neurons, astrocytes and microglia (Stiefel et al. 2005, Unterberg et al. 2004). The pathobiology of CCI injury reproduces brain edema in different head regions including hippocampus and cortex (Baskaya et al. 1997, Obenaus et al. 2007). Moreover rodent model of TBI results in a significant loss of hippocampal synaptic plasticity (Miyazaki et al. 1992, De Beaumont et al. 2012). Other studies have elucidated the inhibition of LTP in the CA1 region of the hippocampus in CCI induced brain injury model (Scheff et al. 2005). The present in vivo animal study demonstrates that Cdk5 cKO mice overcome CCI-induced deficits of synaptic transmission in the hippocampal CA1 region. Moreover, a significant reduction of hyper-intense regions was observed in Cdk5 cKO mice following CCI suggesting that loss of Cdk5 is neuroprotective. Consistent with this result, it has been reported that inhibition of Cdk5/p25, either by the use of pharmacological inhibitors or Cdk5 inhibitory peptide (CIP), can reduce apoptosis in cell culture and neuronal cell death in animal models of neurodegeneration and stroke (Zheng et al. 2005, Zheng et al. 2002, Sundaram et al. 2013, Meyer et al. 2014).
Collectively, the findings reported here support an important role for aberrant Cdk5 activity in mediating TBI and possibly launching the ensuing processes of neurodegeneration. Thus, aberrant Cdk5 may represent an attractive candidate for development of novel therapeutics for the treatment of TBI. Hence it is conceivable that an acute treatment strategy targeting aberrant Cdk5 may limit injury and improve recovery. While this initial study demonstrates that Cdk5 mediates TBI caused by CCI, which models some but not all types of TBI, it will be important to determine if this is the case for other forms of TBI. Also, it will be important to define the post-TBI window when anti-Cdk5 drugs might be most effective. Moreover, future studies aimed at further elucidating the mechanistic action of Cdk5 are essential for the development of selective and potent inhibitors with maximal effectiveness and minimal side effects.
Acknowledgments
We thank G. Mettlach for technical assistance, Dr. P. Davis for phospho-tau antibodies, and the Beatrice Mennen Haggerty Center for Research on Brain Injury and Repair in Strokes for facilitating this research. This work was supported by a Discovery Award from the Texas Institute for Brain Injury and Repair, and National Institutes of Health grants to J.A.B. (MH79710, MH083711, DA018343, DA033485, NS073855) and to S.G.K. (NS083077).
Abbreviation used
- ACSF
artificial cerebrospinal fluid
- APP
amyloid precursor protein
- CCI
controlled cortical impact
- Cdk5
cyclin-dependent kinase 5
- cKO
conditional knockout
- CIP
Cdk5 inhibitory peptide
- fEPSP
field excitatory post synaptic potential
- FJB
Fluoro-Jade B
- FSE
fast spin-echo
- GFAP
glial fibrillary acidic protein
- H&E
hematoxylin and eosin
- Iba1
ionized Ca2+-binding adapter molecule
- LTP
long-term potentiation
- Rb
retinoblastoma
- TBI
traumatic brain injury
Footnotes
Conflict of Interest Disclosure
The authors have no conflict of interest to declare and no competing financial interests exist.
References
- Albert-Weissenberger C, Siren AL. Experimental traumatic brain injury. Exp Transl Stroke Med. 2010;2:16. doi: 10.1186/2040-7378-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem. 2006;99:353–370. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- Baskaya MK, Rao AM, Dogan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia, 44 Suppl. 2003;10:2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol. 2003;182:87–102. doi: 10.1016/s0014-4886(03)00002-5. [DOI] [PubMed] [Google Scholar]
- Chen XH, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Chin WH, Chen Y, Ng YP, Ip NY. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007;5:e63. doi: 10.1371/journal.pbio.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Tremblay S, Poirier J, Lassonde M, Theoret H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb Cortex. 2012;22:112–121. doi: 10.1093/cercor/bhr096. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Jallo JJ, Tuma RF. An investigation of cerebral edema and injury volume assessments for controlled cortical impact injury. J Neurosci Methods. 2008;168:320–324. doi: 10.1016/j.jneumeth.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control 2010 [Google Scholar]
- Fineman I, Hovda DA, Smith M, Yoshino A, Becker DP. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Res. 1993;624:94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb prot4986. [DOI] [PubMed] [Google Scholar]
- Futatsugi A, Utreras E, Rudrabhatla P, Jaffe H, Pant HC, Kulkarni AB. Cyclin-dependent kinase 5 regulates E2F transcription factor through phosphorylation of Rb protein in neurons. Cell Cycle. 2012;11:1603–1610. doi: 10.4161/cc.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hamdane M, Bretteville A, Sambo AV, Schindowski K, Begard S, Delacourte A, Bertrand P, Buee L. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J Cell Sci. 2005;118:1291–1298. doi: 10.1242/jcs.01724. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Saito T, Hisanaga S, Hashiguchi T. Truncation of CDK5 activator p35 induces intensive phosphorylation of Ser202/Thr205 of human tau. J Biol Chem. 2002;277:44525–44530. doi: 10.1074/jbc.M207426200. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits AZ, Davies P. The regulation of tau phosphorylation by PCTAIRE 3: implications for the pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2006;23:398–408. doi: 10.1016/j.nbd.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Hisanaga S, Asada A. Cdk5-induced neuronal cell death: the activation of the conventional Rb-E2F G 1 pathway in post-mitotic neurons. Cell Cycle. 2012;11:2049. doi: 10.4161/cc.20536. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Kusakawa G, Saito T, Onuki R, Ishiguro K, Kishimoto T, Hisanaga S. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- Lee KY, Clark AW, Rosales JL, Chapman K, Fung T, Johnston RN. Elevated neuronal Cdc2-like kinase activity in the Alzheimer disease brain. Neurosci Res. 1999;34:21–29. doi: 10.1016/s0168-0102(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Lee KY, Helbing CC, Choi KS, Johnston RN, Wang JH. Neuronal Cdc2-like kinase (Nclk) binds and phosphorylates the retinoblastoma protein. J Biol Chem. 1997;272:5622–5626. doi: 10.1074/jbc.272.9.5622. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Richer E, Benkovic SA, et al. Striatal dysregulation of Cdk5 alters locomotor responses to cocaine, motor learning, and dendritic morphology. Proc Natl Acad Sci U S A. 2008;105:18561–18566. doi: 10.1073/pnas.0806078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DA, Torres-Altoro MI, Tan Z, et al. Ischemic stroke injury is mediated by aberrant Cdk5. J Neurosci. 2014;34:8259–8267. doi: 10.1523/JNEUROSCI.4368-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Katayama Y, Lyeth BG, Jenkins LW, DeWitt DS, Goldberg SJ, Newlon PG, Hayes RL. Enduring suppression of hippocampal long-term potentiation following traumatic brain injury in rat. Brain Res. 1992;585:335–339. doi: 10.1016/0006-8993(92)91232-4. [DOI] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Nath R, Davis M, Probert AW, Kupina NC, Ren X, Schielke GP, Wang KK. Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem Biophys Res Commun. 2000;274:16–21. doi: 10.1006/bbrc.2000.3070. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Robbins M, Blanco G, Galloway NR, Snissarenko E, Gillard E, Lee S, Curras-Collazo M. Multi-modal magnetic resonance imaging alterations in two rat models of mild neurotrauma. J Neurotrauma. 2007;24:1147–1160. doi: 10.1089/neu.2006.0211. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Marion DW, Botscheller ML, Swedlow PE, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Pike BR, Zhao X, Newcomb JK, Posmantur RM, Wang KK, Hayes RL. Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. Neuroreport. 1998;9:2437–2442. doi: 10.1097/00001756-199808030-00002. [DOI] [PubMed] [Google Scholar]
- Plattner F, Hernandez A, Kistler TM, et al. Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron. 2014;81:1070–1083. doi: 10.1016/j.neuron.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Castro-Rivera E, Tan C, et al. The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell. 2013;24:499–511. doi: 10.1016/j.ccr.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salib E, Hillier V. Head injury and the risk of Alzheimer’s disease: a case control study. Int J Geriatr Psychiatry. 1997;12:363–368. doi: 10.1002/(sici)1099-1166(199703)12:3<363::aid-gps515>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Shapira Y, Yadid G, Cotev S, Shohami E. Accumulation of calcium in the brain following head trauma. Neurol Res. 1989;11:169–172. doi: 10.1080/01616412.1989.11739885. [DOI] [PubMed] [Google Scholar]
- Sinclair RA, Burns J, Dunnill MS. Immunoperoxidase staining of formalin-fixed, paraffin-embedded, human renal biopsies with a comparison of the peroxidase-antiperoxidase (PAP) and indirect methods. J Clin Pathol. 1981;34:859–865. doi: 10.1136/jcp.34.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Smith PD, Crocker SJ, Jackson-Lewis V, et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel MF, Tomita Y, Marmarou A. Secondary ischemia impairing the restoration of ion homeostasis following traumatic brain injury. J Neurosurg. 2005;103:707–714. doi: 10.3171/jns.2005.103.4.0707. [DOI] [PubMed] [Google Scholar]
- Sundaram JR, Poore CP, Sulaimee NH, et al. Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo. J Neurosci. 2013;33:334–343. doi: 10.1523/JNEUROSCI.3593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Uryu K, Chen XH, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM, Trojanowski JQ, Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Byrnes KR, Symes AJ. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front Neurol. 2014;5:82. doi: 10.3389/fneur.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Baumgarten L, Trabold R, Thal S, Back T, Plesnila N. Role of cortical spreading depressions for secondary brain damage after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2008;28:1353–1360. doi: 10.1038/jcbfm.2008.30. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, Amin N, Albers W, Grant P, Pant HC. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Amin ND, Albers W, Pant HC. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]