Abstract
Objectives
About two-thirds of patients will remain on insulin therapy after total pancreatectomy with islet autotransplant (TPIAT) for chronic pancreatitis. We investigated the relationship between measured pancreas volume on computerized tomography (CT) or magnetic resonance imaging (MRI), and features of chronic pancreatiits on imaging, with subsequent islet isolation and diabetes outcomes.
Methods
CT or MRI was reviewed for pancreas volume (Vitrea software), and presence or absence of calcifications, atrophy, and dilated pancreatic duct in 97 patients undergoing TPIAT. Relationship between these features and: (1) islet mass isolated and (2) diabetes status at 1 year post-TPAIT were evaluated.
Results
Pancreas volume correlated with islet mass measured as total islet equivalents (r=0.50, p<0.0001). Mean islet equivalents was reduced by more than half if any one of calcifications, atrophy, or ductal dilatation were observed. Pancreatic calcifications increased the odds of insulin dependence 4.0 fold (1.1, 15). Collectively, the pancreas volume and 3 imaging features strongly associated with 1 year insulin use (p=0.07), islet graft failure (p=0.003), Hemoglobin A1c (p=0.0004), fasting glucose (p=0.027), and fasting C-peptide level (p=0.008).
Conclusions
Measures of pancreatic parenchymal destruction on imaging, including smaller pancreas volume and calcifications associate strongly with impaired islet mass and 1 year diabetes outcomes.
Keywords: total pancreatectomy, islet, islet autotransplant, TPIAT, chronic pancreatitis, pancreas
Introduction
Chronic pancreatitis (CP) is an often debilitating disease with few treatment options. While abdominal pain is the clinical hallmark of CP, inflammation and progressive fibrosis can damage the islets and lead to diabetes [1,2]. When patients fail medical and endoscopic management, surgery may be considered. Total pancreatectomy with islet auto-transplantation (TPIAT) is one treatment option for severely affected patients but presents a real risk of insulin dependent diabetes, particularly in those patients where islet recovery is low [3–5].
Our ability to accurately predict islet mass and subsequent diabetes outcomes before surgery remains quite limited [6]. Current imaging modalities provide detailed information about the structural damage to the exocrine pancreas, but no imaging test exists to directly measure islet mass. However, there is evidence that endocrine tissue loss occurs in parallel with progression to exocrine insufficiency, and thus greater damage to the exocrine pancreas may be associated with islet loss [7,8]. One preliminary study in 20 patients suggested that pre-operative magnetic resonance imaging (MRI) abnormalities, such as delayed interstitial phase, ductal dilation, and atrophy are correlated with diminished islet yield, and may be helpful in predicting risk for diabetes [9]. However, the relationship of these abnormalities to diabetes status was not studied.
We analyzed results of pre-operative imaging (MRI or computerized tomography (CT)), islet isolation, and 1 year diabetes outcomes in nearly 100 patients in our population over a 2 year interval to determine if these studies provided clinically useful information to predict success of islet isolation and diabetes outcomes including insulin independence, glycemic markers, and measures of islet graft function.
Materials and Methods
Subjects
Medical history, imaging, and laboratory data were reviewed for all patients undergoing TPIAT at the University of Minnesota between November 2010 and December 2012. Patients were enrolled in a prospective cohort study of islet transplant outcomes, reviewed and approved by the University of Minnesota Institutional Review Board. Informed consent was obtained from all patients.
The most recent pre-operative MRI or CT scan of the abdomen was identified. Only patients with at least one MRI or CT study with adequate images available in our system were included. There were 104 patients who had undergone TPIAT during the time interval of interest, of whom 97 patients had adequate imaging studies (47 MRI, 50 CT); when more than one imaging study was available, the study closest to the date of transplant was selected. Patients were screened by a multi-disciplinary working group for eligibility for TPIAT as previously described [10]. This included a spectrum of imaging findings of CP (variable abnormalities on CT, MRI, EUS, or pancreatic function tests) as well as patients with acute recurrent pancreatitis without or with early CP on imaging, and thus a wide spectrum of pancreatic parenchymal and/or ductal changes are represented in this CP cohort.
CT/MRI Review
Imaging studies were reviewed using Vitrea imaging software (Vitrea version 6.3, Vital Images, Minnetonka MN) to calculate pancreas volume. The CT and/or MRI study most proximal in time to surgery was selected for each patient. A single radiologist read all studies under the supervision of a certified body radiologist. The volume of the pancreas was calculated in the Vitrea program by tracing the pancreas in serial sections and reconstructing a 3D image. Presence or absence of parenchymal calcifications, atrophy, and dilation of the main pancreatic duct was recorded. Atrophy was based on the assessment of the interpreting radiologist; main pancreatic duct dilatations were conventionally defined by >3mm in the head and >2mm in the tail for adults, and at the discretion of the radiologist for children.
Islet Isolation
Islet isolation was performed in the University of Minnesota Molecular and Cellular Therapeutics GMP facility using previously published methods [10,11]. The trimmed pancreas mass before tissue digestion was recorded. The yield of islets was quantified as islet equivalents (IEQ), which is a volumetric conversion of the islet mass based on a standard 150-μm diameter islet. The IEQ/ kilogram recipient body weight (IEQ/kg) and IEQ per gram of pancreas tissue (IEQ/gm) are also calculated. The digested tissue volume (TV) was recorded.
Post-transplant Islet Function
Following islet transplantation, insulin therapy is initiated in all patients at our institution and only weaned as tolerated after 3 months post-transplant. The following goals are targeted for insulin independence: Hemoglobin A1c (HbA1c) ≤6.5%, fasting blood glucose <126 mg/dL, and 2 hour post-prandial blood glucoses <180 mg/dL.
At 1 year post-transplant, insulin use is collected by clinical follow up visit and/or routine health questionnaires sent to all participants and including questions about insulin use, frequency, and dose. Patients are defined as insulin independent only if free of all exogenous insulin use. Graft function is defined as full function in insulin independent patients, partial in patients with graft function (C-peptide ≥0.6 ng/mL fasting or stimulated, or, in the absence of C-peptide data ability to maintain goal glycemic control on a basal-only insulin regimen), or graft failure (C-peptide <0.6 ng/mL, or basal-bolus insulin regimen when C-peptide is unknown). The following labs are recommended yearly for all patients: HbA1c, fasting glucose, fasting and stimulated C-peptide on 3 sample mixed meal tolerance test (Boost HP 6 mL/kg, C-peptide at fasting, 1 hour, 2 hours).
Statistical Analysis
Patient population demographics are reported as mean ± standard deviation; all other results are presented as mean ± standard error. Mean values were compared using student t-tests. Categorical associations were compared with Fisher exact tests. Linear regression was used to measure impact of pancreas volume on islet mass. Logistic regression analyses were performed to model the continuous predictor variable of pancreas volume against insulin independence at 1 year (yes/no) and Fisher exact test for univariate analyses of categorical predictors (calcifications, ductal dilatation, or atrophy) for insulin independence (yes/no). Because of the intercorrelated nature of the imaging findings, for analysis of 1 year outcomes with imaging features, we also constructed an index from the 4 components—called the “first principal component”. The statistical procedure and rationale for constructing such a model is further described in online Supplement 1.
Sub-analyses were performed using the 50 patients with CT scan only for pancreatic calcifications; and using the 47 patients with MRI scan only for ductal changes. These were undertaken because of the differential sensitivity of these imaging studies for the respective findings, and were performed identical to those for the entire cohort for the specified imaging procedures. Because these sub-analyses yielded nearly identical results as the entire cohort, only the pooled analyses using the entire cohort are presented
P-values ≤0.05 were considered significant. All analyses were performed using JMP software (SAS Institute, Cary, NC).
Results
Patient characteristics
Patient characteristics are displayed in Table 1. Patients had a mean age of 31.7 ± 14.9 years, and 75 (77%) were female. Pancreatitis-predisposing genetic mutations and idiopathic disease were the most common cause of disease. Eight patients (8%) had diabetes diagnosed before TPIAT. Imaging studies were obtained on average 150 ±128 days prior to surgery.
Table 1.
Characteristics of patient population (n=97)
| Age (years) | 31.7 ± 14.9 |
| Gender, Female | 75 (77%) |
| Body Mass Index (BMI, kg/m2) | 23.8 ± 6.3 |
| Etiology | |
| Genetic/ Familial | 37 (38%) |
| Pancreas Divisum | 13 (13%) |
| Sphincter of Oddi dysfunction | 14 (14%) |
| Idiopathic | 27 (28%) |
| Other | 6 (6%) |
| Duration of Disease (years) | 7.1 ± 8.1 |
| Pre-TPIAT Diabetes Mellitus | 8 (8%) |
| Previous pancreatic surgery | 7 (7%) |
| Preoperative HbA1c (%) | 5.4 ± 0.75 |
| Preoperative Fasting Plasma Glucose (mg/d) | 91 ± 16 |
| Islet Equivalents (IEQ) | 262,333 ± 164,288 |
| IEQ/ kg | 4,308 ± 2689 |
Data expressed as mean ± standard deviation
Prevalence of imaging abnormalities
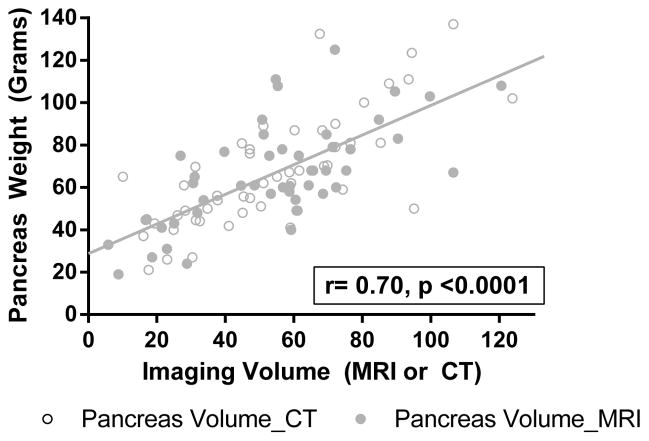
Volume was estimated in all patients by MRI or CT using Vitrea software. The imaging estimated volume correlated strongly with measured pancreas weight (measured by the islet isolation team immediately following pancreatectomy, r= 0.70, p <0.0001, Figure 1) supporting that this measure was an accurate means to estimate the pancreas volume prior to resection. Pancreatic parenchymal calcifications were diagnosed in 23 patients, atrophy in 38 patients, and dilated main pancreatic duct in 30 patients.
Figure 1.
Correlation of pancreas volume by imaging against measured pancreas weight in lab, indicating MRI (grey circle) and CT (open circles).
These features were highly associated with one another; specifically: calcifications were more likely to be seen in an atrophic pancreas (p=0.0002), dilated pancreatic duct was more common in an atrophic pancreas (p<0.0001), and dilated pancreatic duct was more common in a calcified pancreas (p=0.004). Furthermore, mean pancreatic volume measured on imaging was lower when atrophy as present (31.0 ±2.7 mL vs 69.3 ±2.2 mL, p<0.0001), when calcifications were present (43.5 ±5.1 mL vs 57.0 ±2.9 mL, p=0.02), and when the pancreatic duct was dilated (39.4 ±4.2 mL vs 60.4 ±2.8 mL, p<0.0001).
Of note, hereditary/genetic etiology of disease was independently associated with lower pancreas volume (p=0.005), and increased risk for calcifications (p=0.026), atrophy (p=0.0004), and ductal dilatation (p=0.003) compared with other causes of pancreatitis. A longer duration of diagnosed pancreatitis was similarly associated with lower pancreas volume (p=0.007) and in logistic regression modelling with presence of atrophy (p=0.001) and calcifications (p=0.016). Our experienced islet lab personnel assign each pancreas a fibrosis score at the time of pancreatectomy, on a scale of 0–10, with 10 representing the most severe fibrosis. In multivariate modelling adjusted for the age and sex of patient, and etiology and duration of pancreatitis, more severe fibrosis was significantly associated with lower pancreas volume (p=0.014), and with the findings of calcifications (p=0.01) and dilated pancreatic duct (p=0.02). This suggests, as might be expected, that these features are markers for the severity of pancreatic fibrosis.
Relationship between the imaging features and islet isolation outcomes
Pancreas volume measured by imaging was significantly correlated with islet mass as measured by total IEQ isolated (R2= 0.25, p<0.0001) and less strongly with IEQ/kg (R2= 0.156, p<0.0001). For each 10 mL reduction in pancreas volume, islet mass was estimated to be reduced by 33,067 IEQ and 421 IEQ/kg. Presence of atrophy, pancreatic calcifications, and dilation of the main pancreatic duct were all individually associated with lower islet mass (Table 2); in fact, mean total IEQ was reduced by more than half if any of these features were present.
Table 2.
Mean islet mass by presence or absence of atrophy, calcifications, and ductal dilatation
| Total IEQ | p-value | ||
|---|---|---|---|
| Feature Present | Feature Absent | ||
|
| |||
| Atrophy | 154,721 ± 22,842 | 333,950 ± 18,489 | <0.0001 |
| Calcifications | 139,296 ± 31,423 | 300,637 ± 17,368 | <0.0001 |
| Pancreatic Duct Dilatation | 138,159 ± 35,965 | 317,934 ± 17,374 | <0.0001 |
| IEQ/kg | |||
|---|---|---|---|
| Feature Present | Feature Absent | ||
|
| |||
| Atrophy | 2,854 ± 394 | 5,287 ± 319 | <0.0001 |
| Calcifications | 2,563 ± 527 | 4,866 ± 295 | 0.0002 |
| Pancreatic Duct Dilatation | 2,937 ± 463 | 4,921 ± 310 | 0.0006 |
Data expressed as mean ± SE
Subsequently, in a multivariate analysis including volume as a continuous variable, and atrophy, calcifications, and ductal dilatation as categorical variables, the total IEQ remained significantly associated with volume (p=0.03), calcifications (p=0.01), and ductal dilatation (p=0.002). This model could predict approximately 43% of the variability in islet mass (R2 = 0.43). Results were unchanged when adjusted from potential confounding variables of gender, age, duration of disease, and etiology. Interestingly, in a multivariate model for IEQ/kg, only pancreas calcifications remained significantly associated with IEQ/kg (that is, the islet mass for the patient size, p=0.03, model R2= 0.27).
As might be expected, tissue volume correlated with pancreas volume (p<0.0001, R2=0.37), and was also lower in the presence of structural changes of atrophy, calcifications, and fibrosis. The islet mass isolated per gram tissue (IEQ/gm) was not correlated with volume, but was lower in those with atrophy, calcifications, or dilated duct.
Because patients had a variable interval between the preoperative imaging and surgery, we repeated analyses adjusting for the time duration between imaging and surgery. Time from imaging to surgery had little effect on results and did not change the statistical significance.
Relationship between imaging and islet graft function at 1 year after TPIAT
Sufficient outcomes data were available for 91 of 97 patients at 1 year. These 91 patients resembled the original 97 in age, gender, duration, and etiology of disease. Thirty-two patients (35%) were insulin independent; these same 32 patients were considered to have full graft function, 48 (49%) partial graft function, and 11 (12%) graft failure (indicating full insulin dependence with minimal islet function).
Logistic regression modelling was used to determine association of imaging measured pancreas volume before TPIAT with insulin independence and islet graft function at 1 year. Pancreas volume was significantly associated with insulin use, with an OR of 0.59 (95% CI, 0.36–0.95)—that is for each one standard deviation increase in pancreas volume (equivalent to 25 mL), the odds of needing insulin therapy are reduced by a factor of 0.59. For islet graft function, the OR for partial function (vs full function) was 0.67 (0.41–1.1) and the OR for graft failure (vs full function) was 0.26 (0.10–0.66).
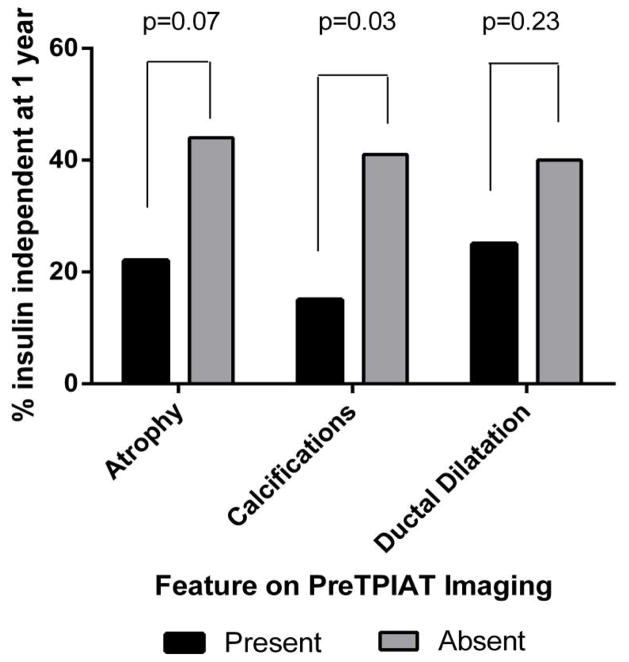
Those with atrophy and calcifications were more likely to require insulin therapy at 1 year than those without these same features: For atrophy OR for insulin dependence was 2.6 (1.0 –6.8), for calcifications OR was 4.0 (1.1–15). Ductal dilatation was not a significant predictor of requiring insulin at 1 year, however, with OR of 2.0 (0.7 – 5.3). The proportions of patients who were insulin independent by imaging feature are displayed in Figure 2.
Figure 2.
Proportion of patients achieving insulin independence by absence or presence of imaging features of atrophy, calcifications, and ductal dilatation
Pancreas volume was weakly and negatively correlated with 1 year HbA1c level (R2= 0.16, p=0.0002). Those with atrophy and/or calcifications had higher HbA1c on average, and lower fasting and stimulated C-peptide at 1 year; those with ductal dilatation had higher HbA1c and lower fasting C-peptide at 1 year (Table 3). These results were not significantly changed by adjusting for the time duration between imaging and TPIAT surgery.
Table 3.
Labs for glycemic control and islet function at 1 year by features on imaging at preoperative baseline
| Atrophy | Calcifications | Ductal Dilitation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Present | Absent | p-value | Present | Absent | p-value | Present | Absent | p-value | |
| HbA1c level (%), n=81 | 7.08 ± 0.21 | 5.87 ± 0.16 | 0.001 | 6.98 ± 0.32 | 6.15 ± 0.16 | 0.06 | 7.00 ± 0.24 | 6.01 ± 0.16 | 0.01 |
| Fasting Glucose (mg/dL), n=78 | 117 ± 5.9 | 101 ± 4.8 | 0.07 | 115 ± 8.0 | 104 ± 3.9 | 0.2 | 124 ± 6.3 | 98 ± 3.9 | 0.03 |
| Fasting C-peptide (ng/mL), n=72 | 0.67 ± 0.11 | 1.16 ± 0.08 | 0.001 | 0.63 ± 0.17 | 1.10 ± 0.08 | 0.02 | 0.77 ± 0.13 | 1.10 ± 0.08 | 0.04 |
| Stimulated C-peptide (ng/mL), n=70 | 2.25 ± 0.30 | 2.98 ± 0.22 | 0.05 | 1.79 ± 0.41 | 2.95 ± 0.19 | 0.01 | 2.27 ± 0.33 | 2.90 ± 0.20 | 0.11 |
Multivariate and adjusted models of imaging with 1 year outcomes: The poor diabetes outcomes seen with more severe disease on imaging are largely mediated by the lower islet mass
When all 4 imaging features—volume, atrophy, calcifications, and ductal dilatation—are considered together collectively, these features are strongly associated with insulin use (p=0.07), islet graft function/failure (p=0.003), HbA1c (p=0.0004), fasting glucose (p=0.027), and fasting C-peptide (p=0.008), and trended towards association with stimulated C-peptide (p=0.079). However, the significance of the individual features is lost, which we postulate is due to the highly associated nature of these for predictor variables (volume, atrophy, calcifications, duct dilatation) with one another. To resolve this apparent paradox, an index was constructed from the 4 components—called the “first principal component” (see supplement 1); this collective measure is highly associated with each outcome and is used for the multivariate analysis. When the models (using the first principal component) were adjusted for other confounding features including duration of disease, etiology, BMI, and islet mass transplanted (IEQ), the significance of the imaging features was lost. Islet mass (IEQ) was a significant predictor in the adjusted analyses for all outcomes. This does not mean that the imaging features were unimportant, but rather, as expected, that they reflect a damaged pancreas with elevated risk of low islet mass at isolation and therefore worse diabetes outcomes.
Discussion
Total pancreatectomy with islet autotransplantation is a valuable therapeutic option for patients with intractable painful chronic pancreatitis who have failed medical and endoscopic therapies [4,12]. However, patients must be willing to accept post-operative diabetes, since as many as two-thirds of patients will remain on insulin for the remainder of life after the procedure [5,13–17]. We postulated that imaging features of more severe disease (i.e. more severe fibrosis and islet loss) could aid in identifying patients at particularly high risk of poor diabetes outcomes. By reviewing clinically available MRI and CT studies performed shortly prior to TPIAT, we demonstrated in this report that: (1) automated software measurements to estimate pancreas volume on CT or MRI imaging are reflective of the actual size of the pancreas as measured after total pancreatectomy; (2) lower pancreas volume or presence of atrophy, calcifications, and/or ductal dilatation are all risks for low islet mass at the time of TPIAT; and (3) these same features predict a higher risk of insulin dependence, worsened glycemic control, and impaired graft function at 1 year, largely mediated by isolated and transplanted islet mass at time of isolation.
These findings build upon previously reports of relationship between MRI findings and islet yield [9], are clinically applicable to counseling the TPIAT recipient, and address an important research gap recently identified by experts in the field of TPIAT, to better define markers for diabetes risk after TPIAT [18]. Calcifications in particular increased the odds of insulin dependence greatly—those with parenchymal calcifications were at 4 fold greater risk of remaining insulin dependent at 1 year after the surgery compared to those without. Only 15% of those patients with calcifications were later insulin independent. Low pancreas volume or atrophy were also a risk for insulin dependence. This is not to say that the patient with calcific pancreas should not have TPIAT; rather these patients should have a realistic expectation of the surgical result. While likely to still have islet graft function (as the majority in our series did), lifelong supplemental insulin is also likely to be required. The most logical explanation for this finding is that the islet mass is compromised by extensive pancreatic fibrosis, as clearly evidenced in our series by the lower islet yields in these patients. However, we cannot exclude that there was also a functional impairment of the remaining beta cells, as has been postulated to occur from intra-pancreatic inflammation by some investigators [19].
Reassuringly, we did see in most recipients that islet function and a metabolic benefit of islet function is present even when supplemental insulin is required, as evidenced by the mean values for HbA1c, glucose, and C-peptide at 1 year. Even when evidence of fibrosis and damage are apparent on imaging, these patients maintained, on average, a hemoglobin A1c level below 7%, the target for diabetes control set by the American Diabetes Association, regardless of need for supplemental insulin [20]. Nearly 9 out of every 10 TPIAT recipients have islet graft function, using a relatively conservative definition for islet graft function. Thus, even though these results highlight an advantage to potentially earlier intervention in those patients clearly debilitated by chronic pancreatitis, before pancreatic calcifications and atrophy are present, an IAT is still beneficial to preserve endogenous beta cell function even when extensive parenchymal destruction is already evident.
In this study, we used imaging software to estimate the size of the pancreas. Rarely do we then have the opportunity to measure pancreas size directly, as we do in this case with the pancreas resection. The islet isolation lab routinely weighs the pancreas prior to the islet isolation. We identified a strong correlation between the pancreas volume on imaging (mL) and weight in the lab (grams). Thus, these imaging approaches may be a reasonable tool for an isolation team to estimate the size of the pancreas before surgery, which may be useful to planning for the doses of collagenase / neutral protease enzymes which are dosed on pancreas size for islet isolation, as well as to anticipate where tissue volume may be higher.
For this study, we used the imaging studies that were readily available to us. In some cases, this was a MRI scan, and in others a CT. Recognizing that the sensitivity of MRI or CT differ for various features— in particular calcifications are much more readily observed by CT and ductal changes by MRI, particularly when findings are subtle—we performed subanalyses for these two features. When evaluating calcifications only in the subgroup with CT scans (about half the cohort), we found qualitatively the same results with very little quantitative difference in risk. Similarly, when applying the ductal changes to only those patients with MRI, the same significant findings were present (data not shown). Because these findings were similar, we present results for the entire cohort for brevity, pooling MRI and CT scan results. This certainly apples to the “real world” scenario, where providers seeing a patient in consultation may have only one study or the other available to them. However, one may bear in mind the limitations of detecting features by MRI or CT when applying this information to the TPIAT patient in front of them in clinic.
As this is a retrospective study, additional limitations include the possibility of changes in imaging features during the time from assessment to TPIAT, although the vast majority of the studies were done <7 months prior to TPIAT and so dramatic change seems unlikely. This is supported by the observation that adjusting for time from imaging study to TPIAT surgery resulted in similar results. Such measures could be incorporated into future prospective studies for validation. Interestingly, very few of these patients had both recent CT and MRI images and so we were not able to make direct comparisons of these two measures. While age did not seem to impact results in our cohort, the majority of patients were adults; children have more predominately genetic causes of pancreatitis and may have a different disease course then adults [21], and so could be considered separately from adults in a larger cohort.
In conclusion, we found that pancreas size, atrophy, calcifications, and/or ductal dilatation seen on CT or MRI imaging provided prognostic information for islet isolation results and 1 year diabetes outcomes in patients undergoing TPIAT for chronic or recurrent acute pancreatitis. Calcifications in particular conveyed a 4-fold increased odds of insulin dependence. A smaller measured volume pancreas and/or atrophy also predicted higher rates of insulin use. These findings should not prohibit TPIAT in patients with severe chronic pancreatitis but do highlight the potential detrimental effect of delaying surgery as more progressive structural damage occurs in the pancreatic parenchyma, and raises some simple risk factors that can aid in counseling the patient referred for consideration of TPIAT.
Supplementary Material
Acknowledgments
Funding: Support was provided by NIDDK grant K23DK084315 (PI Bellin)
Footnotes
Disclosures: The authors declare no conflicts of interest.
References
- 1.Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss med weekly. 2006;136:166–174. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- 2.Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19:7276–7281. doi: 10.3748/wjg.v19.i42.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409–424. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellin MD, Balamurugan AN, Pruett TL, et al. No islets left behind: islet autotransplantation for surgery-induced diabetes. Curr Diab Rep. 2012;12:580–586. doi: 10.1007/s11892-012-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson GC, Sutton JM, Abbott DE, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg. 2014;260:659–665. doi: 10.1097/SLA.0000000000000920. discussion 665–657. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg R, Beilman GJ, Dunn TB, et al. Metabolic Assessment Prior to Total Pancreatectomy and Islet Autotransplant: Utility, Limitations and Potential. Am J Transplant. 2013;13:2664–2671. doi: 10.1111/ajt.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domschke S, Stock KP, Pichl J, et al. Beta-cell reserve capacity in chronic pancreatitis. Hepatogastroenterology. 1985;32:27–30. [PubMed] [Google Scholar]
- 8.Nyboe Andersen B, Krarup T, Thorsgaard Pedersen NT, et al. B cell function in patients with chronic pancreatitis and its relation to exocrine pancreatic function. Diabetologia. 1982;23:86–89. doi: 10.1007/BF01271165. [DOI] [PubMed] [Google Scholar]
- 9.Khan KM, Desai CS, Kalb B, et al. MRI Prediction of Islet Yield for Autologous Transplantation After Total Pancreatectomy for Chronic Pancreatitis. Dig Dis Sci. 2013;58:1116–24. doi: 10.1007/s10620-012-2448-1. [DOI] [PubMed] [Google Scholar]
- 10.Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:793–799. doi: 10.1016/j.cgh.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blondet JJ, Carlson AM, Kobayashi T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. 2007;87:1477–1501. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Bellin MD, Freeman ML, Gelrud A, et al. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology. 2014;14:27–35. doi: 10.1016/j.pan.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Desai KD, Dong H, et al. Prior surgery determines islet yield and insulin requirement in patients with chronic pancreatitis. Transplantation. 2013;95:1051–1057. doi: 10.1097/TP.0b013e3182845fbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston PC, Lin YK, Walsh RM, et al. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab. 2015;100:1765–70. doi: 10.1210/jc.2014-4298. [DOI] [PubMed] [Google Scholar]
- 16.Savari O, Golab K, Wang LJ, et al. Preservation of Beta cell function after pancreatic islet autotransplantation: university of chicago experience. Am Surg. 2015;81:421–427. [PubMed] [Google Scholar]
- 17.Webb MA, Illouz SC, Pollard CA, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas. 2008;37:282–287. doi: 10.1097/mpa.0b013e31816fd7b6. [DOI] [PubMed] [Google Scholar]
- 18.Bellin MD, Gelrud A, Arreaza-Rubin G, et al. Total pancreatectomy with islet autotransplantation: summary of a National Institute of Diabetes and Digestive and Kidney diseases workshop. Pancreas. 2014;43:1163–1171. doi: 10.1097/MPA.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasikala M, Talukdar R, Pavan kumar P, et al. beta-Cell dysfunction in chronic pancreatitis. Dig Dis Sci. 2012;57:1764–1772. doi: 10.1007/s10620-012-2086-7. [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr. 2015;166:890–896 e891. doi: 10.1016/j.jpeds.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.