Abstract
Objective
We assessed circulating tumor cells (CTCs) with epithelial and mesenchymal phenotypes as a potential prognostic biomarker for patients with pancreatic adenocarcinoma (PDAC).
Background
PDAC is the fourth leading cause of cancer death in the United States. There is an urgent need to develop biomarkers that predict patient prognosis and allow for better treatment stratification.
Methods
Peripheral and portal blood samples were obtained from 50 patients with PDAC before surgical resection and filtered using the Isolation by Size of Epithelial Tumor cells method. CTCs were identified by immunofluorescence using commercially available antibodies to cytokeratin, vimentin, and CD45.
Results
Thirty-nine patients (78%) had epithelial CTCs that expressed cytokeratin but not CD45. Twenty-six (67%) of the 39 patients had CTCs which also expressed vimentin, a mesenchymal marker. No patients had cytokeratin-negative and vimentin-positive CTCs. The presence of cytokeratin-positive CTCs (P < 0.01), but not mesenchymal-like CTCs (P = 0.39), was associated with poorer survival. The presence of cytokeratin-positive CTCs remained a significant independent predictor of survival by multi-variable analysis after accounting for other prognostic factors (P < 0.01). The detection of CTCs expressing both vimentin and cytokeratin was predictive of recurrence (P = 0.01). Among patients with cancer recurrence, those with vimentin-positive and cytokeratin-expressing CTCs had decreased median time to recurrence compared with patients without CTCs (P = 0.02).
Conclusions
CTCs are an exciting potential strategy for understanding the biology of metastases, and provide prognostic utility for PDAC patients. CTCs exist as heterogeneous populations, and assessment should include phenotypic identification tailored to characterize cells based on epithelial and mesenchymal markers.
Keywords: circulating tumor cells, CTCs, epithelial–mesenchymal transition, metastases, pancreatic adenocarcinoma, prognosis
Pancreatic adenocarcinoma (PDAC) is the fourth leading cause of cancer mortality in the United States, with an estimated 48,960 new cases diagnosed in 2015.1 At this time, surgical resection offers the best chance for meaningful long-term survival with overall 5-year survival rates as high as 25% after resection.2,3 However, most patients are diagnosed only after the tumor has metastasized and as a result are not operative candidates.2 Additional challenges remain even in those patients with early stage disease, as there are currently no methods to stratify a patient’s risk for metastasis to help guide neoadjuvant and adjuvant therapies. The current use and timing of chemoradiation therapy is highly dependent on tumor resectability. Patients with borderline resectable PDAC will often receive chemoradiation therapy before surgery to increase the likelihood of a margin-negative (R0) resection, whereas those with unresectable, nonmetastatic tumors will undergo systemic therapy to prevent disease spread and achieve conversion to surgical resectability.4,5 In contrast, patients with resectable tumors are usually taken immediately for pancreatic resection.6 Even with resection, the majority of patients will progress to local or distant tumor recurrence, and it is often difficult to determine which patients may benefit from neoadjuvant chemotherapy to prevent early recurrence after resection.7,8 One possible strategy to improve outcomes in pancreatic cancer is to understand better the process of metastasis, and to identify biomarkers to stratify patients for treatment based on prognosis and metastatic potential.9
Circulating tumor cells (CTCs) are defined as neoplastic cells shed from a solid tumor into the blood.10–13 Several studies have identified CTCs as a potential minimally invasive mechanism to analyze a patient’s primary tumor and their subsequent risk of developing metastasis.14–17 CTCs have been identified in the blood of many patients with malignant neoplasms, but only rarely in healthy controls.18–20 Given their location in the vasculature, CTCs are believed to be a potential source of distant metastases, and their presence has been associated with poor survival in several tumor types.10,18,19,21 Indeed, a decreased CTC number after chemotherapy portends a more favorable outcome for patients with colorectal and breast cancer.10,22 CTCs have been identified in the blood of patients with all stages of PDAC, and previous studies have found an association between the presence of CTCs and poorer survival.11,13,23–27 However, most studies have identified CTCs using the epithelial marker cytokeratin, with only limited reports of further phenotypic characteristics of CTCs in PDAC.27,28
Cancer cells often lose some of their epithelial characteristics and gain features of a more mesenchymal phenotype, a phenotype termed “epithelial-to-mesenchymal transition.”29,30 Epithelial-to-mesenchymal transition allows for increased mobility and invasion and is thought to facilitate metastasis.29–31 CTCs from patients with several cancer types have been shown to express traditional mesenchymal markers, such as vimentin.32–34 One large study of patients with metastatic prostate and breast cancer found most patients with CTCs had CTCs co-expressing epithelial and mesenchymal markers.34 In addition, a study comparing patients with early-stage vs metastatic breast cancer found a statistically higher detection of vimentin-positive and pan-cytokeratin-positive CTCs in those with metastases, suggesting a strong association between the presence of mesenchymal CTCs and metastatic potential.35
Studies in multiple tumor types including PDAC have demonstrated that vimentin expression in primary tumors is associated with disease recurrence, metastases, and shorter survival.36,37 The majority of studies investigating CTCs in patients with PDAC use epithelial marker-based selection of CTCs, typically using the Cell-Search platform or flow cytometry.24 However, other groups have demonstrated that isolating CTCs based on size and morphology can more accurately detect CTCs.38 The purpose of this study was to characterize CTCs in the blood of patients with clinically resectable PDAC using epithelial and mesenchymal markers and to determine the impact of CTCs on recurrence risk and overall survival (OS).
METHODS
Patient Selection
The study included 50 consecutive patients with PDAC treated at the Johns Hopkins Hospital between June 1, 2013 and October 9, 2014, who consented for peripheral and/or portal blood collection before surgical resection. All patients gave written informed consent for blood sample donation. Between 5 and 10 mL of venous and/or arterial blood was collected before incision for resection by pancreaticoduodenectomy, distal pancreatectomy with splenectomy, or total pancreatectomy. In a subset of patients, between 5 and 10 mL of portal venous blood was also collected after surgical incision but before manipulation and removal of the pancreatic tumor. Portal blood was only collected in patients who signed an additional consent form for its collection. The charts of all 50 patients were reviewed, and information on patient demographics, tumor histopathology, perioperative and surgical factors, and chemoradiation therapy was collected. The pathology of resected tumors was reviewed by a trained pathologist, and included an analysis of tumor stage, grade, nodal status, perineural invasion, perivascular invasion, margin status, and the presence of precursor lesions such as intraductal papillary mucinous neoplasms and pancreatic intraepithelial lesions in the resected pancreas.
Patients were followed with a standard postoperative protocol, with routine postoperative clinic visits every 3 to 6 months with their surgeon in addition to regular visits with a medical oncologist. Patients who were followed postoperatively at the Johns Hopkins Hospital underwent routine imaging every 3 to 6 months including computed tomography of the chest, abdomen, and pelvis to monitor for tumor recurrence, whereas patients receiving treatment at outside institutions underwent imaging at a similar interval based upon guidelines. The decision to receive neoadjuvant or adjuvant therapy was determined by the patient and the medical oncologist, independent of the results of the CTC analyses, with recommendations often given by the surgeon. Recurrence was determined by the presence of clinically recognizable local or metastatic disease on imaging.
CTC Filtration
Samples were processed and filtered using the Isolation by Size of Epithelial Tumor Cells method (Rarecells, France). Blood samples were processed within 6 hours of collection in accordance with a previously described protocol by Rarecells.39 Isolation buffer was prepared by mixing all 3 buffer samples with ultra-filtrated water and brought to a pH between 7.2 and 7.4 with 1 M sodium hydroxide. Blood samples were diluted with isolation buffer and mixed with formaldehyde before undergoing filtration on the ISET machine, which separates components based upon size using 8 μm-sized pores. After filtration, samples were stored at −20°C until staining or analysis.
Hematoxylin and Eosin (H&E) Staining
Membranes were rehydrated in 1X phosphate-buffered saline for 5 minutes before staining. Membranes were submerged in hematoxylin for 3 minutes, which was then removed before washing membranes in deionized water. Membranes were then submerged in eosin for 1 minute, which was then removed before washing membranes in deionized water. Membranes were affixed to a slide with 10% glycerol. Cell counts by H&E were performed by a single pathologist within 2 days of staining the slides. A cell was identified as a CTC if it met the following criteria: CTC diameter over 15 μm in size and greater than 2 times the size of nearby leukocytes, nuclear membrane irregularities, and a lack of cytoplasmic granules.
Immunohistochemistry of Primary Tumors
Immunohistochemistry of paraffin-embedded primary pancreatic tumor tissue was performed by the immunohistochemistry laboratory at the Johns Hopkins Hospital. In brief, paraffin was removed from a sample before cell conditioning and incubation. Commercially available antibodies to p53 (Ventana) and SMAD4 (Santa Cruz Biotechnology) were individually applied to separate samples and allowed to further incubate, before removal and counter-staining with hematoxylin. A Ventana BenchMark ULTRA platform was used to process the p53 slides, whereas a Leica BOND platform was used for SMAD4. A pathologist specializing in pancreatic tumors reviewed all immunolabeled slides to determine the presence or absence of SMAD4 and p53, in addition to whether labeling was normal or abnormal as has been described.40
Immunofluorescence
Immunofluorescence was carried out using a standard protocol and commercially available conjugated antibodies. In brief, ISET membranes were rehydrated in 1X tris-buffered saline before 0.2% triton to permeabilize cell membranes. The triton was removed, and membranes were incubated in a 5% milk-based blocking buffer. The ISET membranes were then incubated with conjugated antibodies to pan-cytokeratin (1:100, Millipore, FITC), vimentin (1:100, Abcam, alexa fluor 594), and CD45 (1:100, Bioss, alexa fluor 647) diluted in the milk-based blocking buffer. Finally, the membranes were washed and affixed to glass microscope slides with DAPI (Life Sciences) before being analyzed under a fluorescence microscope. All slides were viewed using 20× magnification with the entire membrane viewed for CTCs, with CTCs counted manually by a single user across the entire membrane. Initial exposure times were identified automatically by the Nikon NIS Elements imaging program (version 4.20.02-64 bit), corresponding to 600 ms for DAPI, 1 second for cytokeratin, 800 ms for vimentin, and 3 seconds for CD45. These exposure times were the same for each individual patient membrane that was viewed. All sections were observed under each separate wavelength corresponding to DAPI, pan-cytokeratin, vimentin, and CD45, and when a candidate CTC was identified, an image under each wavelength was captured and saved. Epithelial CTCs were defined as cells greater than 15 μm in diameter with cytoplasmic labeling for cytokeratin, with no expression of CD45. Mesenchymal-like CTCs were defined as cells greater than 15 μm in diameter with cytoplasmic labeling for cytokeratin and vimentin, with no expression of CD45. This differentiated CTCs from leukocytes, which expressed vimentin and CD45 but not pan-cytokeratin.
Statistical Analysis
Summary statistics for the patient cohort and for individual patient groups were presented as frequencies and percentages for categorical variables and as mean and median values with ranges for continuous variables. Differences in patient characteristics by CTC group were calculated with linear or logistic regression models that included dichotomous indicators for whether the patient had cytokertin-positive and/or vimentin-positive CTCs. OS was calculated from the date of surgery to the last date of follow-up or the date of death and estimated using the Kaplan Meier method. Differences in OS between patient groups were tested using the log rank test and hazard ratios were estimated from Cox proportional hazards models that adjusted for age and gender. The cumulative incidence of recurrence after surgery was estimated with death considered a competing risk event. Comparisons of time to recurrence between patient groups were summarized using proportional subdistribution hazards calculated using Fine and Gray’s method, adjusting for age and gender. All statistical analyses were carried out using STATA Version 13.0 (StataCorp, College Station, TX) and R version 3.1.2 [R Core Team (2014), Vienna, Austria].41 A P < 0.05 was considered statistically significant.
Institutional Review Board
This study, including all blood collection, was carried out with the approval of the Johns Hopkins Hospital Institutional Review Board.
RESULTS
Patient Demographics and Tumor Histopathology
All 50 patients included in this study had histologically confirmed diagnosis of PDAC (Table 1). The patient cohort was predominantly male (n ¼ 30, 60%) with an average age of 64.9 years (range, 27–86 yrs). Thirty-nine patients (78%) had a preoperative carbohydrate antigen 19-9 (CA19-9) level measured. The mean CA19-9 level was 965.8 units/mL (<1–9032 units/mL), and 26 patients (67%) had a level above 36 units/mL which is considered abnormally elevated. Sixteen patients (32%) underwent neoadjuvant chemotherapy before resection. All patients were taken to the operating room for resection; 6 patients (12%) were found intraoperatively to have unresectable disease (including 4 with distant metastases), and for this reason the resection was aborted. In the remaining patients, the majority underwent resection by pancreaticoduodenectomy (n = 32, 73%), and 9 (20%) had a distal pancreatectomy and splenectomy. Three patients (7%) required total pancreatectomy given the extent of tumor involvement of the pancreas. The majority of patients had tumors located in the head of the pancreas (n = 37, 74%), and average tumor size was 3.5 cm (0.1–8 cm). Most of the adenocarcinomas were either moderately (n = 24, 48%) or poorly differentiated (n = 20, 40%). The primary tumor of 22 patients (48%) lost SMAD4 expression based upon immunohistochemistry and 34 of the 46 evaluable patients (74%) had abnormal expression (either increased or decreased) of p53 (Fig. 1A–D).
TABLE 1.
Clinicopathological Characteristics of Patients With Circulating Tumor Cells With an Epithelial Phenotype
| Variable | All Patients (n = 50) (%) | CK+ CTCs (n = 39) | No CK+ CTCs (n = 11) | P** |
|---|---|---|---|---|
| Average age (yrs) | 64.9 (27–86) | 65.2 (27–85) | 63.8 (40–86) | 0.58 |
| Male sex | 30 (60%) | 25 (64%) | 5 (45%) | 0.97 |
| Resectable disease | 44 (88%) | 33 (92%) | 11 (100%) | 0.16 |
| Average tumor size (cm) | 3 (0.1–8.0) | 3.3 (0.1–8.0) | 3.3 (1.8–6.3) | 0.49 |
| Average CA19-9 level (n = 39) | 966 (0.6–9032) | 1134 (1–9032) | 245 (0.6–1382) | 0.09 |
| CA19-9 level (n = 39) | 0.32 | |||
| High >36 | 26 (67%) | 23 (72%) | 3 (43%) | |
| Low <36 | 13 (33%) | 9 (28%) | 4 (57%) | |
| Tumor grade | 0.57 | |||
| Well/moderate | 26 (57%) | 19 (53%) | 7 (70%) | |
| Poor | 20 (44%) | 17 (47%) | 3 (30%) | |
| Not specified | 4 | 3 | 1 | |
| Perineural invasion (n = 46) | 37 (80%) | 26 (74%) | 11 (100%) | 0.99 |
| Perivascular invasion (n = 46) | 27 (59%) | 18 (51%) | 9 (82%) | 0.16 |
| Positive lymph nodes | 35 (70%) | 24 (62%) | 11 (100%) | 0.99 |
| Positive margin | 11 (22%) | 9 (23%) | 2 (%) | 0.35 |
| Stage* | — | |||
| Stage I | 8 (16%) | 8 (21%) | 0 (0%) | |
| Stage II | 38 (76%) | 27 (69%) | 11 (100%) | |
| Stage III | 0 (0%) | — | — | |
| Stage IV | 4 (8%) | 4 (10%) | 0 (0%) | |
| Neoadjuvant chemotherapy | 16 (32%) | 14 (36%) | 2 (18%) | 0.29 |
| Adjuvant chemotherapy | 36 (72%) | 28 (72%) | 8 (73%) | >0.99 |
| SMAD4 (n = 46) | ||||
| Intact | 24 (52%) | 19 (54%) | 5 (45%) | 0.40 |
| Lost | 22 (48%) | 16 (46%) | 6 (55%) | |
| TP53 (n = 46) | ||||
| Normal expression | 12 (26%) | 10 (28%) | 2 (18%) | >0.99 |
| Abnormal | 34 (74%) | 25 (71%) | 9 (82%) |
Comparisons were performed for patients with and without cytokeratin-positive CTCs (CK+ CTCs). Values are n (%) or mean (range).
P values for stage were not calculated given the small number of patients with stage I and IV disease, and that all patients with no mesenchymal-like CTCs had stage II disease.
P values from linear or logistic regression models for the association between the given patient characteristics and cytokeratin-positive or cytokeratin-negative CTC group while adjusting for vimentin-positive or vimentin-negative status.
FIGURE 1.
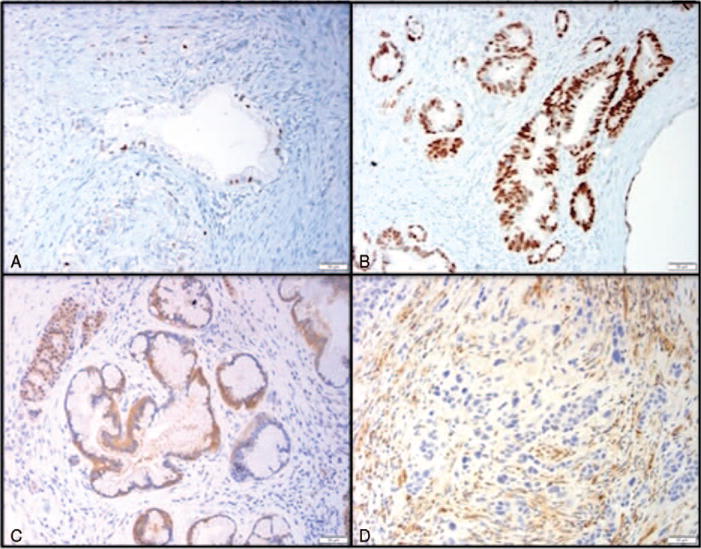
Representative images of primary tumor immunohistochemistry for p53 and SMAD 4. Staining of primary tumors by immunohistochemistry (20×) demonstrating (A) normal expression of p53, (B) abnormal increase in p53 expression, (C) normal expression of SMAD4, and (D) loss of SMAD4.
Blood samples were obtained before surgery from at least one source for all 50 patients (Supplemental Table 1, http://links.lww.com/SLA/A940). Forty patients had a preincision venous blood sample and 46 had a preincision arterial blood sample. Ten patients (20%) also had an intraoperative sample of portal venous blood obtained before resection of the tumor. Of all 50 patients included in this study, 37 had blood collected from more than one source for analysis. Samples were first assessed for CTCs by manual count of H&E stained membranes to identify cells that seemed to be CTCs based upon size and morphologic features. By this method, 45 patients (90%) had CTCs based on identification of cells; only 5 patients had no CTCs isolated from arterial or venous blood samples. The median number of cells per mL blood was 85 (range, 0–300 cells/mL of blood).
Identification of Epithelial CTCs in PDAC
Thirty-nine patients (78%) were found to have CTCs that immunolabeled with antibodies to cytokeratin, but without antibodies to CD45, constituting an epithelial phenotype (Fig. 2A). Thirty of the 39 patients with cytokeratin-positive CTCs had both a venous and arterial blood samples available for evaluation by immunofluorescence; the majority of these 30 patients (29 of 30; 97%) had epithelial CTCs present in the venous sample. By comparison, 27 of 30 patients (90%) had cytokeratin-positive CTCs in the arterial sample. In total, 10 patients had a portal venous sample, and 7 (70%) of these 10 were found to have cytokeratin-positive CTCs. Of the 7 patients with cytokeratin-positive CTCs that had a portal venous sample available, 6 (86%) had epithelial CTCs in the portal sample. One patient with a portal venous blood sample did not have epithelial CTCs despite the presence of cells in both the venous and arterial sample (Supplemental Table 1, http://links.lww.com/SLA/A940). The median number of epithelial CTCs present in venous blood was 30 CTC/mL blood (1–251 CTC/mL blood) compared with 8 CTC/mL blood (1–34 CTC/mL blood) in the arterial blood samples. Among the 10 patients with analyzed portal blood, the median number of epithelial CTCs was 6.5 CTC/mL blood (1–44 CTC/mL blood). There was no correlation between the number of circulating cells identified by H&E and by immunofluorescence (P = 0.65). Six patients with CTCs identified by H&E did not have cytokeratin-positive CTCs identified by immunofluorescence, and 4 patients were found to have CTCs by immunofluorescence but not by H&E; there was no correlation between cytokeratin-positive CTCs and CTCs identified by H&E for an individual patient (P = 0.19).
FIGURE 2.
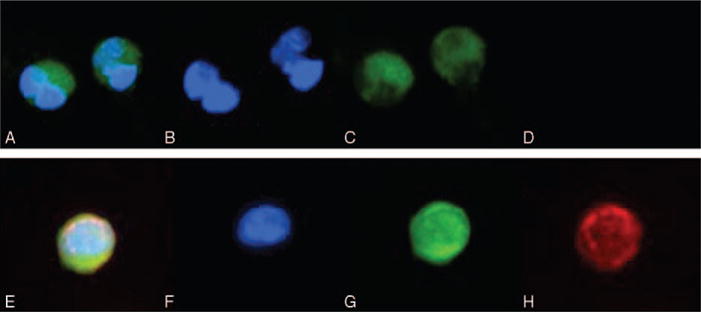
Circulating tumor cells from pancreatic cancer patients. Immunofluorescence microscopy (20×) demonstrating (A) pan-cytokeratin-positive and vimentin-negative CTC (merge), (B) DAPI (blue), (C) pan-cytokeratin (green), (D) absence of vimentin (red); and (E) pan-cytokeratin-positive and vimentin-positive CTC (merge), (F) DAPI (blue), (G) pan-cytokeratin (green), and (H) vimentin (red).
Differences between patients with and without cytokeratin-positive CTCs are shown in Table 1. There were no statistically significant differences with regard to age, sex, stage, and grade between the 2 patient groups. Mean CA19-9 level trended higher for patients with epithelial CTCs compared with those without, but this was not statistically significant (1134 vs 244 units/mL; P = 0.29). Interestingly, all 11 patients without epithelial CTCs had lymph node metastases compared with 24 (71%) patients with epithelial CTCs (P = 0.09). The presence of CTCs did not differ significantly between patients who did or did not receive neoadjuvant chemotherapy (P = 0.47), nor was there an association with tumor recurrence after surgery. Of the patients who were alive at last followup, 9 patients (41%) with epithelial CTCs had recurrences compared with 4 patients (36%) without cytokeratin-positive CTCs. A sensitivity analysis was performed comparing these patient characteristics by the presence or absence of cytokeratin-positive CTCs in only those patients with a venous blood sample (n = 40). There results were similar to the analysis of all fifty patients (Supplemental Table 2, http://links.lww.com/SLA/A940).
Identification of Mesenchymal CTCs in PDAC
Twenty-six patients (52%) had CTCs that were vimentin-positive and CD45-negative, constituting a “mesenchymal-like” phenotype (Fig. 2B). In all cases, these cells also expressed cytokeratin. Twenty of the 26 patients with cytokeratin-positive and vimentin-positive CTCs had both a venous and arterial blood sample for evaluation by immunofluorescence; the majority of patients (19 of 20; 95%) had mesenchymal-like CTCs present in the venous sample. In comparison, in 15 of 20 patients (75%), cytokeratin-positive and vimentin-positive CTCs were found in the arterial sample. In total, 4 (40%) of 10 patients with portal venous blood samples were found to have mesenchymal-like CTCs in that sample. Of the 6 patients with cytokeratin-positive and vimentin-positive CTCs that had a portal venous sample available, 4 (67%) had mesenchymal-like CTCs in the portal sample. Two patients with portal venous blood samples did not have cytokeratin-positive and vimentin-positive CTCs despite the presence of these cells in both the venous and arterial samples (Supplemental Table 1, http://links.lww.com/SLA/A940). In addition, all 26 patients with mesenchymal-like CTCs also were found to have separate cytokeratin-positive, epithelial CTCs found in the same blood sample. The median number of mesenchymal-like CTCs was 3 CTC/mL blood (1–16 CTC/mL blood) in venous blood samples and 2 CTC/mL blood (1–15 CTC/mL blood) in arterial blood samples, among only those patients with mesenchymal-like CTCs. Among the patients with analyzed portal blood, the median number of mesenchymal-like CTCs was 2 CTC/mL blood (1–14 CTC/mL blood).
Differences between patients with and without dual-staining vimentin-positive and cytokeratin-positive CTCs are shown in Table 2. Significantly more males had mesenchymal-like CTCs present in their blood, but otherwise there was no difference in age, tumor size, grade, nodal status, or margin status. Mean CA19-9 level was not statistically different between patients with and without mesenchymal-like CTCs (1188 vs 704 units/mL; P = 0.47). There was no difference in the presence of mesenchymal-like CTCs between patients who did or did not receive neoadjuvant chemotherapy. Patients with cytokeratin-positive and vimentin-positive CTCs were significantly more likely to recur compared with those without any vimentin-positive CTCs. In patients with vimentin-positive and cytokeratin-positive CTCs, local recurrence was seen in 3 patients and 16 had distant metastases, compared with 1 and 3 patients, respectively, in those without dual-staining CTCs.
TABLE 2.
Clinicopathological Characteristics of Patients With Circulating Tumor Cells With a Mesenchymal-like Phenotype
| Variable | CK+Vim+ CTCs (n = 26) | No CK+Vim+ CTCs (n = 24) | P** |
|---|---|---|---|
| Average age (yrs) | 64.5 (27–85) | 65.3 (40–86) | 0.62 |
| Male sex | 19 (73%) | 11 (46%) | 0.11 |
| Resectable disease | 22 (85%) | 22 (92%) | 0.44 |
| Tumor size (cm) | 2.9 (0.1–8) | 3.2 (0.2–6.3) | 0.29 |
| Average CA19-9 level (n = 39) | 1189 (1–9032) | 704 (0.6–7312) | 0.88 |
| CA19-9 level (n = 39) | 0.61 | ||
| High >36 | 15 (58%) | 5 (38%) | |
| Low <36 | 11 (42%) | 8 (62%) | |
| Tumor grade/differentiation | 0.64 | ||
| Well/moderate | 12 (50%) | 14 (64%) | |
| Poor | 12 (50%) | 8 (36%) | |
| Not specified | 2 | 2 | |
| Perineural invasion | 17 (77%) | 20 (83%) | 0.60 |
| Perivascular invasion | 11 (50%) | 16 (67%) | 0.83 |
| Positive lymph nodes | 17 (77%) | 18 (78%) | 0.25 |
| Positive margin | 5 (23%) | 6 (27%) | 0.41 |
| Stage* | — | ||
| Stage I | 4 (15%) | 4 (17%) | |
| Stage II | 18 (70%) | 20 (83%) | |
| Stage III | 0 (0%) | 0 (0%) | |
| Stage IV | 4 (15%) | 0 (0%) | |
| Neoadjuvant chemotherapy | 9 (35%) | 7 (29%) | 0.81 |
| Adjuvant chemotherapy | 19 (73%) | 17 (71%) | 0.97 |
| SMAD4 (n = 46) | 0.45 | ||
| Intact | 12 (50%) | 12 (55%) | |
| Lost | 12 (50%) | 10 (45%) | |
| TP53 (n = 46) | 0.36 | ||
| Normal expression | 8 (33%) | 8 (33%) | |
| Abnormal | 16 (67%) | 16 (67%) |
Comparisons were performed for patients with and without cytokeratin-positive and vimentin-positive CTCs (CK+Vim+CTCs). Values are n (%) or mean (range).
P values for stage were not calculated given the small number of patients with stage I and IV disease, and that all patients with no mesenchymal-like CTCs had stage II disease.
P values from linear or logistic regression models for the association between the given patient characteristics and vimentin group while adjusting for whether the patient had cytokeratin-positive CTCs.
A sensitivity analysis was performed comparing these characteristics between patients with a presence or absence of cytokeratin-positive and vimentin-positive CTCs in only those patients with a venous blood sample (n = 40). There results were similar to the analysis of all 50 patients (Supplemental Table 2, http://links.lww.com/SLA/A940). In addition, we compared the subset of patients with cytokeratin-positive and vimentin-negative CTCs (n = 13) to those with cytokeratin-positive and vimentin-positive CTCs (n = 26) and patients without any CTCs (n = 11). Apart from a difference in the number of patients who had positive lymph nodes between the group without any CTCs (n = 11, 100%) and the group with cytokeratin-positive and vimentin-negative CTCs (n = 7, 58%, P = 0.04), there were no other significant differences between groups when analyzing data for all blood samples or only in patients with a venous blood sample.
Survival Analysis
Of the patients with localized disease at the time of surgery, at last follow-up 23 patients (50%) had local or distant cancer recurrence at a median follow-up of 10.3 months; 4 patients had local recurrence whereas 19 had distant metastases. This excludes 4 patients in whom metastatic disease was found at the time of surgery, as extra-pancreatic spread had already occurred. An analysis of tumor recurrence was performed for the remaining 46 patients to assess for tumor recurrences by patient variables including age, sex, resection margin, nodal status, tumor size, grade, and the presence of any cytokeratin-positive CTCs or only vimentin-positive CTCs. In our patient population, only the presence of vimentin-positive CTCs was significantly associated with cancer recurrence [hazard ratio (HR) 2.78, 95% confidence interval (CI) 1.31–5.88, P = 0.01], adjusting for age and sex. In addition, median time to recurrence was 9.5 months in patients with mesenchymal-like CTCs, compared with 13.5 months in patients without these CTCs (P = 0.02). An analysis of the 3 mutually exclusive cytokeratin–vimentin CTC groups of patients showed that patients with cytokeratin-positive and vimentin-positive CTCs were more likely to recur after surgery compared with patients with CTCs only expressing cytokeratin (HR = 3.7, 95% CI 1.3–10.6, P = 0.02). These patients were also more likely to recur than patients with no CTCs, though the result was not statistically significant (HR = 2.0, 95% CI 0.8–5.0, P = 0.13) (Supplemental Table 3, http://links.lww.com/SLA/A940). All 4 patients found to have metastatic disease at the time of surgery had cytokeratin-positive and vimentin-negative CTCs as well as dual-staining cytokeratin-positive and vimentin-positive CTCs. We further explored the risk for recurrence according to the number of CTCs, measured both continuously and grouped into 1 to 11 vs 12 or more cells, but none of these analyses revealed any association. There was also no association between tumoral loss of SMAD4 or abnormal p53 immunolabeling of the primary pancreatic cancer and tumor recurrence (P = 0.87 and P = 0.43, respectively). There was no difference in the number of cytokeratin-positive CTCs (20.8 vs 33.3 cells, P = 0.35) or dual-staining CTCs (3.2 vs 2.7 cells, P = 0.69) in patients based on SMAD4 protein status (retention vs loss).
Patient characteristics that were significantly associated with OS are shown in Table 3. The median patient follow-up time was 14.0 months. The presence of cytokeratin-positive CTCs and positive operative margins were associated with decreased survival. The detection of epithelial CTCs was significantly associated with worse survival compared with patients without CTCs (median survival 13.7 mo vs not reached, P = 0.008) (Fig. 3A), whereas mesenchymal-like CTCs were not associated with survival (P = 0.39) (Fig. 3B). These results remained significant when accounting for other factors associated with survival by multivariate analysis. The presence of cytokeratin-positive CTCs remained a significant predictor of survival even when evaluating only the 40 patients with venous samples (P = 0.04) or when evaluating only the 34 patients who went immediately to surgery without neoadjuvant therapy (P = 0.01). In addition, the presence of CTCs detected by H&E was not associated with survival (P = 0.47). However, whereas the presence of cytokeratin-positive CTCs conferred worse survival, the number of cytokeratin-positive CTCs (either continuously measured or divided into numerical categories) did not predict OS.
TABLE 3.
Univariate Analysis of Factors Associated With OS, Adjusted for Patient Age and Sex
| Survival Factor | Median OS (mo) | HR | 95% CI | P |
|---|---|---|---|---|
| CK+ CTCs | ||||
| Present | 13.7 | — | (0, infinity) | 0.008 |
| Absent | NR | |||
| CK+Vim+ CTCs | ||||
| Present | 13.7 | 1.52 | 0.5–4.6 | 0.39 |
| Absent | NR | |||
| CTC subgroup | ||||
| CK+Vim+ | 13.7 | 1 | — | 0.02 |
| CK+Vim− | 12.7 | 1.39 | 0.44–4.36 | |
| No CTC | NR | 0 | 0-infinity | |
| CTC density | ||||
| 0 cells | NR | 1 | — | 0.03 |
| 1–11 cells | 13.7 | — | 0-infinity | |
| 12+ cells | 16.4 | — | 0-infinity | |
| CTCs on H&E | ||||
| Yes | 16.2 | 0.62 | 0.16–2.4 | 0.47 |
| No | 12.6 | |||
| Tumor size | ||||
| ≥3 cm | 13.7 | 1.5 | 0.55–4.11 | 0.45 |
| <3 cm | 16.4 | |||
| Positive LN | ||||
| Yes | 14.0 | 2.4 | 0.46–12.5 | 0.45 |
| No | 16.4 | |||
| Positive margin | ||||
| Yes | 10.4 | 28.9 | 4.1–205.6 | 0.0003 |
| No | 16.4 | |||
| Tumor grade | ||||
| Poor | 13.7 | 1.4 | 0.47–4.12 | 0.65 |
| Well/moderate | 14.0 | |||
| Tumor stage | ||||
| Stage 1 | NR | 0.13 | ||
| Stage 2 | 14.0 | 1.8 | 0.21–14.98 | |
| Stage 4 | 8.4 | 7.36 | 0.75–72.6 | |
| Perineural invasion | ||||
| Yes | 16.3 | 1.05 | 0.29–3.87 | 0.86 |
| No | 14.0 | |||
| Perivascular invasion | ||||
| Yes | 16.3 | 2.17 | 0.66–7.15 | 0.22 |
| No | 14.0 | |||
| SMAD4 (n = 46) | ||||
| Intact | 14.0 | 1.17 | 0.39–3.51 | 0.72 |
| Lost | 16.2 | |||
| TP53 (n = 46) | ||||
| Normal | 13.7 | 0.63 | 0.15–2.57 | 0.77 |
| Abnormal | 16.3 | |||
| Neoadjuvant chemotherapy | ||||
| Yes | 14.0 | 1.12 | 0.38–3.31 | 0.66 |
| No | 13.7 | |||
| Adjuvant chemotherapy | ||||
| Yes | 16.2 | 0.17 | 0.03–1.12 | 0.08 |
| No | 10.8 |
FIGURE 3.
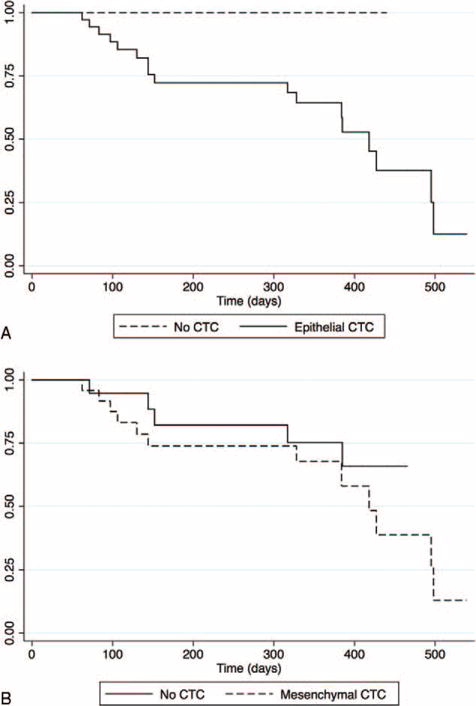
Survival analysis based upon the presence of CTCs. Kaplan-Meier survival analysis in patients with CTCs with an (A) epithelial phenotype or (B) mesenchymal-like phenotype.
DISCUSSION
Given the poor outcome for patients with pancreatic cancer, research has focused on new methods for early disease detection, stratification based upon prognosis, and prediction of distant tumor recurrence. CTCs are of particular interest given their presence in patients with various forms of malignancy and their location in the vasculature, allowing for easy sampling and analysis. Recent studies have demonstrated the presence of CTCs in the blood of patients with all stages of PDAC, not only patients with metastatic disease, indicating CTCs as a possible source of and not only a result of metastatic disease.11,13,23–27 In addition, several studies have found an association between the presence of CTCs and poorer survival.23,25 Our study investigated the prognostic significance of phenotypic subtypes of CTCs.
We identified cytokeratin-positive and CD45-negative circulating cells in the blood of the majority of pancreatic cancer patients. These cells were not limited to patients with advanced-stage or unresectable disease. We also found that patients with epithelial CTCs had poorer survival compared with those patients without epithelial CTCs, confirming results of prior studies.23,24 In addition, whereas the rate of recurrence was similar in patients with and without CTCs, recurrence was significantly earlier in patients with epithelial CTCs. The relationship of cytokeratin-positive CTCs to survival but not recurrence could be due to the relatively small number of patients involved in this study. The lack of association with recurrence could also reflect the limitations of clinical tests to detect recurrent pancreatic cancer, particularly in its early stages. However, CTCs may also be indicative of a separate feature of tumor biology that influences survival but is not captured by the other clinical variables (such as recurrence) analyzed in this study.
Interestingly, tumor characteristics were similar among the patients with and without epithelial CTCs. The exception was lymph node metastasis, which was more frequent in the group of patients without CTCs, suggesting different mechanisms of tumor invasion between patients. Both lymphatic and venous invasion have been described in PDAC: lymphatic invasion involves tumor spread to lymph nodes, whereas in venous invasion the cancer is presumed to metastasize directly to distant organs via the blood stream. Our data raise the possibility of separate invasion mechanisms in at least a subset of patients, with more frequent lymphatic invasion (positive lymph nodes) in patients without vascular invasion (no CTCs). Thus, direct venous invasion to distant organs without involvement of lymph nodes may play an important role in the metastasis of PDAC in some patients.
The lack of correlation between CTCs and clinicopathological tumor characteristics demonstrates the difficulty in predicting which patients will have CTCs without direct observation of the blood. Furthermore, this finding fits with other studies that have shown the presence of CTCs in patients with all stages of PDAC and not only metastatic cancer.11,13,24 The association of CTCs with survival remained significant by multivariate analysis even when accounting for traditional prognostic factors such as positive margin and grade, suggesting that epithelial CTCs may be useful as an independent prognostic factor before resection to identify patients with more aggressive tumors.
Our study identifies mesenchymal-like CTCs in patients with PDAC. Clusters of CTCs have been shown to have elevated expression of vimentin compared with the primary tumor in a mouse model of PDAC.28 In our study, vimentin-positive and cytokeratin-positive CTCs were present in blood samples in patients with PDAC, but these cells were not observed in as many patients as were cytokeratin-positive and vimentin-negative CTCs. This indicates that not all CTCs are alike, but are instead a heterogeneous population. The ISET platform identifies CTCs by size and does not rely on the expression of epithelial markers, allowing for different phenotypes of CTCs to be identified. Thus, this study demonstrates the importance of using a platform that allows for the identification of both epithelial and mesenchymal characteristics, as epithelial markers alone may not be sufficient. Unlike studies in other tumor types, purely mesenchymal CTCs (those that express mesenchymal markers and are cytokeratin-negative) were not identified in our study of PDAC. This may be related to the patients included in the study or the mesenchymal marker chosen. A more comprehensive study involving other mesenchymal markers may be useful to determine the presence and significance of purely mesenchymal CTCs in PDAC.
In our cohort, the presence of vimentin-positive CTCs was not predictive of decreased OS but was associated strongly with early tumor recurrence, predominantly to distant organs such as the liver and lung. As vimentin is a marker of the “mesenchymal phenotype,” these findings support the idea of “epithelial-to-mesenchymal transition,” in which the acquisition of mesenchymal features may facilitate dissemination of neoplastic cells as a mechanism for distant metastasis.29,30,42 The absence of a relationship with OS may be related to the number of patients included in this study, and further analysis in a larger group of PDAC patients will be needed to confirm these findings. As our understanding of the heterogeneity of CTCs is enhanced, additional work regarding the genetics and gene expression patterns in individual cells will be needed to better characterize epithelial and mesenchymal CTCs and determine their relationship to primary and metastatic tumors. These findings would clarify which CTCs are responsible for tumor recurrence and identify genetic factors that contribute to decreased survival in PDAC.
In this study we assessed CTCs from multiple different blood sources. We found that CTCs were most likely to be detected in peripheral venous blood. Of the patients with either vimentin-positive or cytokeratin-positive cells, only 1 patient was found to have CTCs in an arterial and portal venous sample, but not in a venous blood sample. This patient, however, had only one cell found in each of the other 2 sources. Our findings suggest that venous blood is adequate and would be recommended for the detection of CTCs in patients with PDAC, especially given its ease of collection. In addition, the lack of correlation between counts based on H&E morphology and immunofluorescence phenotype suggests that morphology alone is not sufficient to accurately quantify CTCs. H&E seems to be an unreliable method for CTC identification, given the dependence on subjective interpretation of the cellular morphology rather than more objective variables such as the presence or absence of expression of specific markers. Thus, identification of CTCs should be based on expression of epithelial, mesenchymal, and hematopoietic markers in addition to the assessment of cell morphology.
This study, although a prospective analysis of CTCs, has several limitations. This study only describes the analysis of blood obtained from patients before surgical intervention, most of whom had either stage 1 or 2 disease. Thus, our results may not be representative of those obtained from patients with more advanced disease. Still, CTCs are most likely to be a useful prognostic biomarker in early-stage patients, as these patients have the most variability in outcome and treatment options. By comparison, the prognosis for patients with advanced disease is almost universally poor, indicating that prognostic biomarkers have less utility in this patient population. In addition, our study design did not include the collection of samples at multiple time points to observe how CTC characteristics predict outcome over time and with treatment. Although such studies will help guide the use of CTCs in evaluating responses to treatment, our study does provide data on the utility of CTCs as a biomarker at a time in the patient’s clinical care when key treatment decisions are made.
Our results indicate that the evaluation of CTCs in patients with PDAC can be used to predict outcome. The presence of epithelial CTCs was associated with worse OS, whereas mesenchymal-like CTCs may predict early distant tumor recurrence after resection and may be a useful component in the stratification of patients for neoadjuvant chemotherapy. This study confirms that CTC populations are heterogeneous, and assessment in PDAC patients should include methods tailored for the identification of CTCs by both epithelial and mesenchymal markers.
Supplementary Material
Acknowledgments
Funding for this study was provided by NIH grants CA126607-06A1 (KEP, JFG), T32DK007713 (VV), CA179991 (CAID), NIH SPORE grant CA62924, the Kaya Tuncer Career Development Award in GI Cancer Prevention (LDW), the AGA-Bernard Lee Schwartz Research Scholar Award in Pancreatic Cancer (LDW), Sigma Beta Sorority, The Natalie and David Lederman Foundation, Joseph C. Monastra Foundation, Michael Rolfe Pancreatic Cancer Foundation, and the Sol Goldman Pancreatic Cancer Research Center.
Footnotes
The author reports no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Stojadinovic A, Brooks A, Hoos A, et al. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. J Am Coll Surg. 2003;196:954–964. doi: 10.1016/S1072-7515(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 7.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 8.Fischer R, Breidert M, Keck T, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol. 2012;18:118–121. doi: 10.4103/1319-3767.93815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poruk KE, Firpo MA, Adler DG, et al. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17–26. doi: 10.1097/SLA.0b013e31825ffbfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 11.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 13.Chausovsky G, Luchansky M, Figer A, et al. Expression of cytokeratin 20 in the blood of patients with disseminated carcinoma of the pancreas, colon, stomach, and lung. Cancer. 1999;86:2398–2405. [PubMed] [Google Scholar]
- 14.Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 15.Marrinucci D, Bethel K, Kolatkar A, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012;9:016003. doi: 10.1088/1478-3975/9/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitzer E, Auer M, Ulz P, et al. Circulating tumor cells and DNA as liquid biopsies. Genome Med. 2013;5:73. doi: 10.1186/gm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinzani P, Salvadori B, Simi L, et al. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol. 2006;37:711–718. doi: 10.1016/j.humpath.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Vona G, Estepa L, Beroud C, et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39:792–797. doi: 10.1002/hep.20091. [DOI] [PubMed] [Google Scholar]
- 19.Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17:827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 20.Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. 2012;23:30–38. doi: 10.1111/j.1365-2303.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 21.Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14:7004–7010. doi: 10.1158/1078-0432.CCR-08-0030. [DOI] [PubMed] [Google Scholar]
- 22.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 23.Kurihara T, Itoi T, Sofuni A, et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15:189–195. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 24.Tjensvoll K, Nordgard O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer. 2014;134:1–8. doi: 10.1002/ijc.28134. [DOI] [PubMed] [Google Scholar]
- 25.Z’Graggen K, Centeno BA, Fernandez-del Castillo C, et al. Biological implications of tumor cells in blood and bone marrow of pancreatic cancer patients. Surgery. 2001;129:537–546. doi: 10.1067/msy.2001.113819. [DOI] [PubMed] [Google Scholar]
- 26.Soeth E, Grigoleit U, Moellmann B, et al. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol. 2005;131:669–676. doi: 10.1007/s00432-005-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Albuquerque A, Kubisch I, Breier G, et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology. 2012;82:3–10. doi: 10.1159/000335479. [DOI] [PubMed] [Google Scholar]
- 28.Ting DT, Wittner BS, Ligorio M, et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 33.Lecharpentier A, Vielh P, Perez-Moreno P, et al. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105:1338–1341. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong AJ, Marengo MS, Oltean S, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallergi G, Papadaki MA, Politaki E, et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13:R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vora HH, Patel NA, Rajvik KN, et al. Cytokeratin and vimentin expression in breast cancer. Int J Biol Markers. 2009;24:38–46. doi: 10.1177/172460080902400106. [DOI] [PubMed] [Google Scholar]
- 37.Handra-Luca A, Hong SM, Walter K, et al. Tumour epithelial vimentin expression and outcome of pancreatic ductal adenocarcinomas. Br J Cancer. 2011;104:1296–1302. doi: 10.1038/bjc.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508–516. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilentz RE, Su GH, Dai JL, et al. Immunohistochemical labeling for DPC4 mirrors genetic status in pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am J Pathol. 2000;156:37–43. doi: 10.1016/S0002-9440(10)64703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Core Team. R: A Language and Environment for statistical computing. 2014 Available at: http://www.R-project.org.
- 42.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


