Abstract
Objective
Determine the long-term cardiovascular-specific mortality in patients with acute kidney injury (AKI) or chronic kidney disease (CKD) after major surgery.
Summary Background Data
In surgical patients, preexisting CKD and postoperative AKI are associated with increases in all-cause mortality.
Methods
In a single-center cohort of 51,457 adult surgical patients undergoing major inpatient surgery, long-term cardiovascular-specific mortality was modeled using a multivariable subdistributional hazards model while treating any other cause of death as a competing risk and accounting for the progression to end-stage renal disease (ESRD) after discharge. Preexisting CKD and ESRD and postoperative AKI were the main independent predictors.
Results
Prior to the admission, 4% and 8% of the cohort had preexisting ESRD and CKD not requiring renal replacement therapy, respectively. During hospitalization, 39% developed AKI. At 10-year follow-up, adjusted cardiovascular-specific mortality estimates were 6%, 11%, 12%, 19% and 27% for patients with no kidney disease, AKI with no CKD, CKD with no AKI, AKI with CKD, and ESRD, respectively (P<0.001). This association remained after excluding 916 patients who progressed to ESRD after discharge although it was significantly amplified among them. Compared to patients with no kidney disease, adjusted hazard ratios for cardiovascular mortality were significantly higher among patients with kidney disease ranging from 1.95 (95% CI, 1.80-2.11) for patients with de novo AKI to 5.70 (CI, 5.00-6.49) for patients with preexisting ESRD.
Conclusions
Both acute kidney injury and chronic kidney disease were associated with higher long-term cardiovascular-specific mortality compared to patients with no kidney disease.
Keywords: Acute Kidney Injury, chronic kidney disease, competing risk, cardiovascular-specific mortality, survival, cause of death, end-stage renal disease progression
INTRODUCTION
Acute kidney injury (AKI) is independently associated with the development of chronic kidney disease (CKD), end-stage renal disease (ESRD), and increased all-cause mortality.1-8 The severity of the renal insult and the development of multiple AKI episodes increase the risk of occurrence of these outcomes.5, 7, 8
All severity stages of CKD, from mild disease to the dialysis dependent ESRD, are associated with an increase in cardiovascular risk and mortality.9 Furthermore, both a decline in estimated glomerular filtration rate (eGFR) and albuminuria are important determinants of longevity in the general population.10 Recent studies suggested AKI as a risk factor for cardiovascular disease either through progression to CKD or through independent mechanisms.11, 12 Patients with AKI had increased risk for subsequent congestive heart failure, coronary artery disease, and de novo stroke, regardless of progression to CKD.13-15 Patients hospitalized for myocardial infarctions whose stay was complicated by the AKI were more likely to have a subsequent admission for an adverse cardiac event compared to those patients who did not develop AKI.11
In surgical patients, CKD is an important risk factor for thirty-day mortality16 and postoperative AKI is associated with increase in all-cause mortality.17-20 Previous studies in surgical patients were focused on all-cause mortality rather than cardiovascular causes with assumption that progression to ESRD is the underlying mechanism for observed mortality increase.12 Furthermore, since patients with kidney disease often have a decrease in hemoglobin levels, and chronic and acute anemia in the perioperative setting increase the risk for adverse outcomes,21 it is important to delineate whether their effects are interrelated.22
In a large, single center cohort of surgical patients, we examined the long-term cardiovascular-specific mortality in patients with either acute or chronic kidney disease while adjusting for demographic characteristics, comorbidity burden, operative variables, admission hemoglobin levels, progression to ESRD and other competing causes of death.
MATERIALS AND METHODS
Data source and participants
Using the University of Florida Integrated Data Repository we have previously assembled a single-center cohort of patients age greater or equal to 18 years admitted to the hospital for longer than 24 hours following any type of inpatient operative procedure between January 1, 2000 and November 30, 2010.17 For patients with multiple surgeries we chose the first procedure. After excluding patients with missing serum creatinine (n=6636) the final cohort consisted of 51,457 patients. The study was approved by the Institutional Review Board and Privacy Office of the University of Florida.
Deaths
The main outcome of the study was the cardiovascular-specific death with any other cause of death being treated as a competing risk. For the secondary analysis the cancer-specific death was treated as the main cause of death in a competing risk model. The date of death was determined using hospital records, the Social Security Death Index (SSDI) and Florida Bureau of Vital Statistics. The survival time was calculated from the admission date to the date of death from any cause or the date at which the patient was last known alive. We used full name, birth date, and social security number to search the SSDI in July 2014 to assess survival through January 31, 2014 that was also the date of data censoring for patients who were last known to be alive. Primary cause of death was obtained from death certificates from the Florida Bureau of Vital Statistics using a matching algorithm that utilized full name, date of birth and date of death. About 10% of non-survivors did not have matching cause of death likely reflecting death records from other states. Since November 2011 Social Security Administration has imposed changes mandating that states were no longer permitted to share data on deaths in SSDI.23 To determine whether this change affected our analyses we performed sensitivity analysis by censoring all patients known to be alive or who had died after October 31, 2011 on that day and by excluding records without cause of death from analysis. Using the International Classification of Diseases, Tenth Revision (ICD 10) for the primary cause of death on the death certificate we classified deaths into cardiovascular-specific (ICD-10 codes I00-I99, Q20-Q28, E10-E14, N00-N08, N10-N16, N17-N19), cancer-specific (codes C00-C97), and all other causes. We included diabetes and kidney disease into the expanded cardiovascular-specific category to capture deaths related to CKD but traditionally not classified as cardiovascular as previously reported.9 For sensitivity analysis we used alternative approach reported for general population.24, 25
Definition of kidney disease and covariates
The main covariate of interest was the occurrence of acute or chronic kidney disease during the index hospitalization. For all patients we calculated a reference eGFR using standardized reference serum creatinine, sex, race, and age.26 For the reference creatinine we used the minimum of values available within six months prior to admission or the minimum and mean of the creatinine values available within seven days prior to admission (used for sensitivity analyses).27 Patients with CKD and ESRD prior to admission were identified using the validated combination of ICD-9-CM codes.28 Patients with CKD were stratified using reference eGFR without criteria for albuminuria into mild to moderate (eGFR ≥ 30 ml/min/1.73 m2) and severe chronic kidney disease (eGFR < 30 ml/min/1.73 m2) according to guidelines.29 We defined AKI using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria as at least a 50% or 0.3 mg/dl increase in serum creatinine relative to the reference value and stratified AKI severity based on the maximum change in serum creatinine during hospitalization.30 Progression to ESRD after discharge was determined by linking records of discharged patients with the United States Renal Data System (USRDS) database using their matching algorithm. Time to ESRD was calculated from the discharge date to the date of the first ESRD Service.31 Patients whose primary surgery was renal transplant were excluded from analysis. The presence of underlying comorbidities was identified by validated ICD-9-CM codes and using the Charlson-Deyo comorbidity index.32 We defined postoperative complications using previously described criteria.18 We classified all surgeries as cardiothoracic, non-cardiac general and vascular, neurologic, specialty (orthopedic, gynecological, otorhinolaryngology, urology and plastic) and other surgery (burn, transplantation and trauma). We categorized admission hemoglobin values into missing, < 8 g/dl, 8-9.9 g/dl, 10-11.9 g/dl and ≥12 g/dl. The thresholds were developed after constructing spline function of hemoglobin values and the risk of mortality in univariate analysis and are similar to previously used values in patients with kidney disease.22
Statistical Analysis
The analytical plan followed the STROBE recommendations.33 Kaplan-Meier estimates were used to calculate cumulative survival probabilities for all-cause mortality. We used the proportional subdistribution hazards regression analyses to model a) cardiovascular and cancer-specific mortality while treating any other cause of death as a competing risk and b) progression to ESRD while treating death from any cause prior to ESRD as a competing risk.34 The application of the regression modeling directly on a cumulative incidence function allows the best estimation of the effect of covariates in the model.35 In addition to the occurrence of kidney disease each model was adjusted for preoperative demographics covariates, Charlson comorbidity index, emergent surgery status, surgery type, and admission day hemoglobin level. Adjusted hazards ratios with 95% confidence intervals (95% CI) were reported for each covariate in the model. We plotted unadjusted and adjusted model-based cumulative incidence functions of the cardiovascular and cancer-specific mortality and ESRD progression by kidney disease and severity stages for the entire cohort and for patients grouped by gender and age and by surgery type. We used independent validation datasets created with a bootstrap cross-validation method for internal validation and to assess prediction accuracy of the models. The models were trained on 100 bootstrap samples that were drawn with replacement of 60% of the original data and were validated in the bootstrap samples that did not contain any observations from the training datasets. We compared the discriminative power of competing risk models between training and cross-validation datasets using the adaptation of Harrell's concordance probability to the competing risk setting by calculating the C-index with 95% CI for both training and validation datasets.36 Sensitivity analyses were performed by running the competing risk models after censoring all patients who were last known to be alive or who had died after October 31, 2011, using alternative classification for cardiovascular cause of death, excluding patients with unknown cause of death, using different methods for missing hemoglobin values and reference serum creatinine, by including individual comorbidities instead of Charlson-Deyo comorbidity index and by adding total number of postoperative complications as a covariate. All significance tests were two-sided with a p-value<0.05 considered statistically significant. Statistical analyses were performed with R 3.2.0 (cmprsk and pec packages)37 and SAS (v.9.3, Cary, N.C.).
RESULTS
Baseline characteristics
Overall 44% of the 51,457 surgical patients had evidence of either chronic or acute kidney disease during hospitalization (Table 1 and SDC Table 1 and 2). At the time of admission, 4% and 8% of the cohort had documented history of ESRD and CKD not requiring renal replacement therapy, respectively. Among patients with CKD, 30% (1215/4024) presented with severe disease (eGFR < 30 ml/min per 1.73m2). During hospitalization 39% of patients developed AKI and the majority of them did not have a history of CKD prior to admission. Patients with severe CKD were more likely to develop AKI compared to those with mild to moderate disease (p<0.001). African-American ethnicity was more common among patients with CKD and ESRD prior to admission. Patients with acute or chronic kidney disease were more likely to be older males and to have multiple comorbidities compared to patients with no kidney disease. Emergent surgery was more common among patients with AKI and those who had ESRD prior to admission.
Table 1.
Clinical Characteristics for all patients stratified by kidney disease.
| Variables | No known kidney disease (n=28644, 56%) | Acute kidney injury without chronic kidney disease (n=16854, 33%) | Acute kidney injury with chronic kidney disease (n=3171, 6%) | Chronic kidney disease without acute kidney injury (n=853, 2%) | End stage renal disease (n=1935, 4%) |
|---|---|---|---|---|---|
| Age (years), Mean (SD) | 53 (17) | 57 (17)a | 62 (15)a | 65 (16)a | 53 (15) |
| Age >=65 years, n (%) | 7864 (27) | 6326 (38)a | 1491 (47)a | 493 (58)a | 481 (25) |
| Female sex, n (%) | 15012 (52) | 7658 (45)a | 11224 (39)a | 415 (49) | 811 (42)a |
| African-American ethnicity, n (%) | 3345 (12) | 1859 (11) | 591 (19)a | 118 (14) | 671 (35)a |
| Rural area residency, n (%) | 9277 (32) | 5311 (32) | 1008 (32) | 274 (32) | 534 (28)a |
| Distance from residing neighborhood to hospital (km), Median (25th,75th) | 52 (23, 117) | 56 (27, 120)a | 57 (27, 120) | 49 (23, 118) | 76 (28, 171) |
| Population living in poverty in residing neighborhood, % (SD) | 14.9 (8.4) | 14.6 (8.1)a | 15.0 (8.4) | 14.7 (8.1) | 15.4 (8.6)a |
| Primary Insurance, n (%) | |||||
| Medicare | 9310 (33) | 7173 (43)a | 1897 (60)a | 560 (66)a | 1280 (66)a |
| Medicaid | 3835 (13) | 2233 (13) | 307 (10)a | 74 (9)a | 133 (7)a |
| Private | 12972 (45) | 6343 (38)a | 879 (28)a | 204 (24) a | 510 (26)a |
| Uninsured | 2527 (9) | 1105 (7)a | 88 (3)a | 15 (2)a | 12 (1)a |
| Emergent surgery, n (%) | 10543 (37) | 9545 (57)a | 1909 (60)a | 318 (37) | 1276 (66)a |
| Weekend admission, n (%) | 3175 (11) | 2940 (17)a | 533 (17)a | 89 (10) | 378 (20)a |
| Charlson-Deyo Comorbidity Score, n (%) | |||||
| 0 | 13297 (46) | 4782 (28)a | 267 (8)a | 141 (17)a | 5 (0.3)a |
| 1 | 5420 (19) | 3932 (23)a | 386 (12)a | 107 (13)a | 8 (0.4)a |
| 2 | 4898 (17) | 3534 (21)a | 688 (22)a | 185 (22)a | 643 (33)a |
| >=3 | 5029 (18) | 4606 (27)a | 1830 (58)a | 420 (49)a | 1279 (66)a |
| Comorbidities, n (%) | |||||
| Hypertension | 10953 (38) | 7386 (44)a | 1781 (56)a | 550 (64)a | 1184 (61)a |
| Cancer | 5901 (21) | 3553 (21) | 455 (14)a | 171 (20) | 72 (4)a |
| Diabetes | 3956 (14) | 2863 (17)a | 968 (31)a | 268 (31)a | 728 (38)a |
| Chronic Pulmonary Disease | 3962 (14) | 3247 (19)a | 694 (22)a | 165 (19)a | 235 (12) |
| Peripheral Vascular Disease | 2427 (8) | 2558 (15)a | 696 (22)a | 168 (20)a | 218 (11)a |
| Cerebrovascular Disease | 1786 (6) | 1945 (12)a | 315 (10)a | 76 (9)a | 119 (6) |
| Congestive Heart Failure | 984 (3) | 1931 (11)a | 787 (25)a | 128 (15)a | 274 (14)a |
| Myocardial Infarction | 1276 (4) | 1451 (9)a | 380 (12)a | 99 (12)a | 170 (9)a |
| Liver Disease | 871 (3) | 1209 (7)a | 312 (10)a | 29 (3) | 104 (5)a |
| Surgery Type, n (%) | |||||
| Cardiothoracic Surgery | 2354 (8) | 3329 (20)a | 842 (27)a | 114 (13)a | 251 (13)a |
| Neurologic Surgery | 5667 (20) | 2340 (14)a | 240 (8)a | 112 (13)a | 63 (3)a |
| Non-Cardiac General Surgery | 5901 (22) | 3799 (23)a | 646 (20) | 196 (23) | 439 (23) |
| Specialty Surgeriesb | 10733 (37) | 3469 (21)a | 613 (19)a | 350 (41) | 120 (6)a |
| Other Surgeriesc | 3989 (14) | 3917 (23)a | 830 (26)a | 81 (10)a | 1062 (55)a |
| Admission Hemoglobin, g/dL, n (%) | |||||
| Missing | 10371 (36) | 3268 (19)a | 564 (18)a | 291 (34) | 374 (19)a |
| <10 | 3201 (11) | 3320 (20)a | 813 (26)a | 146 (17)a | 355 (18)a |
| [10,12) | 5912 (21) | 4438 (26)a | 962 (30)a | 238 (28)a | 589 (30)a |
| >=12 | 9160 (32) | 5828 (35) | 832 (26)a | 178 (21)a | 617 (32) |
| Chronic Kidney Disease, n (%) | |||||
| Mild to moderate (eGFR≥30 ml/min per 1.73m2) | NA | NA | 2022 (64) | 787 (92) | NA |
| Severe (eGFR<30 ml/min per 1.73m2) | NA | NA | 1149 (36)d | 66 (8) | NA |
Abbreviations. SD, standard deviation; eGFR, estimated glomerular filtration rate.
p-value <0.05 for comparison with respect to no known kidney disease group using Bonferroni adjustment.
Specialty surgeries include orthopedic, gynecological, ear-nose-throat, urology and plastic surgeries.
Other surgeries include trauma, burn and transplant surgeries.
p-value <0.05 for comparison with respect to mild to moderate chronic kidney disease group.
All-cause mortality, cause of death, and progression to end-stage renal disease
The median follow-up time for the cohort was seven years (interquartile range 5 to 10 years). Overall survival rates for all-cause mortality among patients with any type of kidney disease were significantly lower compared to patients with no kidney disease (p<0.0001). At 10-year follow-up, cumulative survival probability for the group with no kidney disease was 75%, whereas it ranged between 39% and 55% for patients with kidney disease (SDC Figure 1). The top two causes of all deaths were cancer (5051/15247, 33%) and cardiovascular diseases (4269/15247, 28%). Cardiovascular disease accounted for more deaths among patients with any type of kidney disease compared to 18% among patients with no kidney disease (p<0.0001) ranging from 29% for patients with AKI and no CKD, 35% for patients with CKD but no AKI, 45% for those with CKD and AKI during admission, and 55% for ESRD patients when). In contrast, cancer accounted for fewer deaths among patients with kidney disease compared to those without (SDC Figure 1).
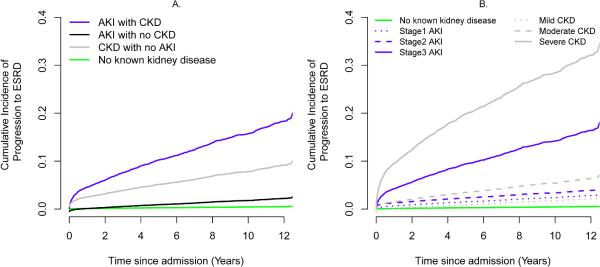
Among patients without prior history of ESRD who were discharged alive, 916 (1.9%) progressed to ESRD after discharge: 0.3% among patients with no known kidney disease, 2.0% among patients with de novo AKI, 7.4% among patients with CKD and no AKI during admission and 18.9% among CKD patient who developed AKI after surgery. When death from any cause was treated as a competing risk, adjusted ESRD progression estimates at 10-year follow-up were 0.4%, 2.3%, 7.3% and 15.7% for patients with no kidney disease, AKI with no CKD, CKD with no AKI, and AKI with CKD, respectively (P<0.001 compared to no kidney disease group, Figure 1A). The rates of progression to ESRD were increased with increasing severity of CKD and AKI (Figure 1B).
Figure 1.
Adjusted cumulative incidence curves for progression to end stage renal disease (A) by kidney disease status (B) by severity stages (adjusted for age, gender, ethnicity, Charlson comorbidity index, emergent surgery status, surgery type, and admission day hemoglobin level as described in Methods). All groups with acute or chronic kidney disease have significantly higher hazards ratios compared to no known kidney disease group with p<0.001.
Cardiovascular-specific mortality and Competing Risk Models
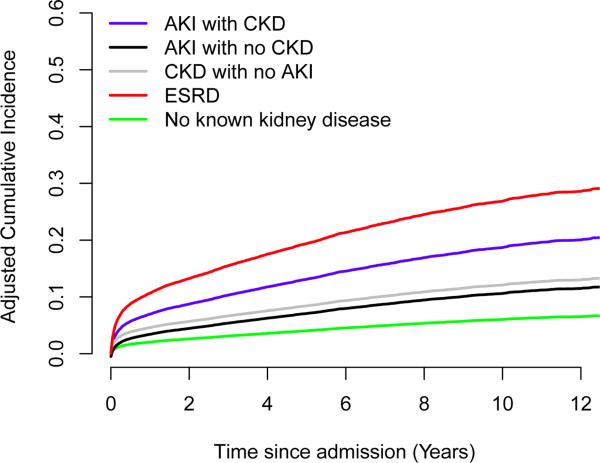
Both unadjusted and adjusted cumulative cardiovascular-specific mortality rates were significantly higher among patients with any type of kidney disease compared to those without (Figure 2 and SDC Figure 2). This association remained after excluding the patients who progressed to ESRD (SDC Figure 3A). At 10-year follow-up, adjusted cardiovascular-specific mortality estimates were 6%, 11%, 12%, 19% and 27% for patients with no kidney disease, AKI with no CKD, CKD with no AKI, AKI with CKD, and ESRD respectively (P<0.001 compared to no kidney disease group). Among small proportion of cohort who progressed to ESRD cardiovascular-specific mortality rates were amplified in all groups and comparable to the rates for patients with ESRD prior to surgery (SDC Figure 3B). In contrast, at 10-year follow-up adjusted cancer-specific mortality rates were lower among patients with kidney disease and ranged from 13%, 13%, 7%, 5% and 2% for patients with no kidney disease, AKI with no CKD, CKD with no AKI, AKI with CKD, and ESRD, respectively (SDC Figure 4). At 10 years, cumulative incidence rates for progression to ESRD and cardiovascular mortality among patients with CKD and AKI exceeded the cumulative incidence rate for cancer mortality (Table 2).
Figure 2.
Adjusted cumulative incidence curves for cardiovascular-specific mortality by kidney disease status (adjusted for age, gender, ethnicity, Charlson comorbidity index, emergent surgery status, surgery type, and admission day hemoglobin level as described in Methods). All groups with acute or chronic kidney disease have significantly higher hazards ratios compared to no known kidney disease group with p<0.001.
Table 2.
Adjusted 10-year cumulative incidence rates using multivariable subdistributional hazards models.
| Group | Patients, numbera | Cardiovascular-specific mortality (%) | Cancer-specific mortality (%) | Progression to ESRD (%) |
|---|---|---|---|---|
| No known kidney disease | 28644 | 6 | 13 | 0.4 |
| Acute kidney injury without chronic kidney disease | 16854 | 11 | 13 | 2.3 |
| Acute kidney injury with chronic kidney disease | 3171 | 19 | 5 | 15.7 |
| Chronic kidney disease without acute kidney injury | 853 | 12 | 7 | 7.3 |
| End stage renal disease | 1935 | 27 | 2 |
Number of patients for the analysis of progression to ESRD were 28490, 15495, 2445, and 810 for no known kidney disease, AKI with no CKD, AKI with CKD, and CKD with no AKI groups respectively, as patients with prior history of ESRD, renal transplant as primary surgery, and in-hospital mortality were excluded.
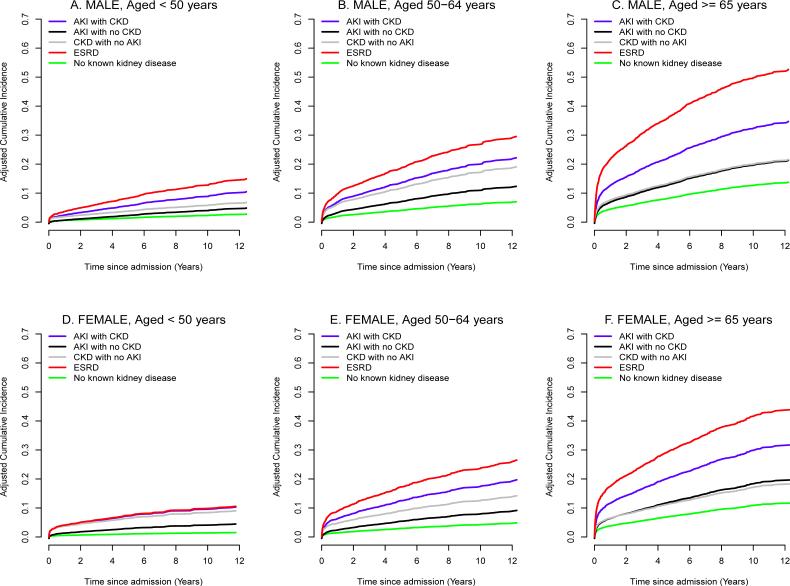
The unadjusted and adjusted cardiovascular-specific mortality rates associated with kidney disease remained increased when cohort was stratified by age, gender and surgery type (Figure 3 and SDC Figure 5 and 6). Even younger, premenopausal women with kidney disease had increase in cardiovascular mortality (at 10-year follow-up, adjusted cardiovascular-specific mortality estimates were 1%, 5%, 8%, 10% and 10% for patients with no kidney disease, AKI with no CKD, CKD with no AKI, AKI with CKD, and ESRD, respectively, P<0.001). The risk for cardiovascular-specific mortality was amplified by age for both women and men. At 10-year follow-up among patients ≥ 65 years, adjusted cardiovascular-specific mortality estimates for females and males were 11-13%, 19-20%, 17-20%, 30-32% and 42-50% for patients with no kidney disease, AKI with no CKD, CKD with no AKI, AKI with CKD, and ESRD respectively (P<0.001 compared to no kidney disease group).
Figure 3.
Adjusted cumulative incidence curves for cardiovascular mortality by kidney disease status after stratification by age and gender (A) Male, aged<50 years (B) Female, aged<50 years (C) Male, aged 50 to 64 years (D) Female, aged 50 to 64 years (E) Male, aged ≥ 65 years (F) Female, aged ≥ 65 years. All groups with acute or chronic kidney disease have significantly higher hazards ratios compared to no known kidney disease group within each strata with p<0.001 except for CKD with no AKI group for females with age between 50 and 64 years and males with age<50 years which have p<0.05.
Compared to patients with no kidney disease, the adjusted hazard ratios for cardiovascular-specific mortality were significantly higher among patients with kidney disease, ranging from 1.95 (95% CI, 1.80-2.11) for patients with de novo AKI to 5.70 (95% CI, 5.00-6.49) for patients with ESRD prior to admission. This significant association was preserved in sensitivity analyses with inclusion of individual comorbidities and postoperative complications in the model (Table 3). The adjusted hazards of cardiovascular-specific mortality increased with increasing severity of both AKI and CKD (Table 4). Age, admission comorbidities and hemoglobin level below 12 g/dl, emergent surgery status and surgery type were significantly associated with cardiovascular mortality.
Table 3.
Adjusted hazard ratios for cardiovascular-specific mortality using multivariable subdistributional hazards models.
| Model with Charlson Comorbidity Index Score | Model with Individual Comorbidities | Model with postoperative complications | |
|---|---|---|---|
| Variables | Adjusted Hazard Ratio | Adjusted Hazard Ratio | Adjusted Hazard Ratio |
| (95% Confidence Interval) | (95% Confidence Interval) | (95% Confidence Interval) | |
| Kidney Disease | |||
| No known kidney disease (reference group) | 1 | 1 | 1 |
| Acute kidney injury without chronic kidney disease | 1.95 (1.80, 2.11)a | 1.86 (1.71, 2.01)a | 1.58 (1.45, 1.72)a |
| Acute kidney injury with chronic kidney disease | 3.58 (3.22, 3.98)a | 3.24 (2.91, 3.61)a | 2.86 (2.56, 3.20)a |
| Chronic kidney disease without acute kidney injury | 2.04 (1.68, 2.48)a | 1.93 (1.59, 2.34)a | 2.02 (1.67, 2.45)a |
| End stage renal disease | 5.70 (5.00, 6.49)a | 6.05 (5.33, 6.87)a | 5.11 (4.49, 5.83)a |
| Age, per 1-year increase | 1.04 (1.04, 1.04)a | 1.04 (1.03, 1.04)a | 1.04 (1.04, 1.04)a |
| Male (vs. Female) | 1.08 (1.02, 1.15)a | 1.09 (1.02, 1.16)a | 1.07 (1.003, 1.13)a |
| African-American Ethnicity (vs. others) | 1.13 (1.03, 1.24)a | 1.08 (0.99, 1.19) | 1.16 (1.06, 1.27)a |
| Charlson Comorbidity Index Score | |||
| 0 (reference group) | 1 | NA | 1 |
| 1 | 2.05 (1.83, 2.31)a | NA | 1.97 (1.75, 2.22)a |
| 2 | 2.17 (1.94, 2.43)a | NA | 2.12 (1.89, 2.38)a |
| >=3 | 2.45 (2.19, 2.73)a | NA | 2.40 (2.15, 2.68)a |
| Comorbidities | |||
| Hypertension (Yes vs. No) | NA | 1.10 (1.02, 1.17)a | NA |
| Diabetes (Yes vs. No) | NA | 1.41 (1.31, 1.52)a | NA |
| Chronic Pulmonary Disease (Yes vs. No) | NA | 1.09 (1.01, 1.17)a | NA |
| Peripheral Vascular Disease (Yes vs. No) | NA | 1.65 (1.53, 1.78)a | NA |
| Cerebrovascular Disease (Yes vs. No) | NA | 2.07 (1.89, 2.26)a | NA |
| Congestive Heart Failure (Yes vs. No) | NA | 2.00 (1.85, 2.17)a | NA |
| Myocardial Infarction (Yes vs. No) | NA | 1.44 (1.32, 1.57)a | NA |
| Emergent surgery (vs. Elective) | 1.58 (1.48, 1.68)a | 1.46 (1.37, 1.56)a | 1.50 (1.40, 1.60)a |
| Surgery Type, n (%) | |||
| Specialty Surgeriesb (reference group) | 1 | 1 | 1 |
| Cardiothoracic Surgery | 2.28 (2.08, 2.5)a | 1.76 (1.59, 1.94)a | 1.86 (1.69, 2.05)a |
| Neurologic Surgery | 1.53 (1.38, 1.7)a | 1.21 (1.09, 1.36)a | 1.28 (1.15, 1.43)a |
| Non-Cardiac General Surgery | 1.46 (1.33, 1.6)a | 1.26 (1.15, 1.39)a | 1.39 (1.27, 1.53)a |
| Other Surgeriesc | 0.59 (0.52, 0.67)a | 0.66 (0.58, 0.75)a | 0.57 (0.50, 0.65)a |
| Admission Hemoglobin, g/dl | |||
| >=12 g/dl (reference group) | 1 | 1 | 1 |
| <10 g/dl | 1.09 (0.99, 1.19) | 1.12 (1.02, 1.23)a | 1.03 (0.94, 1.14) |
| [10,12) g/dl | 1.13 (1.04, 1.22)a | 1.13 (1.04, 1.23)a | 1.11 (1.03, 1.21)a |
| Missing | 0.97 (0.89, 1.06) | 1.02 (0.93, 1.11) | 0.99 (0.92, 1.09)a |
| Number of postoperative complicationsd | |||
| 0 (reference group) | NA | NA | 1 |
| 1 | NA | NA | 1.15 (1.05, 1.25)a |
| 2 | NA | NA | 1.37 (1.24, 1.51)a |
| >= 3 | NA | NA | 2.08 (1.91, 2.27)a |
p-value <0.05
Specialty surgeries include orthopedic, gynecological, ear-nose-throat, urology and plastic surgeries.
Other surgeries include trauma, burn and transplant surgeries.
Number of postoperative complications sums six major complications including mechanical ventilation for longer than 48 hours, intensive care unit admission, severe sepsis, cardiovascular complications, neurological complications and wound complications (including mechanical wound complications and surgical infections).
Table 4.
Adjusted hazard ratios for cardiovascular-specific mortality for acute and chronic kidneys disease stratified by severity stages using multivariable subdistributional hazards models.
| Model with Charlson Comorbidity Index Score | Model with Individual Comorbidities | |
|---|---|---|
| Variables | Adjusted Hazard Ratio | Adjusted Hazard Ratio |
| (95% Confidence Interval) | (95% Confidence Interval) | |
| Kidney Disease stratified by severity stages | ||
| No known kidney disease | Reference group Hazard ratio 1 | Reference group Hazard ratio 1 |
| Stage 1 Acute kidney injury | 1.76 (1.62, 1.92)a | 1.66 (1.52, 1.82)a |
| Stage 2 Acute kidney injury | 2.14 (1.92, 2.39)a | 1.97 (1.76, 2.20)a |
| Stage 3 Acute kidney injury | 4.01 (3.62, 4.43)a | 3.92 (3.53, 4.35)a |
| Mild chronic kidney disease | 1.94 (1.37, 2.75)a | 1.78 (1.26, 2.51)a |
| Moderate chronic kidney disease | 1.92 (1.51, 2.44)a | 1.79 (1.40, 2.29)a |
| Severe chronic kidney disease | 2.87 (1.66, 4.97)a | 3.16 (1.85, 5.39)a |
| End stage renal disease | 5.73 (5.03, 6.53)a | 6.06 (5.34, 6.88)a |
| Age, per 1-year increase | 1.04 (1.04, 1.04)a | 1.04 (1.03, 1.04)a |
| Male (vs. Female) | 1.10 (1.03, 1.17)a | 1.11 (1.04, 1.18)a |
| African-American Ethnicity (vs. others) | 1.15 (1.05, 1.26)a | 1.09 (0.997, 1.20) |
| Charlson Comorbidity Index Score | ||
| 0 | Reference group Hazard ratio 1 | NA |
| 1 | 2.01 (1.79, 2.26)a | NA |
| 2 | 2.13 (1.90, 2.39)a | NA |
| >=3 | 2.52 (2.26, 2.81)a | NA |
| Comorbidities | ||
| Hypertension (Yes vs. No) | NA | 1.17 (1.09, 1.26)a |
| Diabetes (Yes vs. No) | NA | 1.46 (1.36, 1.57)a |
| Chronic Pulmonary Disease (Yes vs. No) | NA | 1.09 (1.01, 1.17)a |
| Peripheral Vascular Disease (Yes vs. No) | NA | 1.70 (1.57, 1.83)a |
| Cerebrovascular Disease (Yes vs. No) | NA | 2.06 (1.89, 2.25)a |
| Congestive Heart Failure (Yes vs. No) | NA | 2.00 (1.84, 2.17)a |
| Myocardial Infarction (Yes vs. No) | NA | 1.44 (1.32, 1.57)a |
| Emergent surgery (vs. Elective) | 1.51 (1.42, 1.62)a | 1.4 (1.31, 1.50)a |
| Surgery Type, n (%) | ||
| Specialty Surgeriesb | Reference group Hazard ratio 1 | Reference group Hazard ratio 1 |
| Cardiothoracic Surgery | 2.21 (2.02, 2.43)a | 1.67 (1.51, 1.84)a |
| Neurologic Surgery | 1.52 (1.36, 1.69)a | 1.20 (1.07, 1.34)a |
| Non-Cardiac General Surgery | 1.42 (1.29, 1.55)a | 1.21 (1.10, 1.33)a |
| Other Surgeriesc | 0.57 (0.50, 0.64)a | 0.63 (0.56, 0.72)a |
| Admission Hemoglobin, g/dl | ||
| >=12 g/dl | Reference group Hazard ratio 1 | Reference group Hazard ratio 1 |
| <10 g/dl | 1.10 (1.01, 1.21) | 1.13 (1.03, 1.24)a |
| [10, 12) g/dl | 1.14 (1.05, 1.23)a | 1.14 (1.05, 1.24)a |
| Missing | 0.98 (0.9, 1.07) | 1.03 (0.95, 1.13) |
p-value <0.05
Specialty surgeries include orthopedic, gynecological, ear-nose-throat, urology and plastic surgeries.
Other surgeries include trauma, burn and transplant surgeries.
For internal validation of the study we tested the performance of the models in validation cohorts and performed several sensitivity analyses. The multivariable competing risk models performed well with C-index values of 0.84 (95% CI, 0.83-0.85), 0.81 (95% CI, 0.80-0.82), and 0.79 (95% CI, 0.78-0.80) at 1, 5, and 10 years, respectively in the validation datasets. Similar performance was observed for the competing risk models that included severity of kidney disease stages with C-index values of 0.85 (95% CI, 0.84-0.86), 0.81 (95% CI, 0.80-0.83), and 0.79 (95% CI, 0.78-0.80) at 1, 5, and 10 years, respectively. No significant difference was found between the C-indices of competing risk models applied to training and validation cohorts (p>0.05). The slight decrease in C-index over time was likely due to the fact that earlier events are easier to predict than later events.36 Sensitivity analyses demonstrated no significant difference among multivariable models after censoring patients on October 31, 2011, excluding missing causes of death or after using different definitions for cardiovascular cause of death and reference creatinine or after imputing missing hemoglobin values (P>0.05 for all comparisons).
DISCUSSION
In a large single-center cohort of surgical patients, both acute kidney injury and chronic kidney disease were associated with up to a four-fold increase in long-term cardiovascular-specific mortality compared to patients with no kidney disease. This association was independent of the progression to ESRD after discharge although patients who progressed had larger increase in cardiovascular mortality. The increasing cardiovascular mortality was proportional to the severity of kidney disease independent of patients’ age, gender, comorbidity burden on admission, other postoperative complications or the type of operation in the cohort that included a wide range of major surgical procedures, including non-cardiac and specialty surgeries. The development of postoperative AKI, in either the presence or absence of underlying CKD, was independently associated with long-term cardiovascular-specific mortality. Even patients with mild AKI had a 76% increase in the adjusted hazard for cardiovascular-specific mortality. While de novo AKI was four times more common than CKD, occurring in 33% of the cohort, it was associated with a comparable two-fold increase in cardiovascular-specific mortality compared to patients with no kidney disease. Patients with AKI superimposed on underlying CKD comprised a smaller proportion of AKI patients but had a markedly increased cumulative cardiovascular-specific mortality rate of 19%, almost as high as patients with ESRD. AKI survivors with no previous CKD had five-fold increase in the rates of ESRD progression compared to patients with no kidney disease and this association was strengthen by the presence of preexisting CKD as previously reported among different patient cohorts.3, 8, 11, 38 To the best of our knowledge this is the first study to demonstrate that the cardiovascular-specific mortality even in the absence of the progression to ESRD is the significant contributor to the excessive long-term mortality after surgery in patients with kidney disease.
Chronic kidney disease, from earlier stages to ESRD, is a well-known risk factor for cardiovascular disease. The absolute risk for death increases exponentially with decreasing renal function even among patients without manifest cardiovascular disease. 39, 40 After adjusting for traditional cardiovascular risk factors, the risk gradient for cardiovascular mortality increased linearly when eGFR decreased below 75 mL/min per 1.73 m2. 10, 41 Men and women with lower levels of kidney function, reflective of CKD, have a substantial and progressive reduction in overall life expectancy ranging from 1.3 to 21.3 years depending on age and eGFR, specifically due to cardiovascular disease.9, 42, 43 Importantly, individuals with earlier stages of CKD are more likely to die of cardiovascular disease than to develop kidney failure and require dialysis.9, 10, 41 Once developed, ESRD is associated with an up to thirty times higher cardiovascular mortality compared to the general population.44 Unfortunately, commonly used guidelines on the management of cardiovascular risk have paid only limited attention to CKD as a notable risk factor rendering cardiovascular disease frequently underdiagnosed and undertreated in these patients.9
Our data highlight that patients with postoperative AKI, even in the absence of preexisting CKD, are susceptible to die from cardiovascular disease at levels comparable to patients with CKD. The relationship between AKI and cardiovascular disease has been recognized in hospitalized, cardiac, and vascular patient populations. Hospitalized patients who recovered from de novo dialysis-requiring AKI are at a high risk of developing nonfatal myocardial infarctions and of needing coronary angiography procedures and coronary artery bypass grafting surgery, independent of their progression to CKD and ESRD.13 Studies evaluating outcomes after isolated coronary artery bypass grafting surgery accounted for preoperative renal dysfunction in their multivariate survival analyses and have demonstrated that AKI is independently associated with incident congestive heart failure hospitalization and a composite outcome consisting of myocardial infarction, heart failure, stroke, and long-term all-cause mortality.15, 45 Similarly, the occurrence of AKI following coronary angiography is significantly associated with the next hospitalization for congestive heart failure, independent of baseline kidney function and proteinuria, but this association was not significant when myocardial infarctions or cerebrovascular accidents were considered as outcomes 6. Olsson and colleagues recently demonstrated that AKI without preexisting CKD 15 was the strongest risk factor for incident hospitalization for congestive heart failure in isolated coronary artery bypass grafting surgical patients. When superimposed on preexisting CKD, AKI may exacerbate renal dysfunction and expedite the progression to ESRD 12 rendering these patients more susceptible to cardiovascular death with mortality rates comparable to ESRD patients, as demonstrated in our study. Interestingly, the risk for cardiovascular-specific mortality associated with kidney disease was more pronounced among older individuals but similar across genders. Liotta and colleagues demonstrated that the associations between AKI and all-cause long-term mortality was even stronger for women than for men in cardiac surgical patients who underwent primary, isolated coronary artery bypass grafting surgery.45
Since the kidneys act as key regulators of multiple homeostatic mechanisms, kidney-specific risk factors for cardiovascular disease become more relevant with acutely or chronically failing kidneys. Apart from hypertension, renal anemia and increased vascular stiffness, endothelial dysfunction manifested by reduced cardiac capillary density and impaired coronary dilatory response may be contributory mechanisms for the increased prevalence of left ventricular hypertrophy, myocardial fibrosis, impaired contractility and the more than fifty fold increase in incidence of sudden cardiac death in chronic kidney disease patients. Increased activity of the renin–angiotensin system and sympathetic nerve activity further impair endothelial function and worsen systemic inflammation and malnutrition.46 Unfortunately standardized follow-up after an episode of AKI is suboptimal in contemporary clinical practice. Less than 50% of patients with the most severe AKI will have a follow-up creatinine measured within the first three months of hospitalization, and it is even less likely that follow-up will be obtained after less severe AKI.47 Among AKI survivors with persistent renal dysfunction at discharge the referral rates for outpatient nephrology consultation are as low as 11%. 47, 48
We acknowledge the limitation of the retrospective nature of the cohort but with the use of multivariable adjustments and evaluation of model discrimination on validation datasets we have attempted to increase the internal validity of the competing risk models. Longitudinal studies involving determination of long-term mortality, and especially cause of death, are not only costly but increasingly difficult to perform 23. Although this is a single-center study which may elicit questions of generalizability, the site of the study is a large tertiary care center that receives a large number of referrals from all over the state and hence has a very heterogeneous patient profile over a wide range of procedures. Furthermore the application of standard survival analysis leads to bias and risk over-estimation if competing risks are present and specialized methods as one we used are needed.35, 36. Our multivariable modeling technique is attractive when potentially competing causes of death are present as it allows subject-specific estimates of the absolute risk of the cardiovascular-specific death based on a set of covariates.34 To our knowledge no prospective surgical cohort of this size and heterogeneity had concomitant data on both kidney disease and cardiovascular cause of death. We had only limited data on urine output or on patients with AKI and preoperative proteinuria among patients with CKD that could have strengthened our analysis. We used a combination of ICD-9-CM administrative codes and eGFR on admission to define CKD status. A recent systematic review demonstrated that although sensitivity for coded CKD covariates was highly variable, specificity was high, with all studies reporting values above 0.90.49 We have demonstrated that, although increased, progression to ESRD among AKI patients may not be an only determinant of cardiovascular mortality, just as patients with mild CKD are more likely to die from cardiovascular disease that to progress to ESRD.9, 10, 41 Some of traditional cardiovascular risk factors were not recorded in our database – i.e., systolic blood pressure, total cholesterol, HDL and smoking history – and thus were unable to be included as covariates.50 Patient cardiovascular comorbidity information, however, was available for previous myocardial infarctions, congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes and hypertension, and each patients’ Charlson-Deyo comorbidity index was calculated and included as a model covariate.
CONCLUSIONS
In summary, both acute kidney injury and chronic kidney disease commonly occurred during hospitalization for major surgical procedures and were associated with up to a four-fold increase in long-term cardiovascular-specific mortality compared to patients with no kidney disease. This association was proportional to the severity of kidney disease, independent of gender and amplified by age and progression to ESRD. This information speaks to the importance of the preoperative determination of kidney health by applying consensus staging criteria for CKD that uses both eGFR and proteinuria. Similarly, both preoperative and postoperative risk stratification for AKI using clinical scores and urinary biomarkers can allow implementation of simple and inexpensive preventive strategies in the perioperative period that could prevent or mitigate further decline in kidney function. The appropriate transition of follow-up to the outpatient setting, with emphasis on the prevention of kidney disease progression and mitigation of cardiovascular risk, is almost absent in contemporary practice. Our data present compelling evidence that such an effort is both warranted and justifiable.
Supplementary Material
Acknowledgments
Source of Funding: AB and TB are supported by Center for Sepsis and Critical Illness Award P50 GM-111152 from the National Institute of General Medical Sciences and has received research grants from Society of Critical Care Medicine and Astute Medical, Inc. Part of the cost for data acquisition for this study was supported by the award to Dr. Bihorac by I Heermann Anesthesia Foundation, Inc and Vision Grant by Society of Critical Care Medicine. MH was supported by the University of Florida Medical Student Summer Research fellowship from NIH T35 funds. This work was supported in part by the NIH/NCATS Clinical and Translational Sciences Award to the University of Florida UL1 TR000064. The preliminary report from this research was presented as a poster presentation at Kidney Week 2014 by American Society of Nephrology in Philadelphia, PA. The data reported have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the United States government.
Footnotes
Conflicts of Interest:
The authors report no conflict of interest.
REFERENCES
- 1.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 2.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 3.Wald R, Quinn RR, Luo J. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 4.van Kuijk JP, Flu WJ, Chonchol M, et al. Temporary perioperative decline of renal function is an independent predictor for chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:1198–1204. doi: 10.2215/CJN.00020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 6.James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–416. doi: 10.1161/CIRCULATIONAHA.110.970160. [DOI] [PubMed] [Google Scholar]
- 7.Thakar CV, Christianson A, Himmelfarb J, et al. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. The Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. The Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla LS, Amdur RL, Shaw AD, et al. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9:448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu VC, Wu CH, Huang TM, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsson D, Sartipy U, Braunschweig F, et al. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail. 2013;6:83–90. doi: 10.1161/CIRCHEARTFAILURE.112.971705. [DOI] [PubMed] [Google Scholar]
- 16.Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg. 2013;258:169–177. doi: 10.1097/SLA.0b013e318288e18e. [DOI] [PubMed] [Google Scholar]
- 17.Bihorac A, Brennan M, Ozrazgat-Baslanti T, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013;41:2570–2583. doi: 10.1097/CCM.0b013e31829860fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobson CE, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakar CV. Perioperative Acute Kidney Injury. Adv Chronic Kidney Dis. 2013;20:67–75. doi: 10.1053/j.ackd.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, et al. The Pattern of Longitudinal Change in Serum Creatinine and 90-Day Mortality After Major Surgery. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 22.Shavit L, Hitti S, Silberman S, et al. Preoperative hemoglobin and outcomes in patients with CKD undergoing cardiac surgery. Clin J Am Soc Nephrol. 2014;9:1536–1544. doi: 10.2215/CJN.00110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winn D. [11/19, 2014];Could the recent change in the availability of the Death Master File affect your research? 2012 Cancer Epidemiology Matters Blog web site. Available at: http://blogepi.grants.cancer.gov/2012/05/24/could-the-recent-change-in-availability-of-death-master-file-data-affect-your-research.
- 24.Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–51. [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siew ED, Ikizler T, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. J Am Soc Nephrol. 2012;7:8. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wald R, Waikar SS, Liangos O, et al. Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. J Vasc Surg. 2006;43:460–466. doi: 10.1016/j.jvs.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 29.Kidney Disease: Improving Global Outcomes ( KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney inter. Suppl. 2013. 3:1–150. [Google Scholar]
- 30.Kidney Disease: Improving Global Outcomes ( KDIGO) Acute Kidney Injury Work Group Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl. 2012;2:1–138. [Google Scholar]
- 31.U.S. Renal Data System Coordinating Center USRDS 2013 Researcher USRDS Database. 2013 [Google Scholar]
- 32.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity Measures for Use with Administrative Data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 34.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 35.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolbers M, Blanche P, Koller MT, et al. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. doi: 10.1093/biostatistics/kxt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2-2. 2011 [computer program]. Version 2.2-2. [Google Scholar]
- 38.Rimes-Stigare C, Frumento P, Bottai M, et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care. 2015;19:221. doi: 10.1186/s13054-015-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonelli M, Wiebe N, Culleton B, et al. Chronic Kidney Disease and Mortality Risk: A Systematic Review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 40.Di Angelantonio E, Chowdhury R, Sarwar N, et al. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. Br Med J. 2010;341:c4986. doi: 10.1136/bmj.c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 42.Turin TC, Tonelli M, Manns BJ, et al. Chronic kidney disease and life expectancy. Nephrol Dial Transplant. 2012;27:3182–3186. doi: 10.1093/ndt/gfs052. [DOI] [PubMed] [Google Scholar]
- 43.Turin TC, Tonelli M, Manns BJ, et al. Proteinuria and life expectancy. Am J Kidney Dis. 2013;61:646–648. doi: 10.1053/j.ajkd.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 44.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease: A Statement From the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 45.Liotta M, Olsson D, Sartipy U, et al. Minimal Changes in Postoperative Creatinine Values and Early and Late Mortality and Cardiovascular Events After Coronary Artery Bypass Grafting. Am J Cardiol. 2014;113:70–75. doi: 10.1016/j.amjcard.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.U.S. Renal Data System . USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. [Google Scholar]
- 48.Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23:305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grams ME, Plantinga LC, Hedgeman E, et al. Validation of CKD and related conditions in existing data sets: A systematic review. Am J Kidney Dis. 2011;57:44–54. doi: 10.1053/j.ajkd.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.