Abstract
Standard therapies used for the treatment of Acute Myeloid Leukemia (AML) are cytotoxic agents that target rapidly proliferating cells. Unfortunately, this therapeutic approach has limited efficacy and significant toxicity and the majority of AML patients still die of their disease. In contrast to the poor prognosis of most AML patients, most individuals with a rare subtype of AML, Acute Promyelocytic Leukemia (APL), can be cured by differentiation therapy using regimens containing all-trans retinoic acid. GSK3 has previously been identified as a therapeutic target in AML where its inhibition can lead to the differentiation and growth arrest of leukemic cells. Unfortunately, existing GSK3 inhibitors lead to suboptimal differentiation activity making them less useful as clinical AML differentiation agents. Here we describe the discovery of a novel GSK3 inhibitor, GS87. GS87 was discovered in efforts to optimize GSK3 inhibition for AML differentiation activity. Despite GS87's dramatic ability to induce AML differentiation, kinase profiling reveals its high specificity in targeting GSK3 as compared to other kinases. GS87 demonstrates high efficacy in a mouse AML model system and unlike current AML therapeutics, exhibits little effect on normal bone marrow cells. GS87 induces potent differentiation by more effectively activating GSK3-dependent signaling components including MAPK signaling as compared to other GSK3 inhibitors. GS87 is a novel GSK3 inhibitor with therapeutic potential as a differentiation agent for non-promyelocytic AML.
Keywords: Leukemia, GSK3, Differentiation
Introduction
Acute myeloid leukemia (AML), the most common form of acute leukemia in adults, is a heterogeneous disorder defined by the arrest of proliferation of immature hematopoietic cells (1). Standard AML chemotherapy leads to significant toxicity and is frequently ineffective. Despite the fact that AML is characterized by a differentiation arrest, standard AML treatment targets the rapid proliferation of leukemic cells. In contrast to the poor prognosis of the majority of AML patients, most individuals with a rare subtype of AML, acute promyelocytic leukemia (APL), can be cured by differentiation therapy using regimens containing all-trans retinoic acid (ATRA) (2).
In efforts to find novel strategies for inducing differentiation in non-APL leukemic cells, we and others identified that inhibiting GSK3 leads to AML differentiation (3-5). GSK3 is a serine/threonine kinase involved in multiple signaling pathways. Originally identified for its role in regulating glycogen synthesis, it has been found to play roles in many biological processes such as inflammation, stem cell maintenance, cell proliferation, and regulation of normal and aberrant hematopoiesis (6). In addition, GSK3 plays important roles in a variety of key signaling pathways such as Wnt/β-catenin, PI3K, mTOR, and MAPK signaling, (6).
While pharmacologic inhibition of GSK3 has historically been studied in a variety of diseases (7), in recent years, GSK3 has been found to play an important role in cancer. GSK3 has been demonstrated to be overexpressed and hyperactivated in a variety of solid cancers. Additionally, overexpression of GSK3 has been associated with resistance to therapy and poor prognosis (6, 8). Prior studies have identified that inhibition of GSK3 results in AML cell growth inhibition and monocytic differentiation (3-5, 9-12). Importantly, several studies have reported that targeting GSK3 specifically impairs leukemic cell growth, but not normal bone marrow proliferation. Due to the role of GSK3 in the molecular pathology of many diseases, development of specific inhibitors of this kinase with therapeutic intentions have been pursued. While investigational GSK3 inhibitors exist, the potency and specificity of GSK3 inhibition is variable. Lithium (Li), the only FDA approved GSK3 inhibitor, has long been used clinically to treat bipolar disorder. Lithium directly binds GSK3 at a Mg2+ binding site, leading to reversible enzyme inhibiton and inhibitory serine-9 phosphorylation (13-15). LY2090314 and Tideglusib are more specific GSK3 inhibitory small molecules utilized in humans in clinical trials (16, 17). Other small molecule GSK3 inhibitors have been described, such as SB415285 (SB), which inhibits GSK3 by competitive binding to the ATP binding site (18). In general, the GSK3 inhibitors described to date also inhibit many other kinases such as CDK2-cyclin A (19).
Although GSK3 inhibition has been demonstrated to induce evidence of AML differentiation, existing GSK3 inhibitors lead to sub-optimal levels of AML differentiation that would likely prohibit their use as clinically useful differentiation agents. Due to the promise of differentiation therapy for AML, we aimed to identify a novel GSK3 inhibitor that was optimzed for AML differentiation activity. Here, we report the identification of a novel and highly specific GSK3 inhibitor, GS87, that induces extensive AML differentiation with selective growth inhibition of leukemic cells and exhibits promise as an AML differentiation therapeutic.
Materials and Methods
Reagents and cells
The 2000 compound small molecule library was custom designed based upon similarities to known chemical and structural features of previously reported GSK3 inhibitors by Enamine (Ukraine). Additional quantities of GS87 (T5729983) were obtained from Enamine. SB415286 (SB) was obtained from Tocris Biosciences (Minneapolis, MN, USA). Lithium (Li) was obtained from Sigma (St. Louis, MO, USA). SB203580 (SB203), PD08959 (PD) and SP600125 (SP) were obtained from Selleck Chem (Boston, MA, USA). MTT reagent was obtained from the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). Phospho-Ser9 GSK3β (5558), GAPDH (5174), phospho-p38 (4511), phospho-ERK (4370), and phospho-JNK (4668) was obtained from Cell Signaling Technologies (Beverly, MA, USA). β-catenin, p21 (397), c-myc (40), c-MYB (7874), and MAFB (74521) antibodies were obtained from Santa Cruz (Santa Cruz, CA, USA). OCI-AML3 cells were obtained from DSMZ (Braunschweig, Germany), and HL-60, NB4, THP-1, U937 and Hela cells were obtained from ATCC (Manassas, VA, USA). All cell lines were obtained from ATCC or DSMZ and cultured for fewer than 6 months after resuscitation. OCI-AML3 shβcatenin cells were previously reported (20). Primary AML and normal donor bone marrow cells were obtained from Case Western Reserve University (CWRU) Hematopoietic Stem Cell Core Facility (Cleveland, OH).
Cell culture
Cells were cultured in RPMI 1640 media (Hyclone, Waltham, MA,). All media were supplemented with 10% FBS and penicillin/streptomycin. Cells were cultured in a humidified atmosphere of 95% (v/v) air and 5% (v/v) CO2 at 37 °C.
Differentiation
The NBT reduction assay was performed as described previously (21). Immunophenotyping was performed by staining cells with CD11b-PE (Becton Dickinson, Franklin Lakes, NJ,) or CD14-PE (eBiosciences, San Diego, CA,). Stained samples were run on a Beckman Coulter Cytomics FC 500 cytometer (Becton Coulter, Brea, CA,). For morphologic assessment, cells were adhered to a slide using cytospin and then stained with Wright-Giemsa. Images were taken using the built in EVOS XL core camera and acquisition software using a 100x oil objective. Pictures were changed to gray scale using Powerpoint.
Proliferation and colony assay
Equal numbers of THP-1, HL-60, NB4, U937, and OCI cells were plated and treated with Li, SB or GS87 for 72 hours. Cells were analyzed for proliferation using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega) as recommended by the manufacturer using a Molecular Q3 Devices Spectramax M2 plate reader. For the colony assay, after 72hr the cells were washed with PBS, an equal number of viable cells were suspended in 0.03% noble agar (Sigma), the cells were incubated for one week and number of colonies was counted.
Cell cycle analysis
Cells were treated with Li, SB, or GS87 for 24 hours, fixed in 90% methanol and incubated overnight at −20°C. Cells were then washed with PBS, treated with RNase (0.5μg/ml) and propidium iodide (50μg/ml) in the dark for 60-90 minutes at room temperature and analyzed by flow cytometry.
Western blot
Cells treated as indicated were washed with PBS, centrifuged and lysed with a triton-containing lysis buffer for whole cell extracts. Protein lysates (50 μg/lane) were resolved on appropriate SDS-PAGE gel and transferred to PVDF membrane (Millipore, Billerica, MA,) using Bio-Rad transfer apparatus (Bio-Rad, Hercules, CA,). The membranes were blocked, incubated with the indicated primary antibodies at 4 °C overnight, and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies. Immunoreactive protein bands were detected by enhanced chemiluminescence (Pierce, Waltham, MA,) using XAR-5 film.
RNA Microarray
HL-60 cells were treated with Li, SB, or GS87 for 48 hours and total RNA was isolated using Trizol (Invitrogen). The cRNA was prepared using the TargetAmp-Nano kit (Epicenter) and hybridized to the human HT-12 Expression BeadChip (Illumina). Microarray results were evaluated using Genomestudio (Illumina) and R (version 3.1.3) software. Differential expression was assessed using the lumi package for R. False Discovery Rate (FDR) was used to correct for multiple comparisons. Pathway analysis was performed using Ingenuity Pathway Analysis software (Qiagen, Redwood, CA) for genes with significantly dysregulated expression (FDR adjusted p-value < 0.05) and an absolute log2 fold change greater than or equal to 1.5). Micorarray data was submitted to Arrayexpress (accession number E-MTAB-3690).
Real time qRT-PCR
Total RNA was isolated from cells treated with Li, SB or GS87 for 48 h using TRIzol reagent (Invitrogen). RNA was transcribed into cDNA using the Enhanced Avian RT First Strand Synthesis Kit (Sigma). Relative quantitative RT-PCR was performed in triplicate using the FastStart SYBR Green Master (Roche Diagnostics) on an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Primers used for confirmation of microarray data are listed in Supplemental Table 1 and were purchased from Sigma.
Kinase Assays
Kinases assays were performed by Reaction Biology Corporation using their standard 33P-ATP based protocol (Malvern, PA). For kinase profiling, GS87 (1μM) was utilized for radioactive in vitro kinase assays on a panel of 183 kinases as shown in the supplementary data. All assays were carried out using 10μM ATP and staurosporine as a positive control. For the IC50 determination, a 10-dose 3-fold serial dilution assay was performed starting at 100 μM.
Mouse xenograft studies
6 week old female Nod Scid IL-2γR−/− (NSG) mice (Jackson Labs, Bar Harbor, ME) were injected i.v. with 5X106 primary human AML cells or HL-60 cells (n=5 mice per group). Drug treatment was started 3 days after cell injection. GS87 (50mg/kg), Cytarabine (50mg/kg), or vehicle (20μL of DMSO and 80μl of water) were injected as indicated i.p. 3x a week for 3 weeks. The mice were either assessed for survival (primary patient sample group) or sacrificed when the vehicle mice became moribund at 4 weeks after cell injection (HL-60 group). The mice were sacrificed when moribund or at the end of the study period and analyzed by flow cytometry for human leukemia cells in the bone marrow using human CD45 specific antibody (BD Biosciences) as well as CD11b in the HL-60 group. The CWRU Animal Research Committee approved the animal protocols used in this study.
Statistics
Group means were compared using two-tailed analysis of variance (ANOVA). p-values < 0.05 were considered significant. Error bars represent the root mean square error and experiments were performed in triplicate.
Results
Identification of GS87
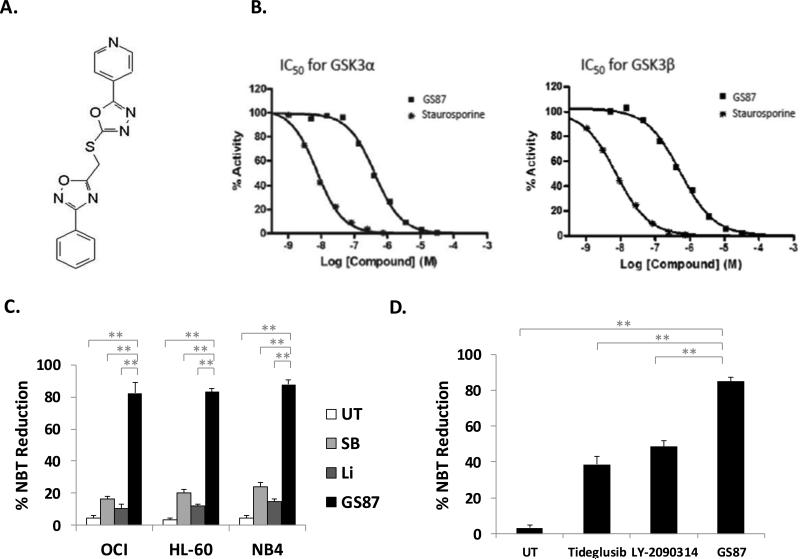
GSK3 has been demonstrated to be an excellent target for AML as inhibiting this kinase can lead to the differentiation and irreversible growth arrest of AML cells yet exhibit little activity or even increase the growth of normal bone marrow cells (3, 22). Unfortunately, the existing GSK3 inhibitors demonstrate only moderate AML differentiation activity leading to suboptimal differentiation. To identify novel GSK3 inhibitors optimized for high AML differentiation activity, a focused library of approximately 2000 novel small molecules was compiled (designed using structural and chemical similarities to existing GSK3 inhibitors). From screening this chemical library we identified not only a novel and highly specific GSK3 inhibitor, but also an inhibitor capable of leading to dramatically higher rates of AML differentiation than previously tested GSK3 inhibitors. The screen involved treating OCI-AML3 cells with the small molecule library and measuring differentiation using the Nitrotetrazolium blue (NBT) reduction assay. NBT reduction measures the respiratory burst activity of cells, a function specific to differentiated cells (23, 24). From this library, GS87 was as the only compound capable of inducing greater than 50% differentiation. The structure of GS87 which represents a novel scaffold for a GSK3 inhibitor is illustrated in Figure 1A.
Figure 1. GS87 is a highly specific and potent GSK3 inhibitor that induces AML cell differentiation.
(A) Molecular structure of GS87. (B) GS87 is a potent GSK3 inhibitor. In vitro kinase assay for GSK3α and GSK3β. (C) GS87 leads to extensive AML differentiation as measured by respiratory burst capacity. OCI, HL-60, and NB4 cells were treated with GS87 (30μM), Li (10mM) or SB (20μM) for 72 hours and differentiation capacity was measured using the NBT reduction assay. (D) GS87 leads to more effective AML differentiation than other clinically used GSK3 inhibitors. HL-60 cells were treated with GS87 (30μM), Tideglusib (30μM) or LY-2090314 (30μM) for 72 hours and differentiation was measured by the NBT assay. * p<.05; **p<.01
GS87 is a highly specific GSK3 inhibitor
As GS87 has a novel scaffold, we confirmed that it is truly a GSK3 inhibitor using radioactive in vitro kinase assays. GS87 was found to demonstrate significant inhibition of both GSK3α and GSK3β (IC50 415nM and 521nM respectively) as seen in Figure 1B. As previously reported, GSK3 inhibitors also tend to inhibit other kinases such as Cyclin-dependent kinase 2/Cyclin A (CDK2A), we also performed kinase profiling to assess GS87's specificity in inhibiting GSK3 (19). This screening demonstrated GS87 is among the most specific GSK3 inhibitors reported as it had little activity on a panel of 187 other kinases at 1uM using in vitro kinase assays including CDK2-CyclinA (Supplemental Table 2).
GS87 induces AML cell differentiation
To confirm the high level of GS87-mediated differentiation, we compared its ability to induce AML differentiation in a variety of cell lines as compared to the widely used GSK3 inhibitors, SB415286 (SB) and Lithium (Li). Importantly all agents were used at optimal doses for inducing differentiation without leading to significant cell death. Lithium was chosen as it is the only currently FDA approved GSK3 inhibitor. OCI-AML3 (OCI), HL-60 and NB4 cell lines showed a dramatically higher level of NBT reduction after treatment with GS87 (~80%) as compared to those treated with SB (~20%) or Li (~10%) (Figure 1C). These levels of differentiation in response to GSK3 inhibition as measured by NBT reduction are similar to previous studies describing these agents as well as to other GSK3 inhibitors such as TWS116, 6-bromoindirubin-3'-oxime, and CHIR9902 (3). Of note, the doses used for differentiation induction due not lead to any appreciable cell death effects on AML cells when assessed at 72 hours after treatment (Supplementary figure 1).
In addition to Li which is used clinically, tideglusib and LY-2090314 are two small molecule GSK3 inhibitors which are in clinical trials and were also compared to GS87 (7, 25) Treatment with GS87 also induced significantly more differentiation as measured by NBT reduction than either tideglusib or LY-2090314 (Figure 1D), identifying GS87 as a dramatically more effective AML differentiation agent.
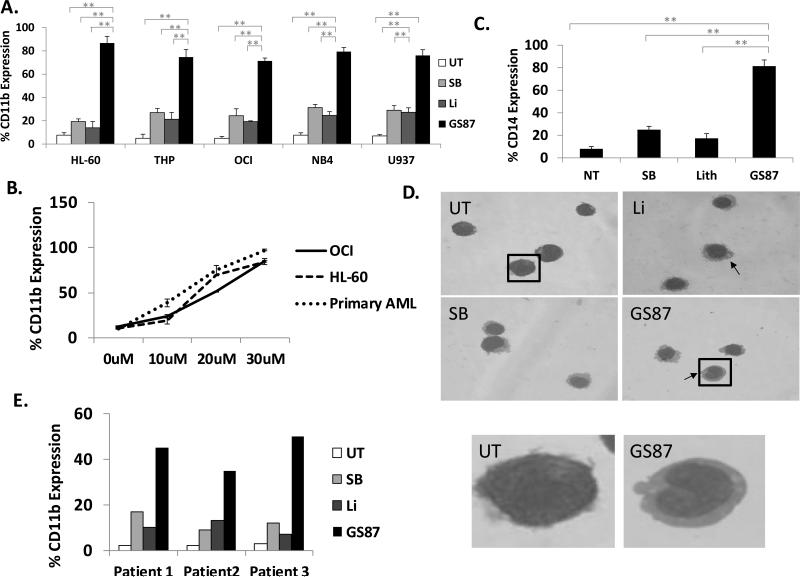
In addition to NBT reduction, differentiation was measured by immunophenotyping for the cell surface markers CD11b and CD14, which are associated with general granulocytic and monocytic differentiation respectively. Similarly to the NBT reduction studies, CD11b and CD14 were induced significantly more with GS87 as compared to other GSK3 inhibitors in a variety of AML cell lines. For example, CD11b was more strongly upregulated by GS87 (~75%) as compared to treatment with SB (~25%) and Li (~20%) in multiple AML cell lines (Figure 2A-B and Supplementary Figure 2). As HL-60 cells can differentiate into multiple lineages such as monocytes and neutrophils, we tested for CD14 induction, a specific marker of monocytic differentiation, in this cell line. CD14 expression in HL-60 cells was highest after GS87 treatment (81%) as compared to treatment with SB (25%) or Li (17%) (Figure 2C). Additionally, morphologic assessment of cells after treatment with GS87 confirmed high level of monocytic differentiation as evidenced by condensation of the nucleus, change in nuclear shape and appearance of cytoplasmic vacuoles (Figure 2D). Of note, while both cell lines treated with Li, SB and GS87 all exhibited features of monocytic differentiation as expected, GS87 treated cells demonstrated noticeably more nuclear indention.
Figure 2. GS87 promotes monocytic differentiation of AML cells.
(A-C) GS87 leads to extensive AML differentiation as measured by immunophenotyping. AML cell lines were treated with GS87 (30μM or as indicated), Li (10mM) or SB (20μM) for 72 hours and cell surface expression of CD11b and CD14 (HL-60 cells) were measured by flow cytometry. *p<.05, **p<.01 (D) Treatment with GS87 induces morphologic changes consistent with monocytic differentiation in HL-60 cells compared to untreated cells. Cells were stained using Wright-Giemsa stain. Images were taken using the built in EVOS XL core camera and acquisition software using a 100x oil objective. The arrows indicate representative cells with marked nuclear condensation or vacuoles. The boxed cells are shown expanded to better illustrate the morphology. (E) GS87 leads to AML differentiation in primary patient leukemic cells. Primary patient leukemia cells treated with GS87, Li, or SB showed the highest increase in the cell surface expression of CD11b after GS87 treatment.
We next studied the differentiation effects of GS87 on primary leukemic cells derived directly from non-APL leukemia patients. Compared to untreated samples, AML cells treated with SB, Li, and GS87 displayed increased differentiation characterized by CD11b expression on flow cytometry. As observed with AML cell lines, the greatest percentage of CD11b expression was observed after GS87 treatment as compared to the other GSK3 inhibitors tested (Figure 2E). Of note, GS87 is able to exhibit potent AML differentiation in a wide range of diverse primary AML samples and cell lines in contrast to the clinically used differentiation agent ATRA that is specifically potent for APL cells (Figures 1-2 and Supplementary Figure 3).
GS87 inhibits AML proliferation and causes terminal differentiation
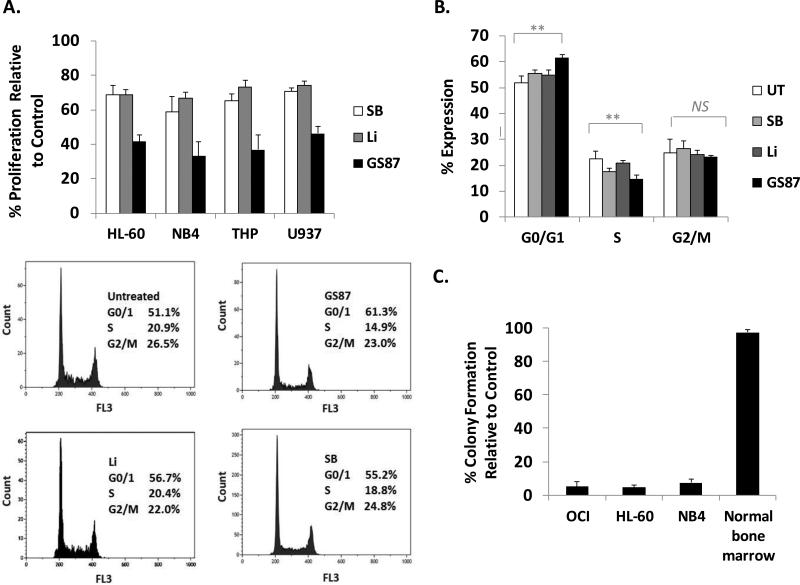
We next evaluated the effects of GS87 on leukemic cell growth. Using the MTT assay, we compared cell proliferation following treatment with SB, Li, and GS87 for 72 hours using doses leading to maximal differentiation without significant cytotoxic effects. GS87 treatment resulted in 35-47% cell proliferation relative to untreated controls in THP-1, HL-60, NB4, and U937 cells whereas SB and Li treatment showed 60-70% and 68-74% proliferation relative to vehicle control cells, respectively (Figure 3A). Furthermore, cell cycle analysis in HL-60 cells showed an increased accumulation of cells in the G0/G1 phase and a reduction in the S phase 24 hours after treatment with GS87 consistent with the observed growth inhibition (Figure 3B).
Figure 3. GS87 inhibits AML cell proliferation and causes irreversible growth inhibition.
(A) GS87 impairs AML proliferation. Equal numbers of HL-60, U937, THP-1, and NB4 cells were treated with GS87 (30μM), Li (10mM) or SB (20μM) for 72 hours and the MTT assay was performed. The MTT signal was normalized to the vehicle treated control. (B) GS87 modulates the cell cycle of AML cells. HL-60 cells were treated with GS87, Li, or SB for 24 hours and assessed for cell cycle status. Representative flow results are shown and the histogram is an average of 3 independent studies. **p<.01 NS indicates no significant difference. (C) GS87 inhibits colony formation of AML cells but not normal marrow mononuclear cells. OCI, HL-60, and NB4 cells and normal marrow mononuclear cells were treated with GS87 for 72 hours, after which drug was washed away and equal numbers of viable cells were cultured in semisolid soft agar medium for 7-10 days. Colony formation of cells were normalized to a vehicle treated control.
The purpose of differentiation therapy for AML is to induce terminal differentiation such that differentiated cells can no longer divide and propagate disease. Thus we evaluated the irreversibility of AML cell growth arrest by limited treatments of GS87 using colony assays. After a 72 hour treatment of OCI, HL-60, and NB4 cells with GS87, the drug was washed away, an equal number of viable cells was plated in soft agar and subsequent colony formation was assessed. Consistent with extensive terminal differentiation, limited treatment with GS87 was found to lead to a near complete inhibition of colony formation with only 4-9% colony growth relative to the vehicle treated control in all cell lines tested (Figure 3C). Next, we tested the effects of GS87 on the colony formation of normal hematopoietic progenitor cells to investigate the potential for hematologic toxicity as this is an extremely common side effect of most AML therapeutics. Similar to previous reports of inhibiting GSK3 in normal bone marrow, GS87 treatment did not significantly inhibit colony growth of cells taken from normal human bone marrow samples (Figure 3C). In addition, mice treated with GS87 did not show any impact on normal hematopoiesis as measured by changes in white blood cell, red blood cell or platelet counts (Supplementary Figure 4).
GS87 modulates key GSK3 target proteins involved in cell proliferation and differentiation more effectively than Lithium and SB
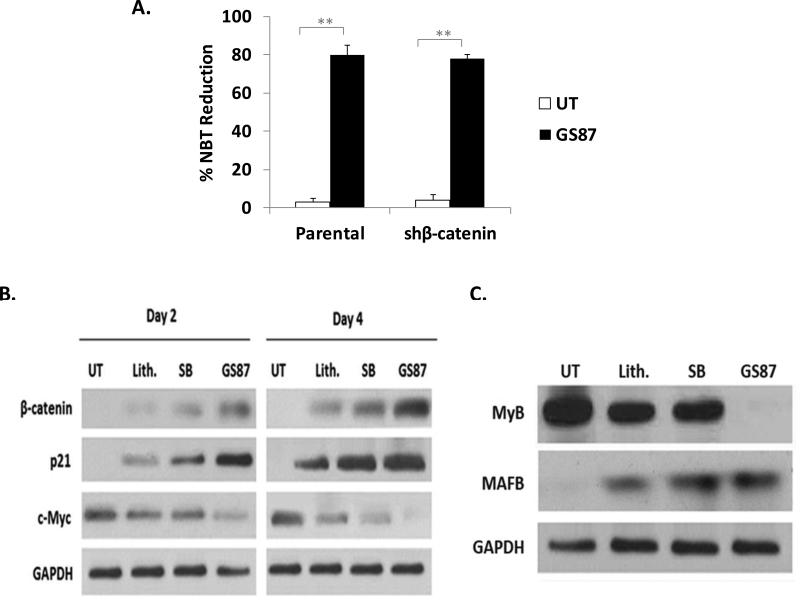
To explore mechanistic insights that may explain GS87's enhanced differentiation activity over other GSK3 inhibitors, we investigated the impact of GS87 on known GSK3 dependent pathways that are important for AML differentiation and growth in leukemic cells including β-catenin, p21, c-myc, c-MYB, and MAFB. One of the major direct target proteins of GSK3 is β-catenin, which is phosphorylated by GSK3 and targeted for degradation. Therefore, we investigated the role of β-catenin in mediating the differentiation effects of GS87. GS87 treatment of OCI cells with stable knockdown of β-catenin showed similar differentiation compared to cells transfected with empty vector (Figure 4A), demonstrating that the differentiation effect of GS87 is independent of β-catenin. Though β-catenin does not appear to be important for GS87-mediated differentiation, GS87 did lead to a more pronounced induction of this GSK3 target protein than the other GSK3 inhibitors tested (figure 4B).
Figure 4. GS87 effectively modulates GSK3-dependent proteins.
(A) GS87-induced differentiation is independent of β-catenin. OCI cells with stable knockdown of β-catenin showed similar differentiation as compared to cells transfected with empty vector after treatment with GS87 for 3 days as measured by NBT reduction. (B-C) GS87 modulates GSK3 target proteins important in cell proliferation and differentiation more effectively than Li and SB. HL-60 cells were treated as indicated for 2 and 4 days (B) or 4 days (C) and assessed by western blot for key AML growth and differentiation proteins that can be modulated by GSK3.
p21 is a key regulator of the cell cycle and is induced during AML differentiation (26) while c-myc is a promoter of cell proliferation whose presence prevents terminal cell differentiation in cancer cells and is downregulated during AML differentiation (27). p21 is also known to be a direct GSK3 target protein that can be degraded in a GSK3-dependent fashion (28). As shown in Figure 4B, GS87 treatment increased p21 while it reduced c-Myc protein levels more robustly than either Li or SB reinforcing the cell proliferation analysis. Importantly, the ability of GS87 to more effectively modulate GSK3 dependent pathways than SB and Li occurs at doses optimized for differentiation for all 3 agents. We also investigated levels of c-MYB and MAFB, protooncogenes over-expressed in immature hematopoietic cells and in some hematologic malignancies that are regulated by GSK3. c-MYB has previously been demonstrated to be a key target of GSK3 in mediating its cell growth effects in AML cells and MAFB is also regulated by GSK3 phosphorylation and plays a key role in myeloid differentiation (29-32). GSK3 inhibition has been shown to directly down regulate c-Myb and upregulate MAFB expression. GS87 more effectively modulated protein expression of both GSK3 target proteins c-MYB and MAFB than SB and Li further establishing the ability of GS87 to strongly impact GSK3 dependent pathways (Figure 4C).
GS87-mediated differentiation is dependent upon MAPK signaling
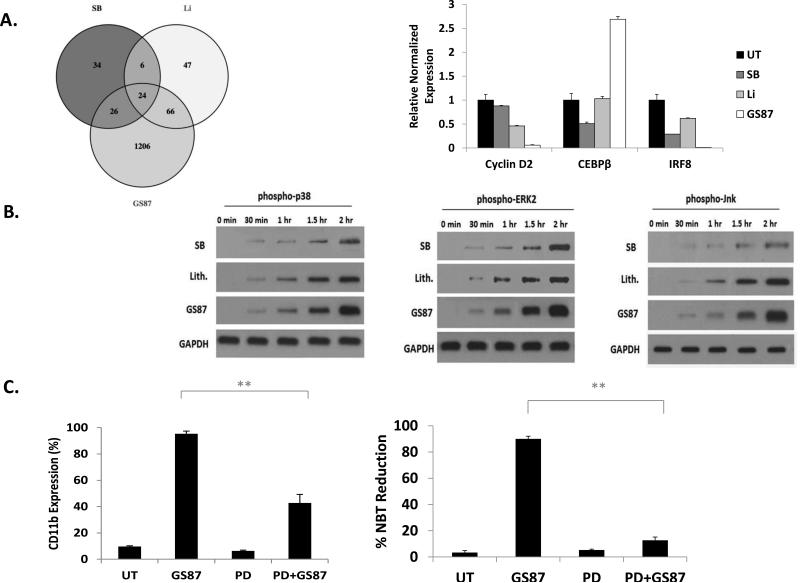
To further explore the cellular pathways involved in mediating the differentiation effects of GS87 as compared to other GSK3 inhibitors, we performed RNA expression analysis using a gene microarray after treatment of OCI cells with GS87, Li, and SB. The cells were treated using a short treatment (48 hours) to enable the identification of mediators of differentiation instead of simply to appreciate the phenotype of the differentiated cells. After False Discovery Rate correction, 1,322 genes were significantly (p-value < 0.05) differentially expressed with an absolute log2 fold change greater than or equal to 1.5 after GS87 treatment, 90 genes after treatment with SB, and 143 genes after treatment with Li (Supplementary Figure 5 and Supplemental Table 3). 34 genes were only significantly dysregulated by SB treatment, 47 genes were only significantly dysregulated by Li treatment, and a total of 1,206 genes were only significantly dysregulated by treatment with GS87 (Supplemental Table 4 and Figure 5A).
Figure 5. GS87-induced AML differentiation is dependent on MAPK signaling.
(A) Comparison of GSK3 inhibitor modulation of gene expression in AML cells. HL-60 cells were treated for 48 hours and the expression of the indicated genes was assessed. Left Panel: Venn diagram of significantly regulated genes. Right Panel: Validation of microarray results by real-time PCR. (B) Multiple components of the MAPK signaling cascade are activated by GS87 treatment. HL-60 cells were treated with GS87, Li, or SB and the activation of p38, ERK, and JNK were analyzed by Western blot at various time points using phospho-specific antibodies. (C) Chemical inhibition of ERK (PD08959 10μM) abrogates the differentiation capacity of GS87 in HL-60 cells. **p<.01.
Next, we performed pathway analysis on genes that were significantly modulated (FDR adjusted p-value < 0.05, |log2 fold change| ≥ 1.5) in the GS87, Li, and SB treatment groups. This analysis identified key pathways known to be involved in AML differentiation and growth such as MAPK signaling as being more significantly upregulated by GS87 treatment as compared to the other treatments (Table 1 and Supplemental Table 5) (33-36).
Table 1.
List of upstream regulators related to MAPK signaling identified from pathway analysis of genes significantly modulated (>2 fold) after treatment with GS87 (30μM), Li (10mM) or SB (20μM) for 48 hours.
| MAPK pathway – GS87 | ||
|---|---|---|
| Upstream regulator | Activation Fold Change | p-value |
| SB203580 | −5.84 | 5.43E-13 |
| PD98059 | −5.231 | 7.30E-26 |
| U0126 | −4.754 | 5.18E-15 |
| SP600125 | −3.885 | 1.92E-09 |
| SB202190 | −3.024 | 1.36E-10 |
| MAPK10 | 2.197 | 1.37E-04 |
| MAPK | 2.287 | 6.33E-09 |
| MAP2K1/2 | 2.365 | 6.66E-09 |
| MAPK13 | 2.404 | 4.15E-04 |
| MAPK12 | 2.418 | 2.17E-04 |
| MAP3K7 | 2.494 | 1.23E-06 |
| MAP2K3 | 2.760 | 1.10E-05 |
| MAPK7 | 2.768 | 2.01E-05 |
| MAPK3 | 3.056 | 1.45E-06 |
| MAP2K1 | 3.092 | 4.83E-10 |
| MAPK14 | 3.429 | 8.35E-04 |
| MAP3K8 | 3.765 | 2.11E-06 |
| ERK1/2 | 3.805 | 8.31E-11 |
| ERK | 3.961 | 8.06E-17 |
| JNK | 3.966 | 7.79E-07 |
| MAPK pathway – SB | ||
|---|---|---|
| Upstream regulator | Activation Fold Change | p-value |
| U0126 | -2.949 | 9.97E-07 |
| LY294002 | -2.681 | 6.23E-05 |
| PD98059 | -2.662 | 2.13E-05 |
| SB203580 | -2.153 | 2.32E-04 |
| ERK | 2.000 | 1.70E-05 |
| MAPK pathway – Li | ||
|---|---|---|
| Upstream regulator | Activation Fold Change | p-value |
| JNK | 2.221 | 4.88E-03 |
| ERK | 2.592 | 3.47E-04 |
To validate the results of the microarray, genes that exhibited significant changes that are also involved in myeloid differentiation and growth were tested by RT-PCR. For example, CEBPβ expression was upregulated after GS87 treatment whereas IRF8 and CyclinD2 expression were down-regulated with GS87 treatment (Figure 5A). CEBPβ is a key regulator in myeloid differentiation, (37, 38) whereas CyclinD2 has roles in leukemia proliferation (39-41). IRF8 is a regulator of myeloid differentiation and high expression has been linked to poor prognosis in AML (42).
Due to the known major role of MAPK signaling in AML differentiation, we investigated components of the MAPK signaling cascade in mediating GS87-induced differentiation. Consistent with our pathway analysis, we found that p38, ERK, and JNK were all activated more strongly by GS87 than Li and SB particularly at 2 hours after treatment (Figure 5B). To investigate the importance of these pathways in GS87-mediated differentiation, we utilized chemical inhibitors. The ERK inhibitor (but not JNK and p38 inhibitors) significantly impaired the differentiation effects of GS87 (Figure 5C and data not shown), establishing the role of the MEK/ERK branch of the MAPK signaling cascade as a key mediator of GS87-induced differentiation. This work also provided mechanistic insights into why GS87 may be a more effective differentiation agent.
GS87 demonstrates efficacy in an AML mouse model system
In order to explore the potential of GS87 as a therapeutic, we utilizied two mouse models of circulating human AML. In the first model primary AML cells derived from a patient with relapsed and treatment refractory disease (AML M2 subtype) were injected into immunodeficient mice. GS87 demonstrated significant efficacy as compared to mice treated with vehicle as well as the standard AML therapeutic, cytarabine. While the majority of the GS87 mice showed longer term survival, those treated with vehicle or cytarabine died due to leukemic cell infiltration of the bone marrow by approximately 45 days after cell injection (Figure 6A). Examination of the bone marrow in mice sacrificed due to moribound state or at the study endpoint of 90 days (as in the case of GS87 treated mice) showed significantly higher percentage of blasts in the vehicle and cytarabine groups (93% ±7.5% and 65% ±8.2%) compared with the surviving mice from the GS87 group (0.32% ±0.53%) (Figure 6B). In the second model, the ability of GS87 to induce the differentiation of the leukemic cells was assessed. In this model, GS87 was found not only to significantly reduce the buden of leukemic cells in the bone marrow at the end of the study period but also to lead to evidence of leukemic cell differentiation as measured by CD11b staining (Figure 6C-D). Finally, in order to confirm that GS87 can inhibit GSK3 in vivo, peripheral blood cells were shown to induce the expresson of β-catenin after drug treatment (Supplementary Figure 6).
Figure 6. GS87 demonstrates efficacy in a circulating AML mouse model system.
(A) Mice treated with GS87 showed longer survival compared to those treated with vehicle or the standard AML therapeutic cytarabine in a mouse model of human primary AML. (B) Bone marrow blast percentage in mice with primary human AML sacrificed due to moribound state or at study endpoint of 90 days showed significantly higher percentage of blasts in the vehicle and cytarabine groups compared with the GS87 group. Note the GS87 mice tested were the surviving mice at the end of the study period. (C-D). Mouse model of circulating HL-60 cells showing a reduced disease burden in the bone marrow (C) and evidence of AML differentiation (D) in the GS87 treated mice.
Discussion
Here we report the identification of a novel and highly specific inhibitor of GSK3 that induces superior AML cell differentiation and growth arrest compared to existing GSK3 inhibitors without affecting normal hematopoietic cell proliferation. The discovery of novel differentiation therapies for AML is important due to the poor efficacy and high toxicities of existing agents. Differentiation therapy has demonstrated remarkable clinical efficacy for a small subset of AML patients with APL reinforcing its potential as an attractive therapeutic strategy for this disease (2).
Numerous groups have implicated GSK3 as a target for AML growth inhibition and differentiation (3-5, 9-12). In addition, it has been reported that GSK3 inhibition may also target leukemia initiating cells that are poorly targeted by standard AML chemotherapy (43). One of the major advantages of targeting GSK3 as compared to the vast majority of agents is the selective targeting of AML cells as compared to normal hematopoietic cells. Our studies with GS87 have further validated the selective effects on leukemic cells suggesting that this approach will not lead to myelotoxicity, a major side effect of most leukemia therapeutics.
GS87 exhibits dramatically more differentiation activity as compared to other reported GSK3 inhibitors. GS87 induces differentiation and growth inhibition of AML cell lines and primary patient samples and exhibits high efficacy using aggressive mouse models of human AML. Though GS87 exhibits extensive differentiation activity as compared to other GSK3 inhibitors, interestingly it leads to GSK3 inhibition at similar levels as measured by in vitro kinase assays to SB and many other reported GSK3 inhibitors. Further, despite these differences in AML differentiation activity, GS87 was found to be a highly specific kinase inhibitor through kinase profiling. GS87, however, leads to more dramatic effects on GSK3 target proteins as compared to other GSK3 inhibitors when used at optimal differentiation doses. For example, GS87 leads to larger changes in the expression of c-myb, MAFB, p21, and β-catenin in AML cells as compared to SB and Li. In addition, GS87 leads to a stronger activation of MAPK signaling which was found to be important for its differentiation activity.
The reasons for the enhanced activation of GSK3-dependent signaling with GS87 are not known. GSK3 activity is controlled in a relatively complex manner by multiple post-translational modifications, cellular localization, and interactions with other proteins (6). In addition, GSK3 can target substrates primed by phosphorylation from other kinases or non-primed substrates in different manners. It is possible that GS87 impairs GSK3 activity in a distinct fashion as compared to other agents. Additionally, despite the fact that GS87 is a specific kinase inhibitor, it is also likely that it regulates other proteins that mediate pathways that can synergize with GSK3-dependent differentiation. Several pathways such as retinoic acid signaling have been found to cooperate with GSK3-mediated differentiation (3).
Further pre-clinical optimization of GS87 is needed to determine pharmacokinetics, pharmacodynamics and drug formulation prior to human testing. Whether clinically effective plasma concentrations of GS87 can be achieved in humans is unknown. However, given its high anti-leukemic efficacy with limited toxicity in our AML mouse model, GS87 has tremendous potential for drug development and first-in-human testing in persons with AML. Further testing will be necessary to determine if GS87, like ATRA, requires co-administration of chemotherapy or other agents for optimal disease control (44, 45).
In conclusion, GS87 is a novel GSK3 inhibitor identified in efforts to discover a small molecule GSK3 inhibitor optimized for AML differentiation activity. Due to its high efficacy in cell and animal models and its minimal apparent toxicity, GS87 is a promising new agent for clinical AML differentiation therapy.
Supplementary Material
Acknowledgements
The work was supported by a contract from the National Cancer Institute to DNW and MKA and Core Facilities of the Case Comprehensive Cancer Center (P30 CA43703)
Financial Support: The work was supported by a contract from the National Cancer Institute to David Wald and Mukesh Agrawal (NIH/NCI HHSN261201100093C and Core Facilities of the Case Comprehensive Cancer Center (P30 CA43703)
Footnotes
Conflicts of Interest: DNW and MKA have filed intellectual property related to GS87
References
- 1.Society AC. American Cancer Society. Cancer Facts & Figures 2014. American Cancer Society; Atlanta: 2014. 2014. [Google Scholar]
- 2.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. The New England journal of medicine. 2013;369:111–21. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Gulen F, Sun L, Aguilera R, Chakrabarti A, Kiselar J, et al. GSK3 is a regulator of RAR-mediated differentiation. Leukemia. 2012;26:1277–85. doi: 10.1038/leu.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Si J, Mueller L, Collins SJ. GSK3 inhibitors enhance retinoic acid receptor activity and induce the differentiation of retinoic acid-sensitive myeloid leukemia cells. Leukemia. 2011;25:1914–8. doi: 10.1038/leu.2011.171. [DOI] [PubMed] [Google Scholar]
- 5.Banerji V, Frumm SM, Ross KN, Li LS, Schinzel AC, Hahn CK, et al. The intersection of genetic and chemical genomic screens identifies GSK-3alpha as a target in human acute myeloid leukemia. The Journal of clinical investigation. 2012;122:935–47. doi: 10.1172/JCI46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Abrams SL, Montalto G, et al. Multifaceted roles of GSK-3 and Wnt/beta-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia. 2014;28:15–33. doi: 10.1038/leu.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolosa E, Litvan I, Hoglinger GU, Burn D, Lees A, Andres MV, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2014;29:470–8. doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 8.Zeng J, Liu D, Qiu Z, Huang Y, Chen B, Wang L, et al. GSK3beta overexpression indicates poor prognosis and its inhibition reduces cell proliferation and survival of non-small cell lung cancer cells. PloS one. 2014;9:e91231. doi: 10.1371/journal.pone.0091231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes T, O'Brien TA, Knight R, Lindeman R, Shen S, Song E, et al. Glycogen synthase kinase-3beta inhibition preserves hematopoietic stem cell activity and inhibits leukemic cell growth. Stem cells. 2008;26:1288–97. doi: 10.1634/stemcells.2007-0600. [DOI] [PubMed] [Google Scholar]
- 10.Song EY, Palladinetti P, Klamer G, Ko KH, Lindeman R, O'Brien TA, et al. Glycogen synthase kinase--3beta inhibitors suppress leukemia cell growth. Experimental hematology. 2010;38:908–21. e1. doi: 10.1016/j.exphem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Wu CH, Yeh SH, Tsay YG, Shieh YH, Kao CL, Chen YS, et al. Glycogen synthase kinase-3 regulates the phosphorylation of severe acute respiratory syndrome coronavirus nucleocapsid protein and viral replication. The Journal of biological chemistry. 2009;284:5229–39. doi: 10.1074/jbc.M805747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008;455:1205–9. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Current biology : CB. 1996;6:1664–8. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 14.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8455–9. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacology & therapeutics. 2014 doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Ser T, Steinwachs KC, Gertz HJ, Andres MV, Gomez-Carrillo B, Medina M, et al. Treatment of Alzheimer's disease with the GSK-3 inhibitor tideglusib: a pilot study. Journal of Alzheimer's disease : JAD. 2013;33:205–15. doi: 10.3233/JAD-2012-120805. [DOI] [PubMed] [Google Scholar]
- 17.Zamek-Gliszczynski MJ, Abraham TL, Alberts JJ, Kulanthaivel P, Jackson KA, Chow KH, et al. Pharmacokinetics, metabolism, and excretion of the glycogen synthase kinase-3 inhibitor LY2090314 in rats, dogs, and humans: a case study in rapid clearance by extensive metabolism with low circulating metabolite exposure. Drug metabolism and disposition: the biological fate of chemicals. 2013;41:714–26. doi: 10.1124/dmd.112.048488. [DOI] [PubMed] [Google Scholar]
- 18.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chemistry & biology. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 19.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. The Biochemical journal. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta K, Gulen F, Sun L, Aguilera R, Chakrabarti A, Kiselar J, et al. GSK3 is a regulator of RAR-mediated differentiation. Leukemia. 2012 doi: 10.1038/leu.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald DN, Vermaat HM, Zang S, Lavik A, Kang Z, Peleg G, et al. Identification of 6-benzylthioinosine as a myeloid leukemia differentiation-inducing compound. Cancer research. 2008;68:4369–76. doi: 10.1158/0008-5472.CAN-07-6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Zhang Y, Bersenev A, O'Brien WT, Tong W, Emerson SG, et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. The Journal of clinical investigation. 2009;119:3519–29. doi: 10.1172/JCI40572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins SJ, Bodner A, Ting R, Gallo RC. Induction of morphological and functional differentiation of human promyelocytic leukemia cells (HL-60) by componuds which induce differentiation of murine leukemia cells. International journal of cancer Journal international du cancer. 1980;25:213–8. doi: 10.1002/ijc.2910250208. [DOI] [PubMed] [Google Scholar]
- 24.Newburger PE, Chovaniec ME, Greenberger JS, Cohen HJ. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. The Journal of cell biology. 1979;82:315–22. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21:2555–63. doi: 10.1038/sj.onc.1205332. [DOI] [PubMed] [Google Scholar]
- 26.Steinman RA. Cell cycle regulators and hematopoiesis. Oncogene. 2002;21:3403–13. doi: 10.1038/sj.onc.1205325. [DOI] [PubMed] [Google Scholar]
- 27.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nature reviews Cancer. 2002;2:764–76. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 28.Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. The Journal of biological chemistry. 2002;277:9684–9. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- 29.Zhou F, Zhang L, van Laar T, van Dam H, Ten Dijke P. GSK3beta inactivation induces apoptosis of leukemia cells by repressing the function of c-Myb. Molecular biology of the cell. 2011;22:3533–40. doi: 10.1091/mbc.E11-06-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocques N, Abou Zeid N, Sii-Felice K, Lecoin L, Felder-Schmittbuhl MP, Eychene A, et al. GSK-3-mediated phosphorylation enhances Maf-transforming activity. Molecular cell. 2007;28:584–97. doi: 10.1016/j.molcel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Herath NI, Rocques N, Garancher A, Eychene A, Pouponnot C. GSK3-mediated MAF phosphorylation in multiple myeloma as a potential therapeutic target. Blood cancer journal. 2014;4:e175. doi: 10.1038/bcj.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T. MafB is an inducer of monocytic differentiation. The EMBO journal. 2000;19:1987–97. doi: 10.1093/emboj/19.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia Z, Baer MR, Block AW, Baumann H, Wetzler M. Expression of signal transducers and activators of transcription proteins in acute myeloid leukemia blasts. Cancer research. 1998;58:3173–80. [PubMed] [Google Scholar]
- 34.Wang X, Pesakhov S, Weng A, Kafka M, Gocek E, Nguyen M, et al. ERK 5/MAPK pathway has a major role in 1alpha,25-(OH)2 vitamin D3-induced terminal differentiation of myeloid leukemia cells. The Journal of steroid biochemistry and molecular biology. 2014;144(Pt A):223–7. doi: 10.1016/j.jsbmb.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, Fang Y, Jing H, Zhong L, Luo P, Song H, et al. Involvement of mitogen-activated protein kinase in signal transducer and activator of transcription-1 mediated differentiation induced by bortezomib in acute myeloid leukemia cells. Molecular carcinogenesis. 2013;52:18–28. doi: 10.1002/mc.20873. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Harrison JS, Studzinski GP. Isoforms of p38MAPK gamma and delta contribute to differentiation of human AML cells induced by 1,25-dihydroxyvitamin D(3). Experimental cell research. 2011;317:117–30. doi: 10.1016/j.yexcr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott LM, Civin CI, Rorth P, Friedman AD. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992;80:1725–35. [PubMed] [Google Scholar]
- 38.Natsuka S, Akira S, Nishio Y, Hashimoto S, Sugita T, Isshiki H, et al. Macrophage differentiation-specific expression of NF-IL6, a transcription factor for interleukin-6. Blood. 1992;79:460–6. [PubMed] [Google Scholar]
- 39.Delmer A, Ajchenbaum-Cymbalista F, Tang R, Ramond S, Faussat AM, Marie JP, et al. Overexpression of cyclin D2 in chronic B-cell malignancies. Blood. 1995;85:2870–6. [PubMed] [Google Scholar]
- 40.Buchakjian MR, Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nature reviews Molecular cell biology. 2010;11:715–27. doi: 10.1038/nrm2972. [DOI] [PubMed] [Google Scholar]
- 41.Chen BB, Glasser JR, Coon TA, Zou C, Miller HL, Fenton M, et al. F-box protein FBXL2 targets cyclin D2 for ubiquitination and degradation to inhibit leukemic cell proliferation. Blood. 2012;119:3132–41. doi: 10.1182/blood-2011-06-358911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pogosova-Agadjanyan EL, Kopecky KJ, Ostronoff F, Appelbaum FR, Godwin J, Lee H, et al. The prognostic significance of IRF8 transcripts in adult patients with acute myeloid leukemia. PloS one. 2013;8:e70812. doi: 10.1371/journal.pone.0070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Iwasaki M, Ficara F, Lin C, Matheny C, Wong SH, et al. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer cell. 2010;17:597–608. doi: 10.1016/j.ccr.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ablain J, Leiva M, Peres L, Fonsart J, Anthony E, de The H. Uncoupling RARA transcriptional activation and degradation clarifies the bases for APL response to therapies. The Journal of experimental medicine. 2013;210:647–53. doi: 10.1084/jem.20122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coombs CC, Tavakkoli M, Tallman MS. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood cancer journal. 2015;5:e304. doi: 10.1038/bcj.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.