Abstract
Objectives
People in prison may be at high risk for infectious diseases and have an elevated risk of death immediately after release compared with later; their risk of death is elevated for at least a decade after release. We compared rates, characteristics, and prison-related risk factors for infectious disease–related mortality among people released from prisons in Queensland, Australia, and Washington State, United States, regions with analogous available data.
Methods
We analyzed data from retrospective cohort studies of people released from prison in Queensland (1997–2007, n=37,180) and Washington State (1999–2009, n=76,208) and linked identifiers from each cohort to its respective national death index. We estimated infectious disease–related mortality rates (deaths per person-years in community) and examined associations using Cox proportional hazard models.
Results
The most frequent infectious disease–related underlying cause of death after release from prison was pneumonia (43%, 23/54 deaths) in the Australian cohort and viral hepatitis (40%, 69/171 deaths) in the U.S. cohort. The infectious disease–related mortality rate was significantly higher in the U.S. cohort than in the Australian cohort (51.2 vs. 26.5 deaths per 100,000 person-years; incidence rate ratio = 1.93, 95% confidence interval 1.42, 2.62). In both cohorts, increasing age was strongly associated with mortality from infectious diseases.
Conclusion
Differences in the epidemiology of infectious disease–related mortality among people released from prison may reflect differences in patterns of community health service delivery in each region. These findings highlight the importance of preventing and treating hepatitis C and other infectious diseases during the transition from prison to the community.
Globally, infectious diseases, including human immunodeficiency virus (HIV), hepatitis C virus (HCV), tuberculosis, methicillin-resistant Staphylococcus aureus, and influenza, are important public health problems among prison populations.1–8 Prison environmental factors that increase the risk of infectious disease transmission include congregate living, poor sanitation and hygiene, limited access to vaccinations and treatment, prohibition of needle exchange, and frequent population movement between facilities and in and out of prison.1,2,5 Countries differ in the demographic characteristics of incarcerated populations, correctional policies, prison and community health services, and national health policies, and little is known about how infectious disease risks differ between prison populations by country. This information could help public health experts understand how such factors influence health outcomes to guide future improvements.
A growing body of literature has documented an elevated risk of mortality after release from prison, particularly from drug-related causes and in the first weeks after release.9–14 However, many preventable deaths result from causes other than substance use and occur after the immediate post-release period.13,15 Risk of death is elevated for at least a decade after release.11,12 Some of these deaths result from infectious disease,11 yet little is known about the mortality rates and risk factors for infectious disease–related deaths. Such information is a prerequisite for developing targeted, evidence-based preventive interventions.
In a study of 15,673 men released from prison in North Carolina, 10.3% of deaths were infectious disease–related compared with 4.7% of deaths among unincarcerated North Carolina residents.12 Similarly, 85,203 men and women in prison and after release from prison in an Australian study had a subtantially higher risk of infectious disease–related death than their age-matched peers in the community.11 Other studies in the United States16 and Australia17 show similar results, although differences in definitions of cause of death and in approaches to data analysis and reporting make between-study comparisons difficult.13 Few studies of people released from prison have directly compared findings across multiple jurisdictions.
The goals of this study were to quantify and compare the rates, timing, and risk factors of infectious disease–related mortality among cohorts of people released from prison in Australia and the United States. The incidence of infectious diseases differs by geographic region, underlying population characteristics, and health services context. Although Australia and the United States both represent high-income countries with relatively affluent populations, the incarcerated populations in each country tend to include large numbers of socioeconomically and ethnically disadvantaged populations, as well as people who have a history of risky drug use. These incarcerated populations face a higher-than-average risk of acquiring infections such as HIV and hepatitis C. In all countries, prisons, because of the congregate living environment, represent particular challenges for the prevention and control of infectious diseases. However, the organization of prison health services, infection-control practices, and policies that affect access to health services in the community differ between the United States and Australia.
We selected two large cohorts of people released from prison: one in Queensland, Australia (1997–2007), and the other in Washington State, United States (1999–2009), to compare the epidemiology of infectious diseases. These cohorts were comparable because they (1) included representative cohorts of people released from prison in similar years, (2) had information about deaths and causes of death after release from prison based on probabilistic linkage to their respective national death index (NDI), and (3) were available for research purposes. In these cohorts, we aimed to (1) quantify infectious disease–related mortality and the percentage of deaths in which mental disorders or substance use contributed to cause of death and (2) examine and compare demographic and prison-related factors as potential risk factors for infectious disease–related deaths. Comparing the epidemiology of infectious disease–related mortality in distinct country contexts could help generate hypotheses about how to address the risk of infectious diseases among people released from prison.
METHODS
Study design
We analyzed data from two large, retrospective cohort studies (Table 1). The Australian cohort included all men and women (aged $17 years) released from prison in Queensland from January 1, 1997, through December 31, 2007 (n=37,180). The U.S. cohort included adult men and women (aged $18 years) released from state prisons in Washington State from July 1, 1999, through December 31, 2009 (n=76,208). Both cohorts included people released $1 time from prison.9,10,18 In the United States, prisons generally hold people sentenced for a crime and serving a sentence of $1 year, or people who have violated the terms of their probation or parole (also called “community supervision”). We excluded people who were released because of grave illness (compassionate release).
Table 1.
Characteristics of people released from prison in Queensland, Australia, 1997–2007, and Washington State, United States, 1999–2009
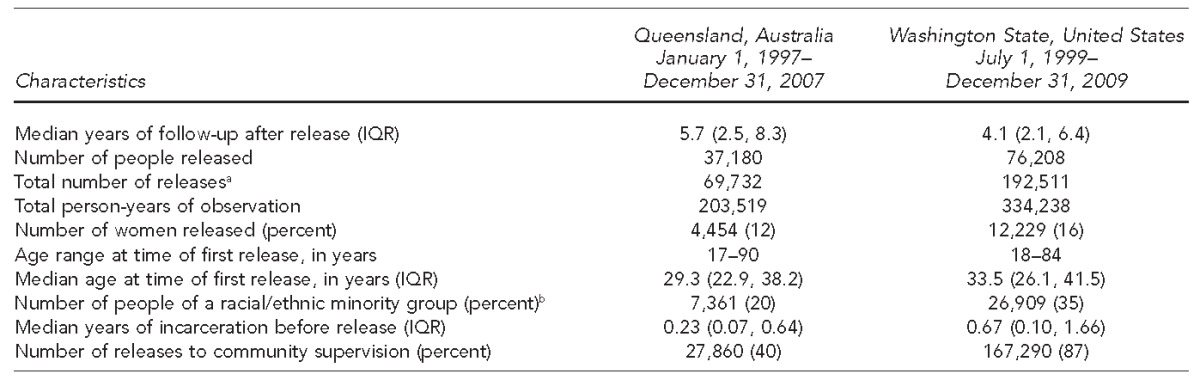
aThe sum of each person's releases from prison during the follow-up period
bIncludes nonwhite and/or Hispanic in Washington State and Indigenous people in Queensland
IQR = interquartile range
Variables
We linked personal identifiers probabilistically to the relevant NDI to identify deaths and their causes using International Classification of Diseases, Tenth Revision (ICD-10) codes.19 Personal identifiers obtained from the prison administrative databases (e.g., first name, last name, social security number, date of birth) were sent to the respective NDI for matching to deaths. All known aliases were submitted for both cohorts, given evidence that doing so increases sensitivity without adversely affecting specificity of linkage.20,21 The NDI provided an indicator of a true match based on probabilistic scores, which account for the number and types of matched identifiers.22
In Australia and the United States, the NDI provides codes for the underlying cause of death (defined as “[a] the disease or injury which initiated the train of morbid events leading directly to death, or [b] the circumstances of the accident or violence which produced the fatal injury”) and all other causes mentioned on the death certificate, consistent with World Health Organization guidelines.19 Similar to Australia, the U.S. NDI-Plus identifies the underlying cause of death and names up to 20 other ICD-10 codes that represent contributing conditions.23,24
We defined an infectious disease–related death as one for which an infectious disease was identified as the underlying cause of death or contributing cause of death or both. Infectious disease–related ICD-10 codes included all codes starting with A or B and G00–G03 (meningitis); I33 (endocarditis); J00–06 (acute upper respiratory infections); J10–J22 (influenza, pneumonia, and other acute lower respiratory infections); J85–J86 (suppurative and necrotic infections of the lower respiratory tract); L00–L08 (infections of skin and subcutaneous tissue); N10–N12, N13.6, and N15.1 (kidney infections); N39.0 (urinary tract infection); and P35–P39 (infections in the perinatal period). Viral hepatitis included acute and chronic hepatitis A, B, and C, as well as unspecified viral hepatitis (B15–B19).
We obtained data on potential demographic and prison-related risk factors for infectious disease–related mortality from routinely collected prison records, including sex, age at each release from prison (for multiple releases), racial/ethnic minority status (yes/no), time served in prison prior to each release, and supervision status at each release (supervised/unsupervised). Community supervision refers primarily to probation or parole but also to community service or home detention. We defined racial/ethnic minority as Indigenous Australians for Australia and nonwhite and Hispanic people for the United States. In the U.S. cohort, we also examined data on American Indian/Alaska Natives to approximate the Australian Indigenous group.
Statistical analysis
Because the study's focus was death in the community after release from prison, we excluded data on subsequent person-years in custody for those who were reincarcerated after their first release and deaths in custody. Death rates in prison are generally lower than death rates after release from prison.10,11 We calculated crude mortality rates (CMRs) per 100,000 person-years during the following time periods after each release: (1) 0–14 days, (2) 15–90 days, (3) 91–180 days, (4) .180 days, and (5) entire observation period.
We constructed Cox proportional hazard models to assess the association between demographic and prison-related factors and infectious disease–related death. We selected potential risk factors based on available data on factors known to be associated with infectious diseases (e.g., sex, age), proxies for socioeconomic status (e.g., racial/ethnic minority status, marital status), proxies for known risk factors for infection (e.g., drug offense for drug use), and factors that could be associated with the acquisition, management, or follow-up of infectious disease (e.g., length of incarceration, supervision upon release). For example, the prevalence of infectious disease is higher in older people in the general population,25 who constitute an increasing proportion of the prison population in many countries;26 thus, we hypothesized that older adults would be at higher risk of infectious disease–related death in both cohorts.
For each cohort, we constructed a multivariable model using factors common to both cohorts (i.e., sex, age by decade, racial/ethnic minority status, time served in prison, supervision status) and generated hazard ratios (HRs) and 95% confidence intervals (CIs) to model time to infectious disease–related death. Next, we constructed a multivariable Cox regression model for each cohort. In the Australian cohort only, we included the following four covariates: (1) marital status at sentence start, (2) whether each incarceration included sentencing for a drug-related offense, (3) total number of adult prison terms served at each release, and (4) total time served in adult prisons at each release. In the U.S. cohort only, we included a covariate for type of incarceration (a new sentence vs. a sentence resulting from a violation of the terms of community supervision) and a more detailed categorization of race/ethnicity (non-Hispanic white, non-Hispanic black, Asian, and American Indian/Alaska Native, or Hispanic/Latino) than was available for the Australian cohort.
In both cohorts, many individuals had more than one release (45% of the U.S. cohort, 41% of the Australian cohort) through the end of follow-up (end of 2009 for the U.S. cohort; end of 2007 for the Australian cohort). We accounted for potential statistical correlation between characteristics associated with releases by the same person using SAS® options that summed the score residuals for each individual in the Lin and Wei robust sandwich estimate of the covariance matrix.27 We conducted all analyses using SAS® version 9.3.28
RESULTS
The Australian cohort consisted of 69,732 releases, with a median length of follow-up in the community of 5.7 years and 203,519 person-years of observation. Of these 69,732 releases, 4,454 (12%) were female, 7,361 (20%) were from a racial/ethnic minority group, and the median age at first release was 29.3 years (range: 17 to 90 years) (Table 1). For those who returned to prison during follow-up, the median number of years in prison was 0.74 years (interquartile range 0.25, 1.95).
The U.S. cohort had 192,511 releases, with a median length of follow-up in the community of 4.1 years and 334,238 person-years of observation. Of the 192,511 releases, 12,229 (16%) were female, 26,909 (35%) were from a racial/ethnic minority group, and the median age at first release was 33.5 years (range: 18 to 84 years (Table 1).
In the Australian cohort, 1,563 people died from any cause, of whom 54 (3%) had an underlying infectious disease–related cause and 174 (11%) had a contributing infectious disease–related cause. The three most frequent individual underlying infectious disease–related causes of death were pneumonia, viral hepatitis, and septicemia (no single cause in the “other” category exceeded septicemia). Of the 54 deaths with an underlying infectious disease–related cause, substance use was a contributing cause in 14 deaths. In the U.S. cohort, 2,462 people died from any cause, of whom 171 (7%) had an underlying infectious disease–related cause and 254 (10%) had a contributing infectious disease–related cause. The three most frequent individual underlying infectious disease–related causes of death in the U.S. cohort were viral hepatitis, HIV, and septicemia (no single cause in the “other” category exceeded septicemia). Substance use was a contributing cause in 49 (29%) infectious disease–related deaths and mental illness in one death. HIV was the underlying cause in two (4%) infectious disease–related deaths in the Australian cohort and 37 (22%) infectious disease–related deaths in the U.S. cohort (Table 2).
Table 2.
Number and proportion of all deaths caused by infection among people released from prison in Queensland, Australia (1997–2007), and Washington State, United States (1999–2009)
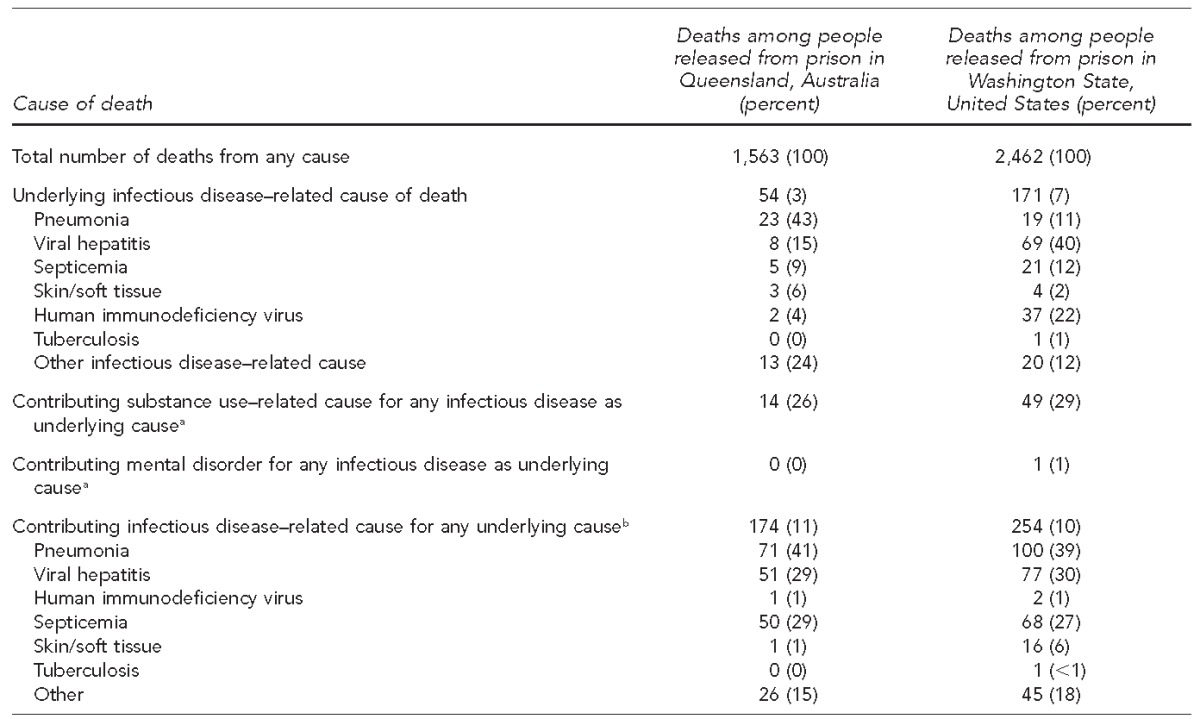
aNot mutually exclusive (i.e., an individual could have both a mental disorder and a substance-related cause, but he or she will be represented in only one category, even if he or she had more than one condition in that category)
bPercentages may exceed 100% because a death may have more than one contributing cause.
During follow-up, the all-cause death rate was similar in the Australian cohort (CMR5768.0 per 100,000 person-years, 95% CI 730.8, 807.0) and the U.S. cohort (CMR5736.6 per 100,000 person-years, 95% CI 707.5, 765.7). In both cohorts, the all-cause death rate was substantially higher in the first two weeks after release than later. In contrast, in both cohorts the infectious disease–related death rate did not vary substantially by time since release (Table 3). During follow-up, the infectious disease–related death rate was significantly higher in the U.S. cohort than in the Australian cohort (incidence rate ratio = 1.93, 95% CI 1.42, 2.62).
Table 3.
Number of deaths and crude mortality rates among people released from prison, for all causes and infectious disease–related causes, by time since release, Queensland, Australia (1997–2007), and Washington State, United States (1999–2009)
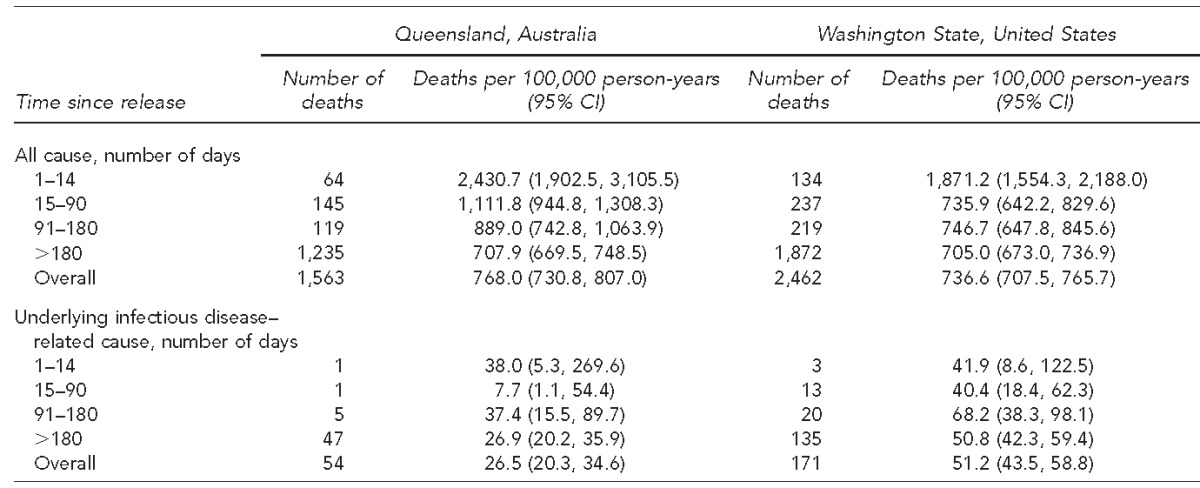
CI = confidence interval
In the multivariable analyses, the adjusted hazard of infectious disease–related death increased with each decade of age in both cohorts. In Australia, the risk of infectious disease–related death was higher for Indigenous people than for non-Indigenous people. The association between age and infectious disease–related death was stronger in the United States (adjusted HR52.53, 95% CI 2.36, 2.70) than in Australia (adjusted HR51.96, 95% CI 1.78, 2.15). Post-release supervision protected against infectious disease–related death in Australia, whereas more time in prison was protective in the United States (Table 4).
Table 4.
Unadjusted and adjusted Cox proportional hazards regression model of factors associated with infectious disease–related death among people released from prison, Queensland, Australia (1997–2007), and Washington State, United States (1999–2009)
aAdjusted for sex, age, racial/ethnic minority, length of time incarcerated, and release under community supervision
bIncludes nonwhite and/or Hispanic for the Washington State cohort and Indigenous for the Queensland, Australia, cohort
cNumber of years served in prison for the incarceration immediately before release
CI = confidence interval
Ref. = reference group
In the Australian cohort, inclusion of cohort-specific variables in the adjusted model revealed three additional risk factors for infectious disease–related death: incarceration for a drug offense (adjusted HR51.68, 95% CI 1.21, 2.34), being unmarried at prison entry (adjusted HR51.88, 95% CI 1.35, 2.63), and number of prior imprisonments (adjusted HR51.09, 95% CI 1.03, 1.15), adjusted for sex, indigenous status, age, length of time incarcerated, and release under community supervision. In the U.S. cohort, additional factors significantly associated with increasing risk of infectious disease–related death included being American Indian/Alaska Native compared with being non-Hispanic white (adjusted HR52.04, 95% CI 1.40, 8.98), adjusted for sex, age, length of time incarcerated, and release under community supervision.
DISCUSSION
In this study of mortality among people after release from prison, infectious disease contributed to approximately one in 10 deaths, suggesting that infectious disease is an important driver of preventable mortality among this population. It is likely that many more people released from prison experience nonfatal sequelae of infectious diseases. The public health impact of infectious diseases extends to the communities to which people released from prison return.29 Future research should assess whether or not differences in correctional and community health-care services and policies contribute to post-release mortality between countries.
Consistent with the high prevalence of HCV infection in the prison populations of both countries (31%–55% in Australia and 17% in the United States),30–32 viral hepatitis was a major contributor to infectious disease–related death in our study. Some deaths caused by viral hepatitis may not have been classified as such; thus, our estimates are likely to be conservative. Treatment of HCV infection during incarceration may prevent or delay death in people released from prison,33 particularly with the advent of more effective, simpler, and safer treatments. Despite the value of new HCV treatments, their availability may be limited without expansion of health-care budgets by local legislatures, federal funding, or prison discounts from pharmaceutical companies.34,35 In Australia, federal funding is available for new HCV treatments,36 and such funding could be replicated in the United States. In many countries, treatment after prison release may be complicated by poor housing and the need to obtain employment. However, when viewed from a public health perspective, preventing HCV infection by expanding access to treatment in prison,37 particularly for people who inject drugs,38 is essential to reducing the burden of this disease. To this end, several successful models of care, such as a nurse-led and specialist-supported assessment and treatment model and a telemedicine and distance-learning program, have been developed for HCV treatment delivery in correctional care environments.38,39 Evidence-based harm-reduction measures, including pharmacotherapy and syringe exchange programs,40 will be central to this effort.
The high prevalence of substance use as a contributing cause of death (26%–29%) among people released from prison who died of infectious diseases suggests the possibility of inadequate access to treatment for substance use disorders. Substance use–related risk behaviors that lead to infection often occur during and immediately after imprisonment;29,41–43 as such, prevention should be targeted to these same time periods. In contrast, mental disorders were infrequently identified as contributing causes, although they may have contributed to death through behavioral mechanisms that were not recorded.44
Our transnational comparison highlights differences between the United States and Australia in the role of HIV as a contributor to mortality after release from prison. HIV infection contributed to approximately one-fifth of infectious disease–related deaths in the United States but few such deaths in Australia. This finding may be related to the benefits derived from Australian community-based harm-reduction efforts45 and access to universal health care. Other research has found poor adherence to antiretroviral therapy among people living with HIV after release from prison in the United States,46 suggesting that efforts to improve treatment adherence may prevent infection and mortality.
In both countries, other leading causes of death included pneumonia and septicemia, which may be related to the poor health of people released from prison,26 compromised immune function, delayed access to care, and/or poor living conditions. These findings highlight the importance of facilitating access to health care after release from prison.47,48
As prison populations age,26 infectious disease, particularly HCV infection, may cause an increasing proportion of deaths. Indigenous people in both countries were at increased risk of infectious disease–related death. Given the overrepresentation of indigenous people, this increased risk compounds the impact of incarceration on racial/ethnic disparities. Supervision after release from prison protected against infectious disease–related death in Australia. Further research is needed to understand the aspects of supervision that can be strengthened to improve health outcomes. Finally, in the Australian cohort, marital status protected against infectious disease–related death, suggesting a role for prosocial supports in post-release outcomes.49
Although far from conclusive, transnational comparisons of infectious disease epidemiology in prison populations can be used to hypothesize the effects of various correctional and community health-care policies for these populations. The United States has longer sentences and more supervision; Australia has universal health care and well-established community harm-reduction programs to diminish the risks of drug use. Universal health care and harm-reduction programs could contribute to the lower rates of infectious disease observed in the U.S. cohort. Further epidemiologic research is needed in resource-poor settings. Meta-analyses of this literature suggest heterogeneity in post-release estimates of death across studies and systems, probably because of methodological differences such as inconsistent operational definitions of exposure and outcome variables, and between-study variation in design and approach to data analysis.13,50 In this study, we sought to minimize such heterogeneity by analyzing data similarly across cohorts.
Limitations
This study had several limitations. First, the years studied were similar but not identical; given that the incidence of disease can change over time, secular trends could have influenced observed differences. Second, cause of death could be coded differently in each country, because cause-of-death certification practices differ between countries. Third, the use of underlying and multiple cause-of-death codes has limitations.44 For example, certain causes of infectious disease–related death may have been underestimated, particularly chronic infections such as HCV. Fourth, from 2001 to 2008, about half of people with HCV infection in the United States were unaware of their infection status.51 Additionally, some HCV deaths may have been classified as deaths resulting from cirrhosis, hepatocellular carcinoma, or alcoholic hepatitis (e.g., in co-occurring alcohol use disorder).16 Fifth, we did not examine infectious disease–related deaths in prison, because our focus was on deaths in the community. In countries with different patterns of infectious disease in the community (e.g., a higher incidence of active tuberculosis), we would expect to observe different patterns of death after release. Sixth, several factors limited our ability to merge our data for analysis, including data use restrictions and differences in variables available across correctional systems. Future work should develop ways to harmonize and share data across jurisdictions and countries through distributed research networks or individual data meta-analysis. Such approaches would permit exploration of rarer, but important, causes of death.52
CONCLUSION
Our findings highlight the need for interventions to improve the health of men and women released from prison, especially immediately after release. Prisons should provide evidence-based care for infectious diseases, including vaccinations, screening, diagnostic testing, and treatment. Many questions about health care in correctional environments and at release are unanswered. For example, should community-level guidelines for the prevention and treatment of infectious diseases be modified for prison or post-release environments, given the risks of poor post-release outcomes? The Centers for Disease Control and Prevention and other organizations periodically issue guidelines that either include recommendations for correctional facilities or focus on them.53 However, epidemiologic data on prison populations or trials to inform these clinical guidelines are limited. Future research should assess misclassification of infectious disease–related death. Additionally, further research on how to prevent or manage infectious disease among prison populations, both in custody and after release, is needed.
Footnotes
The University of Queensland Behavioural and Social Sciences Ethical Review Committee, the Queensland Corrective Services Research Committee, and the Australian Institute of Health and Welfare Ethics Committee approved the Australian study. The Colorado Multiple Institutions Review Board and the Washington State Department of Corrections Review Board approved the U.S. study. Neither site required additional approval to compare findings between sites, because analyses took place at each site according to similar specifications.
REFERENCES
- 1.Macalino GE, Hou JC, Kumar MS, Taylor LE, Sumantera IG, Rich JD. Hepatitis C infection and incarcerated populations. Int J Drug Policy. 2004;15:103–14. [Google Scholar]
- 2.Jürgens R, Ball A, Verster A. Interventions to reduce HIV transmission related to injecting drug use in prison. Lancet Infect Dis. 2009;9:57–66. doi: 10.1016/S1473-3099(08)70305-0. [DOI] [PubMed] [Google Scholar]
- 3.Hammett TM, Harmon MP, Rhodes W. The burden of infectious disease among inmates of and releasees from US correctional facilities, 1997. Am J Public Health. 2002;92:1789–94. doi: 10.2105/ajph.92.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young LC, Dwyer DE, Harris M, Guse Z, Noel V, Levy MH, et al. Summer outbreak of respiratory disease in an Australian prison due to an influenza A/Fujian/411/2002(H3N2)-like virus. Epidemiol Infect. 2005;133:107–12. doi: 10.1017/s0950268804003243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson S, Smith P, Sears SD, Shubert J, Reed C, Manning SE. Influenza outbreaks at two correctional facilities—Maine, March 2011. MMWR Morb Mortal Wkly Rep. 2012;61(13):229–32. [PubMed] [Google Scholar]
- 6.Butler T, Lim D, Callander D. Darlinghurts (New South Wales): National Drug Research Institute & Kirby Institute; 2011. National Prison Entrants' Bloodborne Virus and Risk Behaviour Survey report 2004, 2007 and 2010: prevalence of HIV, hepatitis C, hepatitis B, sexually transmissible infections, and risk behaviours among Australian prison entrants. [Google Scholar]
- 7.Baillargeon J, Black SA, Leach CT, Jenson H, Pulvino J, Bradshaw P, et al. The infectious disease profile of Texas prison inmates. Prev Med. 2004;38:607–12. doi: 10.1016/j.ypmed.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Baillargeon J, Kelley MF, Leach CT, Baillargeon G, Pollock BH. Methicillin-resistant Staphylococcus aureus infection in the Texas prison system. Clin Infect Dis. 2004;38:e92–5. doi: 10.1086/383146. [DOI] [PubMed] [Google Scholar]
- 9.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159:592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356:157–65. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kariminia A, Butler TG, Corben SP, Levy MH, Grant L, Kaldor JM, et al. Extreme cause-specific mortality in a cohort of adult prisoners—1998 to 2002: a data-linkage study. Int J Epidemiol. 2007;36:310–6. doi: 10.1093/ije/dyl225. [DOI] [PubMed] [Google Scholar]
- 12.Rosen DL, Schoenbach VJ, Wohl DA. All-cause and cause-specific mortality among men released from state prison, 1980–2005. Am J Public Health. 2008;98:2278–84. doi: 10.2105/AJPH.2007.121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinner SA, Forsyth S, Williams G. Systematic review of record linkage studies of mortality in ex-prisoners: why (good) methods matter. Addiction. 2013;108:38–49. doi: 10.1111/add.12010. [DOI] [PubMed] [Google Scholar]
- 14.Merrall EL, Bird SM, Hutchinson SJ. A record-linkage study of drug-related death and suicide after hospital discharge among drug--treatment clients in Scotland, 1996–2006. Addiction. 2013;108:377–84. doi: 10.1111/j.1360-0443.2012.04066.x. [DOI] [PubMed] [Google Scholar]
- 15.Kinner SA. Commentary on Merrall et al. (2010): understanding mortality and health outcomes for ex-prisoners—first steps on a long road. Addiction. 2010;105:1555–6. doi: 10.1111/j.1360-0443.2010.03030.x. [DOI] [PubMed] [Google Scholar]
- 16.Spaulding AC, Allen SA, Stone A. Mortality after release from prison. N Engl J Med. 2007;356:1785–7. doi: 10.1056/NEJMc070267. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs M, Krazlan K, Ridout S, Mai Q, Knuiman M, Chapman R. Canberra (New South Wales): Australian Institute of Criminology; 2006. Mortality and morbidity in prisoners after release from prison in Western Australia 1995–2003. Report No. 71. [Google Scholar]
- 18.van Dooren K, Kinner SA, Forsyth S. Risk of death for young ex-prisoners in the year following release from adult prison. Aust N Z J Public Health. 2013;37:377–82. doi: 10.1111/1753-6405.12087. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva: WHO; 1999. International statistical classification of diseases and related health problems, 10th revision. [Google Scholar]
- 20.Larney S, Burns L. Evaluating health outcomes of criminal justice populations using record linkage: the importance of aliases. Eval Rev. 2011;35:118–28. doi: 10.1177/0193841X11401695. [DOI] [PubMed] [Google Scholar]
- 21.Kariminia A, Butler T, Corben S, Kaidor J, Levy M, Law M. Mortality among prisoners: how accurate is the Australian National Death Index? Aust N Z J Public Health. 2005;29:572–5. doi: 10.1111/j.1467-842x.2005.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 22.Horm JW. Hyattsville (MD): National Center for Health Statistics (US); 1996. Assignment of probabilistic scores to National Death Index record matches. [Google Scholar]
- 23.METeOR (Metadata Online Registry) Person—underlying cause of death, code (ICD-10 2nd edn) ANN-ANN. 2013 [cited 2013 Oct 17] Available from: http://meteor.aihw.gov.au/content/index.phtml/itemId/307931.
- 24.National Center for Health Statistics (US) Hyattsville (MD): NCHS; 1999. National Death Index Plus: coded causes of death: supplement to the NDI user's manual. [Google Scholar]
- 25.van Dooren K, Kinner SA, Hellard M. A comparison of risk factors for hepatitis C among young and older adult prisoners. J Correct Health Care. 2014;20:280–91. doi: 10.1177/1078345814541536. [DOI] [PubMed] [Google Scholar]
- 26.Fazel S, Baillargeon J. The health of prisoners. Lancet. 2011;377:956–65. doi: 10.1016/S0140-6736(10)61053-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 28.SAS Institute, Inc. SAS®: Version 9.3. Cary (NC): SAS Institute, Inc.; 2011. [Google Scholar]
- 29.Binswanger IA, Mueller SR, Beaty BL, Min SJ, Corsi KF. Gender and risk behaviors for HIV and sexually transmitted infections among recently released inmates: a prospective cohort study. AIDS Care. 2014;26:872–81. doi: 10.1080/09540121.2013.859650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Australian Institute of Health and Welfare. Canberra (Australia): Australian Institute of Health and Welfare; 2015. The health of Australia's prisoners, 2015. [Google Scholar]
- 31.Snow KJ, Young JT, Preen DB, Lennox NG, Kinner SA. Incidence and correlates of hepatitis C virus infection in a large cohort of prisoners who have injected drugs. BMC Public Health. 2014;14:830. doi: 10.1186/1471-2458-14-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C sero-prevalence among prison inmates since 2001: still high but declining. Public Health Rep. 2014;129:187–95. doi: 10.1177/003335491412900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cramp ME, Rosenberg WM, Ryder SD, Blach S, Parkes J. Modelling the impact of improving screening and treatment of chronic hepatitis C virus infection on future hepatocellular carcinoma rates and liver-related mortality. BMC Gastroenterol. 2014;14:137. doi: 10.1186/1471-230X-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Watcha D, Holodniy M, Goldhaber-Fiebert JD. Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in U.S. incarcerated populations: a cost-effectiveness analysis. Ann Intern Med. 2014;161:546–53. doi: 10.7326/M14-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He T, Li K, Roberts MS, Spaulding AC, Ayer T, Grefenstette JJ, et al. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med. 2016;164:84–92. doi: 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Commonwealth of Australia. Turnbull government invests over $1 billion to cure hep C [2015 Dec 22] press release [cited 2016 Apr 28] Available from: https://www.health.gov.au/internet/ministers/publishing.nsf/Content/health-mediarel-yr2015-ley154.htm.
- 37.Martin NK, Vickerman P, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol. 2011;54:1137–44. doi: 10.1016/j.jhep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd AR, Clegg J, Lange J, Stevenson A, Post JJ, Lloyd D, et al. Safety and effectiveness of a nurse-led outreach program for assessment and treatment of chronic hepatitis C in the custodial setting. Clin Infect Dis. 2013;56:1078–84. doi: 10.1093/cid/cis1202. [DOI] [PubMed] [Google Scholar]
- 39.Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(Suppl 2):74–7. doi: 10.1177/00333549071220S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolan K, Rutter S, Wodak AD. Prison-based syringe exchange programmes: a review of international research and development. Addiction. 2003;98:153–8. doi: 10.1046/j.1360-0443.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 41.Kinner SA. Continuity of health impairment and substance misuse among adult prisoners in Queensland, Australia. Int J Prison Health. 2006;2:101–13. [Google Scholar]
- 42.Dolan K, Teutsch S, Scheuer N, Levy M, Rawlinson W, Kaldor J, et al. Incidence and risk for acute hepatitis C infection during imprisonment in Australia. Eur J Epidemiol. 2010;25:143–8. doi: 10.1007/s10654-009-9421-0. [DOI] [PubMed] [Google Scholar]
- 43.Kinner SA, George J, Campbell G, Degenhardt L. Crime, drugs and distress: patterns of drug use and harm among criminally involved injecting drug users in Australia. Aust N Z J Public Health. 2009;33:223–7. doi: 10.1111/j.1753-6405.2009.00379.x. [DOI] [PubMed] [Google Scholar]
- 44.Israel RA, Rosenberg HM, Curtin LR. Analytical potential for multiple cause-of-death data. Am J Epidemiol. 1986;124:161–79. doi: 10.1093/oxfordjournals.aje.a114375. [DOI] [PubMed] [Google Scholar]
- 45.Iversen J, Wand H, Topp L, Kaldor J, Maher L. Extremely low and sustained HIV incidence among people who inject drugs in a setting of harm reduction. AIDS. 2014;28:275–8. doi: 10.1097/QAD.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 46.Westergaard RP, Kirk GD, Richesson DR, Galai N, Mehta SH. Incarceration predicts virologic failure for HIV-infected injection drug users receiving antiretroviral therapy. Clin Infect Dis. 2011;53:725–31. doi: 10.1093/cid/cir491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinner SA, Lennox N, Williams GM, Carroll M, Quinn B, Boyle FM, et al. Randomised controlled trial of a service brokerage intervention for ex-prisoners in Australia. Contemp Clin Trials. 2013;36:198–206. doi: 10.1016/j.cct.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Kinner SA, Young JT, Carroll M. The pivotal role of primary care in meeting the health needs of people recently released from prison. Australas Psychiatry. 2015;23:650–3. doi: 10.1177/1039856215613008. [DOI] [PubMed] [Google Scholar]
- 49.Naser RL, La Vigne NG. Family support in the prisoner reentry process. J Offender Rehabil. 2006;43:93–106. [Google Scholar]
- 50.Merrall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction (Abingdon, England) 2010;105:1545–54. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55:1652–61. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbert K, Plugge E, Foster C, Doll H. Prevalence of risk factors for non-communicable diseases in prison populations worldwide: a systematic review. Lancet. 2012;379:1975–82. doi: 10.1016/S0140-6736(12)60319-5. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Prevention and Control (US) Prevention and control of tuberculosis in correctional and detention facilities: recommendations from CDC. MMWR Recomm Rep. 2006;55(RR-9):1–44. [PubMed] [Google Scholar]


