Abstract
The biological properties of mesenchymal stem cells (MSCs) are influenced by donor age, gender and/or tissue sources. The present study investigated the cellular and molecular properties of porcine mesenchymal stromal/stem cells (MSCs) isolated from different tissues (adipose & dermal skin) and sex at different ages (1 week & 8 months after birth) with similar genetic and environmental backgrounds. MSCs were analyzed for alkaline phosphatase (AP) activity, CD90 and Oct3/4 expression, in vitro differentiation ability, senescence-associated β-galactosidase (SA-β-Gal) activity, telomeric properties, cell cycle status and expression of senescence (IL6, c-myc, TGFβ, p53 and p21)- and apoptosis (Bak and Bcl2)-related proteins. An age-dependent decline in AP activity and adipogenesis was observed in all MSCs, except for male A-MSCs. CD90 expression did not change, but SA-β-Gal activity increased with advancement in age, except in A-MSCs. Telomeric properties were similar in all MSCs, whereas expression levels of Oct3/4 protein declined with the advancement in age. p21 expression was increased with increase in donor age. Male derived cells have shown higher IL6 expression. The expression of p53 was slightly lower in MSCs of dermal tissue than in adipose tissue. Bak was expressed in all MSCs regardless of age, but up regulation of Bcl2 was observed in DS-MSCs derived at 1 week after birth. In conclusion, adipose tissue-derived MSCs from young female individuals were found to be more resistant to senescence under in vitro culture conditions.
Keywords: adipose MSCs (A-MSCs), age, apoptosis, dermal skin MSCs (DS-MSCs), senescence
Mesenchymal stromal/stem cells (MSCs) possess self-renewal capacity and multipotency with the ability to differentiate into various lineages [26, 31, 46]. Currently, MSCs have gained an increased attention not only due to their immunomodulatory properties but also with the advancement in culture techniques to improve their in vitro expansion. The broad range of clinical applications of MSCs includes many diseases, such as graft-versus-host disease, periodontitis, severe chronic myocardial ischemia, liver cirrhosis, multiple sclerosis and diabetes [3, 14, 29, 37, 43].
Although MSCs are considered to be important candidates for regenerative medicine, their procurement with minimal discomfort and in vitro expansion with desired quality as well as quantity for clinical application is still challenging. It is observed that the number and frequency of stem cells decline with the advancement in age [4], which not only contribute to human and animal aging but also associated with number of age-related diseases [38]. Several studies on MSCs have shown that aging induces many changes in their biological properties, such as proliferation and differentiation [45, 51]. Furthermore, previous reports have also demonstrated an age-related decline in the number of proliferative MSCs in the bone marrow of rodents, monkeys and humans [42, 45, 51]. However, most of these previous studies have evaluated the cells from individuals with different life histories under uncontrolled conditions.
The faster growth rate was observed in in vitro fertilized male embryos compared to female embryos before implantation, indicating the existence of genetic and cellular differences among sex before the induction of hormonal stimulation [2, 27, 36, 49]. Particularly, gene expression profiles during early embryo development differ among sex [20, 35]. These observations clearly suggest that the difference in cellular characteristics among sex will arise from the early embryo and may have an impact on the regenerative capacity of both sexes [7, 21, 33]. Recently, it has been reported that stem cells isolated from the animals of different sexes have shown varying cellular characteristics and stemness [16, 17, 23].
After initial identification of MSCs in the bone marrow’s stromal component [18], these cells are also isolated from other tissues, such as synovium, muscle, periosteum and adipose tissue in humans and other animal species. However, differentiation potential of these MSCs differs based on the anatomical harvest site [1].
All primary cells, including MSCs, undergo only a limited number of cell divisions under standard culture conditions due to a process called cellular senescence. Several molecular pathways are involved in senescence, such as DNA damage, accumulation of cyclin-dependent proteins, tumor suppressor factors and progressive shortening of telomeres. Alteration of telomeric structures has been implicated in replicative senescence, as the number of telomere repeats decreases with every cell division [5]. Despite the progressive telomere shortening in long-term-cultured MSCs in vitro, little is known about the relationship between the MSC donor age and telomere shortening [8, 30, 44].
Several recent studies have used pigs as preclinical models for cell therapy, because of their anatomical and physiological resemblance with humans. The present study investigated the effect of sex and tissue source according to donor age on the biological properties of porcine MSCs at cellular and molecular levels in vitro. Pigs from the same litter were used as MSC donors at different ages to eliminate genetic and environmental variations.
MATERIALS AND METHODS
Reagents and media: Unless otherwise specified, all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.), and media were from Gibco (Invitrogen, Burlington, ON, Canada).
Isolation and culture: All experiments were authorized by the Animal Center for Biomedical Experimentation at Gyeongsang National University. Tissues of three female and three male pigs from the same litter were recovered under standard surgical procedures at both 1 week and 8 months after birth. Porcine MSCs from subcutaneous adipose tissue (A-MSCs) were isolated following a previously described method by digestion with 0.075% collagenase type I (Millipore, Temecula, CA, U.S.A.) and subsequent separation by filtration with 100- and 40-µm cell strainers [10]. Porcine dermal skin MSCs (DS-MSCs) were isolated after removal of the epidermis of the ear skin as previously described [39]. As a control, MSCs were isolated from porcine bone marrow (BM-MSCs) as described previously [28]. All cells were isolated from each sample in triplicates and cultured in advanced Dulbecco’s Modified Eagle Medium (ADMEM) supplemented with 10% fetal calf serum (FCS) and 1% penicillin-streptomycin (10,000 IU and 10,000 µg/ml) at 38.5°C in a humidified atmosphere of 5% CO2 in air. After cells reaching 70% confluence, they were sub-cultured/passaged for further analysis using 0.25% trypsin-EDTA solution. MSCs at passage 3 were used in all the experimentations unless otherwise specified.
Alkaline phosphatase (AP) activity: To detect AP activity, porcine MSCs grown on 35-mm dishes for 2 weeks were stained with an AP chromogen kit (BCIP/NBT) (Abcam Inc., Cambridge, MA, U.S.A.) after fixing with 4% formaldehyde. Positive AP reactivity was indicated by purple-brown staining.
CD marker analysis: The expression of cell surface marker CD90 in pMSCs was analyzed by flow cytometer as previously described [41]. In brief, 1 × 105 cells at passage 3 were suspended in 100 µl of Dulbecco’s phosphate-buffered saline (DPBS) and labeled with fluorescein isothiocyanate (FITC) conjugated mouse anti-human CD90 (Thy-1, BD Biosciences, San Jose, CA, U.S.A.) or isotype-matched control antibodies (mouse IgG1, BD Biosciences). A total of 10,000 cells were analyzed by flow cytometer using CellQuest software (Becton Dickinson, Frankin Lakes, NJ, U.S.A.).
In vitro differentiation potential: Cells at ~80% confluence were induced toward adipogenic or osteogenic differentiation by culturing in ADMEM supplemented with lineage specific constituents for 3 weeks in triplicates. Adipogenic differentiation medium consisted of 10 µM insulin, 200 µM indomethacin, 500 µM isobutylmethylxanthine and 1 µM dexamethasone [46]. Accumulation of lipid droplets was evaluated by Oil red O staining. Osteogenic differentiation medium consisted of 1 µM dexamethasone, 100 µM ascorbic acid and 10 mM β-glycerophosphate [9]. The formation of osteoblasts was evaluated by Alizarin red and von Kossa staining. The cultures were replaced with fresh medium at every 3 days.
Senescence-associated β-galactosidase (SA-β-Gal) staining: To evaluate cellular senescence, an SA-β-Gal assay was performed according to manufacturer’s protocol using SA-β-Gal Staining Kit (Cell Signaling Technology, Cambridge, MA, U.S.A.). Briefly, Cells at 60% confluence grown in 35-mm dishes in triplicates were fixed with 3.7% formalin for 10 min at room temperature. After washing twice with DPBS, 1 ml of SA-β-Gal staining solution was added to each dish and incubated at 37°C overnight in a dry incubator (no CO2). The Cells were observed under a microscope for the development of blue color, while the SA-β-Gal staining solution was still on the dishes.
Analysis of telomere length: Genomic DNA from each sample was extracted using the GENE ALLTM Tissue SV plus mini kit (General Biosystem, Seoul, Korea). The DNA was subjected to telomere length analysis using the Telo TAGGG Telomere Length Assay kit (Roche, Mannheim, Germany) following manufacturer’s instructions. Briefly, genomic DNA was digested with Rsa1 and Hinf1 for 2 hr at 37°C and separated by 0.8% agarose gel electrophoresis at 30 V/cm for 5 hr. After gel depurination and denaturation, DNA fragments were transferred overnight by Southern blotting in 20× SSC buffer to a positively charged nylon membrane. DNA was fixed to the membrane by UV cross-linking, and the membrane was probed using a DIG-labeled telomere probe, washed and blocked. Bands were detected using AP anti-DIG antibody and CDP-Star chemiluminescent substrate. The telomere length was calculated by exposing the X-ray film for 1–5 min and then imaging it. The signals were scanned and evaluated using the Unok-8000 Gel Manager System Gel Viewer 1.5 system (Biotechnology, Seoul, Korea). The mean telomere length was calculated using the formula: mean TRF ∑(ODi)/ ∑(ODi/Li), where ODi is the optical density at a position i on the lane and Li is the corresponding terminal restriction fragment (TRF) length at position i on the lane. This formula takes into account the higher signal intensity generated by larger TRF fragments. Female BM-MSCs derived from the same litter both at 1 week and 8 months after birth were used for an internal control, whereas cells derived from the testis tissue of 1-year-old pig were used for a positive control.
Cell cycle analysis: For cell cycle analysis by flow cytometry, the cells were fixed with 70% ethanol, washed twice with DPBS and stained with 10 µg/ml propidium iodide. The cell cycle analysis was performed in triplicate using 10,000 cells per sample each time.
Western blot analysis: The expression of proliferation, senescence and apoptosis associated proteins was analyzed using Western blot. Briefly, protein lysate was prepared from respective MSCs at passage 3 using RIPA buffer (all samples were isolated from three female and three male pigs from the same litter) (Thermo Scientific, Rockford, IL, U.S.A.) and then centrifuged at 14,000 × g for 5 min at 4°C. The supernatant was used for determining protein concentration using bicinchoninic acid (BCA) protein assay reagent kit (Pierce Biotechnology, Rockford, IL, U.S.A.). A total of 25 µg each protein sample was separated on 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE, Mini Protean, BioRad, Hercules, CA, U.S.A.) and electroblotted onto polyvinylidene difluoride (PVDF, Millipore) membranes. Female BM-MSCs derived from 1 week old pig were used as an internal control for MSCs. HeLa and F9 whole cell lysates (Santa Cruz, CA) were used as positive controls for telomerase reverse transcriptase (TERT; HeLa), p21 (HeLa) and Oct 3/4 (F9), whereas lysates from human fibroblast (MCF 5) and cancer cells (MDA-MB-231) were used as negative controls for Oct 3/4 protein expression. The membranes were blocked and incubated with anti-TERT (1:200 dilution; Santa Cruz Biotechnology, CA, U.S.A.), anti-Oct 3/4 (1:100 dilution; Santa Cruz Biotechnology), anti-IL6 (1:500 dilution; Abcam, Cambridge, UK), anti-P53 (1:100 dilution; Santa Cruz Biotechnology), anti-c-myc (1:500 dilution; Abcam), anti-TGFβ (1;500 dilution; Abcam), anti-p21 (1:200 dilution; Santa Cruz Biotechnology), anti-Bak (1:1,000 dilution; Abcam), anti-Bcl2α (1:200 dilution; Abcam) and anti-β-actin (1:1,000 dilution; Cell Signaling Technology, Cambridge, MA, U.S.A.) antibodies overnight at 4°C. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies, such as goat anti-mouse (1:1,000 dilution; p53 and Bcl2α), goat anti-rabbit (1:5,000 dilution; p21, IL6, c-Myc, TGFβ, Bax and β-actin) and donkey anti-goat (1:5,000 dilution; Oct3/4 and TERT) for 1 hr at room temperature. Protein bands were detected by enhanced chemiluminescence (ECL) (Amersham Biosciences Corp., Piscataway, NJ, U.S.A.).
Statistical analysis: Data were analyzed by one-way analysis of variance (ANOVA) using SPSS 12.0 (SPSS Inc., Chicago, IL, U.S.A.) followed by Tukey’s multiple comparisons test. Data are expressed as mean ± standard deviation (SD). Differences were considered significant at P<0.05.
RESULTS
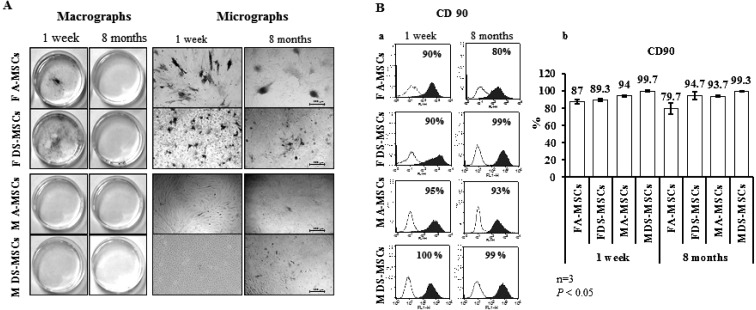
General observations for MSCs: All MSCs have shown fibroblast-like morphology in monolayer culture and formed colonies regardless of donor sex and age. As shown in Fig. 1A, MSCs have shown reduced AP activity with the advancement in age. Female-derived MSCs obtained at 1 week after birth showed higher AP activity than male-derived MSCs obtained at the same age. However, the AP activity was decreased in MSCs harvested at 8 months after birth regardless of gender compared to that in MSCs harvested at 1 week after birth. There was no notable difference in AP activity in MSCs of both ages with respect to the source of tissue. All MSCs were strongly positive for CD90 with staining in 87% of cells, except for adult female A-MSCs, which showed CD90 expression in –80% of cells (Fig. 1Ba). CD90 expression was maintained in cells isolated at 8 months after birth without any significant differences (P<0.05), indicating the mesodermal lineage of the cells (Fig. 1Bb).
Fig. 1.
Analysis of alkaline phosphatase (AP) activity and CD90 expression of MSCs harvested at 1 week and 8 months after birth. (A) AP activity. Scale bars=500 µm. (B) CD90 expression determined by flow cytometry. Ba, open and filled blue histograms display isotype-matched controls and positive reaction, respectively. Bb, CD90 expression levels of MSCs. Significant differences were considered when P<0.05.
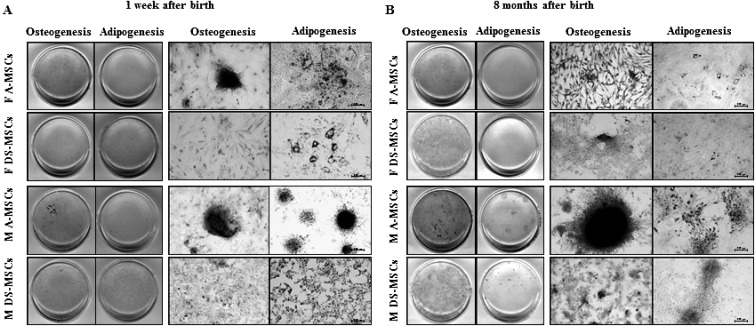
Analysis of MSC differentiation potential: The stemness of MSCs harvested at 1 week and 8 months after birth was assessed by in vitro differentiation into osteocytes and adipocytes. All MSCs differentiated into osteocytes and adipocytes in vitro regardless of donor age, sex and tissue source with varying differentiation capacity (Fig. 2A and 2B ).
Fig. 2.
In vitro differentiation of MSCs. (A) MSCs harvested at 1 week after birth. (B) MSCs harvested at 8 months after birth. Scale bars=200 µm.
MSCs harvested at 8 months after birth showed enhanced osteogenic differentiation, where male-derived cells have shown higher propensity toward osteogenesis than female-derived cells. Furthermore, MSCs from adipose tissue displayed a higher differentiation capacity than those from dermal skin.
In contrast, all MSCs harvested at 8 months after birth showed markedly diminished adipogenic differentiation, except for male-derived A-MSCs which maintained their differentiation capacity with the advancement in age. On the other hand, A-MSCs harvested at 1 week after birth regardless of their sex have shown higher adipogenic differentiation ability when compared to both male and female derived DS-MSCs harvested at the same age.
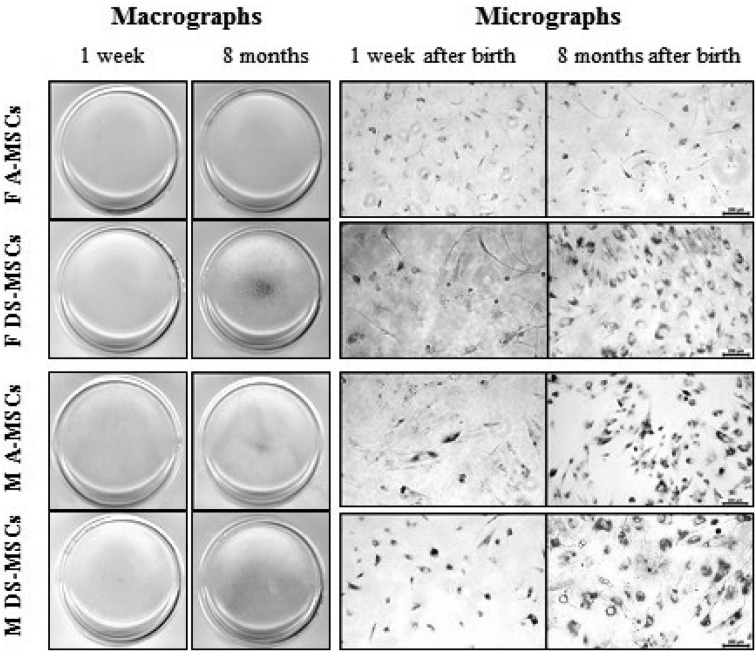
Analysis of SA-β-galactosidase activity: The results of SA-β-Gal staining for the detection of cell aging are displayed in Fig. 3. All MSCs harvested at 8 months after birth were more positive for SA-β-Gal staining, except for female A-MSCs, when compared to those harvested at 1 week after birth (macrographs of Fig. 3). Furthermore, DS-MSCs showed stronger SA-β-Gal staining than A-MSCs harvested at 8 months after birth. However, there was no difference found in SA-β-Gal staining among MSCs harvested at 1 week age regardless of their sex and tissue source.
Fig. 3.
Senescence-associated (SA)-β-galactosidase activity of MSCs obtained at 1 week and 8 months after birth. Scale bars=200 µm. The blue color indicates positivity for cell senescence.
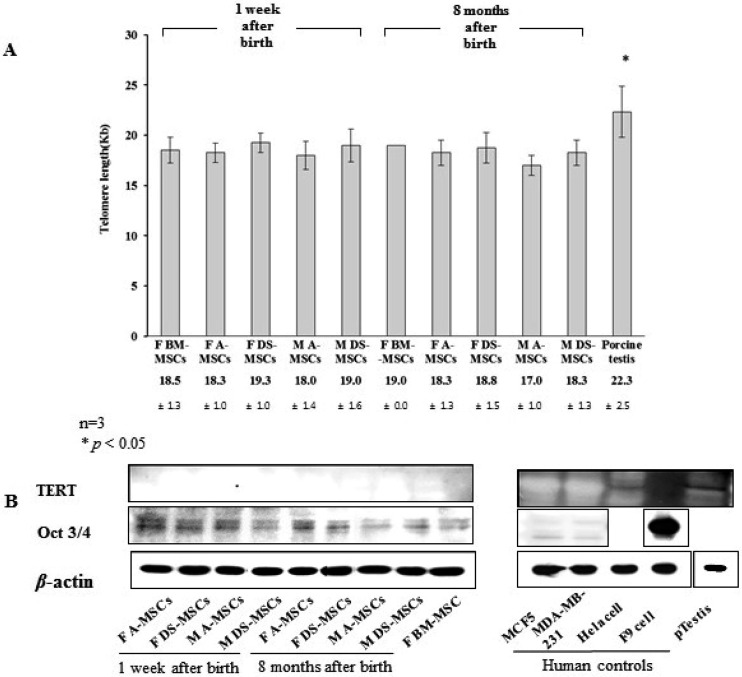
Analysis of MSC life-span and proliferation: MSC life-span was analyzed according to donor sex, age and tissue source based on telomere length (Southern blotting) and telomerase activity (TERT; Western blotting) (Fig. 4). The telomere length of female-derived BM-MSCs was 18.5 ± 1.3 and 19.0 ± 0.0 Kb at 1 week and 8 months of age, respectively. The telomere length ranged from 17.0 ± 1.0 to 19.3 ± 1.0 Kb in all MSCs with no significant differences among donor sex, age or tissue source. The telomere length in the testis tissue of 1-year-old pig was 22.3 ± 2.5 Kb, which was significantly higher than that in all other MSCs under current investigation. TERT protein expression was not detected in any of the MSCs evaluated.
Fig. 4.
Analysis of telomere length, telomere activity-related TERT protein expression and Oct 3/4 protein expression in MSCs harvested at 1 week and 8 months after birth. (A) Telomere restriction fragment lengths determined by Southern blotting using a telomere length assay. Significant differences were considered when P<0.05. (B) TERT and Oct3/4 protein expression determined by western blot analysis.
Expression of Oct 3/4 protein, an early stem cell transcription factor and a potent indicator of proliferation capacity, was observed to be diminished with the advancement in age. Female-derived MSCs showed slightly stronger Oct 3/4 protein expression than male-derived MSCs at both the ages. Further, its expression was slightly higher in A-MSCs than in DS-MSCs.
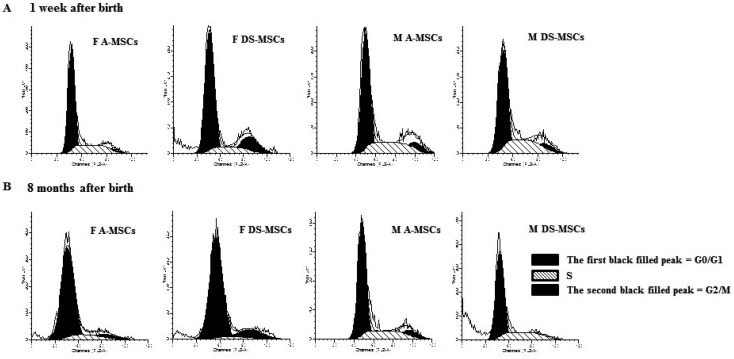
Cell cycle analysis: The cell cycle distribution of MSCs is presented in Table 1 and Fig. 5. A higher proportion of MSCs harvested at 8 months after birth was in the G0/G1 phase compared to that of MSCs harvested at 1 week after birth.
Table 1. Analysis of cell cycle status in MSCs harvested at 1 week and 8 months after birth.
| Groups* | Mean ± S.D. (%) |
|||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| 1 week after birth | FA-MSCs | 68.8 ± 4.8 a) | 27.1 ± 3.3 a) | 4.1 ± 2.0 a) |
| FDS-MSCs | 67.8 ± 2.9 a) | 16.8 ± 5.6 b) | 15.4 ± 3.5 b) | |
| MA-MSCs | 62.7 ± 10.1 b) | 25.6 ± 5.2 a) | 11.7 ± 5.0 b) | |
| MDS-MSCs | 61.6 ± 4.3 b) | 25.0 ± 7.0 a) | 13.4 ± 2.6 b) | |
| 8 months after birth | FA-MSCs | 78.5 ± 0.8 a) | 16.8 ± 5.6 a) | 4.7 ± 4.9 a) |
| FDS-MSCs | 77.9 ± 2.8 a) | 7.3 ± 4.7 b) | 14.8 ± 5.1 b) | |
| MA-MSCs | 71.6 ± 4.0 b) | 21.0 ± 5.0 a,b) | 7.4 ± 4.4 a,b) | |
| MDS-MSCs | 70.5 ± 1.5 b) | 27.3 ± 3.7 c) | 2.2 ± 2.2 c) | |
This experiment was performed in triplicate, and significant differences are shown within columns. a, b, c) P<0.05. Each sample was analyzed with 10,000 cells at a time by flow cytometry. *FA-MSC, MSC derived from female adipose tissue; FDS-MSC, MSC derived from female dermal skin tissue; MA-MSC, MSC derived from male adipose tissue; MDS-MSC, MSC derived from male dermal skin tissue.
Fig. 5.
Cell cycle analysis of MSCs harvested at 1 week (A) and 8 months after birth (B). The first and second black filled peaks indicate the G0/G1 and G2/M phases, respectively, and the black oblique line-filled peak in the middle indicates the S phase.
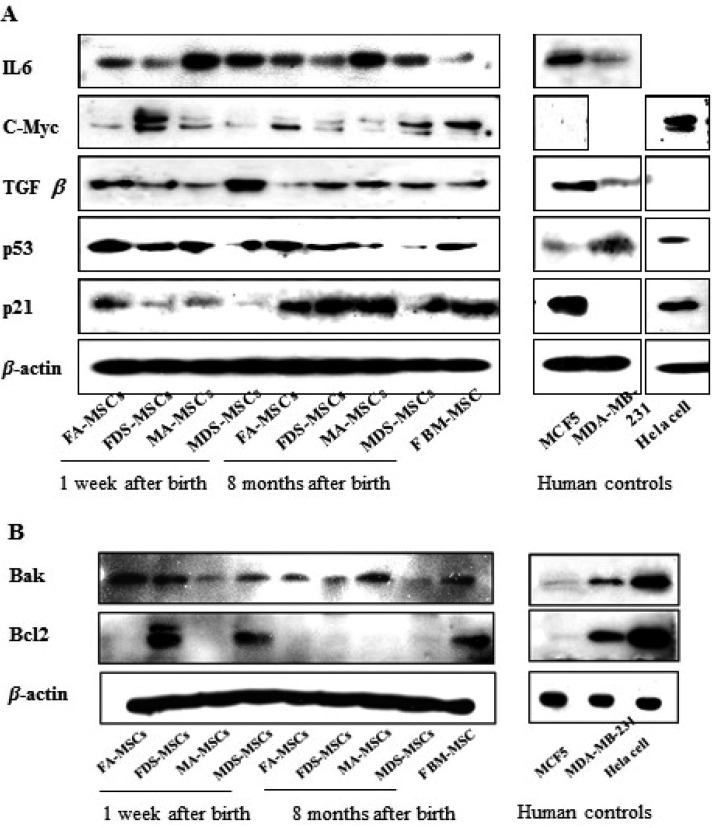
Analysis of cell-aging-related proteins: The expression of proteins related to cell proliferation, differentiation and apoptosis was analyzed by western blotting (Fig. 6A). The pro-inflammatory cytokine IL6 was expressed in all types of MSCs without change in its expression with the advancement in age. However, IL6 expression was higher in male-derived MSCs than in female-derived MSCs, wherein adipose-derived MSCs have shown slightly higher expression than dermal skin-derived MSCs at both the ages. c-Myc, a cell proliferation factor and oncogene, was expressed at higher levels in female DS-MSCs derived at one week after birth than that in other types of MSCs. Expression of TGFβ, a cell proliferation and differentiation factor, was found to be elevated in male DS-MSCs derived at one week after birth compared to other types of MSCs. p53, an antitumor molecule and stress factor, was expressed in all types of MSCs and did not vary according to donor age, sex and tissue source, except in male DS-MSCs harvested at 8 months after birth. Further, p53 expression was higher in A-MSCs compared to that in DS-MSCs regardless of donor age and sex. The expression of p21 was increased in MSCs harvested at 8 months after birth and was similar to that of female-derived BM-MSCs.
Fig. 6.
Senescence-related proteins expression in MSCs harvested at 1 week and 8 months after birth. (A) IL6 as an immunomodulator, c-myc as a cell proliferation factor and oncoprotein, TGF β as a differentiation and cell proliferation factor, p53 as an external stress and antitumor factor, and p21 as a cell cycle arrest protein were assayed. (B) Bak as a proapoptotic protein and Bcl2 as an antiapoptotic protein were analyzed.
Analysis of apoptosis-related proteins: Expression of Bak and Bcl2 is known to be directly correlated with apoptosis, and the expression patterns of these proteins in MSCs are shown in Fig. 6B. Bak, a pro-apoptotic protein, was expressed in all MSC types, although with varying expression levels, female-derived MSCs harvested at 1 week after birth showed higher expression. In contrast, Bcl2 protein was expressed at extremely low levels in all MSCs, except DS-MSCs harvested at 1 week after birth. Bcl2 expression in female-derived DS-MSCs and male-derived DS-MSCs harvested 1 week after birth was similar to that of female BM-MSCs.
DISCUSSION
Here, we report that the donor age, gender and tissue source are linked to the change in physiological, functional and molecular characteristics of porcine MSCs. Both human and rat MSCs are previously reported to be associated with age-dependent change in biological properties [45, 50], and especially, the proliferative nature of MSCs may vary upon in vitro cultivation depending on the tissue source as well as the donor age [25, 40].
The present study also demonstrated the characteristics of porcine MSCs, such as stemness, proliferation, senescence and apoptosis, were affected by donor age, gender and tissue source. Stemness was evaluated by AP activity, CD marker expression and differentiation ability of MSCs. In porcine, AP expression has been reported as a marker for assessing pluripotency of stem cells including porcine umbilical cord (PUCs) [13] and skin-derived cells [28], and its expression was found to be affected by donor age. Our study showed the decreased AP activity with aging regardless of donor sex and tissue source, indicating the progressive loss of differentiation capacity of MSCs with the advancement in age of porcine.
CD90 is a representative MSC-specific marker to confirm mesodermal origin of porcine MSCs. Donor aging has been reported to be associated with the decline in number of CD90-positive cells [12, 45]. However, in the present study, its expression was not altered by either donor age, sex or tissue source. This may be due to the species specific variability. As CD90 also participates in immunosuppression by reducing the levels of HLA-G or IL10 [12], our results indicate that porcine MSCs are thought to maintain their immunosuppressive capacity at least for up to 8 months after birth.
To address whether donor age, sex and tissue source will affect the multipotency of porcine MSCs, we have evaluated in vitro osteogenesis and adipogenesis. Previously, it has been reported that donor aging will affect multipotency of MSCs, and especially, osteogenic propensity will decline with the advancement in age [48, 51, 52]. However, in this study, donor aging had only affected adipogenic ability while maintaining osteogenic ability of porcine MSCs. The reason for conflicting results obtained in this study may possibly be due to the early procurement of MSCs at 8 months after birth when compared to previous studies where MSCs were derived from the bone marrow of elderly humans ( >50 years old) and rats (26 months old) [48, 52]. Further, adipose tissue derived MSCs showed higher multipotency than those derived from dermal skin, indicating MSCs present in adipose tissue may be more primitive than those in dermal skin. Nevertheless, higher adipogenic differentiation of A-MSCs obtained at 1 week after birth regardless of their sex suggests that the epigenetic memory could also play an important role in the differentiation ability of MSCs.
Embryonic stem cells possess long telomeres and high telomerase activity and expression of the early stem cell transcription factor Oct 3/4 [19, 22]. These factors are involved in cell proliferation and maintenance of cellular aging in stem cells. However, in adult stem cells, such as MSCs, the expression of telomerase and Oct 3/4 is still unclear as some studies have reported very low telomerase activity and the absence or extremely low levels of Oct 3/4 expression [19, 24, 34]. In this study, telomere length was unaffected by the effect of donor age, sex and tissue source. However, telomerase activity was absent or extremely low in all types of MSCs. The proliferative capacity of porcine MSCs is found to be declined with the advancement of donor age as indicated by the higher expression of p21 protein with higher proportion of cells in G0/G1 phase of cell cycle in MSCs harvested at 8 months after birth compared to that in MSCs harvested at 1 week after birth. Apart from donor age, it is also evident that sex and tissue source can affect proliferative capacity of MSCs as revealed by the expression of Oct 3/4 protein. Female-, adipose-derived MSCs are more likely to maintain their proliferative capacity than male-, dermal skin-derived MSCs based on their expression of Oct 3/4 protein. Further, lower c-Myc expression in A-MSCs compared to DS-MSCs indicates that the porcine adipose tissue may be the best source of MSCs in terms of safety for in vivo transplantation. As short-term activation of c-Myc promotes cell cycle progression, its prolonged activation may result in the initial hyper-proliferation of cells to subsequently undergo cellular senescence [11].
SA-β-Galactosidase is known to specifically indicate the senescence of cells, whereas IL6 acts as both pro-inflammatory and anti-inflammatory cytokine. It also represents an important protein related to cellular senescence. Its role in human aging is plausible, though largely obscure. Adult mouse BM-MSCs were found to secrete significantly higher levels of IL6 than neonatal mouse BM-MSCs, and this elevated IL6 levels have been linked to various pathologies [15, 32, 47]. These findings indicate an increased secretion of IL6 might directly be associated with stem cell aging. Although MSCs were obtained at a relatively younger age of 8 months, positive staining was observed in the present study. MSCs isolated at 8 months of age displayed reduced proliferation with higher SA-β-Galactosidase activity indicating donor age may directly affect the cellular characteristics. Female-, adipose-derived MSCs with reduced expression of SA-β-Galactosidase and IL6 protein even at 8 months of age suggest that these cells might be more resistant to senescence in vitro compared to male-, DS-derived MSCs in porcine. This increased resistance to senescence may possibly due to their higher expression of Oct3/4 protein, which might contribute to prolonged proliferative capacity under in vitro condition. In the present study, apoptotic behavior of MSCs was not influenced by sex. Bcl2 is an anti-apoptotic protein that extends the cell life-span and facilitates tumorigenesis [6, 42], and therefore, our study suggests that the DS-MSCs may possess tumorigenic properties due to their higher Bcl2 protein expression observed at 1 week after birth.
In conclusion, biological characteristics of porcine MSCs were affected by both donor age, gender and tissue source. Stemness and proliferative capacity of porcine MSCs were declined with donor aging. Higher differentiation potential was noted in adipose-derived MSCs compared to dermal skin-derived MSCs. Female adipose-derived MSCs displayed prolonged proliferation potential. Increased c-Myc and Bcl2 levels in DS-MSCs at 1 week after birth suggested their tumorigenic potential. Although we did not use very old porcine donors, our results suggest that MSCs display a cellular and molecular variability depending on the donor age, sex and tissue source even under similar genetic and environmental conditions. Based on these findings, it is concluded that adipose-tissue-derived MSCs from young female individuals tend to have higher resistance to senescence under in vitro culture conditions.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (no. PJ01131102), Rural Development Administration and Bio-industry Technology Development Program (IPET, 312060–5) of the Ministry for Food, Agriculture, Forestry and Fisheries and the Republic of Korea. The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Aksu A. E., Rubin J. P., Dudas J. R., Marra K. G.2008. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann. Plast. Surg. 60: 306–322. doi: 10.1097/SAP.0b013e3180621ff0 [DOI] [PubMed] [Google Scholar]
- 2.Avery B., Jørgensen C. B., Madison V., Greve T.1992. Morphological development and sex of bovine in vitro-fertilized embryos. Mol. Reprod. Dev. 32: 265–270. doi: 10.1002/mrd.1080320312 [DOI] [PubMed] [Google Scholar]
- 3.Bai L., Lennon D. P., Caplan A. I., DeChant A., Hecker J., Kranso J., Zaremba A., Miller R. H.2012. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 15: 862–870. doi: 10.1038/nn.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter M. A., Wynn R. F., Jowitt S. N., Wraith J. E., Fairbairn L. J., Bellantuono I.2004. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22: 675–682. doi: 10.1634/stemcells.22-5-675 [DOI] [PubMed] [Google Scholar]
- 5.Ben-Porath I., Weinberg R. A.2005. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37: 961–976. doi: 10.1016/j.biocel.2004.10.013 [DOI] [PubMed] [Google Scholar]
- 6.Bhatavdekar J. M., Patel D. D., Ghosh N., Chikhlikar P. R., Trivedi T. I., Suthar T. P., Doctor S. S., Shah N. G., Balar D. B.1997. Coexpression of Bcl-2, c-Myc, and p53 oncoproteins as prognostic discriminants in patients with colorectal carcinoma. Dis. Colon Rectum 40: 785–790. doi: 10.1007/BF02055433 [DOI] [PubMed] [Google Scholar]
- 7.Blankenhorn E. P., Troutman S., Clark L. D., Zhang X. M., Chen P., Heber-Katz E.2003. Sexually dimorphic genes regulate healing and regeneration in MRL mice. Mamm. Genome 14: 250–260. doi: 10.1007/s00335-002-2222-3 [DOI] [PubMed] [Google Scholar]
- 8.Bonab M. M., Alimoghaddam K., Talebian F., Ghaffari S. H., Ghavamzadeh A., Nikbin B.2006. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 8: 14. doi: 10.1186/1471-2121-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch P., Pratt S. L., Stice S. L.2006. Isolation, characterization, gene modification, and nuclear reprogramming of porcine mesenchymal stem cells. Biol. Reprod. 74: 46–57. doi: 10.1095/biolreprod.105.045138 [DOI] [PubMed] [Google Scholar]
- 10.Bunnell B. A., Flaat M., Gagliardi C., Patel B., Ripoll C.2008. Adipose-derived stem cells: isolation, expansion and differentiation. Methods 45: 115–120. doi: 10.1016/j.ymeth.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campaner S., Doni M., Hydbring P., Verrecchia A., Bianchi L., Sardella D., Schleker T., Perna D., Tronnersjö S., Murga M., Fernandez-Capetillo O., Barbacid M., Larsson L. G., Amati B.2010. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nat. Cell Biol. 12: 54–59, 1–14. doi: 10.1038/ncb2004 [DOI] [PubMed] [Google Scholar]
- 12.Campioni D., Lanza F., Moretti S., Ferrari L., Cuneo A.2008. Loss of Thy-1 (CD90) antigen expression on mesenchymal stromal cells from hematologic malignancies is induced by in vitro angiogenic stimuli and is associated with peculiar functional and phenotypic characteristics. Cytotherapy 10: 69–82. doi: 10.1080/14653240701762364 [DOI] [PubMed] [Google Scholar]
- 13.Carlin R., Davis D., Weiss M., Schultz B., Troyer D.2006. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod. Biol. Endocrinol. 4: 8. doi: 10.1186/1477-7827-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang C., Wang X., Niu D., Zhang Z., Zhao H., Gong F.2009. Mesenchymal stem cells adopt beta-cell fate upon diabetic pancreatic microenvironment. Pancreas 38: 275–281. doi: 10.1097/MPA.0b013e318191521c [DOI] [PubMed] [Google Scholar]
- 15.Crisostomo P. R., Wang M., Herring C. M., Markel T. A., Meldrum K. K., Lillemoe K. D., Meldrum D. R.2007. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J. Mol. Cell. Cardiol. 42: 142–149. doi: 10.1016/j.yjmcc.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deasy B. M., Lu A., Tebbets J. C., Feduska J. M., Schugar R. C., Pollett J. B., Sun B., Urish K. L., Gharaibeh B. M., Cao B., Rubin R. T., Huard J.2007. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J. Cell Biol. 177: 73–86. doi: 10.1083/jcb.200612094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faiola B., Fuller E. S., Wong V. A., Pluta L., Abernethy D. J., Rose J., Recio L.2004. Exposure of hematopoietic stem cells to benzene or 1,4-benzoquinone induces gender-specific gene expression. Stem Cells 22: 750–758. doi: 10.1634/stemcells.22-5-750 [DOI] [PubMed] [Google Scholar]
- 18.Friedenstein A. J.1976. Precursor cells of mechanocytes. Int. Rev. Cytol. 47: 327–359. doi: 10.1016/S0074-7696(08)60092-3 [DOI] [PubMed] [Google Scholar]
- 19.Greco S. J., Liu K., Rameshwar P.2007. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells 25: 3143–3154. doi: 10.1634/stemcells.2007-0351 [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez-Adán A., Oter M., Martínez-Madrid B., Pintado B., De La Fuente J.2000. Differential expression of two genes located on the X chromosome between male and female in vitro-produced bovine embryos at the blastocyst stage. Mol. Reprod. Dev. 55: 146–151. doi: [DOI] [PubMed] [Google Scholar]
- 21.Harada H., Pavlick K. P., Hines I. N., Lefer D. J., Hoffman J. M., Bharwani S., Wolf R. E., Grisham M. B.2003. Sexual dimorphism in reduced-size liver ischemia and reperfusion injury in mice: role of endothelial cell nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 100: 739–744. doi: 10.1073/pnas.0235680100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiyama E., Hiyama K.2007. Telomere and telomerase in stem cells. Br. J. Cancer 96: 1020–1024. doi: 10.1038/sj.bjc.6603671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hörner S., Pasternak G., Hehlmann R.1997. A statistically significant sex difference in the number of colony-forming cells from human peripheral blood. Ann. Hematol. 74: 259–263. doi: 10.1007/s002770050296 [DOI] [PubMed] [Google Scholar]
- 24.Jeon B. G., Kumar B. M., Kang E. J., Ock S. A., Lee S. L., Kwack D. O., Byun J. H., Park B. W., Rho G. J.2011. Characterization and comparison of telomere length, telomerase and reverse transcriptase activity and gene expression in human mesenchymal stem cells and cancer cells of various origins. Cell Tissue Res. 345: 149–161. doi: 10.1007/s00441-011-1191-9 [DOI] [PubMed] [Google Scholar]
- 25.Katsara O., Mahaira L. G., Iliopoulou E. G., Moustaki A., Antsaklis A., Loutradis D., Stefanidis K., Baxevanis C. N., Papamichail M., Perez S. A.2011. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 20: 1549–1561. doi: 10.1089/scd.2010.0280 [DOI] [PubMed] [Google Scholar]
- 26.Keating A.2012. Mesenchymal stromal cells: new directions. Cell Stem Cell 10: 709–716. doi: 10.1016/j.stem.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 27.Kochhar H. S., Kochhar K. P., Basrur P. K., King W. A.2003. Influence of the duration of gamete interaction on cleavage, growth rate and sex distribution of in vitro produced bovine embryos. Anim. Reprod. Sci. 77: 33–49. doi: 10.1016/S0378-4320(03)00006-X [DOI] [PubMed] [Google Scholar]
- 28.Kumar B. M., Jin H. F., Kim J. G., Ock S. A., Hong Y., Balasubramanian S., Choe S. Y., Rho G. J.2007. Differential gene expression patterns in porcine nuclear transfer embryos reconstructed with fetal fibroblasts and mesenchymal stem cells. Dev. Dyn. 236: 435–446. doi: 10.1002/dvdy.21042 [DOI] [PubMed] [Google Scholar]
- 29.Kuo Y. R., Goto S., Shih H. S., Wang F. S., Lin C. C., Wang C. T., Huang E. Y., Chen C. L., Wei F. C., Zheng X. X., Lee W. P.2009. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation 87: 1769–1777. doi: 10.1097/TP.0b013e3181a664f1 [DOI] [PubMed] [Google Scholar]
- 30.Lansdorp P. M.2008. Telomeres, stem cells, and hematology. Blood 111: 1759–1766. doi: 10.1182/blood-2007-09-084913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lermen D., Gorjup E., Dyce P. W., von Briesen H., Müller P.2010. Neuro-muscular differentiation of adult porcine skin derived stem cell-like cells. PLoS ONE 5: e8968. doi: 10.1371/journal.pone.0008968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markel T. A., Wang M., Crisostomo P. R., Manukyan M. C., Poynter J. A., Meldrum D. R.2008. Neonatal stem cells exhibit specific characteristics in function, proliferation, and cellular signaling that distinguish them from their adult counterparts. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294: R1491–R1497. doi: 10.1152/ajpregu.00031.2008 [DOI] [PubMed] [Google Scholar]
- 33.Mulroney S. E., Woda C., Johnson M., Pesce C.1999. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int. 56: 944–953. doi: 10.1046/j.1523-1755.1999.00647.x [DOI] [PubMed] [Google Scholar]
- 34.Ock S. A., Jeon B. G., Rho G. J.2010. Comparative characterization of porcine mesenchymal stem cells derived from bone marrow extract and skin tissues. Tissue Eng. Part C Methods 16: 1481–1491. doi: 10.1089/ten.tec.2010.0149 [DOI] [PubMed] [Google Scholar]
- 35.Peippo J., Farazmand A., Kurkilahti M., Markkula M., Basrur P. K., King W. A.2002. Sex-chromosome linked gene expression in in-vitro produced bovine embryos. Mol. Hum. Reprod. 8: 923–929. doi: 10.1093/molehr/8.10.923 [DOI] [PubMed] [Google Scholar]
- 36.Pergament E., Fiddler M., Cho N., Johnson D., Holmgren W. J.1994. Sexual differentiation and preimplantation cell growth. Hum. Reprod. 9: 1730–1732. [DOI] [PubMed] [Google Scholar]
- 37.Quevedo H. C., Hatzistergos K. E., Oskouei B. N., Feigenbaum G. S., Rodriguez J. E., Valdes D., Pattany P. M., Zambrano J. P., Hu Q., McNiece I., Heldman A. W., Hare J. M.2009. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. U.S.A. 106: 14022–14027. doi: 10.1073/pnas.0903201106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rando T. A.2006. Stem cells, ageing and the quest for immortality. Nature 441: 1080–1086. doi: 10.1038/nature04958 [DOI] [PubMed] [Google Scholar]
- 39.Riekstina U., Muceniece R., Cakstina I., Muiznieks I., Ancans J.2008. Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology 58: 153–162. doi: 10.1007/s10616-009-9183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sethe S., Scutt A., Stolzing A.2006. Aging of mesenchymal stem cells. Ageing Res. Rev. 5: 91–116. doi: 10.1016/j.arr.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 41.Shivakumar S. B., Bharti D., Jang S. J., Hwang S. C., Park J. K., Shin J. K., Byun J. H., Park B. W., Rho G. J.2015. Cryopreservation of human Wharton’s jelly-derived mesenchymal stem cells following controlled rate freezing protocol using different cryoprotectants; A comparative study. Int. J. Stem Cells 8: 155–169. doi: 10.15283/ijsc.2015.8.2.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shortt J., Johnstone R. W.2012. Oncogenes in cell survival and cell death. Cold Spring Harb. Perspect. Biol. 4: a009829. doi: 10.1101/cshperspect.a009829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Souza B. S., Nogueira R. C., de Oliveira S. A., de Freitas L. A., Lyra L. G., Ribeiro dos Santos R., Lyra A. C., Soares M. B.2009. Current status of stem cell therapy for liver diseases. Cell Transplant. 18: 1261–1279. doi: 10.3727/096368909X470522 [DOI] [PubMed] [Google Scholar]
- 44.Stenderup K., Justesen J., Clausen C., Kassem M.2003. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33: 919–926. doi: 10.1016/j.bone.2003.07.005 [DOI] [PubMed] [Google Scholar]
- 45.Tokalov S. V., Grüner S., Schindler S., Wolf G., Baumann M., Abolmaali N.2007. Age-related changes in the frequency of mesenchymal stem cells in the bone marrow of rats. Stem Cells Dev. 16: 439–446. doi: 10.1089/scd.2006.0078 [DOI] [PubMed] [Google Scholar]
- 46.Vacanti V., Kong E., Suzuki G., Sato K., Canty J. M., Lee T.2005. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J. Cell. Physiol. 205: 194–201. doi: 10.1002/jcp.20376 [DOI] [PubMed] [Google Scholar]
- 47.Wang M., Markel T., Crisostomo P., Herring C., Meldrum K. K., Lillemoe K. D., Meldrum D. R.2007. Deficiency of TNFR1 protects myocardium through SOCS3 and IL-6 but not p38 MAPK or IL-1beta. Am. J. Physiol. Heart Circ. Physiol. 292: H1694–H1699. doi: 10.1152/ajpheart.01063.2006 [DOI] [PubMed] [Google Scholar]
- 48.Wilson A., Shehadeh L. A., Yu H., Webster K. A.2010. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics 11: 229. doi: 10.1186/1471-2164-11-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu K. P., Yadav B. R., King W. A., Betteridge K. J.1992. Sex-related differences in developmental rates of bovine embryos produced and cultured in vitro. Mol. Reprod. Dev. 31: 249–252. doi: 10.1002/mrd.1080310404 [DOI] [PubMed] [Google Scholar]
- 50.Yew T. L., Chiu F. Y., Tsai C. C., Chen H. L., Lee W. P., Chen Y. J., Chang M. C., Hung S. C.2011. Knockdown of p21(Cip1/Waf1) enhances proliferation, the expression of stemness markers, and osteogenic potential in human mesenchymal stem cells. Aging Cell 10: 349–361. doi: 10.1111/j.1474-9726.2011.00676.x [DOI] [PubMed] [Google Scholar]
- 51.Yu J. M., Wu X., Gimble J. M., Guan X., Freitas M. A., Bunnell B. A.2011. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell 10: 66–79. doi: 10.1111/j.1474-9726.2010.00646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou S., Greenberger J. S., Epperly M. W., Goff J. P., Adler C., Leboff M. S., Glowacki J.2008. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 7: 335–343. doi: 10.1111/j.1474-9726.2008.00377.x [DOI] [PMC free article] [PubMed] [Google Scholar]