Abstract
Background
Trimethylamine‐N‐oxide (TMAO), a metabolite derived from gut microbes and dietary phosphatidylcholine, is linked to both coronary artery disease pathogenesis and increased cardiovascular risks. The ability of plasma TMAO to predict 5‐year mortality risk in patients with stable coronary artery disease has not been reported. This study examined the clinical prognostic value of TMAO in patients with stable coronary artery disease who met eligibility criteria for a patient cohort similar to that of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial.
Methods and Results
We examined the relationship between fasting plasma TMAO and all‐cause mortality over 5‐year follow‐up in sequential patients with stable coronary artery disease (n=2235) who underwent elective coronary angiography. We identified the COURAGE‐like patient cohort as patients who had evidence of significant coronary artery stenosis and who were managed with optimal medical treatment. Higher plasma TMAO levels were associated with a 4‐fold increased mortality risk. Following adjustments for traditional risk factors, high‐sensitivity C‐reactive protein, and estimated glomerular filtration rate, elevated TMAO levels remained predictive of 5‐year all‐cause mortality risk (quartile 4 versus 1, adjusted hazard ratio 1.95, 95% CI 1.33–2.86; P=0.003). TMAO remained predictive of incident mortality risk following cardiorenal and inflammatory biomarker adjustments to the model (adjusted hazard ratio 1.71, 95% CI 1.11–2.61; P=0.0138) and provided significant incremental prognostic value for all‐cause mortality (net reclassification index 42.37%, P<0.001; improvement in area under receiver operator characteristic curve 70.6–73.76%, P<0.001).
Conclusions
Elevated plasma TMAO levels portended higher long‐term mortality risk among patients with stable coronary artery disease managed with optimal medical treatment.
Keywords: optimal medical treatment, prognosis, stable coronary artery disease, trimethylamine N‐oxide
Subject Categories: Chronic Ischemic Heart Disease, Clinical Studies, Pathophysiology, Angiography
Introduction
The prevalence of coronary artery disease (CAD) continues to increase and remains the leading cause of death worldwide.1 Despite advances in medical treatment, contemporary coronary revascularization techniques, and risk factor modification, the mortality risk of CAD remains high.1, 2, 3 There is a pressing need to identify residual risk factors to improve our understanding of the processes contributing to CAD and vulnerable plaque pathogenesis and to identify novel therapeutic targets in CAD. Recently, our group identified a novel mechanistic link between trimethylamine‐N‐oxide (TMAO), an intestinal microbiota‐generated metabolite, and cardiovascular disease (CVD) pathogenesis by a pathway involving dietary ingestion of nutrients containing trimethylamine, including phosphatidylcholine, choline, and l‐carnitine.4, 5, 6, 7, 8, 9, 10 These nutrients are more abundant in a Western diet rich in red meat, egg yolk, and meat products—a diet strongly associated with increased incident CVD risks.5, 11, 12 Elevated plasma TMAO levels have shown TMAO to be a proatherogenic biomarker and predictor of adverse CVD risks in multiple cohorts.4, 6, 7, 8, 9, 13, 14, 15, 16, 17
The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial showed no significant difference in death and myocardial infarction risks for stable CAD patients who were treated with revascularization plus optimal medical treatment (OMT) versus OMT alone.18 We sought to examine the relationship between fasting plasma TMAO levels and long‐term clinical prognosis in patients with stable CAD who had significant coronary artery stenosis detected on elective coronary angiography and were managed with OMT alone (COURAGE‐like patient cohort).
Methods
Study Population
This single‐center prospective cohort study was approved by the Cleveland Clinic institutional review board. All participants provided written informed consent. We included adult participants (aged ≥18 years) with symptoms or signs of CAD who underwent elective nonurgent coronary angiography at the Cleveland Clinic between 2001 and 2007 without evidence of acute coronary syndrome (cardiac troponin I <0.03 ng/mL). Patients who had experienced acute coronary syndrome or revascularization procedures within 30 days before enrollment were excluded. A COURAGE‐like patient cohort was identified for the present studies by using COURAGE inclusion and exclusion criteria,18 as follows: (1) patients who had an elective diagnostic coronary angiography procedure, as described earlier; (2) patients with evidence of significant CAD defined by ≥70% diameter stenosis in at least 1 epicardial coronary artery; and (3) patients assigned to receive OMT alone, without a revascularization procedure within 30 days after enrollment in the present study. All angiogram interpretations and the decision of whether to undergo revascularization plus OMT or OMT alone were determined at the discretion of the board‐certified cardiology staff at the Cleveland Clinic. A total of 2235 consecutive patients who fulfilled criteria for the COURAGE‐like patient cohort were included in this study and represent a subset of the previously reported study cohort6 of medically managed patients that fulfilled COURAGE trial criteria for having significant coronary artery disease. All‐cause mortality at 5 years was tracked by electronic chart review and Social Security Death Index up to 2011 and confirmed by telephone interviews, official hospital records, or death certificates.
Laboratory Testing
Fasting blood samples were collected using EDTA tubes at the time of cardiac catheterization, immediately prior to heparin injection and catheterization procedure. Samples were maintained on ice or at 4°C, immediately processed within 3 hours of collection, and frozen at−80°C until analysis. Routine laboratory tests including high‐sensitivity C‐reactive protein (hsCRP) and cardiac‐specific troponin I were performed on samples using the Abbott Architect platform (Abbott Laboratories). An estimated glomerular filtration rate (eGFR; in mL/min per 1.73 m2) was calculated using the Modification of Diet in Renal Disease study equation.19 Myeloperoxidase was measured using the CardioMPO assay (Cleveland HeartLab). TMAO levels in plasma were determined using stable isotope dilution high‐performance liquid chromatography with online electrospray ionization tandem mass spectrometry on an AB Sciex API 5000 mass spectrometer using d9‐(trimethyl)‐labeled internal standards, as described previously.4, 20
Statistical Analyses
Continuous data are presented as mean (standard deviation) or median (interquartile range) and compared with Student t test or nonparametric test when appropriate. Categorical variables are presented as number (percentage) and compared between groups with chi‐square tests. We divided plasma TMAO levels into quartiles. Kaplan–Meier analysis with Cox proportional hazards regression was used for the time‐to‐event analysis to determine hazard ratios (HRs) and 95% CIs for 5‐year all‐cause mortality stratified according to TMAO quartiles. Adjustments were made for individual traditional cardiovascular risk factors (age, sex, systolic blood pressure, diabetes mellitus, low‐ and high‐density lipoprotein cholesterol levels, and smoking status), number of diseased vessels, medications (angiotensin‐converting enzyme inhibitor, angiotensin‐receptor blocker, aspirin, or statin), log‐transformed hsCRP levels, eGFR, myeloperoxidase, and B‐type natriuretic peptide to predict all‐cause mortality. Category‐free net reclassification and area under the receiver operating characteristic curve were calculated according to mortality risk estimated using Cox models adjusted for the above‐mentioned traditional risk factors with versus without TMAO, as described previously.21 All analyses were performed used R 2.15.1 (R Foundation for Statistical Computing). P<0.05 indicated statistical significance.
Results
Baseline Characteristics
The baseline characteristics of our study cohort are provided in Table 1. The median TMAO level was 3.8 μmol/L (interquartile range 2.5–6.5 μmol/L). Participants with higher TMAO levels were more likely to be older, to have hypertension or diabetes, and to have lower eGFR.
Table 1.
Baseline Characteristics
| Variable | All (n=2235) | Trimethylamine‐N‐Oxide Quartiles | P Value | |||
|---|---|---|---|---|---|---|
| Quartile 1, <2.5 | Quartile 2, 2.5–3.8 | Quartile 3, 3.81–6.5 | Quartile 4, >6.5 | |||
| Age, y | 63±11 | 60±11 | 62±10 | 65±10 | 66±10 | <0.001 |
| Female, % | 29 | 23 | 27 | 31 | 36 | <0.001 |
| Diabetes mellitus, % | 35 | 27 | 31 | 34 | 49 | <0.001 |
| Hypertension, % | 76 | 72 | 73 | 75 | 83 | <0.001 |
| Former/current smokers, % | 70 | 73 | 71 | 68 | 67 | 0.106 |
| History of coronary artery disease, % | 95 | 96 | 95 | 94 | 96 | 0.43 |
| Framingham ATP III risk score | 9 (6–12) | 8 (5–10) | 9 (6–11) | 9 (7–12) | 10 (7–13) | <0.001 |
| Low‐density lipoprotein cholesterol, mg/dL | 92 (76–112) | 93 (77–112) | 95 (77–115) | 92 (76–112) | 89 (71–109) | <0.001 |
| High‐density lipoprotein cholesterol, mg/dL | 33 (27–39) | 33 (28–39) | 33 (28–40) | 34 (28–39) | 31 (26–38) | <0.001 |
| Triglycerides, mg/dL | 124 (89–180) | 123 (90–175) | 120 (88–167) | 128 (90–182) | 128 (89–195) | <0.001 |
| High‐sensitivity C‐reactive protein, mg/L | 2.6 (1.1–6.4) | 2.6 (0.9–7.3) | 2.4 (1.1–6.0) | 2.5 (1.2–5.4) | 3.1 (1.3–7.6) | <0.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 98.7 (74.4–125) | 113.9 (92.5–135.2) | 106.3 (83.5–131.2) | 94.8 (72.5–119.6) | 77.9 (52.9–104) | <0.001 |
| Apolipoprotein B, mg/dL | 81 (68–96) | 83 (69–97) | 82 (69–98) | 80 (68.2–94) | 80 (67–93.2) | <0.001 |
| Apolipoprotein A1, mg/dL | 113 (100–129) | 112 (99–127) | 114 (102–129) | 115 (103–131) | 112 (98–128) | <0.001 |
| Triglycerides/high‐density lipoprotein | 3.9 (2.5–5.9) | 3.7 (2.5–5.8) | 3.7 (2.4–5.5) | 3.9 (2.6–5.9) | 4.2 (2.6–6.6) | <0.001 |
| Myeloperoxidase, pmol/L | 112.5 (74.5–239.6) | 125.1 (75.5–278.2) | 113.5 (76.5–268.9) | 112 (77.6–210.8) | 104.5 (71.5–204.4) | <0.001 |
| White blood cell count, per mm3 | 6.2 (5.1–7.6) | 6.3 (5.3–7.9) | 6.1 (5.1–7.5) | 6.1 (5.1–7.5) | 6.1 (5.1–7.4) | <0.001 |
| Medication | ||||||
| Angiotensin‐converting enzyme inhibitor or angiotensin‐receptor blocker, % | 55 | 50 | 52 | 57 | 59 | 0.004 |
| Beta‐blocker, % | 70 | 71 | 69 | 72 | 69 | 0.702 |
| Statin, % | 71 | 75 | 70 | 70 | 67 | 0.029 |
| Aspirin, % | 81 | 83 | 83 | 81 | 76 | 0.008 |
| Trimethylamine‐N‐oxide, μmol/L | 3.8 (2.5–6.5) | 1.8 (1.4–2.2) | 3.1 (2.8–3.4) | 4.9 (4.3–5.6) | 9.7 (7.7–14.9) | <0.001 |
Values expressed as mean±SD, percentage or median (interquartile range).
Baseline Fasting Plasma TMAO and All‐Cause Mortality
Over 5 years of follow‐up, 338 deaths occurred in our study cohort. Figure 1 represents the Kaplan–Meier analysis of TMAO stratified by quartiles, which illustrate a graded increased risk for all‐cause mortality observed with increasing plasma TMAO levels (log‐rank, P<0.001). Importantly, the highest TMAO quartile (>6.5 μmol/L) was associated with a significant 3.9‐fold increased risk for all‐cause mortality compared with the lowest TMAO quartile (quartile 4 versus 1, unadjusted HR 3.90, 95% CI 2.78–5.48; P<0.0001) (Table 2). The significant prognostic value of elevated plasma TMAO level was preserved when adjusted for traditional risk factors (adjusted HR 2.61, 95% CI 1.82–3.76; P<0.0001), with the addition of hsCRP and eGFR (adjusted HR 1.95, 95% CI 1.33–2.86; P=0.003), and following the addition of B‐type natriuretic peptide, myeloperoxidase, number of diseased vessels, and medications to the model (adjusted HR 1.71, 95% CI 1.11–2.61; P=0.0138) (Table 2). The addition of TMAO to a model of traditional cardiovascular risk factors showed that elevated TMAO level significantly improved net reclassification for all‐cause mortality (net reclassification index 42.37%; P<0.001) and area under the receiver operating characteristic curve (70.6–73.76%; P<0.001). As illustrated by the cubic spline curve, the risk of future mortality appeared to be steep and linear, with HR significantly above unity, particularly for plasma TMAO levels >4.5 μmol/L (Figure 2). Moreover, higher TMAO levels also predicted higher future risk of 5‐year all‐cause mortality regardless of age, sex, smoking status, diabetes mellitus, and CVD risk markers including myeloperoxidase, lipids and lipoproteins, hsCRP, eGFR, or B‐type natriuretic peptide (Figure 3).
Figure 1.
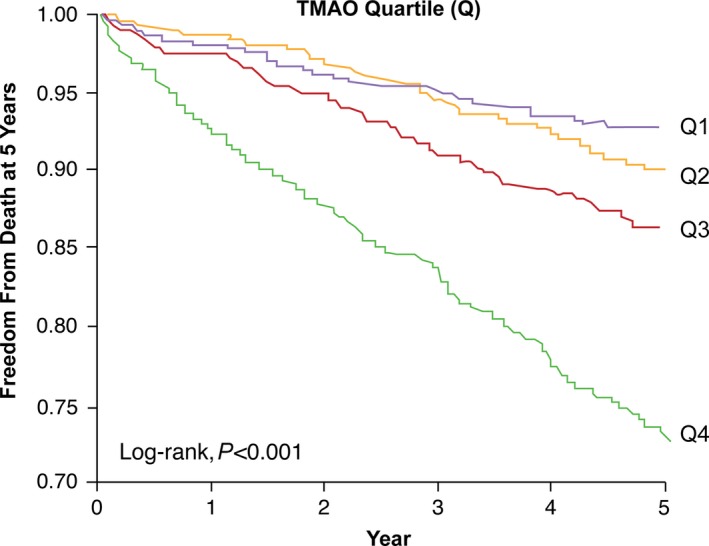
Unadjusted Kaplan–Meier estimates of risk of all‐cause mortality according to the quartiles of plasma TMAO levels. TMAO indicates trimethylamine‐N‐oxide.
Table 2.
Cox Proportional Hazards Analyses of Fasting Plasma Trimethylamine‐N‐Oxide Levels for 5‐Year All‐Cause Mortality
| Model | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Unadjusted | 3.90 (2.78–5.48) | <0.0001 |
| Adjusted Model 1 | 2.61 (1.82–3.76) | <0.0001 |
| Adjusted Model 2 | 1.95 (1.33–2.86) | 0.003 |
| Adjusted Model 3 | 1.71 (1.11–2.61) | 0.0138 |
Hazard ratios shown are for quartile 4 vs 1. Model 1: adjusted for traditional risk factors including age, sex, systolic blood pressure, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, smoking, and diabetes mellitus. Model 2: adjusted for all factors in model 1 plus high‐sensitivity C‐reactive protein (log‐transformed) and estimated glomerular filtration rate. Model 3: adjusted for all factors in model 1 plus medications (angiotensin‐converting enzyme inhibitor, angiotensin‐receptor blocker, β‐blocker, aspirin, or statin), number of stenotic vessels, high‐sensitivity C‐reactive protein (log‐transformed), myeloperoxidase (log‐transformed), estimated glomerular filtration rate (log‐transformed), and B‐type natriuretic peptide (log‐transformed).
Figure 2.
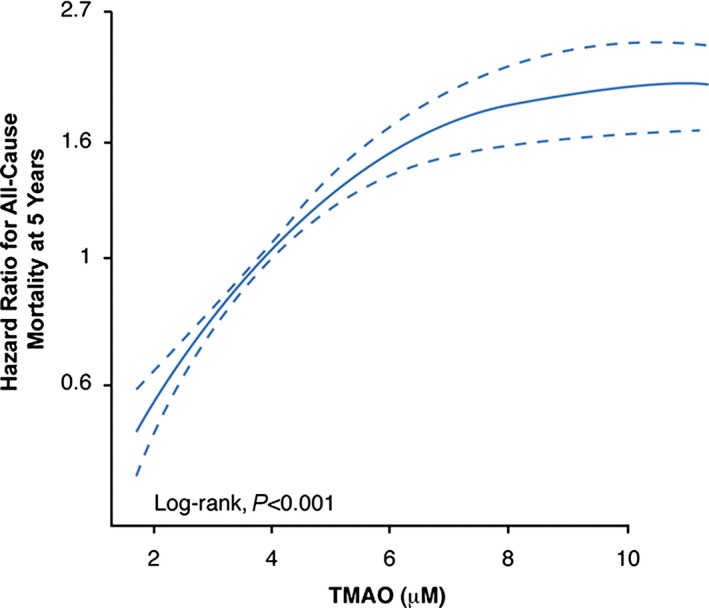
Cubic spline curve for hazard ratios for all‐cause mortality at 5 years with plasma TMAO levels. TMAO indicates trimethylamine‐N‐oxide.
Figure 3.
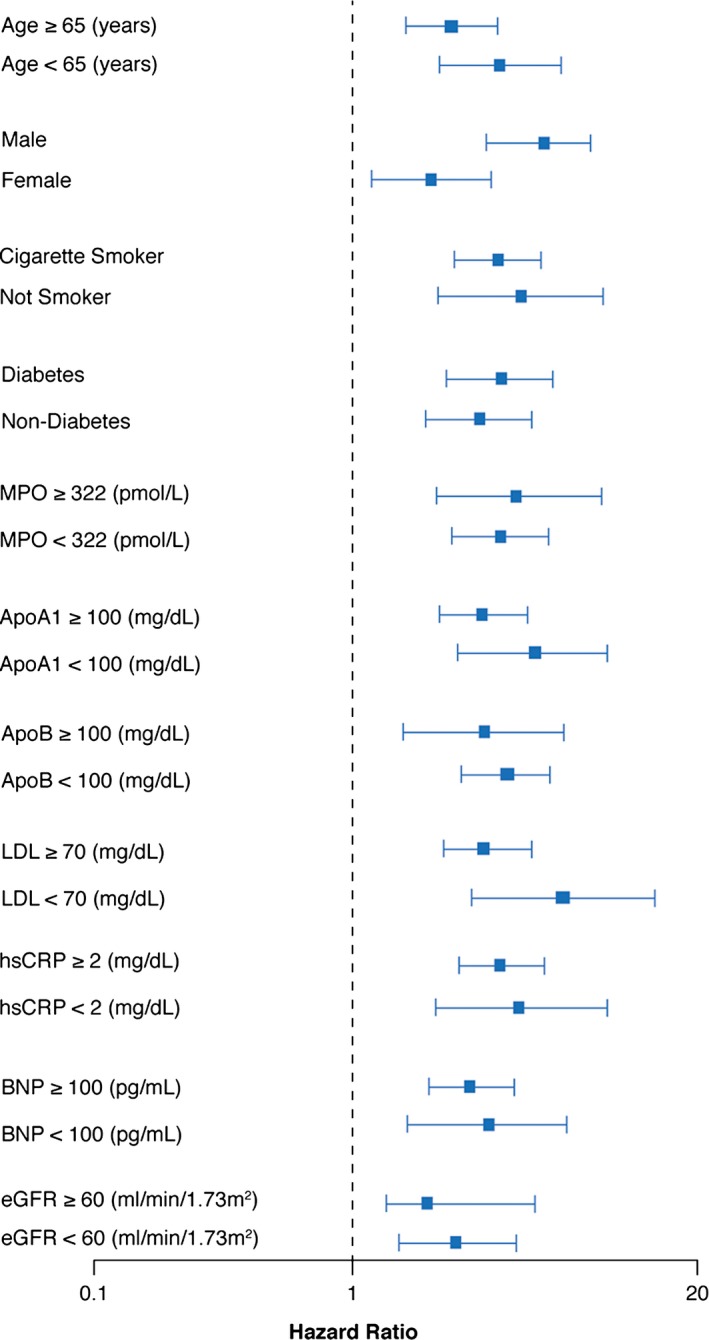
Relationship between plasma TMAO concentration and mortality risk stratified according to clinical and laboratory subgroups. Forest plot of hazard ratio (squares) of 5‐year all‐cause mortality comparing fourth and first quartiles of plasma TMAO levels. Bars represent 95% CIs. ApoA1 indicates apolipoprotein A1; ApoB, apolipoprotein B; BNP, B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; MPO, myeloperoxidase; TMAO indicates trimethylamine‐N‐oxide.
Discussion
We observed that baseline fasting plasma TMAO levels provided strong prognostic information about 5‐year all‐cause mortality in stable CAD patients with significant epicardial coronary artery stenosis who were managed with OMT (COURAGE‐like patient cohort). The strong prognostic value associated with elevated TMAO level was independent of traditional risk factors, cardioprotective medication history (angiotensin‐converting enzyme inhibitor, angiotensin‐receptor blocker, aspirin, or statin), markers of systemic and vascular inflammation (hsCRP, myeloperoxidase), and cardiorenal indices (B‐type natriuretic peptide and eGFR). The present findings confirm incremental prognostic value for TMAO in a patient population with established atherosclerotic burden, raising the possibility that interventions designed to target this pathway may provide independent risk‐reduction value.
Our recent studies demonstrated that TMAO is a proatherogenic metabolite produced by gut microbes, and increased levels of plasma TMAO are associated with CVD risks and greater numbers of diseased major coronary vessels observed by angiography.4 We also reported a strong association between elevated plasma TMAO levels and increased risk of adverse cardiovascular events in stable patients undergoing elective diagnostic cardiac catheterization, which included participants both with and without angiographic evidence of CAD.6 In the present prospective study, we identified stable CAD patients who fulfilled criteria for the COURAGE trial‐like patient cohort18 to evaluate the clinical prognostic value of TMAO levels among participants for whom OMT was selected and for whom no revascularization events occurred within 30 days of the index coronary angiographic study. In addition to COURAGE, other trials involving patients with stable CAD have supported initial medical treatment as an appropriate first approach2, 18, 22; however, all of those studies revealed significant residual cardiovascular risks, and methods to both identify those at increased risk despite optimal medical therapy and potential novel therapeutic targets are needed. In the present study, we included only participants with significant stenosis of ≥70% ≥1 epicardial coronary artery, similar to inclusion criterion used in the COURAGE trial18 and widely accepted for anatomically significant stenosis assessed by angiogram.2 Despite adjustments for known CVD risk factors, medications, and markers of both inflammation and cardiorenal indices, TMAO levels were found to provide significant additive prognostic value. Moreover, addition of TMAO to traditional risk factors and laboratory tests resulted in significant net reclassification of participants (42.4%; P<0.001). Our present study showed, for the first time, that plasma TMAO levels are a powerful prognostic marker among patients with stable CAD who opt for medical therapy (ie, in a COURAGE‐like patient cohort). This study suggests that use of TMAO among stable CAD patients should help identify those with significant residual CVD risks. Because TMAO itself is proatherogenic, one can speculate that the present study also suggests that elevated TMAO levels may help identify patients for whom a TMAO‐lowering intervention (eg, dietary, drug, probiotic) may provide benefit. In addition, the present study suggests that use of TMAO as a screening criterion for enrollment into future CVD outcome trials among stable CAD patients may help enrich the study to contain participants at increased incident mortality risk.
TMAO has been shown previously to directly promote proatherosclerotic effects and to have a direct mechanistic link to adverse cardiovascular outcomes in both animal models and human studies.4, 6, 7, 8, 9, 13, 14, 15, 16, 17, 23 TMAO is cleared by the kidney, and a higher plasma TMAO level was associated with worse long‐term survival in patients with chronic kidney disease8, 15, 16 and with heart failure.7, 10, 17, 24 Interestingly, the association between TMAO and long‐term mortality in the present study cohort remained robust after adjustment for eGFR or cardiorenal indices, although the kidneys may be physiologically impaired in eliminating TMAO. The precise mechanisms through which TMAO is linked to enhanced cardiovascular disease risks are unclear; however, prior mechanistic studies provide some potential insights. Provision of TMAO, for example, in the diet or enhanced production via dietary intake of nutritional precursors including choline and carnitine has been shown to both enhance aortic root atherosclerotic plaque development and to inhibit reverse cholesterol transport.4, 5 TMAO also significantly affected macrophage phenotype, bile acid synthesis, pool size and composition, and multiple bile and sterol transporters in both the liver and the intestines.4, 5 The metaorganismal pathway responsible for TMAO production has similarly been linked mechanistically to multiple cardiovascular and metabolic processes associated with CVD pathogenesis. Several recent studies suggest, for example, that flavin‐containing monooxygenase 3, the host enzyme primarily responsible for converting intestinal microbiota‐generated trimethylamine into TMAO, is an important regulator of body cholesterol and sterol metabolism as well as additional lipid and metabolic pathways.25, 26, 27, 28 In addition, knockdown of hepatic flavin‐containing monooxygenase 3 in low‐density lipoprotein receptor knockout mice using an antisense oligonucleotide resulted in decreased circulating TMAO levels and atherosclerosis, providing further mechanistic support of the importance of the TMAO pathway to vascular disease pathogenesis in animal models.25 TMAO may also exacerbate impaired glucose tolerance and promote adipose tissue inflammation, which are related to the complexity and degree of atherosclerosis burden of CAD.25, 29 Recently, exciting new animal data also suggested that targeting gut microbial TMAO production can inhibit dietary‐induced atherosclerosis.30 Furthermore, a role for gut microbiota and TMAO in modulating platelet hyperresponsiveness and thrombosis potential was discovered recently.31
Many factors may influence circulating TMAO levels, and there have also been reports with discrepant findings. Mueller and colleagues, for example, observed in a cohort of 339 patients undergoing angiographic evaluation that TMAO levels were confounded by impaired kidney function and poor metabolic control and were not associated with history, presence, or incidence of symptoms or events of CAD.32 Interestingly, levels reported in the study by Mueller et al were markedly lower than those described in our current study (median TMAO 1.74 μmol/L; third‐quartile TMAO 3.48 μmol/L). Furthermore, some of the most accepted cardiovascular risk biomarkers such as low‐density lipoprotein cholesterol, triglyceride, and glucose and hsCRP can be highly variable, more so even than TMAO. Further study is needed to determine whether interventions that reduced TMAO levels also improved prognosis among patients with high atherosclerotic burden.
Study Limitations
This single tertiary care center study recruited patients at the time of cardiac evaluation for coronary angiography; therefore, we cannot exclude the presence of selection bias for patients undergoing evaluation and treatment for CAD. Because plasma TMAO levels were measured at only 1 point in time, we were unable to evaluate the prognostic value of changing plasma TMAO concentration over time. Similarly, only fasting plasma levels were examined, and it remains unknown if nonfasting TMAO levels provide similar prognostic value.
Conclusions
Among participants with angiographic evidence of significant (≥70% stenosis) CAD and for whom medical management was chosen, elevated plasma TMAO levels portended higher long‐term mortality risk independent of traditional risk factors, inflammation markers, B‐type natriuretic peptide levels, and renal function.
Sources of Funding
This research was supported by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000). The GeneBank study has been supported by NIH grants P01HL076491, R01HL103931, and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439). Dr Wang was partially supported by an American Heart Association Scientist Development Grant. Dr Hazen is also partially supported by a gift from the Leonard Krieger Endowment. Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Innovations Award by AB SCIEX.
Disclosures
Dr Wang and Dr Hazen are named as coinventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr Wang and Dr Hazen report that they have the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics from Cleveland Heart Lab. Dr Hazen also reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Esperion, Frantz Biomarkers, LLC and Siemens. Dr Hazen reports having been paid as a consultant for the following companies: Esperion and P&G. Dr Hazen reports receiving research funds from Astra Zeneca, P&G, Pfizer Inc., Roche Diagnostics, and Takeda. All other authors have no relationships to disclose.
(J Am Heart Assoc. 2016;5:e002816 doi: 10.1161/JAHA.115.002816)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130:1749–1767. [DOI] [PubMed] [Google Scholar]
- 3. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. Deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 4. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe‐generated metabolite trimethylamine‐N‐oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa‐Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota‐dependent trimethylamine N‐oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota‐generated metabolite trimethylamine‐N‐oxide. Eur Heart J. 2014;35:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL. Intestinal microbiota‐dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O'Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine‐N‐oxide production in humans: a randomized, controlled, dose‐response study. Am J Clin Nutr. 2014;100:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsson SC, Orsini N. Red meat and processed meat consumption and all‐cause mortality: a meta‐analysis. Am J Epidemiol. 2014;179:282–289. [DOI] [PubMed] [Google Scholar]
- 13. Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SL, Troughton RW, Frampton CM, Richards AM, Chambers ST. Betaine and trimethylamine‐N‐oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One. 2014;9:e114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ. The relationship between trimethylamine‐N‐oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015;31:1189–1194. [DOI] [PubMed] [Google Scholar]
- 15. Missailidis C, Hallqvist J, Qureshi AR, Barany P, Heimburger O, Lindholm B, Stenvinkel P, Bergman P. Serum trimethylamine‐N‐oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11:e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS. Serum trimethylamine‐N‐oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjorndal B, Halvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A. Microbiota‐dependent metabolite trimethylamine‐N‐oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. [DOI] [PubMed] [Google Scholar]
- 18. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine‐N‐oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 22. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skagen K, Troseid M, Ueland T, Holm S, Abbas A, Gregersen I, Kummen M, Bjerkeli V, Reier‐Nilsen F, Russell D, Svardal A, Karlsen TH, Aukrust P, Berge RK, Hov JE, Halvorsen B, Skjelland M. The carnitine‐butyrobetaine‐trimethylamine‐N‐oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis. 2016;247:64–69. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N‐oxide and prognosis in acute heart failure. Heart. 2016; doi: 10.1136/heartjnl‐2015‐308826. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25. Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB, Vicent D, Biddinger SB. Flavin‐containing monooxygenase 3 as a potential player in diabetes‐associated atherosclerosis. Nat Commun. 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The TMAO‐generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N‐oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103:703–711. [DOI] [PubMed] [Google Scholar]
- 29. Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N‐oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–481. [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian‐Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non‐lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A. Plasma levels of trimethylamine‐N‐oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–644. [DOI] [PubMed] [Google Scholar]


