Abstract
Background
High‐sensitivity troponin (hs‐TNT), a biomarker of myocardial damage, might be useful for assessing fibrosis in Fabry cardiomyopathy. We performed a prospective analysis of hs‐TNT as a biomarker for myocardial changes in Fabry patients and a retrospective longitudinal follow‐up study to assess longitudinal hs‐TNT changes relative to fibrosis and cardiomyopathy progression.
Methods and Results
For the prospective analysis, hs‐TNT from 75 consecutive patients with genetically confirmed Fabry disease was analyzed relative to typical Fabry‐associated echocardiographic findings and total myocardial fibrosis as measured by late gadolinium enhancement (LE) on magnetic resonance imaging. Longitudinal data (3.9±2.0 years), including hs‐TNT, LE, and echocardiographic findings from 58 Fabry patients, were retrospectively collected. Hs‐TNT level positively correlated with LE (linear correlation coefficient, 0.72; odds ratio, 32.81 [95% CI, 3.56–302.59]; P=0.002); patients with elevated baseline hs‐TNT (>14 ng/L) showed significantly increased LE (median: baseline, 1.9 [1.1–3.3] %; follow‐up, 3.2 [2.3–4.9] %; P<0.001) and slightly elevated hs‐TNT (baseline, 44.7 [30.1–65.3] ng/L; follow‐up, 49.1 [27.6–69.5] ng/L; P=0.116) during follow‐up. Left ventricular wall thickness and EF of patients with elevated hs‐TNT were decreased during follow‐up, indicating potential cardiomyopathy progression.
Conclusions
hs‐TNT is an accurate, easily accessible clinical blood biomarker for detecting replacement fibrosis in patients with Fabry disease and a qualified predictor of cardiomyopathy progression. Thus, hs‐TNT could be helpful for staging and follow‐up of Fabry patients.
Keywords: biomarker, cardiomyopathy, fabry disease, myocardial fibrosis, troponin T
Subject Categories: Cardiomyopathy, Echocardiography, Magnetic Resonance Imaging (MRI)
Introduction
Fabry disease (FD) is a rare X‐linked lysosomal storage disorder caused by a mutation in the gene encoding hydrolase alpha‐galactosidase A, leading to a deficiency of this enzyme. The consequence is a progressive intracellular accumulation of globotriaosylceramide (Gb3) in lysosomes of various tissues and organs. The most commonly affected organs are heart, vascular endothelium of the kidney, nervous system, eyes, and skin.1, 2, 3 Typical clinical manifestations of FD include angiokeratoma, hypohydrosis, acroparesthesia, pain crises, and gastrointestinal symptoms such as diarrhea. Progressive Gb3 accumulation in the microvasculature causes ischemic damages, especially affecting the kidney, brain, and heart, which may cause symptoms related to renal failure, stroke, and cardiovascular diseases.4 Cardiac manifestations, including arrhythmias, chronic heart failure, and small vessel disease, occur frequently. Storage of Gb3 in myocytes causes a regional concentric left ventricular (LV) hypertrophy (LVH) and results in intramural replacement fibrosis, first limited to the mid‐myocardial layers of the posterolateral wall and then spreading to transmural fibrosis.5, 6, 7, 8 This myocardial fibrosis is a typical finding of more‐advanced stages of Fabry cardiomyopathy5, 6 and seems to be a major factor in cardiac arrhythmias and heart failure, which increase morbidity and reduce life expectancy.5, 7, 9, 10, 11 The most reliable noninvasive method of detecting myocardial fibrosis is by late gadolinium‐enhanced (LE) magnetic resonance imaging (MRI).6, 12
Biomarkers such as cardiac troponin T (TNT) and brain natriuretic peptide (BNP) are established in the diagnosis of myocardial infarction (MI) and chronic heart failure,13, 14, 15, 16 and high‐sensitivity (hs) TNT has been used to detect myocardial tissue damage. To date, however, no detailed study has assessed the use of biomarkers to detect myocyte turnover toward myocardial fibrosis in patients with FD. We hypothesized that level of hs‐TNT correlates with myocardial fibrosis and investigated biomarkers, such as hs‐TNT and N‐terminal proBNP (NT‐proBNP), subsequently correlating the findings with myocardial fibrosis as measured by LE MRI.
Methods
Study Protocol
The present study comprises 2 analyses: a prospective analysis to evaluate the relevance of biomarkers such as hs‐TNT and NT‐proBNP for detecting myocardial changes in Fabry patients and a retrospective longitudinal follow‐up study to evaluate longitudinal changes in hs‐TNT in relation to fibrosis, investigate the impact of hs‐TNT on progression of cardiomyopathy, and evaluate the use of frozen blood for measuring hs‐TNT.
For the prospective part of the study, all patients with genetically confirmed FD who were observed between February 2013 and June 2014 at the Fabry center in Wuerzburg were screened. Criteria for inclusion were (1) genetically proven FD, (2) availability of blood samples for measurement of hs‐TNT and NT‐proBNP, (3) no contraindications to contrast agent‐enhanced MRI, and (4) informed consent for examination and participation in the study. Exclusion criteria were contraindications to LE‐ MRI, such as claustrophobia, an implanted cardiac pacemaker, or end‐stage renal dysfunction requiring chronic dialysis. Overall, 75 patients were included for the prospective analyses. In the context of a routine appointment as part of a clinical investigation program for Fabry patients, blood was taken and analyzed for hs‐TNT and NT‐proBNP. To evaluate the stage of Fabry cardiomyopathy, MRI with LE and echocardiography were performed within 24 hours. Typical Fabry‐related parameters, symptoms, and comorbidities were determined by patient interviews and clinical record. A clinical examination was done by a doctor specialized in FD. In addition, 24‐hour urine samples were collected.
The retrospective cohort consisted of 58 Fabry patients for whom frozen blood samples were available for assessment of hs‐TNT, as were patient records of MRI and echocardiography at baseline and after 3.9±2.0 years.
For the analyses, we separated the cohorts into 4 groups according to stage of cardiomyopathy, depending on myocardial fibrosis as measured by LE imaging and LVH and myocardial function as assessed by echocardiography, as follows: group 1, no cardiomyopathy, meaning no LE findings, no LVH (interventricular septal thickness at diastole [IVSd] <12 mm), and normal ejection fraction (EF; ≥55%); group 2, mild cardiomyopathy, consisting of LV hypertrophy (IVSd ≥12 mm) but no LE and normal EF (≥55%); group 3, intermediate cardiomyopathy, consisting of patients who were LE positive but had normal EF (≥55%); and group 4, end‐stage cardiomyopathy, with reduced EF (<55%) and with fibrosis (LE positive).
Written informed consent was obtained from all patients or their guardians. The study was approved by the Local Ethics Committee at the University of Würzburg and conducted in accord to the Declaration of Helsinki.
Assessment of hs‐TNT and NT‐proBNP
All serum concentrations of hs‐TNT and NT‐proBNP were measured in the laboratory of the Wuerzburg University Hospital using the Novel Electrochemiluminescence (ECL) technology on Roche Cobas e411 (Roche Diagnostics GmbH, Mannheim, Germany). Peripheral venous blood was taken from our cohort collected in serum tubes. In the prospective part of this study, fresh blood samples were immediately centrifuged at 5000×U/min for 10 minutes; afterward, serum was analyzed within 1 hour. For the retrospective part, frozen serum was used to measure the level of the biomarkers. In this cohort, the blood samples were taken, at their first visit to our Fabry center (baseline) and at a follow‐up visit at the latest time point possible. Serum tubes were also centrifuged at 5000×U/min for 10 minutes, divided into aliquots, and stored at −80°C until analysis. After defrosting at room temperature to around 20°C degrees, samples were again centrifuged at 5000×U/min for 10 minutes and analyzed. The reference ranges for hs‐TNT were as follows: <5 ng/L, below the detection limit; 5 to 13.9 ng/L, detectable but normal; and ≥14 ng/L, elevated.17, 18
N‐terminal of the prohormone brain natriuretic peptide (NT‐proBNP) was applied as a “rule‐out” test for heart failure. Cut‐off values were based on the recommendations by previous studies in acute and chronic health care settings (acute heart failure, 300 pg/mL; chronic heart failure: <60 years, 50 pg/mL; 60–75 years, 100 pg/mL; >75 years, 250 pg/mL).19, 20
Standard Echocardiographic Measurements
All echocardiographic examinations were performed using standard ultrasound equipment (3.5 MHz; GE VingMed Vivid 7; GE VingMed, Horten, Norway). LV end‐diastolic diameter (LVEDD), LV end‐systolic diameter (LVESD), and end‐diastolic wall thickness of the LV posterior wall (LVPWd) and the septum (IVSd) were manually measured from parasternal long‐axis images using standard M‐mode. Furthermore, left atrial diameter was measured, and the LV fraction shortening was calculated from LVEDD and LVESD. LV ejection fraction (LVEF) was measured with the biplane Simpson method in apical 4‐ and 2‐chamber views. By placing a blood pool–pulsed Doppler between the tips of the mitral valve leaflets, the peak in early (E) and late (A) diastolic flow velocity was measured, to yield the E/A ratio, and the deceleration time was extracted. Another parameter to evaluate diastolic function was the ratio of mitral peak velocity (E/E′) calculated by measuring the ratio between early transmitral flow and peak early tissue Doppler velocity. LV mass, indexed to body surface area (LVMI), was estimated by LV cavity dimension and wall thickness at end diastole: LV mass (g)=0.8×[1.04×(LVEDD+LVPWd+IVSd)3−LVEDD3]+0.6. Systolic pulmonary artery pressure (SPAP) was derived from peak tricuspid regurgitation (TR) jet velocity, using the simplified Bernoulli equation in combination with an estimated right atrial pressure (RAP): SPAP=4V2+RAP, where V is the peak TR jet velocity. RAP was estimated from inferior vena cava diameter and respiratory changes.
Assessment of Fibrosis
Cardiac MRI was performed in all patients using a 1.5 Tesla scanner (Magnetom Symphony Quantum; Siemens Medical Systems, Erlangen, Germany), after intravenous injection of gadopentetate dimeglumine 0.2 mmol/kg (Magnevist; Bayer HealthCare AG, Leverkusen, Germany). LE images were obtained with T1 inversion recovery sequences (8‐mm slice thickness, breath hold, field of view 240×320 mm2, matrix size 165×256, repetition time 7.5 ms, echo time 3.4 ms, and flip angle 258). Areas with pathological mid‐ or transmural LE were measured manually in the basal, mid, and apical segments, covering the entire ventricle using continuous short‐axis views. The sum of all enhanced areas was multiplied by the slice thickness and then set in relation to the LV myocardial volume. With this technique, every LV segment was scanned separately for occurrence of pathological LE. Attention was paid to a similar setting for the follow‐up scan.
Statistical Analysis
Continuous data are presented as mean±SD or median (25th, 75th percentiles) and categorical variables as numbers (percentages). Differences in continuous data among 3 groups were compared using 1‐way ANOVA after normalization, if indicated, followed by appropriate post‐hoc tests for multiple comparisons (Tukey's if equal variances assumed; Games‐Howell if equal variances not assumed). Pair‐wise comparisons were only conducted if the global test was significant. Statistical significance was assumed at Bonferroni‐adjusted P<0.025 (3 groups). Differences between baseline and follow up among 3 groups were compared using repeated‐measures ANOVA followed by the appropriate post‐hoc test for multiple comparisons. Non‐normally distributed variables (hs‐TNT and NT‐proBNP) were normalized before analysis using natural logarithm values (Ln). Correlation between hs‐TNT categories and other clinical and echocardiographic parameters were studied with the Spearman rank correlation (r s). The difference between groups was compared using independent‐samples t test. Categorical data were compared across groups using the chi‐square test. Logistic regression was used to assess significant determinants of elevated hs‐TNT. A P<0.05 (2 groups) was considered statistically significant, and all statistical analysis was performed using IBM SPSS software (version 22 for Windows; SPSS, Inc., Chicago, IL).
Results
Prospective Cohort
Our prospective cohort of 75 patients with a mean age of 43±14 years included 47 women and 28 men. Average body mass index (BMI) was normal (mean 25±5 kg/m2). No patient had a history of MI, only 1 patient had undergone a coronary bypass operation, and 3 patients had coronary artery disease (CAD). Three patients had chronic heart failure corresponding with New York Heart Association (NYHA) class III or IV. Mean glomerular filtration rate (GFR) was in normal range (83±23 mL/min), 29.3% (n=22) of patients had proteinuria, 84.2% had normal renal function, 7.9% (n=6) had a moderately reduced kidney function (stage III of chronic kidney disease [CKD], GFR between 30 and 59 mL/min), and 4.0% (n=3) had severely reduced kidney function (stage IV of CKD, GFR 15–29 mL/min). Patients with end‐stage renal disease (ESRD; 1.33%; n=1) were excluded.
The median hs‐TNT of the cohort was 7.7 ng/L, with a wide range of 4 to 278 ng/L. Of the 75 patients, 30 (40%) had an elevated hs‐TNT (>14 ng/L). The general clinical data for all patients adjusted to the level of hs‐TNT (<5, 5–14, and >14 ng/L) are presented in Table 1. Of note are some correlations between level of hs‐TNT and other typical Fabry symptoms.
Table 1.
Clinical Characteristics Among Subgroups According to hs‐TNT Level in the Prospective Cohort (n=75)
| Total | hs‐TNT <5 ng/L | hs‐TNT 5 to 14 ng/L | hs‐TNT >14 ng/L | |
|---|---|---|---|---|
| n=75 | n=29 | n=16 | n=30 | |
| Age, y | 43±14 | 45±13 | 41±12 | 43±15 |
| Female, n (%) | 47 (62.7) | 19 (65.5) | 12 (75) | 16 (53.3) |
| BMI, kg/m² | 25±5 | 24±4 | 25±4 | 25±6 |
| hs‐TNT*, ng/L | 7.7 (4–20.8) | 4 | 7.7 (6.7–8.4)† | 24.2 (19.1–44.1)†, ‡ |
| NT‐proBNP*, pg/mL | 99 (41–224) | 49.5 (28.5–77.5) | 80.5 (33.5–153.5) | 221 (106–910)†, ‡ |
| Cardiovascular, n (%) | ||||
| NYHA class III/IV | 3 (4.0) | 1 (3.4) | 0 (0) | 2 (6.7) |
| CAD | 3 (4.0) | 0 | 0 | 3 (10.0) |
| MI | 0 | 0 | 0 | 0 |
| Bypass | 1 (1.3) | 0 | 0 | 1 (3.3) |
| ICD/pacemaker | 7 (6.7) | 0 | 0 | 5 (16.7)†, ‡ |
| Renal | ||||
| eGFR, mL/min | 83±23 | 97±22 | 80±18† | 70±19† |
| Creatinine, mg/dL | 1.0±0.3 | 0.8±0.2 | 0.9±0.2 | 1.1±0.3† |
| Proteinuria, n (%) | 22 (29.3) | 6 (20.7) | 2 (12.5) | 14 (46.7)†, ‡ |
| Renal insufficiency: 0/I/II/III/IV, % | 64/0/2/6/3 (84.2/0/2.6/7.9/4.0) | 28/0/1/0/0 (96.6/0/3.4/0/0) | 14/0/0/2/0 (87.5/0/0/12.5/0) | 22/0/1/4/3 (71/0/3.2/12.9/10.0) |
| Renal transplantation, n (%) | 3 (4.0) | 0 | 0 | 3 (10.0) |
| Neurological, n (%) | ||||
| Vertigo | 37 (49.3) | 11 (37.9) | 10 (62.5) | 16 (53.3) |
| Tinnitus | 24 (32.0) | 5 (17.2) | 4 (25.0) | 15 (50)† |
| Hearing loss | 11 (14.7) | 1 (3.4) | 3 (18.8) | 7 (23.3) |
| Depression | 7 (9.3) | 2 (6.9) | 2 (12.5) | 3 (10.0) |
| Dysarthria | 6 (8.0) | 2 (6.9) | 1 (6.3) | 3 (10.0) |
| TIA | 6 (8.0) | 3 (10.3) | 1 (6.3) | 2 (6.7) |
| Stroke | 7 (9.3) | 1 (3.4) | 1 (6.3) | 5 (16.7) |
| Gastrointestinal, n (%) | ||||
| Nausea | 7 (9.3) | 3 (10.3) | 1 (6.3) | 3 (10.0) |
| Diarrhea | 24 (32.0) | 5 (17.2) | 5 (31.3) | 14 (46.7) |
| Abdominal pain | 21 (28.0) | 5 (17.2) | 5 (31.3) | 11 (36.7) |
| Acroparesthesia, n (%) | 43 (57.3) | 16 (55.2) | 8 (50) | 19 (63.3) |
| Chronic pain, n (%) | 20 (26.7) | 8 (27.6) | 2 (12.5) | 10 (33.3) |
| Pain crisis, n (%) | 24 (32.0) | 10 (34.5) | 3 (18.8) | 11 (36.7) |
| Medication, n (%) | ||||
| ACE/AT1 | 46 (61.3) | 8 (27.6) | 12 (75)† | 26 (86.7)† |
| ß‐blockers | 10 (13.3) | 3 (10.3) | 2 (12.5) | 5 (16.7) |
| Calcium antagonists | 3 (4.0) | 1 (3.4) | 0 (0) | 2 (6.7) |
| Anticoagulant | 4 (5.3) | 0 | 0 | 4 (13.3) |
| Regular pain medication | 13 (17.3) | 6 (20.7) | 1 (6.3) | 6 (20.0) |
ACE indicates angiotensin‐converting enzyme inhibitor; AT1, angiotensin II type 1 antagonists; BMI, body mass index; CAD, coronary arterial disease; eGFR, estimated glomerular filtration rate; hs‐TNT, high‐sensitivity cardiac troponin T; ICD, implantable cardioverter‐defibrillator; MI, myocardial infarction; NT‐proBNP, N‐terminal of the prohormone brain natriuretic peptide; NYHA, New York Heart Association; TIA, transient ischemic attack.
*Natural logarithm (Ln) transformed.
Bonferroni‐adjusted † P<0.025 vs hs‐TNT <5 ng/L; ‡ P<0.025 vs hs‐TNT 5 to 14 ng/L.
Separation of the cohort into the stages of Fabry cardiomyopathy according to the above‐mentioned criteria resulted in 38 patients with no cardiomyopathy, 3 with mild cardiomyopathy, 31 with intermediate cardiomyopathy, and 3 with end‐stage cardiomyopathy (Figure 1). Patients with no or mild cardiomyopathy had low levels of hs‐TNT, whereas those with intermediate and end‐stage cardiomyopathy had elevated levels (Figure 1).
Figure 1.
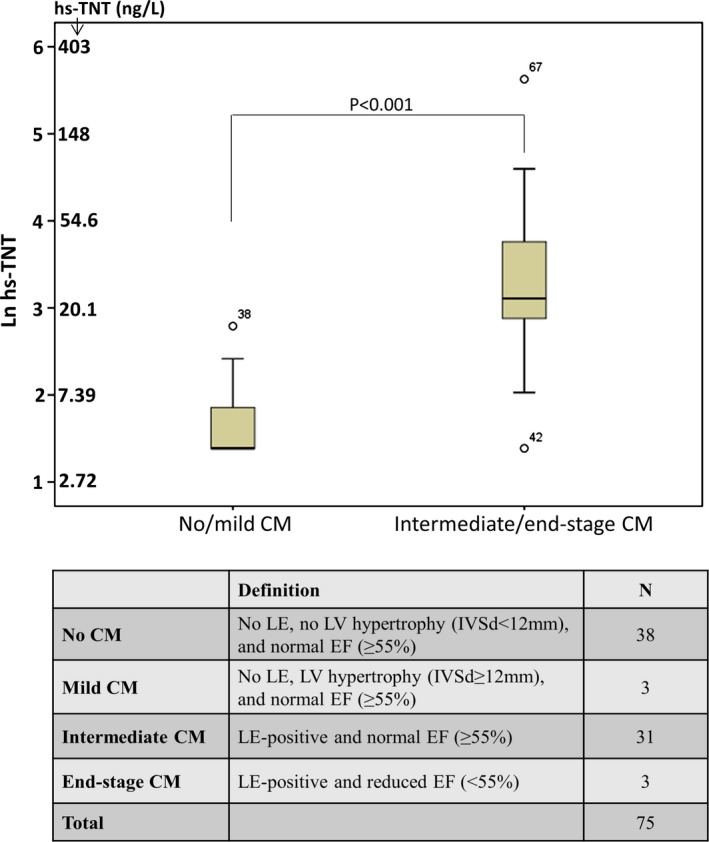
High‐sensitivity cardiac troponin T (hs‐TNT) levels in Fabry patients with different stages of cardiomyopathy (CM). Box plots represent median levels with 25th and 75th percentiles of observed data whereas whiskers indicate the 5th and 95th percentiles in each group. The y‐axis ranges from 1 to 6 representing natural logarithm values of hs‐TNT (Ln hs‐TNT). EF indicates ejection fraction; IVSd, end‐diastolic interventricular septal wall thicknes; LE, late enhancement; LV, left ventricle.
Table 2 shows echocardiographic and MRI characteristics of the prospective cohort. LV wall thickness, LVMI on echocardiography, and MRI, E/E′, and LE‐positive myocardial volume gradually increased with increasing hs‐TNT. All but 1 patient (97%) with an elevated hs‐TNT >14 ng/L showed fibrosis as assessed by LE on MRI.
Table 2.
Echocardiography and Cardiac Magnetic Resonance Imaging in the Prospective Cohort (n=75)
| Total | hs‐TNT <5 ng/L | hs‐TNT 5 to 14 ng/L | hs‐TNT >14 ng/L | |
|---|---|---|---|---|
| n=75 | n=29 | n=16 | n=30 | |
| Echocardiography | ||||
| LV end‐diastolic dimension, mm | 47±6 | 47±5 | 45±6 | 48±7 |
| IVSd, mm | 10.2±2.5 | 8.2±1.4 | 9.9±1.7* | 12.2±2.1*, † |
| LVPWd, mm | 10.0±2.3 | 8.3±1.3 | 9.8±1.5* | 11.7±2.1*, † |
| LV fractional shortening, mm | 36±7 | 35±8 | 39±6 | 35±6 |
| End‐diastolic LV volume, mL | 107±32 | 110±22 | 93±28 | 111±39 |
| End‐systolic LV volume, mL | 38±18 | 40±15 | 30±12 | 41±21 |
| Stroke volume, mL | 69±21 | 69±17 | 64±21 | 71±26 |
| LV mass index, g/m² | 96±43 | 91±38 | 96±36 | 102±51 |
| Left atrial diameter, mm | 35±5 | 33±4 | 35±3 | 38±5*, † |
| LVEF, % | 65±6 | 64±6 | 65±6 | 66±6 |
| E/A | 1.5±0.6 | 1.5±0.5 | 1.4±0.5 | 1.6±0.7 |
| E/E′ | 10.4±4.6 | 7.0±1.8 | 9.7±2.6* | 13.9±4.8*, † |
| DT, ms | 208±49 | 201±56 | 207±37 | 216±49 |
| SPAP, mm Hg | 25±6 | 24±5 | 25±5 | 27±7 |
| Magnetic resonance imaging | ||||
| LE positive, n (%) | 34 (45.3) | 1 (3.4) | 4 (25.0) | 29 (96.7) |
| LE‐positive myocardial volume, % | 0 (0–8.5) | 0 (0–0.71) | 0 (0–1.2)* | 1.4 (0–8.5)*, † |
| LVMI, g/m² | 88±36 | 69±19 | 79±24 | 111±41*, † |
| End‐diastolic LV volume, mL | 142±45 | 140±37 | 130±31 | 150±57 |
| End‐systolic LV volume, mL | 51±24 | 55±21 | 39±13 | 55±29 |
| Stroke volume, mL | 92±25 | 91±23 | 92±22 | 93±28 |
| LVEF, % | 66±10 | 64±8 | 70±6 | 65±13 |
DT indicates deceleration time of early filling; E/A, early diastolic filling velocity (E) to late diastolic filling velocity (A) ratio; E/E′, mitral inflow velocity (E) to tissue Doppler E′ ratio; hs‐TNT, high‐sensitivity cardiac troponin T; IVSd, end‐diastolic interventricular septal wall thickness; LE, late enhancement; LV, left ventricle; LVEF, LV ejection fraction; LVMI, LV mass indexed to body surface area; LVPWd, end‐diastolic posterior wall thickness; SPAP, systolic pulmonary artery pressure.
*P<0.05 vs hs‐TNT <5 ng/L.
† P<0.05 vs hs‐TNT 5 to 14 ng/L.
Correlation analysis showed that hs‐TNT categories positively correlated with NYHA functional class, NT‐proBNP, creatinine, LE‐positive myocardial volume, LV wall thickness, LVMI, LA diameter, and E/E′ and negatively correlated with estimated GFR (eGFR; Table 3). Multivariable logistic regression analysis suggested that only LE‐positive myocardial volume was independently associated with hs‐TNT elevation (Table 4; odds ratio [OR], 32.81; 95% CI, 3.56–302.59; P=0.002). Comparison of hs‐TNT with LVH (septal wall thickness, R 2 linear=0.58; r=0.76; P<0.001; Figure 2A) and amount of LV fibrosis (LE‐positive myocardial volume, R 2 linear=0.66; r=0.72; P<0.001; Figure 2B) showed a strong positive correlation. A moderate positive correlation also was noted between NT‐proBNP level and amount of LV fibrosis (R 2 linear=0.22; r=0.66; P<0.001; Figure 2C).
Table 3.
Spearman's Rank Correlation Between hs‐TNT Categories and Clinical and Echocardiographic Variables in the Prospective Cohort (n=75)
| Spearman's Rank Correlation Coefficient (r s) | P Value | |
|---|---|---|
| Age, y | −0.07 | 0.57 |
| Sex | −0.11 (χ²=2.26) | 0.33 |
| BMI, kg/m² | 0.07 | 0.56 |
| NYHA class | 0.38 (χ²=12.97) | 0.001 |
| NT‐proBNP*, mg/dL | 0.55 | <0.001 |
| eGFR, mL/min | −0.51 | <0.001 |
| Creatinine, mg/dL | 0.36 | 0.001 |
| LE‐positive myocardial volume, % | 0.82 | <0.001 |
| MRI‐LVMI, g/m² | 0.55 | <0.001 |
| Echo‐LVMI, g/m² | 0.09 | 0.31 |
| IVSd, mm | 0.74 | <0.001 |
| LVPWd, mm | 0.70 | <0.001 |
| LV end‐diastolic dimension, mm | −0.05 | 0.77 |
| EF, % | 0.17 | 0.15 |
| Left atrial diameter, mm | 0.44 | <0.001 |
| E/e′ | 0.69 | <0.001 |
| DT | 0.19 | 0.10 |
| SPAP | 0.20 | 0.10 |
BMI indicates body mass index; DT, deceleration time of early filling; E/E′, mitral inflow velocity (E) to tissue Doppler E′ ratio; EF, ejection fraction; eGFR, estimated glomerular filtration rate; hs‐TNT, high‐sensitivity cardiac troponin T; IVSd, end‐diastolic interventricular septal wall thickness; LE, late enhancement; LV, left ventricle; LVMI, LV mass indexed to body surface area; LVPWd, end‐diastolic posterior wall thickness; NT‐proBNP, N‐terminal of the prohormone brain natriuretic peptide; NYHA, New York Heart Association; SPAP, systolic pulmonary artery pressure.
*Ln‐transformed.
Table 4.
Determinants of Elevated hs‐TNT (>14 ng/L) Assessed by Logistic Regression Analysis in the Prospective Cohort (n=75)
| Adjusted* | Multivariate* | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| eGFR, mL/min | 0.95 (0.92–0.98) | 0.001 | 1.00 (0.94–1.06) | 0.946 |
| NT‐proBNP†, pg/mL | 2.96 (1.71–5.21) | <0.001 | 1.79 (0.59–5.40) | 0.304 |
| LE‐positive myocardial volume, % | 97.2 (12.9–734.1) | <0.001 | 32.81 (3.56–302.59) | 0.002 |
| IVSd, mm | 2.62 (1.71–4.00) | <0.001 | 2.49 (0.96–6.47) | 0.061 |
| Left atrial diameter, mm | 1.27 (1.10–1.47) | 0.001 | 1.15 (0.82–1.61) | 0.431 |
eGFR indicates estimated glomerular filtration rate; hs‐TNT, high‐sensitivity cardiac troponin T; IVSd, end‐diastolic interventricular septal wall thickness; LE, late enhancement; NT‐proBNP, N‐terminal of the prohormone brain natriuretic peptide; OR, odds ratio.
*Adjusted for age, sex, and body mass index.
†Ln‐transformed.
Figure 2.
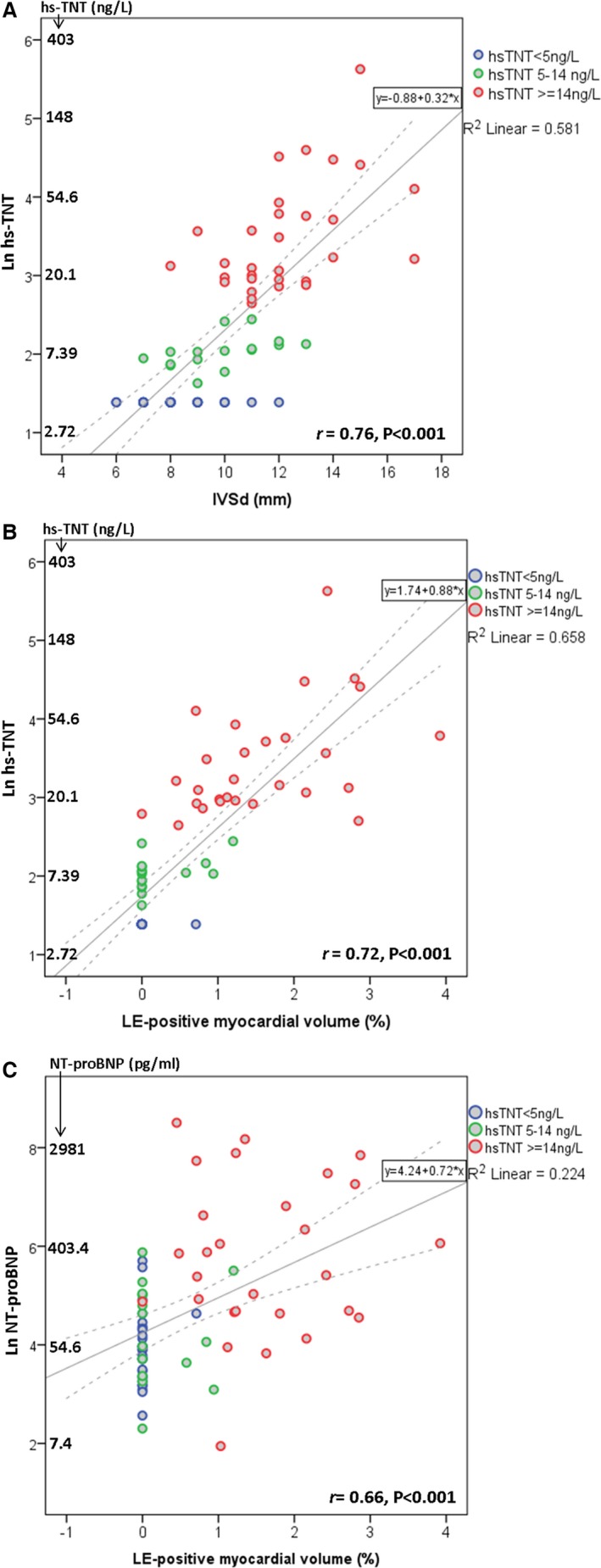
Scatterplots demonstrating the correlation of (A) high‐sensitivity cardiac troponin T (hs‐TNT) with septal wall thickness (IVSd, mm); (B) hs‐TNT with late enhancement (LE) positive myocardial volume (%); and (C) NT‐proBNP with LE‐positive myocardial volume (%). Natural logarithm (Ln) values of hs‐TNT (A and B) and NT‐proBNP (C) are used as the Y‐axis scale. NT‐proBNP indicates N‐terminal of the prohormone brain natriuretic peptide.
A closer look at patients with renal insufficiency showed that CKD stage III to IV was more frequently present in patients with hs‐TNT >14 ng/L compared to those with hs‐TNT <5 and 5 to 14 ng/L. Advanced kidney dysfunction was significantly associated with an elevated hs‐TNT level (median: CKD stage III–IV, 32.7 [18.6–44.1] ng/L vs stage 0–II, 6.7 [4–19.2] ng/L; P=0.003). There was a strong correlation between hs‐TNT and LE‐positive myocardial volume both in patients with CKD stage I to II (n=66; r=0.805; P<0.001) and in patients with stage III to IV (n=9; r=0.797; P=0.010).
Longitudinal, Retrospective Data
For follow‐up, 58 patients were screened for hs‐TNT, NT‐proBNP, and typical features of Fabry cardiomyopathy on MRI and echocardiography at baseline and at the latest possible time. As shown in Table 5, over a follow‐up period of 3.9±2.0 years, patients with an elevated hs‐TNT (>14 ng/L) at baseline had significantly increasing replacement fibrosis (median: baseline, 1.9 [1.1–3.3] %; follow‐up, 3.2 [2.3–4.9] %; P<0.001) and slightly increasing hs‐TNT (baseline, 44.7 [30.1–65.3] ng/L; follow‐up, 49.1 [27.6–69.5] ng/L; P=0.116). In addition, LV wall thickness of patients with elevated hs‐TNT was significantly decreased (baseline, 14.5±3.1 mm; follow‐up, 13.7±2.3 mm; P=0.043) and EF tended to be reduced (baseline, 66±6%; follow‐up, 63±8%; P>0.05) during follow‐up. LV wall thickness thinning might be associated with intramural replacement fibrosis and progression of heart failure and usually occurs in the late stage of disease.21 Global systolic function (=EF) in patients with elevated hs‐TNT was decreased during follow‐up, indicating potential cardiomyopathy progression as well.
Table 5.
Changes in hs‐TNT Level, LE‐Positive Myocardium, Cardiac Morphology, and Function During Follow‐up (n=58)
| hs‐TNT <5 ng/L | hs‐TNT 5 to 14 ng/L | hs‐TNT >14 ng/L | |
|---|---|---|---|
| n=26 | n=19 | n=13 | |
| Duration of follow‐up, y | 4.6±1.9 | 3.6±2.0 | 2.9±1.8† |
| hs‐TNT*, ng/L | |||
| Baseline | 4.0 | 8.7 (7.2–10.2)† | 44.7 (30.1–65.3)†, ‡ |
| Follow‐up | 4.0 (4.0–5.9)[Link] | 12.5 (9.0–18.1)†, [Link] | 49.1 (27.6–69.5)†, ‡ |
| LE‐positive myocardial volume, % | |||
| Baseline | 0 (0–0.3) | 0.4 (0–1.6)† | 1.9 (1.1–3.3)†, ‡ |
| Follow‐up | 0.3 (0–1.4)[Link] | 1.5 (0.4–3.1)†, [Link] | 3.2 (2.3–4.9)†, ‡, [Link] |
| IVSd, mm | |||
| Baseline | 9.1±1.1 | 11.5±1.7† | 14.5±3.1†, ‡ |
| Follow‐up | 9.1±1.5 | 11.3±1.4† | 13.7±2.3†, ‡, § |
| EF, % | |||
| Baseline | 64±5 | 65±5 | 66±6 |
| Follow‐up | 63±6 | 65±5 | 63±8 |
Using repeated‐measures ANOVA followed by Bonferroni post‐hoc test for multiple comparisons. EF indicates ejection fraction; hs‐TNT, high‐sensitivity cardiac troponin T; IVSd, end‐diastolic interventricular septal wall thickness; LE, late enhancement.
*Ln‐transformed.
Bonferroni‐adjusted † P<0.025 vs hs‐TNT <5 ng/L; ‡ P<0.025 vs hs‐TNT 5 to 14 ng/L.
§ P<0.05 vs baseline.
Of the 58 patients with longitudinal data, 21 (36.2%) underwent enzyme replacement therapy (ERT). As shown in Table 6, patients in the ERT group had higher hs‐TNT levels and thicker LV wall thickness compared to the no‐ERT group at baseline. LE‐positive myocardial volume was similar between groups at baseline. During follow‐up, hs‐TNT level and LE‐positive myocardial volumes were significantly increased in both groups.
Table 6.
Changes in hs‐TNT Level, LE‐Positive Myocardium, Cardiac Morphology, and Function During Follow‐up (n=58) in Patients With and Without ERT
| No ERT | ERT | |
|---|---|---|
| n=37 | n=21 | |
| hs‐TNT*, ng/L | ||
| Baseline | 4.0 (4.0–10.4) | 8.9 (4.8–30.1)† |
| Follow‐up | 6.1 (4.0–18.9)‡ | 14.6 (6.9–48.0)†, ‡ |
| LE‐positive myocardial volume, % | ||
| Baseline | 0.2 (0–1.2) | 0.6 (0–1.8) |
| Follow‐up | 1.0 (0–2.3)‡ | 2.3 (0.6–3.9)‡ |
| IVSd, mm | ||
| Baseline | 10.3±2.2 | 12.6±3.2† |
| Follow‐up | 10.1±2.2 | 12.1±2.5† |
| EF, % | ||
| Baseline | 65±5 | 65±5 |
| Follow‐up | 64±6 | 62±7 |
Using repeated‐measures ANOVA followed by Tukey's post‐hoc test. EF indicates ejection fraction; ERT, enzyme replacement therapy; hs‐TNT, high‐sensitivity cardiac troponin T; IVSd, end‐diastolic interventricular septal wall thickness; LE, late enhancement.
*Ln‐transformed.
† P<0.05 vs no ERT.
‡ P<0.05 vs baseline.
Discussion
The aim of this study was to investigate the clinical value of hs‐TNT as a blood biomarker for evaluating myocardial replacement fibrosis in patients with FD. The main results are 4‐fold: First, the well‐known myocyte turnover resulting in myocardial replacement fibrosis was associated with plasma hs‐TNT elevation. Second, hs‐TNT level correlated with the total amount of replacement fibrosis. Third, elevated hs‐TNT could predict an increase in the extent of fibrosis. Finally, hs‐TNT was very stable and predictive even in frozen blood stored for more than 5 years.
hs‐TNT is a reliable, highly sensitive marker for myocyte necrosis and myocardial remodeling, both resulting in myocardial fibrosis.22 Moreno et al. showed that patients with hypertrophic cardiomyopathy and LE on MRI have higher levels of hs‐TNT than hypertrophic cardiomyopathy patients without LE.23 In accord with these findings, Kawasaki et al. demonstrated that higher levels of hs‐TNT are associated with LE on MRI in patients with cardiomyopathy and suggested that hs‐TNT is therefore a direct marker for myocardial replacement fibrosis.24 In an initial study, Feustel et al. showed that regular troponin I was continuously elevated in 3 patients with a Fabry cardiomyopathy.25 However, this troponin I was, by far, not as sensitive for myocyte turnover as the hs‐TNT used in the current study. To date, no well‐performed study has investigated the association of hs‐TNT and replacement fibrosis in patients with Fabry cardiomyopathy. In the current work, both the level of hs‐TNT and LE‐positive area were quantified, and absolute values were given for both parameters in all patients. In this way, we showed a clear correlation between the blood biomarkers and morphological finding of replacement fibrosis. However, whether or not the circulated hs‐TNT is finally generated by myocyte turnover in the LE‐positive region remains unclear. It can be speculated that hs‐TNT is generated everywhere in the diseased myocardium of the LV. If so, then the correlation could result from the fact that the amount of replacement fibrosis mirrors the severity of the diseased myocardium.
The current study also investigated NT‐proBNP as a marker of cardiomyopathy. NT‐proBNP is an established biomarker for heart failure and is mainly related to the wall stress acting on the heart.24, 26, 27 In general, only truly end‐stage Fabry cardiomyopathy patients suffer from heart failure;7, 28 in addition, blood pressure (one of the main components of wall stress) is not severely elevated in these patients. Thus, it was predictable that NT‐proBNP was only a weak biomarker for cardiomyopathy and not clinically meaningful in terms of the amount of fibrosis.
Evaluation of myocardial fibrosis by either imaging or biomarkers is clinically important because fibrosis defines the stage of cardiomyopathy, predicts the outcome during ERT, and indicates a poor prognosis.5, 11, 29, 30 The noninvasive gold standard for detecting intramural replacement fibrosis is LE MRI.6 LE imaging is accurate for evaluating the LV and highly sensitive for detecting and quantifying a small amount of fibrosis.31, 32, 33, 34 However, this technique is expensive, time‐consuming, and often not available for routine diagnosis. Furthermore, many Fabry patients have contraindications for undergoing MRI, such as ESRD or an implanted cardiac defibrillator or pacemaker.31 Another indirect way to screen for regional myocardial fibrosis is by functional deformation imaging.27, 28 This method is sensitive for detecting systolic functional abnormalities; however, it requires an experienced investigator. Additionally, this method is also time‐consuming and not easy to postprocess.30, 31, 33, 35
Clinical Implication
The current data suggest that hs‐TNT could be measured in every Fabry patient at every visit because it is widely available and not expensive. If it is in normal range, that in combination with normal echocardiography and 12‐lead electrocardiography means that a typical Fabry cardiomyopathy is very unlikely. If the level of hs‐TNT is borderline, a sophisticated search, including cardiac MRI, for replacement fibrosis should be done. On the other hand, if the level is very high, an advanced cardiomyopathy is likely present, and imaging of the fibrosis is necessary only for confirmation. In addition, in patients with elevated hs‐TNT, the progression of the cardiomyopathy can be anticipated even when these patients are treated with ERT; thus, a careful follow‐up of these patients should be performed.
Limitations
ESRD patients treated with dialysis were excluded from these analyses because LE imaging is contraindicated. It is possible that hs‐TNT could, in part, accumulate because of a decreased renal filtration rate; thus, the suggested biomarker should not be used in hemodialysis patients. In addition, the patient sample was relatively small for the longitudinal cohort and all data were retrospectively collected. The duration between baseline and follow‐up examinations was unable to be standardized. These limitations could have introduced some bias. Prospective studies with larger sample size are certainly warranted to validate the current results.
Conclusion
The present study shows a clinically meaningful correlation between plasma hs‐TNT level and the amount of replacement fibrosis as assessed by LE. These findings indicate that hs‐TNT is a reliable tool for screening for myocardial fibrosis. It is convenient, cost‐effective, easy to determine, and involves few contraindications. Moreover, hs‐TNT can be used as an easy predictor of the progression of Fabry cardiomyopathy. Thus, we suggest that the biomarker hs‐TNT could be helpful for staging and follow‐up of Fabry patients.
Sources of Funding
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF project 01EO1004) and by Genzyme Corporation.
Disclosures
Weidemann and Wanner have received speaker honoraria from Genzyme and Shire corporations. Weidemann and Wanner are members of the Fabry Registry European Board of Advisors and have received travel assistance and speaker honoraria. Research grants were given to the Institution by Genzyme and Shire corporations.
(J Am Heart Assoc. 2016;5:e002839 doi: 10.1161/JAHA.115.002839)
References
- 1. Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR. Fabry disease, an under‐recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–346. [DOI] [PubMed] [Google Scholar]
- 2. Garman SC, Garboczi DN. The molecular defect leading to Fabry disease: structure of human alpha‐galactosidase. J Mol Biol. 2004;337:319–335. [DOI] [PubMed] [Google Scholar]
- 3. De Francesco PN, Mucci JM, Ceci R, Fossati CA, Rozenfeld PA. Fabry disease peripheral blood immune cells release inflammatory cytokines: role of globotriaosylceramide. Mol Genet Metab. 2013;109:93–99. [DOI] [PubMed] [Google Scholar]
- 4. MacDermot KD, Holmes A, Miners AH. Anderson‐Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001;38:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weidemann F, Breunig F, Beer M, Sandstede J, Stork S, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM. The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J. 2005;26:1221–1227. [DOI] [PubMed] [Google Scholar]
- 6. Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson‐Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J. 2003;24:2151–2155. [DOI] [PubMed] [Google Scholar]
- 7. Linhart A, Kampmann C, Zamorano JL, Sunder‐Plassmann G, Beck M, Mehta A, Elliott PM. Cardiac manifestations of Anderson‐Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007;28:1228–1235. [DOI] [PubMed] [Google Scholar]
- 8. Barbey F, Brakch N, Linhart A, Rosenblatt‐Velin N, Jeanrenaud X, Qanadli S, Steinmann B, Burnier M, Palecek T, Bultas J, Hayoz D. Cardiac and vascular hypertrophy in Fabry disease: evidence for a new mechanism independent of blood pressure and glycosphingolipid deposition. Arterioscler Thromb Vasc Biol. 2006;26:839–844. [DOI] [PubMed] [Google Scholar]
- 9. Morrissey RP, Philip KJ, Schwarz ER. Cardiac abnormalities in Anderson‐Fabry disease and Fabry's cardiomyopathy. Cardiovasc J Afr. 2011;22:38–44. [PMC free article] [PubMed] [Google Scholar]
- 10. Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, Sunder‐Plassmann G. Natural course of Fabry disease: changing pattern of causes of death in FOS—Fabry Outcome Survey. J Med Genet. 2009;46:548–552. [DOI] [PubMed] [Google Scholar]
- 11. Kramer J, Niemann M, Stork S, Frantz S, Beer M, Ertl G, Wanner C, Weidemann F. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am J Cardiol. 2014;114:895–900. [DOI] [PubMed] [Google Scholar]
- 12. Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:2260–2264. [DOI] [PubMed] [Google Scholar]
- 13. Moe GW. B‐type natriuretic peptide in heart failure. Curr Opin Cardiol. 2006;21:208–214. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part III: biomarkers of oxidative stress and angiogenic growth factors. Circulation. 2006;113:e289–e292. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part II: acute‐phase reactants and biomarkers of endothelial cell activation. Circulation. 2006;113:e152–e155. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines. Circulation. 2006;113:e72–e75. [DOI] [PubMed] [Google Scholar]
- 17. Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes‐Genis A, Ordonez‐Llanos J, Santalo‐Bel M, Pinto YM, Richards M. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT‐proBNP Study. Eur Heart J. 2006;27:330–337. [DOI] [PubMed] [Google Scholar]
- 20. Hildebrandt P, Richards AM. Amino‐terminal pro‐B‐type natriuretic peptide testing in patients with diabetes mellitus and with systemic hypertension. Am J Cardiol. 2008;101:21–24. [DOI] [PubMed] [Google Scholar]
- 21. Kawano M, Takenaka T, Otsuji Y, Teraguchi H, Yoshifuku S, Yuasa T, Yu B, Miyata M, Hamasaki S, Minagoe S, Kanmura Y, Tei C. Significance of asymmetric basal posterior wall thinning in patients with cardiac Fabry's disease. Am J Cardiol. 2007;99:261–263. [DOI] [PubMed] [Google Scholar]
- 22. Gomes AV, Barnes JA, Harada K, Potter JD. Role of troponin T in disease. Mol Cell Biochem. 2004;263:115–129. [DOI] [PubMed] [Google Scholar]
- 23. Moreno V, Hernandez‐Romero D, Vilchez JA, Garcia‐Honrubia A, Cambronero F, Casas T, Gonzalez J, Martinez P, Climent V, de la Morena G, Valdes M, Marin F. Serum levels of high‐sensitivity troponin T: a novel marker for cardiac remodeling in hypertrophic cardiomyopathy. J Card Fail. 2010;16:950–956. [DOI] [PubMed] [Google Scholar]
- 24. Kawasaki T, Sakai C, Harimoto K, Yamano M, Miki S, Kamitani T. Usefulness of high‐sensitivity cardiac troponin T and brain natriuretic peptide as biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112:867–872. [DOI] [PubMed] [Google Scholar]
- 25. Feustel A, Hahn A, Schneider C, Sieweke N, Franzen W, Gunduz D, Rolfs A, Tanislav C. Continuous cardiac troponin I release in Fabry disease. PLoS One. 2014;9:e91757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogino K, Ogura K, Kinugawa T, Osaki S, Kato M, Furuse Y, Kinugasa Y, Tomikura Y, Igawa O, Hisatome I, Shigemasa C. Neurohumoral profiles in patients with hypertrophic cardiomyopathy: differences to hypertensive left ventricular hypertrophy. Circ J. 2004;68:444–450. [DOI] [PubMed] [Google Scholar]
- 27. Arteaga E, Araujo AQ, Buck P, Ianni BM, Rabello R, Mady C. Plasma amino‐terminal pro‐B‐type natriuretic peptide quantification in hypertrophic cardiomyopathy. Am Heart J. 2005;150:1228–1232. [DOI] [PubMed] [Google Scholar]
- 28. Weidemann F, Linhart A, Monserrat L, Strotmann J. Cardiac challenges in patients with Fabry disease. Int J Cardiol. 2010;141:3–10. [DOI] [PubMed] [Google Scholar]
- 29. Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Stork S, Voelker W, Ertl G, Wanner C, Strotmann J. Long‐term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;119:524–529. [DOI] [PubMed] [Google Scholar]
- 30. Weidemann F, Niemann M, Herrmann S, Kung M, Stork S, Waller C, Beer M, Breunig F, Wanner C, Voelker W, Ertl G, Bijnens B, Strotmann JM. A new echocardiographic approach for the detection of non‐ischaemic fibrosis in hypertrophic myocardium. Eur Heart J. 2007;28:3020–3026. [DOI] [PubMed] [Google Scholar]
- 31. Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation. 2003;107:1978–1984. [DOI] [PubMed] [Google Scholar]
- 32. Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM. Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation. 2003;108:1299–1301. [DOI] [PubMed] [Google Scholar]
- 33. Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch‐Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR III, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–991. [DOI] [PubMed] [Google Scholar]
- 34. Weidemann F, Strotmann JM. Use of tissue Doppler imaging to identify and manage systemic diseases. Clin Res Cardiol. 2008;97:65–73. [DOI] [PubMed] [Google Scholar]
- 35. Kramer J, Niemann M, Liu D, Hu K, Machann W, Beer M, Wanner C, Ertl G, Weidemann F. Two‐dimensional speckle tracking as a non‐invasive tool for identification of myocardial fibrosis in Fabry disease. Eur Heart J. 2013;34:1587–1596. [DOI] [PubMed] [Google Scholar]


