Abstract
Background
Men and women differ in the risk of cardiovascular disease, but the underlying mechanisms are not completely understood. We examined possible sex‐related differences in supine and upright cardiovascular regulation.
Methods and Results
Hemodynamics were recorded from 167 men and 167 women of matching age (≈45 years) and body mass index (≈26.5) during passive head‐up tilt. None had diabetes mellitus or cardiovascular disease other than hypertension or used antihypertensive medication. Whole‐body impedance cardiography, tonometric radial blood pressure, and heart rate variability were analyzed. Results were adjusted for height, smoking, alcohol intake, mean arterial pressure, plasma lipids, and glucose. Supine hemodynamic differences were minor: Men had lower heart rate (−4%) and higher stroke index (+7.5%) than women (P<0.05 for both). Upright systemic vascular resistance was lower (−10%), but stroke index (+15%), cardiac index (+16%), and left cardiac work were clearly higher (+20%) in men than in women (P<0.001 for all). Corresponding results were observed in a subgroup of men and postmenopausal women (n=76, aged >55 years). Heart rate variability analyses showed higher low:high frequency ratios in supine (P<0.001) and upright (P=0.003) positions in men.
Conclusions
The foremost difference in cardiovascular regulation between sexes was higher upright hemodynamic workload for the heart in men, a finding not explained by known cardiovascular risk factors or hormonal differences before menopause. Heart rate variability analyses indicated higher sympathovagal balance in men regardless of body position. The deviations in upright hemodynamics could play a role in the differences in cardiovascular risk between men and women.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01742702.
Keywords: cardiac output, hemodynamics, sex‐specific
Subject Categories: Hemodynamics, Clinical Studies, Autonomic Nervous System
Introduction
The lifetime risk of cardiovascular disease (CVD) is close to 50% for persons aged 30 years without known CVD,1 and it is the most common cause of death for both men and women.2 The risk and the outcome of CVD, however, differ between sexes; particularly before middle age, morbidity and mortality caused by CVDs are higher in men.3 In the Framingham Heart Study, the lifetime risk of coronary heart disease at age 40 years was about 49% for men and 32% for women.4 These differences in CVD risk between sexes have been attributed to hormonal differences and to the fact that most of the risk factors are more favorable for women.5
Distinct differences in cardiovascular regulation have been characterized between sexes. In early adulthood, pulse pressure is higher in men; however, at older ages, pulse pressure becomes higher in women.6 The myocardial remodeling process in response to aging, pressure and volume overload, and myocardial infarction appears to be more favorable in women than in men.7 In patients with ischemic heart disease, women may be more prone to myocardial ischemia in response to mental stress, whereas men may show higher increases in blood pressure (BP).8 Taken together, these findings raise the question of whether further differences in cardiovascular regulation exist between men and women that could influence the hemodynamic load of the heart and subsequently affect the number of cardiovascular end points.
Hypertension is more prevalent in young men than in young women, but after menopause, the risk of hypertension is significantly increased in women.9 The lifetime burden of hypertension on the prevalence of CVDs is substantial.1 In a survey in Finland performed in 2007, only 37% of the drug‐treated hypertensive patients had normal BP (<140/90 mm Hg).10 The suboptimal control of hypertension raises the question of whether individual characteristics and possible sex‐related differences in the regulation of hemodynamics should be more carefully taken into account in the treatment of elevated BP.
Although a major share of the active life is spent in the upright position, most reports on sex‐related differences in hemodynamics have focused on resting conditions.11, 12, 13, 14 Only few studies have compared upright hemodynamics between sexes.15, 16, 17 Similar upright increase in peripheral arterial resistance and decrease in cardiac output was reported in 9 men versus 8 women,15 lower upright splanchnic vasoconstriction but corresponding decrease in cardiac output was reported in 14 women versus 16 men,17 and corresponding upright increase in forearm vascular resistance was observed in 48 women versus 41 men.16 In most of the previous studies, the control of cardiac autonomic tone between sexes was evaluated by the use of heart rate variability (HRV). In the majority of these reports, sympathovagal balance was reported to be higher in men, regardless of whether the participants were in supine, sitting, or standing position.16, 18, 19, 20
The previous studies comparing hemodynamics between sexes were performed with small numbers of participants, and the groups have not been uniform in age and body mass index (BMI).8, 15, 16, 17 Therapies based on individual sex‐related characteristics could improve outcomes in the treatment of CVDs like hypertension,21 and personalized approaches in the treatment of medical conditions are keenly sought.22, 23, 24 Because only limited information exists about the upright regulation of the cardiovascular system between sexes, we compared the hemodynamic responses to passive head‐up tilt in 167 men versus 167 women with corresponding age and BMI. The main findings were verified in a larger group composed of an additional 313 men and 231 women. A total of 480 men and 398 women were examined.
Methods
Study Participants
All participants were in an ongoing study of hemodynamics in volunteers, with the primary aim of examining the hemodynamic changes in primary and secondary hypertension and normotensive control participants in supine and upright positions (DYNAMIC study; ClinicalTrials.gov identifier NCT01742702). Participants were enrolled from the patients treated at Tampere University Hospital. An announcement of recruitment was distributed at Tampere University Hospital, the University of Tampere, offices of local occupational health care providers, and Varala Sports Institute, and 2 announcements were published in a newspaper. Those who agreed to participate were recruited in the order in which their contact information was available to the research nurses.
By the time of the present analysis, 878 participants (480 men and 398 women) had undergone hemodynamic measurements; of those, 615 were without medications with direct influence on cardiovascular function. The remaining participants included medicated patients with hypertension, chronic renal disease, diabetes mellitus, and atherosclerotic vascular disease. All participants were interviewed to record lifestyle habits, family history, and medical history. Clinical examination of cardiovascular status and routine laboratory tests were performed.
For the main analysis of the present study, participants with antihypertensive medication, coronary heart disease, or previous myocardial infarction, diagnosis of diabetes mellitus, cardiac insufficiency, atherosclerotic vascular disease, renal disease, or cerebrovascular disease were excluded. In addition, all participants using medications with potential influence on hemodynamics (eg, α1‐adrenoceptor blockers for prostate problems, β2‐adrenoceptor agonists, digoxin, and topical β‐blockers for glaucoma) were excluded.25 To avoid confounding caused by differences in age and body weight,26, 27, 28, 29 the following inclusion protocol was applied: A female participant was chosen, followed by selection of a male participant with corresponding age (difference in age ≤3 years) and BMI (difference ≤1.5 units). Using this approach, 334 participants aged 21 to 67 years were included (Table 1).
Table 1.
Clinical and Metabolic Characteristics in the Study Groups
| Men (n=167) | Women (n=167) | P Value | |
|---|---|---|---|
| Age, y | 45±12 | 45±11 | 0.967 |
| Body mass index, kg/m2 | 26.5±3.7 | 26.6±3.8 | 0.898 |
| Height, cm | 180±6 | 166±6 | <0.001 |
| Weight, kg | 88±12 | 73±11 | <0.001 |
| Systolic blood pressure, mm Hg | 132±17 | 127±18 | 0.006 |
| Diastolic blood pressure, mm Hg | 75±12 | 72±12 | 0.026 |
| Smoking | |||
| Current | 51 (30.5) | 46 (27.5) | 0.547 |
| Previous | 22 (13.2) | 17 (10.2) | 0.394 |
| Never | 94 (56.3) | 10 (62.3) | 0.265 |
| Alcohol, standard drinks per week | 4 (1–10) | 2 (0–4) | <0.001 |
| Creatinine, μmol/L | 82±12 | 65±9 | <0.001 |
| Cystatin‐C, mg/L | 0.87±0.14 | 0.80±0.14 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 99.2±14.5 | 99.3±14.0 | 0.956 |
| Total cholesterol, mmol/L | 5.1±1.0 | 5.1±1.0 | 0.804 |
| LDL cholesterol, mmol/L | 3.1±1.0 | 2.8±0.9 | 0.021 |
| HDL cholesterol, mmol/L | 1.4±0.3 | 1.8±0.4 | <0.001 |
| Triglycerides, mmol/L | 1.3 (0.7–1.5) | 1.1 (0.7–1.3) | 0.007 |
| Fasting plasma glucose, mmol/L | 5.5±0.5 | 5.3±0.5 | <0.001 |
| Cornell voltage product in ECG, ms×mm | 1638±615 | 1621±509 | 0.779 |
Values are means±SD except for smoking, which shows the number of participants and percentages, and for alcohol intake and triglycerides, which are shown as medians (lower and upper quartiles) due to skewed distribution. eGFR indicates estimated glomerulus filtration rate30; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Overall, 114 (34%) of the 334 persons used some medication (Table 2). Forty female participants used systemic estrogen, progestin, or their combination (for contraception or hormone replacement therapy), and 1 participant used tibolone. Eight participants were taking statins, and 11 euthyroid participants were on a stable dose of thyroid hormone. Other medications (acetylsalicylic acid, drugs for mental problems, antihistamines, proton pump inhibitors, intranasal or inhaled corticosteroid for asthma or allergy) were used by the study population. One participant who was physically well and symptomless was treated with warfarin for antiphospholipid syndrome.
Table 2.
Medications Used Regularly by the Study Participants (Number of Participants With Each Type of Medication)
| Medication | Men (n=167) | Women (n=167) |
|---|---|---|
| Acetylsalicylic acid | 3 | 0 |
| Acyclovir | 0 | 1 |
| Allopurinol | 0 | 1 |
| Amitriptyline | 0 | 1 |
| Amoxicillin | 0 | 1 |
| Antidepressant (SSRI or SNRI) | 3 | 12 |
| Antihistamine | 2 | 5 |
| Doxycycline (low dose) | 1 | 0 |
| Ezetimibe | 1 | 0 |
| Female hormones | ||
| Systemic (including tibolone) | 0 | 41 |
| Topical | 0 | 3 |
| Glucosamine | 3 | 3 |
| Intranasal or inhaled corticosteroid | 2 | 6 |
| Isotretinoin | 0 | 1 |
| Letrozole | 0 | 1 |
| Levomepromazine | 0 | 1 |
| Levonorgestrel via intrauterine device | 0 | 16 |
| Liothyronine | 0 | 1 |
| Lymecycline | 1 | 0 |
| Mefloquine | 0 | 1 |
| Melatonin | 1 | 1 |
| Mepacrine | 1 | 0 |
| Nonsteroidal anti‐inflammatory drug | 1 | 3 |
| Oxybutynine | 0 | 1 |
| Pramipexole | 0 | 1 |
| Pregabalin | 1 | 1 |
| Proton pump inhibitor | 3 | 4 |
| Quetiapine | 0 | 1 |
| Statin | 7 | 1 |
| Thyroxine | 0 | 10 |
| Valproate | 0 | 1 |
| Vitamin D supplementation | 6 | 11 |
| Warfarin | 1 | 0 |
SNRI indicates serotonin–norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Additional analyses were performed in groups in which (1) all participants using systemic female hormones or statins were excluded, (2) all participants were aged ≥55 years, and (3) all 878 participants who participated in the recording of hemodynamics were included. The participants gave written informed consent, and the study was approved by the ethics committee of Tampere University Hospital (study code R06086M).
Hemodynamic Measurements
Hemodynamic measurements were performed in a temperature‐controlled laboratory. The participants were advised to refrain from using caffeine‐containing products, smoking, and eating heavy meals for at least 4 hours and from drinking alcohol for at least 24 hours prior to the recording. While the participants were resting supine on a tilt table, the electrodes for impedance cardiography were placed on the body surface, a tonometric sensor for radial artery BP on the left wrist (Colin BP‐508T; Colin Medical Instruments Corp) and an oscillometric brachial cuff for BP calibration on the right upper arm.25, 26, 31 The left arm with the tonometric sensor was abducted to 90° in a support that held the arm at the level of the heart in supine and upright positions. Estimation of central aortic BP was performed by mathematical transformation of radial tonometry pressure with the SphygmoCor pulse wave monitoring system (SpygmoCor PWMx; Atcor Medical) using a validated transfer function.32
A whole‐body impedance cardiography device (CircMon; JR Medical Ltd) that records changes in body electrical impedance during cardiac cycles was used to determine heart rate, stroke volume, and cardiac output.25, 33, 34, 35 The description of the method and electrode configuration was reported previously.25, 33, 34, 35 The values were normalized to body surface area and expressed as cardiac index, stroke index, systemic vascular resistance index (SVRI), and left cardiac work index (LCWI). SVRI was calculated from the radial BP signal and cardiac index measured by the CircMon device. LCWI (in kg×m/min per m2) was calculated using following formula: 0.0143×(mean aortic pressure–pulmonary artery occlusion pressure)×cardiac index. Pulmonary artery occlusion pressure was assumed to be 6 mm Hg (normal), and 0.0143 is the conversion factor of pressure from millimeters of mercury to centimeters of water, volume to density of blood (in kg/L), and centimeters to meters.36, 37 The stroke volume and cardiac output measured using CircMon whole‐body impedance cardiography agree with values measured using 3‐dimensional ultrasound and the thermodilution method, respectively, and agreement with the latter has been shown in both supine and upright positions.34, 36, 38
An introductory head‐up tilt was performed before the recordings. The actual measurement consisted of 3 consecutive 5‐minute periods with continuous capture of data: 5 minutes supine, 5 minutes of passive head‐up tilt to 60°, and 5 minutes supine. The repeatability and reproducibility of the supine and upright measurements has been shown to be good.31
Evaluation of Cardiac Autonomic Tone
HRV analysis from electrocardiograms was used to assess cardiac autonomic tone. The electrocardiograms were recorded by the CircMon device at a 200‐Hz sampling rate, and data were analyzed using Matlab software (MathWorks Inc). Normal R‐R intervals were recognized, and a beat was considered ectopic if the interval differed >20% from the previous values. The artifacts were processed using the cubic spline interpolation method.39 Because the data were collected from short‐term recordings, the frequency domain method was applied.40 The following variables were calculated from the recordings in supine (0–5 minutes) and upright (5–10 minutes) positions using the fast Fourier transformation method: (1) total power, (2) power in the low‐frequency (LF) range (0.04–0.15 Hz), (3) power in the high‐frequency (HF) range (0.15–0.40 Hz), and (4) LF/HF ratio.40
Laboratory Tests
Blood samples were obtained after about 12 hours of fasting. Plasma glucose, creatinine, cystatin C, triglyceride, and total and high‐ and low‐density lipoprotein cholesterol concentrations were determined using the Cobas Integra 700/800 or Cobas 6000, module c501 (F. Hoffmann‐La Roche Ltd), and blood cell count was performed by an ADVIA 120 or 2120 system (Siemens Healthcare GmbH). In some participants, low‐density lipoprotein was calculated using the Friedewald formula.41 Creatinine‐ and cystatin C–based estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.30 A standard 12‐lead electrocardiogram was recorded, and left ventricular mass was evaluated using the Cornell voltage QRS duration product.21 All laboratory values were missing from 3 participants, and low‐density lipoprotein cholesterol values were missing from an additional 2 participants.
Statistical Analyses
The characteristics of men versus women were compared using an independent samples t test, and smoking habits (current, previous and never) were compared using the Pearson chi‐square test. Hemodynamic differences in supine and upright positions were examined using ANOVA for repeated measures, and the changes in hemodynamic values from supine to upright position (average values of last 3 minutes before and last 3 minutes during the head‐up tilt25) and HRV differences were analyzed using an independent samples t test and ANOVA with possible confounding factors as covariates. Average BP values during the last 3 minutes of the first supine period were applied for the clinical characterization of the BP of participants (Table 1).
The analyses were adjusted for smoking habits, alcohol intake (standard doses per week), fasting plasma glucose, triglycerides, high‐ and low‐density lipoprotein cholesterol, height, and mean arterial pressure, as appropriate (the adjusting variable was not used if it was included in the formula of the variable of interest). HRV analyses were also adjusted for heart rate.42 In the adjusted analyses, current smoking as cigarettes per day was applied as a continuous variable, whereas in the tables, the smoking habits were described as current, previous, and never smoker. Due to missing data among the main group of interest (334 participants), the number of participants in the adjusted analyses ranged from 311 to 324, with at least 153 men and 158 women in all analyses.
The skewed distributions of triglycerides, total power, LF power, HF power, and LF/HF ratio were logarithmically transformed before statistical analyses. Variables with normal distribution were reported as means and standard deviations (SD) or 95% CIs of the means; skewed distributions were reported as medians, lower and upper quartiles, and range; and categorical variables were reported as numbers of participants and percentages. All testing was 2‐sided, and P values <0.05 were considered statistically significant. The data were analyzed using SPSS 17.0 (IBM Corp).
Results
Study Population
The general characteristics of the study participants are presented in Table 1. Because of the inclusion protocol, men and women were well matched for age and BMI. In addition, there were no significant differences in smoking status, estimated glomerular filtration rate,30 total cholesterol, and Cornell voltage product21 between the 167 men and 167 women. Men, however, were characterized by somewhat higher systolic and diastolic BP, alcohol intake, and higher fasting plasma creatinine, cystatin C, low‐density lipoprotein cholesterol, triglyceride, and glucose concentration and lower high‐density lipoprotein cholesterol concentration than women.
Supine and Upright Hemodynamics
Men had higher supine mean arterial pressure, stroke index, and LCWI and lower heart rate than women in unadjusted analyses (Figure 1A–1C and 1E). Supine cardiac index and SVRI did not differ between sexes (Figure 1D and 1F). After adjusting for height, smoking habits, alcohol intake, mean arterial pressure, low‐ and high‐density lipoprotein cholesterol, triglycerides, and glucose, only the differences in supine heart rate and stroke index for men and women remained significant (Figure 1B and 1C).
Figure 1.
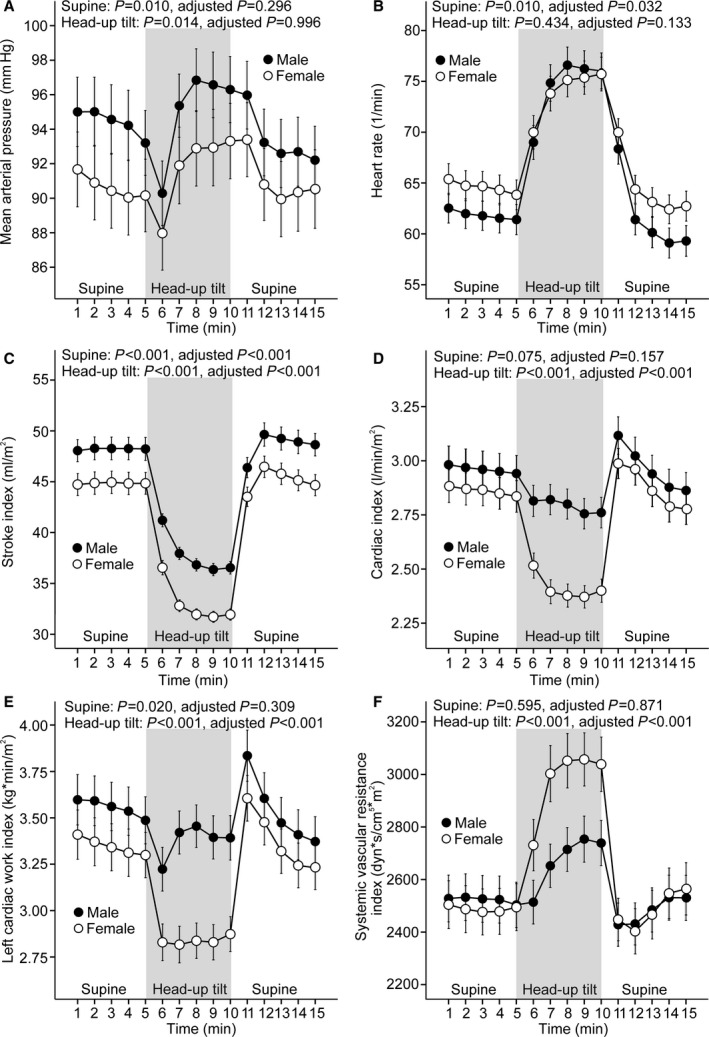
Line graphs show mean arterial pressure (A), heart rate (B), stroke index (C), cardiac index (D), left cardiac work index (E), and systemic vascular resistance index (F) in 167 men and 167 women during supine position and passive head‐up tilt (means and 95% CIs of the mean). P values denote differences between sexes in unadjusted analysis and in analyses adjusted for low‐ and high‐density lipoprotein cholesterol, triglycerides, glucose, mean arterial pressure, smoking habits, alcohol intake, and height.
During passive head‐up tilt to 60°, men had higher mean arterial pressure, stroke index, cardiac index, and LCWI (Figure 1A, 1C, 1D, and 1E) and lower SVRI (Figure 1F) than women in unadjusted analyses. Upright heart rate did not differ between men and women (Figure 1B). In adjusted analyses, with the exception of mean arterial pressure, all of the above differences in upright hemodynamics for men and women remained significant (Figure 1A and 1C–1F).
The magnitude of the changes in hemodynamic variables in response to upright posture was also analyzed (see Methods). Unadjusted analyses showed a greater increase in heart rate (P<0.001); less decrease in stroke index, cardiac index, and LCWI (P<0.05 for all); and less increase in SVRI (P<0.001) in response to head‐up tilt in men than in women. A similar increase in mean arterial pressure was observed in both sexes (P=0.812). In adjusted analyses, the differences in the upright changes in cardiac index, LCWI, and SVRI remained significant (P<0.01 for all), whereas the changes in heart rate and stroke index were no longer different between men and women (P=0.600 and P=0.694, respectively).
HRV in Supine and Upright Positions
In supine and upright positions, there were no significant differences in total power and in power in the LF range in unadjusted analyses (Figure 2A and 2B), whereas men had lower power in the HF range and higher LF/HF ratios than women (Figure 2C and 2D). In adjusted analyses, the upright differences in the HF component were no longer significant (Figure 2C), but supine HF power was lower and both supine and upright LF/HF ratios remained higher in men than in women (Figure 2C and 2D)
Figure 2.
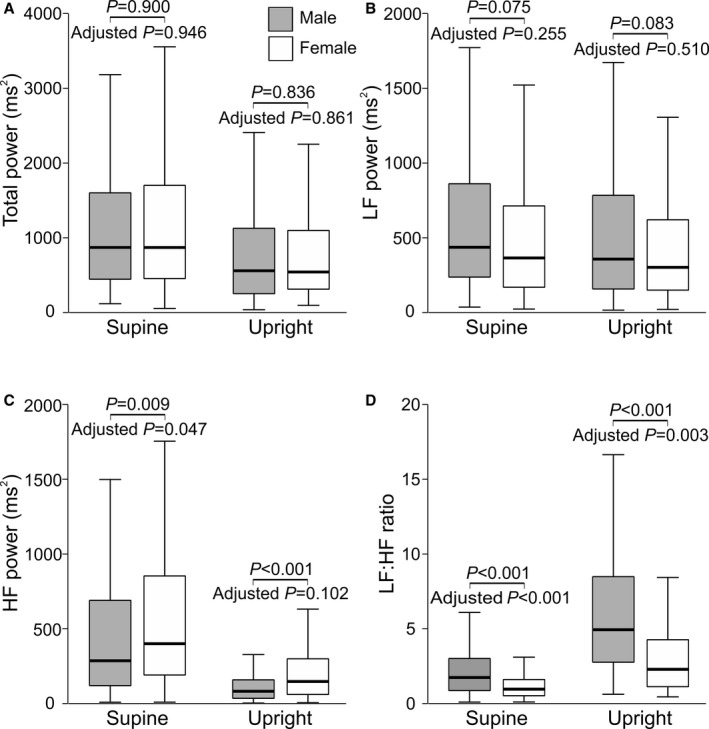
Box plots of heart rate variability in men and women during 5 minutes in supine and upright positions: total power (A), LF power (B), HF power (C), and LF/HF ratio (D) (median [line inside box], 25th to 75th percentile [box], and range [whiskers); outliers were excluded from the figure but were included in the statistics). P values denote differences between sexes in unadjusted analysis and in analyses adjusted for low‐ and high‐density lipoprotein cholesterol, triglycerides, glucose, heart rate,42 mean arterial pressure, smoking habits, alcohol intake, and height. HR indicates high frequency; LF, low frequency.
Supine and Upright Hemodynamics in Additional Analyses
Three additional analyses of hemodynamic changes in response to head‐up tilt were performed: (1) All participants using systemic female hormones and statins were excluded (n=285) (Figure 3); (2) all included participants were aged ≥55 years, and none used systemic female hormones (n=76) (Figure 4); (3) all participants who participated in hemodynamic recordings were included (n=878) (Figure 5). The last group was composed of 615 participants who were without medications with direct influence on cardiovascular function and 263 medicated patients with hypertension, chronic renal disease, diabetes mellitus, or atherosclerotic vascular disease. The outcome of all of these additional analyses was that the sex‐related differences in upright hemodynamics were very similar to those observed in the 167 men and 167 women with matching age and BMI. The results clearly showed a higher upright hemodynamic workload for the heart in men (Figures 3, 4 through 5).
Figure 3.
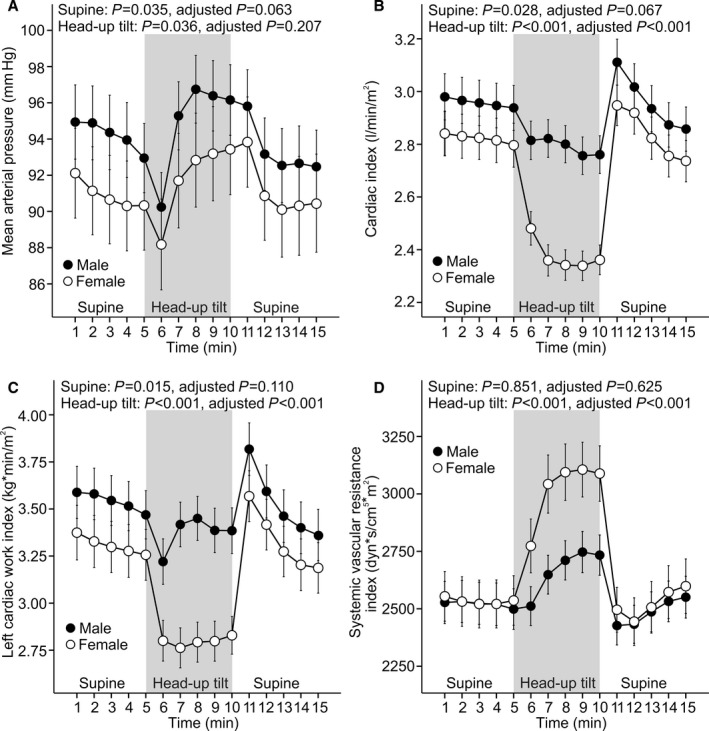
Participants using statins or hormone replacement therapy were excluded. Line graphs show mean arterial pressure (A), cardiac index (B), left cardiac work index (C), and systemic vascular resistance index (D) in 160 men and 125 women during supine position and passive head‐up tilt (means and 95% CIs of the mean). P values denote differences between sexes in unadjusted analysis and in analyses adjusted for low‐ and high‐density lipoprotein cholesterol, triglycerides, glucose, mean arterial pressure, smoking habits, alcohol intake, age, and body mass index.
Figure 4.
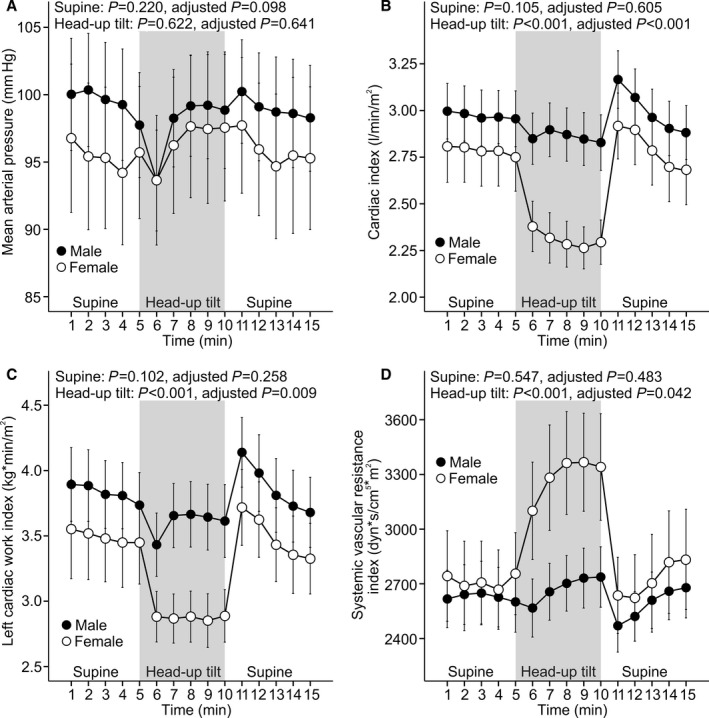
All included participants aged ≥55 years. Line graphs show mean arterial pressure (A), cardiac index (B), left cardiac work index (C), and systemic vascular resistance index (D) in 43 men and 33 women (no hormone replacement therapy in use) during supine position and passive head‐up tilt (means and 95% CIs of the mean). P values denote differences between sexes in unadjusted analysis and in analyses adjusted for low‐ and high‐density lipoprotein cholesterol, triglycerides, glucose, mean arterial pressure, smoking habits, alcohol intake, age, and body mass index.
Figure 5.
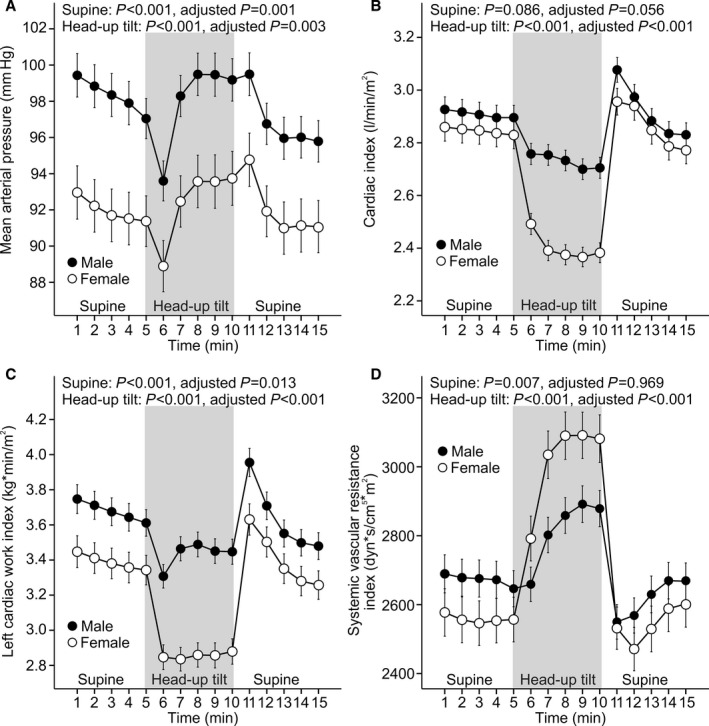
Overall, 878 participants were examined. Line graphs show mean arterial pressure (A), cardiac index (B), left cardiac work index (C), and systemic vascular resistance index (D) in 480 men and 398 women during supine position and passive head‐up tilt (312 men and 303 women were without medications with direct cardiovascular actions; means and 95% CIs of the mean). P values denote differences between sexes in unadjusted analysis and in analyses adjusted for low‐ and high‐density lipoprotein cholesterol, triglycerides, glucose, mean arterial pressure, smoking habits, alcohol intake, age, and body mass index. In adjusted analyses, the numbers of participants ranged from 402 to 411 for men and from 332 to 342 for women.
Discussion
Several previous studies examined the differences in cardiovascular function between sexes,6, 7, 8, 16, 18, 19, 20, 43 but only a few reports compared the upright hemodynamics between sexes.15, 16, 17, 44, 45 Although head‐up tilt for 5 minutes is a short period of observation, we found that upright hemodynamic adaptation differed between men and women. In men, cardiac index and left cardiac work decreased less in the upright position than in women, whereas men also showed lower upright increase in systemic vascular resistance than women. The net outcome was that the upright hemodynamic workload of the heart was higher in men than in women.
To gain functional insight about the cardiovascular system, we applied a head‐up tilt protocol to induce changes in autonomic tone, cardiac function, and peripheral arterial resistance in the study participants.25, 26, 31, 35 Because age and excess body weight have major influences on hemodynamics,26, 27, 28, 29 we initially performed analyses in 167 men and 167 women with corresponding age and BMI; however, the findings showing higher cardiac workload during head‐up tilt in men remained similar in participants not using statins or hormone replacement therapy, in participants aged ≥55 years, and in a large group composed of 878 participants.
We examined cardiac autonomic tone using the frequency domain analyses of HRV.16, 18, 19, 20 The HF component reflects cardiac parasympathetic activity,46, 47, 48 whereas the LF component predominantly reflects sympathetic activity, although it contains parasympathetic contributions.40, 46, 48 The LF/HF ratio is a marker of sympathovagal balance,40, 46 but this matter remains controversial, and conclusions should be drawn with caution.47, 48 In the present study, the frequency domain analysis of HRV showed higher LF/HF ratios in men both supine and upright, suggesting increased sympathovagal balance. The differences in cardiac autonomic tone largely resulted from decreased HF power, namely, lower cardiac parasympathetic tone in men than women. There has been controversy about whether the indices of HRV are higher in men or in women. In participants aged <30 years, the time domain measures of HRV were higher in men than in women49; however, most published reports on frequency domain measures of HRV have found that the values of LF power are higher in men,18, 19, 20, 50 whereas the values of HF power are higher in women.16, 19, 50 Higher LF/HF ratios during supine and standing positions have been reported in 156 men versus 206 women,20 and the majority of reports have suggested that sympathovagal balance is higher in men.15, 16, 18, 19, 20, 43, 50, 51, 52 The present findings stress the role of differences in cardiac parasympathetic regulation between sexes.
Higher sympathovagal balance in men agrees with higher upright cardiac workload but raises questions about lower upright SVRI in men. Both arteries and veins are innervated by autonomic nerves. Autonomic innervation of the resistance arteries consists of sympathetic and sensory–motor nerves, whereas parasympathetic vascular innervation is nonexistent or very limited.53 If higher sympathovagal balance would be transmitted to the resistance arteries similarly in men and women, higher cardiac output would result in an excessive upright rise of arterial pressure in men, with subsequent activation of baroreceptors.54 This would limit the increase in vascular resistance, and the comparison of baroreflexes between men and women is a subject for further studies. Previously, baroreceptor sensitivity during orthostasis has been reported to be higher in men than in women.15 Although sympathetic nerves are predominantly vasoconstrictor nerves, they can induce vasodilation in skeletal muscles,53 and the net effect of sympathetic stimulation on peripheral resistance depends on the circulatory balance between skeletal muscles and other organs. Studies of muscle sympathetic nerve activity have shown that the association of sympathetic tone with hemodynamics is not straightforward.14, 15 In younger men and women (aged <40 years), no relationship was found between muscle sympathetic nerve activity and BP, although a clear relationship was observed in older participants (aged ≥40 years).14 In younger men, muscle sympathetic nerve activity was positively related to peripheral vascular resistance and negatively related to cardiac output, whereas in younger women, no relationship was found between muscle sympathetic nerve activity and peripheral vascular resistance or cardiac output.14 Finally, autonomic innervation is not the sole regulator of resistance arterial tone; endothelium‐dependent mechanisms, myogenic regulation, and humoral factors also contribute to vascular resistance.53, 55
The present findings provide another putative explanation for the higher risk of cardiac events in men. Coronary heart disease and myocardial infarction account for a third to half of the initial presentations of CVD, and male sex particularly predisposes to myocardial infarction in those aged <60 years.56 The more unfavorable upright hemodynamic load of the heart could lead to earlier clinical manifestation of coronary heart disease in men than in women. Of note, the coronary findings in those who experienced sudden death also differ between sexes. In 442 cases of sudden coronary death, the prevalence of acute thrombosis and plaque rupture was higher in men than in women (53% versus 46% and 71% versus 33%, respectively), whereas plaque erosion as a cause of thrombosis was less frequent in men than in women (24% versus 58%).57 Finally, even the prognosis of heart failure has been reported to be worse in men than in women.58, 59 This is not explained by the etiology of the heart failure or by differences in left ventricular ejection fraction.60 Differences in the upright hemodynamic load of the heart could partially explain some of the aforementioned differences in cardiovascular risk between men and women.
An obvious difference between men and women is hormonal function, and both endogenous and exogenous sex hormones have effects on the vasculature.9, 61 Progesterone, for example, has vasodilatory or vasoconstrictive effects depending on the location of the vessel and the level of exposure.9 Exogenous progestins have androgenic properties compared with endogenous progesterone, and baroreflex sensitivity during oral contraceptive pill use may differ from that during the normal menstrual cycle.9 Estradiol may inhibit renin release, whereas testosterone may activate the renin–angiotensin system.61 Estrogens may even acutely enhance endothelium‐dependent vasodilation in postmenopausal women.62 In the present study, the upright differences in hemodynamics between men and women were not confined to premenopausal women but were consistent in participants of postmenopausal age. Consequently, higher left cardiac work in the upright position cannot be explained solely by differences in the sex hormones of men and women.
The sex differences in CVD risk factors and end points are known to diminish with increasing age.5 Although the incidence of myocardial infarction increases in postmenopausal women, after age 70 years, heart failure and stroke are the most common initial presentations of CVD in both sexes.56 A major predisposing factor to heart failure and stroke is the increased prevalence of hypertension.1, 9 The withdrawal of estrogen increases the age‐related cardiovascular risk in postmenopausal women, but chronological aging processes acting on top of biological differences between men and women likely play a more important role in the increase of cardiovascular risk.63
Another difference between men and women is body height. An inverse association between height and the risk of CVD has been found.64, 65 Hemodynamically, height is correlated with higher systolic pressure amplification from central to peripheral arteries and prolonged return of the reflected pressure wave form the peripheral circulation to the aorta.64, 66 Subsequently, shorter body height in women results in less peripheral systolic pressure amplification than in men, with lower peripheral but not central systolic pressure. In premenopausal women, greater arterial distensibility partially offsets the effects of shorter body height, but after menopause, arterial stiffness increases and does not compensate for the smaller stature of women, resulting in higher pressure wave reflections in central arteries.64, 66 In the present study, the estimation of aortic BP was performed using a pulse wave monitoring system that takes into account the reflected pressure waves, and the influences of the reflected waves were included in the estimation of aortic mean pressure that was used for the calculation of left cardiac work.
Our study has several limitations. The noninvasive recordings of cardiac output require mathematical equations and simplification of physiology,38 but invasive measurements are not justified without a clinical reason. The present methods have been validated against invasive methods,32, 34, 67 and we have no reason to suspect that the recordings would be less reliable in the upright position between sexes. Although participants taking medications with direct influence on hemodynamics were excluded from the main group composed of 334 participants, the medications used by the participants may have influenced the results. Selection bias also may have influenced the results in the group composed of 334 participants; however, it is unlikely that the results were observed merely by chance because the outcome in 878 participants (of whom 263 had CVD and were on various medications) correspondingly showed higher upright cardiac load in men than in women. The average BMI (in kg/m2) in the study population was ≈26.5, which corresponds well to the average BMI in Finnish men (27.4) and women (26.9) in a recent survey.68 Finally, the present analyses were adjusted for smoking habits, BP, lipid profile and glucose, and the groups of men and women had similar BMIs and ages. Consequently, the sex‐related differences in upright hemodynamics were not explained by the generally known cardiovascular risk factors.
In conclusion, we found clear differences in upright hemodynamics between men and women. In men, the upright position was associated with higher workload of the heart, whereas in women, the most marked hemodynamic change was a significant rise in peripheral arterial resistance. These hemodynamic differences were not explained by the generally known cardiovascular risk factors like smoking, alcohol use, lipid or glucose disorders, or level of BP. The findings of the current study emphasize that upright hemodynamics should receive special attention when examining cardiovascular differences between men and women. The observed differences in upright hemodynamics could play a role in the higher risk of cardiac events in men than in women.
Sources of Funding
This study was supported by grants from the Finnish Foundation for Cardiovascular Research, Sigrid Jusélius Foundation, Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital, Aarne Koskelo Foundation, Pirkanmaa Regional Fund of Finnish Cultural Foundation, Emil Aaltonen Foundation, Paavo Nurmi Foundation, and Tampere Tuberculosis Foundation.
Disclosures
None.
Acknowledgments
The authors are deeply grateful to Reeta Kulmala, RN, and Paula Erkkilä, RN, for invaluable technical assistance.
(J Am Heart Assoc. 2016;5:e002883 doi: 10.1161/JAHA.115.002883)
References
- 1. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laslett LJ, Alagona P Jr, Clark BA III, Drozda JP Jr, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1–S49. [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Arch Intern Med. 1995;155:57–61. [PubMed] [Google Scholar]
- 4. Lloyd‐Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. [DOI] [PubMed] [Google Scholar]
- 5. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow‐up study of 14 786 middle‐aged men and women in Finland. Circulation. 1999;99:1165–1172. [DOI] [PubMed] [Google Scholar]
- 6. Skurnick JH, Aladjem M, Aviv A. Sex differences in pulse pressure trends with age are cross‐cultural. Hypertension. 2010;55:40–47. [DOI] [PubMed] [Google Scholar]
- 7. Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex‐related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–1065. [DOI] [PubMed] [Google Scholar]
- 8. Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers JG, O'Connor CM, Velazquez EJ, Jiang W. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol. 2012;590:5949–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kastarinen M, Antikainen R, Peltonen M, Laatikainen T, Barengo NC, Jula A, Salomaa V, Jousilahti P, Nissinen A, Vartiainen E, Tuomilehto J. Prevalence, awareness and treatment of hypertension in Finland during 1982–2007. J Hypertens. 2009;27:1552–1559. [DOI] [PubMed] [Google Scholar]
- 11. Denton KM, Hilliard LM, Tare M. Sex‐related differences in hypertension: seek and ye shall find. Hypertension. 2013;62:674–677. [DOI] [PubMed] [Google Scholar]
- 12. Gatzka CD, Kingwell BA, Cameron JD, Berry KL, Liang YL, Dewar EM, Reid CM, Jennings GL, Dart AM. Gender differences in the timing of arterial wave reflection beyond differences in body height. J Hypertens. 2001;19:2197–2203. [DOI] [PubMed] [Google Scholar]
- 13. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural‐hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281:H2028–H2035. [DOI] [PubMed] [Google Scholar]
- 16. Barnett SR, Morin RJ, Kiely DK, Gagnon M, Azhar G, Knight EL, Nelson JC, Lipsitz LA. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension. 1999;33:1195–1200. [DOI] [PubMed] [Google Scholar]
- 17. Jarvis SS, Florian JP, Curren MJ, Pawelczyk JA. Sex differences in vasoconstrictor reserve during 70 deg head‐up tilt. Exp Physiol. 2010;95:184–193. [DOI] [PubMed] [Google Scholar]
- 18. Huikuri HV, Pikkujämsa SM, Airaksinen KE, Ikäheimo MJ, Rantala AO, Kauma H, Lilja M, Kesäniemi YA. Sex‐related differences in autonomic modulation of heart rate in middle‐aged subjects. Circulation. 1996;94:122–125. [DOI] [PubMed] [Google Scholar]
- 19. Stolarz K, Staessen JA, Kuznetsova T, Tikhonoff V, State D, Babeanu S, Casiglia E, Fagard RH, Kawecka‐Jaszcz K, Nikitin Y. Host and environmental determinants of heart rate and heart rate variability in four European populations. J Hypertens. 2003;21:525–535. [DOI] [PubMed] [Google Scholar]
- 20. Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, Pramstaller PP, Schunkert H, Bonnemeier H. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol. 2008;19:1296–1303. [DOI] [PubMed] [Google Scholar]
- 21. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 22. Cooper‐DeHoff RM, Johnson JA. Hypertension pharmacogenomics: in search of personalized treatment approaches. Nat Rev Nephrol. 2016;12:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mann SJ, Ernst ME. Personalizing the diuretic treatment of hypertension: the need for more clinical and research attention. Curr Hypertens Rep. 2015;17:542. [DOI] [PubMed] [Google Scholar]
- 24. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin‐angiotensin‐aldosterone system alterations. Circ Res. 2015;116:960–975. [DOI] [PubMed] [Google Scholar]
- 25. Tikkakoski AJ, Tahvanainen AM, Leskinen MH, Koskela JK, Haring A, Viitala J, Kähönen MA, Koobi T, Niemelä O, Mustonen JT, Pörsti IH. Hemodynamic alterations in hypertensive patients at rest and during passive head‐up tilt. J Hypertens. 2013;31:906–915. [DOI] [PubMed] [Google Scholar]
- 26. Tahvanainen A, Leskinen M, Koskela J, Ilveskoski E, Nordhausen K, Oja H, Kähönen M, Kööbi T, Mustonen J, Pörsti I. Ageing and cardiovascular responses to head‐up tilt in healthy subjects. Atherosclerosis. 2009;207:445–451. [DOI] [PubMed] [Google Scholar]
- 27. Recio‐Rodriguez JI, Gomez‐Marcos MA, Patino‐Alonso MC, Agudo‐Conde C, Rodriguez‐Sanchez E, Garcia‐Ortiz L, Vasorisk‐group . Abdominal obesity vs general obesity for identifying arterial stiffness, subclinical atherosclerosis and wave reflection in healthy, diabetics and hypertensive. BMC Cardiovasc Disord. 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community‐based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Safar ME, Czernichow S, Blacher J. Obesity, arterial stiffness, and cardiovascular risk. J Am Soc Nephrol. 2006;17:S109–S111. [DOI] [PubMed] [Google Scholar]
- 30. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tahvanainen A, Koskela J, Tikkakoski A, Lahtela J, Leskinen M, Kähönen M, Nieminen T, Kööbi T, Mustonen J, Pörsti I. Analysis of cardiovascular responses to passive head‐up tilt using continuous pulse wave analysis and impedance cardiography. Scand J Clin Lab Invest. 2009;69:128–137. [DOI] [PubMed] [Google Scholar]
- 32. Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure, Validation of generalized transfer function. Circulation. 1997;95:1827–1836. [DOI] [PubMed] [Google Scholar]
- 33. Kööbi T, Kähönen M, Iivainen T, Turjanmaa V. Simultaneous non‐invasive assessment of arterial stiffness and haemodynamics ‐ a validation study. Clin Physiol Funct Imaging. 2003;23:31–36. [DOI] [PubMed] [Google Scholar]
- 34. Kööbi T, Kaukinen S, Ahola T, Turjanmaa VM. Non‐invasive measurement of cardiac output: whole‐body impedance cardiography in simultaneous comparison with thermodilution and direct oxygen Fick methods. Intensive Care Med. 1997;23:1132–1137. [DOI] [PubMed] [Google Scholar]
- 35. Tahvanainen AM, Tikkakoski AJ, Leskinen MH, Nordhausen K, Kähönen M, Kööbi T, Mustonen JT, Pörsti IH. Supine and upright haemodynamic effects of sublingual nitroglycerin and inhaled salbutamol: a double‐blind, placebo‐controlled, randomized study. J Hypertens. 2012;30:297–306. [DOI] [PubMed] [Google Scholar]
- 36. Koskela JK, Tahvanainen A, Haring A, Tikkakoski AJ, Ilveskoski E, Viitala J, Leskinen MH, Lehtimaki T, Kahonen MA, Koobi T, Niemela O, Mustonen JT, Porsti IH. Association of resting heart rate with cardiovascular function: a cross‐sectional study in 522 Finnish subjects. BMC Cardiovasc Disord. 2013;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gorlin R, Mc MI, Medd WE, Matthews MB, Daley R. Dynamics of the circulation in aortic valvular disease. Am J Med. 1955;18:855–870. [DOI] [PubMed] [Google Scholar]
- 38. Kööbi T, Kaukinen S, Turjanmaa VM, Uusitalo AJ. Whole‐body impedance cardiography in the measurement of cardiac output. Crit Care Med. 1997;25:779–785. [DOI] [PubMed] [Google Scholar]
- 39. Peltola MA. Role of editing of R‐R intervals in the analysis of heart rate variability. Front Physiol. 2012;3:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Task Force of the European Society of Cardiology, the North American Society of Pacing and Electrophysiology . Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 41. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 42. Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H, Boyett MR. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smetana P, Malik M. Sex differences in cardiac autonomic regulation and in repolarisation electrocardiography. Pflugers Arch. 2013;465:699–717. [DOI] [PubMed] [Google Scholar]
- 44. White DD, Gotshall RW, Tucker A. Women have lower tolerance to lower body negative pressure than men. J Appl Physiol. 1996;80:1138–1143. [DOI] [PubMed] [Google Scholar]
- 45. Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998;275:R1909–R1920. [DOI] [PubMed] [Google Scholar]
- 46. Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Prog Cardiovasc Dis. 2012;55:321–331. [DOI] [PubMed] [Google Scholar]
- 47. Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol. 1999;517(Pt 2):617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–3232. [DOI] [PubMed] [Google Scholar]
- 49. Umetani K, Singer DH, McCraty R, Atkinson M. Twenty‐four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. [DOI] [PubMed] [Google Scholar]
- 50. Dutra SG, Pereira AP, Tezini GC, Mazon JH, Martins‐Pinge MC, Souza HC. Cardiac autonomic modulation is determined by gender and is independent of aerobic physical capacity in healthy subjects. PLoS ONE. 2013;8:e77092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001;91:2611–2618. [DOI] [PubMed] [Google Scholar]
- 52. Cheng YC, Vyas A, Hymen E, Perlmuter LC. Gender differences in orthostatic hypotension. Am J Med Sci. 2011;342:221–225. [DOI] [PubMed] [Google Scholar]
- 53. Storkebaum E, Carmeliet P. Paracrine control of vascular innervation in health and disease. Acta Physiol (Oxf). 2011;203:61–86. [DOI] [PubMed] [Google Scholar]
- 54. Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop‐Busui R, Ziegler D, Kempler P, Freeman R, Low P, Tesfaye S, Valensi P. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev. 2011;27:654–664. [DOI] [PubMed] [Google Scholar]
- 55. Luksha L, Agewall S, Kublickiene K. Endothelium‐derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–344. [DOI] [PubMed] [Google Scholar]
- 56. George J, Rapsomaniki E, Pujades‐Rodriguez M, Shah AD, Denaxas S, Herrett E, Smeeth L, Timmis A, Hemingway H. How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation. 2015;132:1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yahagi K, Davis HR, Arbustini E, Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–267. [DOI] [PubMed] [Google Scholar]
- 58. Martinez‐Selles M, Doughty RN, Poppe K, Whalley GA, Earle N, Tribouilloy C, McMurray JJ, Swedberg K, Kober L, Berry C, Squire I. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta‐analysis. Eur J Heart Fail. 2012;14:473–479. [DOI] [PubMed] [Google Scholar]
- 59. Parashar S, Katz R, Smith NL, Arnold AM, Vaccarino V, Wenger NK, Gottdiener JS. Race, gender, and mortality in adults > or =65 years of age with incident heart failure (from the Cardiovascular Health Study). Am J Cardiol. 2009;103:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Pina IL, Granger CB, Ostergren J, Michelson EL, Solomon SD, Pocock S, Yusuf S, Swedberg K, Pfeffer MA. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–3120. [DOI] [PubMed] [Google Scholar]
- 61. Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. [DOI] [PubMed] [Google Scholar]
- 62. Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO III. Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. [DOI] [PubMed] [Google Scholar]
- 63. Merz AA, Cheng S. Sex differences in cardiovascular ageing. Heart. 2016;102:825–831. doi: 10.1136/heartjnl‐2015‐308769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smulyan H, Marchais SJ, Pannier B, Guerin AP, Safar ME, London GM. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol. 1998;31:1103–1109. [DOI] [PubMed] [Google Scholar]
- 65. Batty GD, Shipley MJ, Gunnell D, Huxley R, Kivimaki M, Woodward M, Lee CM, Smith GD. Height, wealth, and health: an overview with new data from three longitudinal studies. Econ Hum Biol. 2009;7:137–152. [DOI] [PubMed] [Google Scholar]
- 66. London GM, Guerin AP, Pannier B, Marchais SJ, Stimpel M. Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension. 1995;26:514–519. [DOI] [PubMed] [Google Scholar]
- 67. Boer P, Roos JC, Geyskes GG, Mees EJ. Measurement of cardiac output by impedance cardiography under various conditions. Am J Physiol. 1979;237:H491–H496. [DOI] [PubMed] [Google Scholar]
- 68. Peltonen M, Harald K, Männistö S, Saarikoski L, Peltomäki P, Lund L, Sundvall J, Juolevi A, Laatikainen T, Aldén‐Nieminen H, Luoto R, Jousilahti P, Salomaa V, Taimi M, Vartiainen E. The National FINRISK 2007 Study. Publications of the National Public Health Institute, B34/2008 (with English summary). 2008. https://www.julkari.fi/bitstream/handle/10024/78146/2008b34.pdf.


