Abstract
Background
No well‐defined protocols currently exist regarding the optimal rate and duration of normal saline administration to prevent contrast‐induced acute kidney injury (CI‐AKI) in patients with renal insufficiency.
Methods and Results
Hydration volume ratios (hydration volume/weight; HV/W) were calculated in 1406 patients with renal insufficiency (estimated glomerular filtration rate [eGFR], <90 mL/min per 1.73 m2) undergoing percutaneous coronary intervention (PCI) with routine speed hydration (1 or 0.5 mL/kg per hour). We investigated the relationship between hydration volume, risk of CI‐AKI (increase in serum creatinine ≥0.5 mg/dL or 25% within 48–72 hours), and prognosis. Mean follow‐up duration was 2.85±0.88 years. Individuals with higher HV/W were more likely to develop CI‐AKI (quartiles: Q1, Q2, Q3, and Q4: 4.3%, 6.6%, 10.9%, and 15.0%, respectively; P<0.001). After adjusting 12 confounders, including age, sex, eGFR, anemia, emergent PCI, diabetes mellitus, chronic heart failure, diuretics, contrast volume, lesions, smoking status, and number of stents, multivariate analysis showed that a higher HV/W ratio was not associated with a decreased CI‐AKI risk (Q2 vs Q1: adjusted odds ratio [OR], 1.13; Q3 vs Q1: adjusted OR, 1.51; Q4 vs Q1: adjusted OR, 1.87; all P>0.05) and even increased CI‐AKI risk (HV/W >25 mL/kg: adjusted OR, 2.11; 95% CI, 1.24–3.59; P=0.006). Additionally, higher HV/W was significantly associated with an increased risk of death (Q4 vs Q1: adjusted hazard ratio, 3.44; 95% CI, 1.20–9.88; P=0.022).
Conclusions
Excessively high hydration volume at routine speed might be associated with increased risk of CI‐AKI and death post‐PCI in patients with renal insufficiency.
Keywords: contrast‐induced acute kidney injury, hydration, percutaneous coronary intervention, renal insufficiency
Subject Categories: Percutaneous Coronary Intervention, Treatment, Clinical Studies
Introduction
Contrast‐induced acute kidney injury (CI‐AKI) has been shown to be significantly associated with increased mortality, morbidity, and health care costs for patients undergoing percutaneous coronary intervention (PCI),1, 2 especially in patients with renal insufficiency. The most effective methods for controlling risk of CI‐AKI have been reported to include preventive measures aimed at reducing risk of PCI‐induced renal complications. According to existing PCI guidelines, the key prophylaxes include identifying high‐risk patients, individually reducing the dose of the contrast medium, and providing adequate hydration with isotonic saline.3, 4, 5, 6, 7, 8 However, no well‐defined protocols currently exist regarding the optimal rate and duration of normal saline administration to prevent CI‐AKI in patients with renal insufficiency undergoing PCI.9, 10 Recently, randomized trials have failed to identify any benefits of increasing hydration volume in patients with renal insufficiency undergoing coronary angiography.11, 12 In fact, in 1 prospective observational study, the observed benefit of additional hydration with isotonic saline was less than half of that observed in patients receiving no intravenous hydration.13 The uncertain benefit of utilizing standard hydration volumes might explain, at least in part, why volume expansion has not yet been adopted in clinical practice. In addition, few studies have investigated the benefits of higher hydration volumes of isotonic saline using a prospective observational design.
Accordingly, the aim of the present retrospective study was to investigate the potential benefits of higher intravascular hydration volumes of normal saline adjusted by weight (hydration volume/weight [HV/W], mL/kg) for prevention of CI‐AKI in patients with renal insufficiency undergoing PCI.
Methods
Subjects
The prospective observational study was conducted in patients with renal insufficiency (estimated glomerular filtration rate [eGFR], <90 mL/min per 1.73 m2, calculated by the Modification of Diet in Renal Disease [MDRD] formula) undergoing PCI according to institutional protocol between January 2010 and October 2012. Using data from the previous study,6 we included patients ≥18 years of age who agreed to stay in the hospital for 2 to 3 days after coronary angiography. Inclusion criteria were use of low‐osmolarity contrast agents and isotonic saline hydration administration. According to the updated European Society of Urogenital Radiology Contrast Media Safety Committee guidelines,14 the exclusion criteria included pregnancy, lactation, intravascular administration of a contrast medium within the previous 7 days or within 3 days postoperation (n=83), no use of low‐osmolarity contrast agents (n=130), cardiovascular surgery or endovascular repair (n=382), end‐stage renal disease or renal replacement (n=7), missing preoperative or postoperative creatinine values (n=61), malignancy (n=3), no use of isotonic saline for hydration (n=18), patients without renal insufficiency (eGFR, ≥90 mL/min per 1.73 m2) (n=827), and patients undergoing coronary angiography without PCI (n=1128).
Finally, 1406 patients were included in the analysis. Follow‐up events were carefully monitored and recorded by trained nurses through office visits and telephone interviews at 1, 6, 12, 24, and 36 months after coronary angiography. Mean follow‐up time was 2.85±0.88 years. The institutional ethics research committee approved the study, and all patients provided written informed consent to participate.
Percutaneous Coronary Intervention
PCI was performed according to standard clinical practice using standard guide catheters, guidewires, balloon catheters, and stents by the femoral or radial approach. The contrast dose was left to the discretion of each interventional cardiologist. All patients received nonionic, low‐osmolarity contrast agents (either Iopamiron or iopromide, both 370 mg I/mL). Treatment was based on the American Heart Association/American College of Cardiology Foundation guidelines.5 According to the local institutional protocol,6 the serum creatinine concentrations were measured in all patients at hospital admission and 1, 2, and 3 days post‐PCI.
Baseline eGFR was calculated using the MDRD equation,15 and the creatinine clearance (CrCl) was calculated by applying the Cockcroft–Gault formula to the serum creatinine concentration.16 Additionally, HV/W ratios were calculated. All patients received a continuous intravenous infusion of isotonic saline at a rate of 1 mL/kg per hour (0.5 mL/kg per hour in cases of left ventricular ejection fraction [LVEF] <40% or severe congestive heart failure) for at least 2 to 12 hours before and 6 to 24 hours after the procedure.
Study Endpoints
The primary endpoint was CI‐AKI, defined as an increase in serum creatinine of ≥0.5 mg/dL or 25% from the baseline within 48 to 72 hours of contrast exposure.17 Secondary endpoints were in‐hospital death status after a major procedure, acute heart failure (AHF), CI‐AKI requiring renal replacement therapy, and the associated health care costs.
Statistical Analysis
For continuous variables, 1‐way ANOVA was conducted for normally distributed data (expressed as mean±SD), and the Kruskal–Wallis test was used for non‐normal distributions (presented as the median and interquartile range). Pearson's chi‐square test or Fisher's exact test was used, as appropriate, for categorical data, which were expressed as percentages. The odds ratios (ORs) of CI‐AKI for subgroups with different HV/W ratios (cut‐off values determined by the quartiles and previous studies cited by the guidelines [25 mL/kg])4, 5 were estimated using univariate and multivariate logistic regression analyses. After considering the trade‐off between overfitting and control of unbalanced factors, we finally used the following factors with P<0.05 on baseline analysis: age, female sex, smoking status, eGFR, mL/min per 1.73 m2, congestive heart failure, diabetes mellitus, emergent PCI, anemia, contrast volume >200 mL, use of diuretics, lesions, and number of stents implanted for the multivariate logistic regression, along with the clinically important factors. Subsequently, sensitivity analyses that divided the HV/W into 2 groups using a cut‐off value of 25 mL/kg were conducted, and reanalysis of all models after excluding LVEF (missing from >100 cases) was also performed. Propensity‐matched analysis was also used as sensitivity analysis for comparisons of CI‐AKI between these 2 groups. Univariate analyses of mortality were performed using the log‐rank test according to baseline HV/W ratios. In addition, multivariate Cox regression analyses, adjusted for use of an intra‐aortic balloon pump, chronic heart failure, anemia, diabetes mellitus, emergent PCI, and age (>75 years), among other factors, were conducted. Propensity‐matched analysis, after balancing the factors, was also performed. Data were analyzed on an available case basis, and missing data were not included. All data analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC) and R software (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria).18 A 2‐sided P<0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 1406 patients (mean age, 65.2±10.4 years) with renal insufficiency (eGFR, <90 mL/min per 1.73 m2) undergoing PCI were enrolled in this study. Mean serum creatinine level was 1.19±0.41 mg/dL at baseline. A total of 277 (19.7%) patients were women; diabetes mellitus occurred in 344 (24.5%) patients; and 254 (18.1%) patients had congestive heart failure.
Baseline characteristics were calculated according to hydration strength, as measured in HV/W quartiles (Tables 1 and 2). Hydration volume and strength were significantly higher in patients who developed CI‐AKI than in those without CI‐AKI (HV: 1179.2±660.8 vs 834.0±433.5 mL; P25–P75: 750–1500 vs 500–1000 mL; HV/W: 19.73±11.3 vs 13.18±7.45 mL/kg; P25–P75: 11.65–27.27 vs 7.81–16.67 mL/kg). Quartiles of HV/W for the present study population were as follows: Q1 (<7.8 mL/kg), Q2 (7.87–11.03 mL/kg), Q3 (11.11–16.67 mL/kg), and Q4 (>16.78 mL/kg). Patients’ HV/W levels were found to correlate with eGFR (r=−0.303; P<0.001); patients with higher HV/W levels had significantly lower levels of eGFR (Q1, Q2, Q3, and Q4: 73.28±12.51, 71.24±14.82, 67.06±16.54, and 58.81±18.80 mL/min, respectively; P<0.001) were older; had more lesions and stents, more frequently had emergent PCI and anemia; and had a higher frequency of intra‐aortic balloon pumps and diuretic use. Moreover, patients with a higher HV/W level were more likely to have a history of chronic heart failure and a lower LVEF.
Table 1.
Baseline Patient Characteristics According to the HV/W Quartiles
| Characteristics | HV/W Quartiles | P trend | |||
|---|---|---|---|---|---|
| Q1 (n=350) | Q2 (n=351) | Q3 (n=366) | Q4 (n=339) | ||
| Sex, male, n (%) | 319 (91.1) | 274 (78.1) | 297 (81.1) | 239 (70.5) | <0.001 |
| Age, y | 61±10 | 64±10 | 67±10 | 69±9 | <0.001 |
| Age >75 y, n (%) | 31 (8.9) | 37 (10.5) | 87 (23.8) | 90 (26.5) | <0.001 |
| Weight, kg | 73.67±7.57 | 61.42±8.72 | 64.30±10.18 | 60.75±9.50 | <0.001 |
| Systolic blood pressure | 130±19 | 129±21 | 130±22 | 131±21 | 0.783 |
| IABP | 6 (1.7%) | 10 (2.8%) | 19 (5.2%) | 27 (8.0%) | <0.001 |
| LVEF, % | 58.8±11.7 | 58.8±11.9 | 56.4±12.1 | 55.4±12.2 | <0.001 |
| LVEF <40%, n (%) | 5 (1.1) | 9 (1.9) | 37 (7.4) | 145 (28.3) | <0.001 |
| CHF, n (%) | 35 (10.0) | 62 (17.7) | 73 (19.9) | 84 (24.8) | <0.001 |
| Medical history, n (%) | |||||
| Diabetes mellitus | 82 (23.4) | 67 (19.1) | 98 (26.8) | 97 (28.6) | 0.019 |
| Smoker | 168 (48.0) | 138 (39.3) | 149 (40.7) | 127 (37.5) | 0.027 |
| Hypertension | 218 (62.3) | 214 (61.0) | 237 (64.8) | 228 (67.3) | 0.325 |
| Dyslipidemia | 61 (17.4) | 52 (14.8) | 53 (14.5) | 38 (11.2) | 0.144 |
| Past MI | 45 (12.9) | 38 (10.8) | 37 (10.1) | 37 (10.9) | 0.686 |
| History of CABG | 5 (1.4) | 1 (0.3) | 3 (0.8) | 1 (0.3) | 0.226 |
| Medication, n (%) | |||||
| ACEI/ARB | 323 (92.3) | 321 (91.5) | 321 (87.7) | 299 (88.2) | 0.105 |
| β‐blocker | 308 (88.0) | 299 (85.2) | 300 (82.0) | 279 (82.3) | 0.095 |
| CCB | 61 (17.4) | 59 (16.8) | 65 (17.8) | 80 (23.6) | 0.082 |
| Diuretics | 35 (10.0) | 51 (14.5) | 80 (21.9) | 84 (24.8) | <0.001 |
| Laboratory measurements | |||||
| Serum creatinine, mg/dL | 1.1±0.2 | 1.1±0.4 | 1.2±0.4 | 1.4±0.6 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 73.28±12.51 | 71.24±14.82 | 67.06±16.54 | 58.81±18.80 | <0.001 |
| CrCl, mL/min | 72.08±19.04 | 60.80±17.59 | 53.90±16.68 | 47.69±18.21 | <0.001 |
| Total cholesterol, mmol/L | 4.17±1.12 | 4.38±1.16 | 4.31±1.02 | 4.40±1.19 | 0.175 |
| HbA1c, % | 6.44±1.14 | 6.40±1.27 | 6.42±1.28 | 6.68±2.07 | 0.005 |
| hs‐CRP, mg/L | 3.74±6.20 | 5.89±11.00 | 9.88±19.40 | 21.54±31.95 | 0.091 |
| Anemia, n (%) | 94 (26.9) | 108 (30.8) | 117 (32.0) | 145 (42.8) | <0.001 |
| Hematocrit, % | 0.40±0.04 | 0.39±0.05 | 0.39±0.05 | 0.37±0.05 | <0.001 |
| K | 3.79±0.39 | 3.73±0.40 | 3.73±0.46 | 3.75±0.47 | 0.398 |
| Na | 139.14±3.23 | 138.69±3.07 | 138.58±2.97 | 138.39±3.03 | 0.081 |
| Ca | 2.25±0.11 | 2.23±0.12 | 2.23±0.12 | 2.85±7.38 | 0.303 |
| PH | 5.91±0.65 | 5.92±0.66 | 5.86±0.83 | 5.86±0.70 | 0.628 |
ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; CABG, coronary artery bypass grafting; CCB, calcium‐channel blocker; CHF, congestive heart failure; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; hs‐CRP, high sensitivity C‐reactive protein; HV/W, ratio of hydration volume to body weight; LVEF, left ventricular ejection fraction; MI, myocardial infarction; Q, quartile.
Table 2.
Baseline Procedural Characteristics of the HV/W Quartiles
| Characteristics | HV/W Quartiles | P trend | |||
|---|---|---|---|---|---|
| Q1 (n=350) | Q2 (n=351) | Q3 (n=366) | Q4 (n=339) | ||
| Emergent PCI, n (%) | 31 (8.9) | 56 (16.0) | 76 (20.8) | 62 (18.3) | <0.001 |
| No. of lesions | 2.1±0.8 | 2.2±0.9 | 2.4±0.9 | 2.6±0.9 | <0.001 |
| No. of stents | 1.8±1.0 | 1.9±1.1 | 2.0±1.1 | 2.1±1.2 | 0.042 |
| Total length of stent, mm | 45±28 | 46±30 | 48±32 | 51±32 | 0.059 |
| Procedure duration, min | 80±43 | 84±39 | 86±41 | 90±42 | 0.041 |
| Contrast volume, mL | 148±55 | 153±60 | 155±64 | 157±67 | 0.222 |
HV/W indicates ratio of hydration volume to body weight; PCI, percutaneous coronary intervention; Q, quartile.
Associations Between HV/W Ratios and Risk of CI‐AKI, Hospital Stay, Cost, and In‐Hospital Outcomes
Individuals with higher HV/W ratios were more likely to develop CI‐AKI (Q1, Q2, Q3, and Q4: 4.3%, 6.6%, 10.9%, and 15.0%, respectively; P<0.001; Table 3). These differences were consistent for the different definitions of CI‐AKI, including when using cutoffs of an absolute increase of more than 0.3 mg/dL and/or a relative increase of more than 50% in the serum creatinine level from the baseline within 72 hours after contrast administration. The higher HV/W ratio groups stayed in the hospital longer (mean hospital stay: 4, 5, 5, and 6 days, respectively; P<0.001) and had higher in‐hospital costs (median costs: $8314, $8634, $9274, and $10 073 US dollars [USD], respectively; P<0.001). Furthermore, patients with elevated HV/W ratios were more likely to experience major adverse clinical events, including acute heart failure (Q1, Q2, Q3, and Q4: 0.29%, 2.28%, 2.73%, and 5.01%, respectively; P=0.001) and overall death (0.3%, 0.6%, 2.2%, and 4.4%, respectively; P<0.001) and to require dialysis (0.3%, 0.6%, 2.9%, and 1.0%, respectively; P<0.001).
Table 3.
Incidence of Contrast‐Induced Nephropathy and In‐Hospital Clinical Outcomes by HV/W Quartiles
| Characteristics | HV/W Quartiles | P trend | |||
|---|---|---|---|---|---|
| Q1 (n=350) | Q2 (n=351) | Q3 (n=366) | Q4 (n=339) | ||
| Scr increase ≥0.5 mg/dL, n (%) | 5 (1.4) | 8 (2.3) | 20 (5.5) | 29 (8.6) | <0.001 |
| Scr increase ≥0.5 mg/dL or ≥25%, n (%) | 15 (4.3) | 23 (6.6) | 40 (10.9) | 51 (15.0) | <0.001 |
| Scr increase ≥0.3 mg/dL, n (%) | 10 (2.9) | 16 (4.7) | 31 (8.7) | 49 (14.7) | <0.001 |
| Scr increase ≥0.3 mg/dL or ≥50%, n (%) | 10 (2.9) | 16 (4.7) | 31 (8.7) | 49 (14.7) | <0.001 |
| Stay, days, median (P25–P75) | 4 (2–6) | 5 (3–7) | 5 (3–8) | 6 (4–9) | <0.001 |
| Cost, USD, median (P25–P75) | 8314 (39.7–67.7) | 8634 (40.8–73.9) | 9274 (44.6–80.8) | 10 073 (45.2–88.3) | <0.001 |
| Acute heart failure, n (%) | 1 (0.29) | 8 (2.28) | 10 (2.73) | 17 (5.01) | 0.001 |
| Death, n (%) | 1 (0.3) | 2 (0.6) | 8 (2.2) | 15 (4.4) | <0.001 |
| Require RRT, n (%) | 1 (0.3) | 2 (0.6) | 10 (2.9) | 14 (1.0) | <0.001 |
HV/W indicates ratio of hydration volume to body weight; Q, quartile; RRT, renal replacement therapy; Scr, serum creatinine; USD, US dollars.
HV/W Ratios and Other Factors Predicting CI‐AKI
After adjusting 12 confounders, including age, sex, eGFR, anemia, emergent PCI, diabetes mellitus, chronic heart failure, diuretics, contrast volume, lesions, smoking status, and number of stents, multivariate logistic regression analysis revealed that, compared with HV/W Q1, the higher HV/W ratios were not associated with a decreased risk of CI‐AKI (Q2 vs Q1: OR, 1.13; 95% CI, 0.55–2.29; Q3 vs Q1: OR, 1.51; 95% CI, 0.77–2.93; Q4 vs Q1: OR, 1.87; 95% CI, 0.96–3.63; Table 4), and there was no statistical interaction between hydration, stratification of eGFR, and Mehran score.3
Table 4.
Univariate Analyses and Multivariate Associations Between CI‐AKI and HV/W Quartiles
| Risk Factors | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| HV/W ratio Q1 | 1 | Reference | — | 1 | Reference | — |
| HV/W ratio Q2 vs Q1 | 1.57 | 0.80 to 3.05 | 0.188 | 1.13 | 0.55 to 2.29 | 0.740 |
| HV/W ratio Q3 vs Q1 | 2.74 | 1.48 to 5.06 | 0.001 | 1.51 | 0.77 to 2.93 | 0.227 |
| HV/W ratio Q4 vs Q1 | 3.95 | 2.18 to 7.18 | 0.000 | 1.87 | 0.96 to 3.63 | 0.065 |
| Age, y | 1.07 | 1.05 to 1.09 | 0.000 | 1.04 | 1.02 to 1.06 | 0.001 |
| Female sex | 1.59 | 1.05 to 2.41 | 0.027 | 1.13 | 0.70 to 1.83 | 0.609 |
| Smoking | 0.66 | 0.45 to 0.97 | 0.034 | 0.86 | 0.56 to 1.34 | 0.510 |
| eGFR, mL/min per 1.73 m2 | 0.97 | 0.96 to 0.98 | 0.000 | 0.99 | 0.98 to 1.00 | 0.077 |
| Congestive heart failure | 2.74 | 1.85 to 4.05 | 0.000 | 1.54 | 0.98 to 2.44 | 0.063 |
| Diabetes mellitus | 1.27 | 0.85 to 1.90 | 0.243 | 0.93 | 0.60 to 1.45 | 0.751 |
| Emergent PCI | 3.26 | 2.20 to 4.85 | 0.000 | 3.00 | 1.93 to 4.67 | 0.000 |
| Anemia | 1.98 | 1.37 to 2.86 | 0.000 | 1.29 | 0.85 to 1.94 | 0.231 |
| Contrast volume >200 mL | 0.78 | 0.49 to 1.25 | 0.305 | 0.96 | 0.57 to 1.61 | 0.877 |
| Use of diuretics | 2.58 | 1.74 to 3.84 | 0.000 | 1.58 | 1.00 to 2.50 | 0.052 |
| Lesions | 1.24 | 1.04 to 1.47 | 0.016 | 1.16 | 0.95 to 1.42 | 0.148 |
| Stents implanted | 0.90 | 0.76 to 1.07 | 0.234 | 0.91 | 0.74 to 1.11 | 0.350 |
The model against a constant‐only model was statistically significant (χ2=105.0899; P<0.0001). Goodness‐of‐fit test (Hosmer–Lemeshow test: χ2=13.6124; P=0.0924). Nagelkerke's R 2: 0.1604. CI‐AKI indicates contrast‐induced acute kidney injury; eGFR, estimated glomerular filtration rate; HV/W, ratio of hydration volume to body weight; OR, odds ratio; PCI, percutaneous coronary intervention; Q, quartile.
Similarly, in the other multivariate model, the adjusted OR for HV/W >25 mL/kg was found to be 2.11 (95% CI, 1.24–3.59; Table 5). The unadjusted and adjusted ORs for HV/W >25 mL/kg for the different subgroups are shown in Figure 1. The propensity‐matched analysis showed that the OR for HV/W >25 mL/kg was 1.38 (95% CI, 0.82–2.35). After excluding significantly absent factors, the multivariate logistic regression and the propensity score analysis results showed similar trends.
Table 5.
Univariate Analyses and Multivariate Associations Between CI‐AKI and HV/W >25 mL/kg
| Risk Factors | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| HV/W ratio >25 mL/kg | 3.33 | 2.08 to 5.35 | 0.000 | 2.11 | 1.24 to 3.59 | 0.006 |
| Age, y | 1.07 | 1.05 to 1.09 | 0.000 | 1.04 | 1.02 to 1.06 | 0.001 |
| Female sex | 1.59 | 1.05 to 2.41 | 0.027 | 1.13 | 0.70 to 1.83 | 0.610 |
| Smoking | 0.66 | 0.45 to 0.97 | 0.034 | 0.85 | 0.55 to 1.32 | 0.468 |
| eGFR, mL/min per 1.73 m2 | 0.97 | 0.96 to 0.98 | 0.000 | 0.99 | 0.98 to 1.00 | 0.093 |
| Congestive heart failure | 2.74 | 1.85 to 4.05 | 0.000 | 1.54 | 0.98 to 2.44 | 0.063 |
| Diabetes mellitus | 1.27 | 0.85 to 1.90 | 0.243 | 0.98 | 0.63 to 1.53 | 0.930 |
| Emergent PCI | 3.26 | 2.20 to 4.85 | 0.000 | 3.17 | 2.04 to 4.93 | 0.000 |
| Anemia | 1.98 | 1.37 to 2.86 | 0.000 | 1.29 | 0.86 to 1.95 | 0.221 |
| Contrast volume >200 mL | 0.78 | 0.49 to 1.25 | 0.305 | 0.95 | 0.57 to 1.60 | 0.860 |
| Use of diuretics | 2.58 | 1.74 to 3.84 | 0.000 | 1.61 | 1.02 to 2.56 | 0.043 |
| Lesions | 1.24 | 1.04 to 1.47 | 0.016 | 1.17 | 0.96 to 1.43 | 0.120 |
| Stents implanted | 0.90 | 0.76 to 1.07 | 0.234 | 0.93 | 0.76 to 1.13 | 0.480 |
The model against a constant‐only model was statistically significant (χ2=107.0989; P<0.0001). Goodness‐of‐fit test (Hosmer–Lemeshow test: χ2=7.7466; P=0.4586). Nagelkerke's R 2: 0.1633. CI‐AKI indicates contrast‐induced acute kidney injury; eGFR, estimated glomerular filtration rate; HV/W, ratio of hydration volume to body weight; OR, odds ratio; PCI, percutaneous coronary intervention; Q, quartile.
Figure 1.
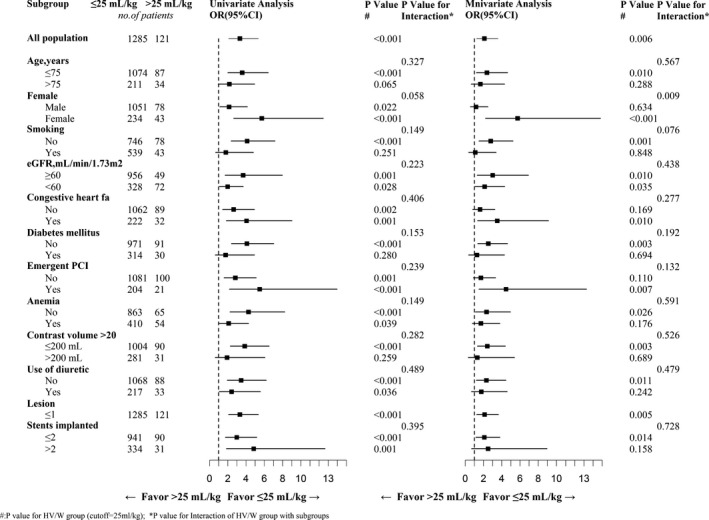
Selected subgroup analyses. eGFR indicates estimated glomerular filtration rate; OR, odds ratio; PCI, percutaneous coronary intervention.
HV/W Ratios and Other Factors for Predicting AHF
After adjusting for age, congestive heart failure, and hypertension, multivariate logistic regression analysis revealed that, compared with HV/W Q1, the higher HV/W ratios were associated with an increased risk of AHF, especially in Q4 (Q2 vs Q1: OR, 5.97; 95% CI, 0.73–48.88; Q3 vs Q1: OR, 5.90; 95% CI, 0.73–47.68; Q4 vs Q1: OR, 9.90; 95% CI, 1.27–77.12; Table S1). Similarly, in the other multivariate model, the adjusted OR for HV/W >25 mL/kg was found to be 2.15 (95% CI, 0.91–5.09; Table S2).
Follow‐up
The median follow‐up period was 2.85±0.88 years (interquartile range, 0.93–4.50), and data were available for all patients who survived to discharge. Log‐rank analyses revealed that patients with higher HV/W ratio quartiles (Q2, Q3, and Q4) showed worse survival rates than patients with HV/W Q1 (P<0.001; Figure 2A), and similar results were obtained for the cut‐off point of HV/W >25 mL/kg (Figure 2B). Moreover, patients with CI‐AKI or AHF were at a higher risk of death (Figure 3).
Figure 2.
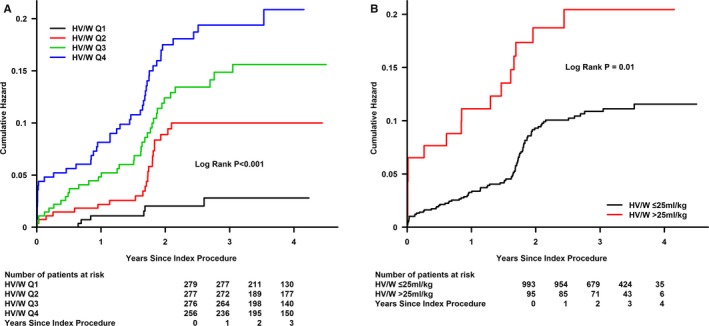
Cumulative mortality as a function of time and HV/W levels. A: HV/W quartiles, B: HV/W>25 ml/kg. HV/W indicates ratio of hydration volume to body weight; Q, quartile.
Figure 3.
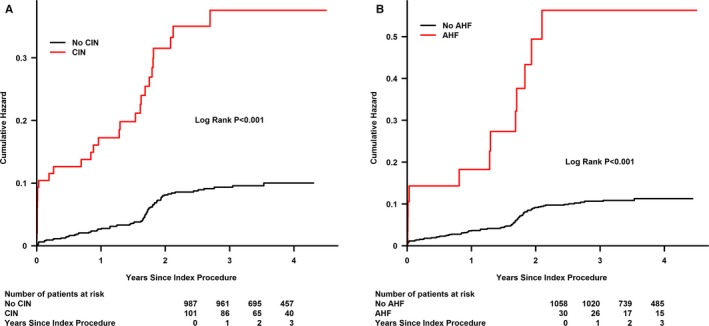
Cumulative mortality as a function of time and contrast‐induced nephropathy (CIN) or acute heart failure (AHF). A: Cumulative mortality as a function of time and CIN. B: Cumulative mortality as a function of time and AHF.
Last, multivariate Cox regression analysis indicated that higher HV/W was significantly associated with an increased risk of death after PCI (Q2 vs Q1: adjusted hazard ratio [HR], 3.01; 95% CI, 1.02–8.87; Q3 vs Q1: adjusted HR, 3.06; 95% CI, 1.06–8.85; Q4 vs Q1: adjusted HR, 3.44; 95% CI, 1.20–9.88; Figure 4). In the other multivariate Cox regression analysis, the adjusted HR for HV/W >25 mL/kg was 1.17 (95% CI, 0.67–2.04; P=0.588; Figure 5).
Figure 4.
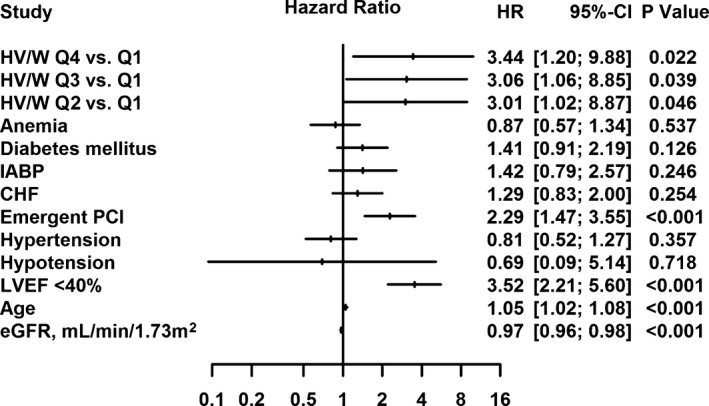
Multivariate Cox regression analysis indicated that the HV/W quartiles were significantly associated with an increased risk of death after percutaneous coronary intervention (PCI). CHF indicates congestive heart failure; eGFR, estimated glomerular filtration rate; HR, hazard ratio; HV/W, ratio of hydration volume to body weight; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction; Q, quartile.
Figure 5.
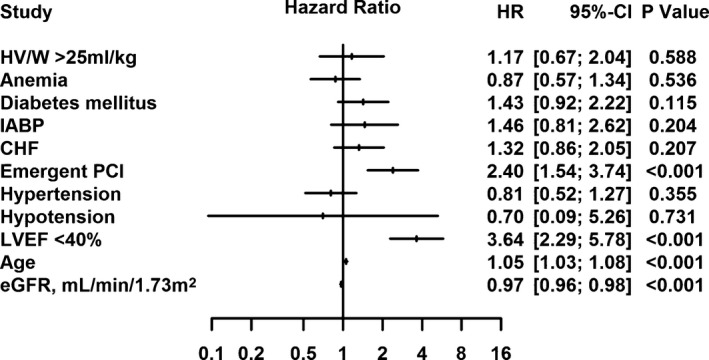
Multivariate Cox regression analysis indicated that excessive hydration (HV/W >25 mL/kg) tended to be associated with an increased risk of death after percutaneous coronary intervention (PCI), although the association was not significant. CHF indicates congestive heart failure; eGFR, estimated glomerular filtration rate; HR, hazard ratio; HV/W, ratio of hydration volume to body weight; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction.
Discussion
To our knowledge, the present study is the first to investigate the potential benefit of a higher hydration volume of normal saline adjusted by weight for the prevention of contrast‐induced nephropathy (CIN) post‐PCI in patients with renal insufficiency. However, we found that a higher hydration volume adjusted by weight and administered at routine speed did not decrease risk of CIN, even after adjustment for other risk factors, such as age, renal function, and contrast dose, and rather appeared to be associated with an increased risk of heart failure and death as well as increased health care costs.
Intravenous hydration with normal saline remains the cornerstone of CI‐AKI prevention in patients with chronic kidney disease, and adequate hydration 12 hours before and 24 hours after PCI, at a speed of 1 mL/kg per hour, has been recommended in the most recent guidelines; however, there is currently very limited clinical evidence supporting this approach.4, 5, 10 Despite being the most frequently recommended method, no well‐defined protocol for hydration with normal saline, especially in terms of the optimal duration and speed for the prevention of CI‐AKI, has been established to guide periprocedural fluid administration in clinical practice. The most frequently used protocol for hydration with saline to prevent CI‐AKI in patients with chronic kidney disease in controlled trials involves fluid supplementation for 12 hours before and after the procedure, rather than 24 hours before and after the procedure, at a speed of 1 mL/kg per hour. In these patients, the CI‐AKI incidence has been reported to be ≈10%, which is similar to that observed in the present study using the same protocol.12, 19, 20, 21, 22 However, more than three quarters of the patients in our real‐world study received a lower hydration volume (<17 mL/kg) than that administered in the above‐mentioned studies. In addition, older patients and those with a higher incidence of diabetes mellitus, worse baseline renal function, and other risk factors of CI‐AKI were prescribed a higher hydration volume in this study. Nevertheless, the physicians could not determine the optimal hydration volume.
In a previous study, radical hydration (>12+1+24 hours, a rate of 1 mL/kg per hour), namely, 20 hours preprocedural and 24 hours postprocedural intravenous hydration with isotonic saline, was found not to be superior to 5 hours preprocedural and 24 hours postprocedural intravenous hydration for the prevention of contrast‐induced increases in serum creatinine (−0.03±0.16 mg/dL vs 0.01±0.13 mg/dL; P=0.16) and cystatin C (−0.06±0.17 mg/dL vs −0.05±0.17 mg/L; P=0.59) in patients with renal insufficiency undergoing elective coronary procedures.11 Furthermore, another study found that more‐radical hydration (3 days, >72 mL/kg) was not better than standard hydration (12+12 hours) for protection of renal function in patients with renal dysfunction undergoing coronary angiography (16.6% vs 10%).12 Both the above‐mentioned studies conducted the hydration at a speed of 1 mL/kg per hour. Similarly, using the same routine hydration speed in the present study, we found that increased hydration strength (HV/W >25 mL/kg) also did not decrease risk of CI‐AKI.
Recently, faster and shorter periprocedural hydration with normal saline (≥3 mL/h) has demonstrated remarkable benefits in reducing risk of CI‐AKI. The REMEDIAL II study showed that the total volume of intravenous hydration associated with a treatment regimen of about 6 hours was higher in the RenalGuard group (PLC Medical Systems, Inc., Franklin, MA) compared to the control group (2312 mL [quartiles 1–3, 1928–2999 mL] vs 1438 mL [quartiles 1–3, 1390–1487 mL]; P<0.001) in patients with an eGFR ≤30 mL/min per 1.73 m2 and/or a risk score of ≤11, whereas the risk of CI‐AKI was lower in the RenalGuard group when an initial bolus (priming) of 250 mL was infused over 30 minutes (preprocedural phase) (11%), as compared to the control group (20.5%).23 Moreover, the MYTHOS study22 also investigated the effects of fast hydration with the RenalGuard system in patients with an eGFR ≤60 mL/min per 1.73 m2. The cumulative intravenous saline hydration volume during the 6‐hour treatment period (3995±1401 mL) was found to be higher than that in the control group (1742±290 mL during 25±2 hours). After the procedure, the short and fast hydration using the RenalGuard system was significantly associated with a decreased risk of CI‐AKI (4.6% vs 18%; P<0.005).21 The POSEIDON study conducted by Brar et al. assessed the efficacy of a new fluid protocol based on the left ventricular end‐diastolic pressure (LVEDP) (5 mL/kg per hour for LVEDP ≤13 mm Hg, 3 mL/kg per hour for <13–18 mm Hg, and 1.5 mL/kg per hour for ≥18 mm Hg), for 4 hours after cardiac catheterization.24 CI‐AKI was found to occur less frequently in patients in the LVEDP‐guided group (6.7% [12 of 178]) than in the control group (16.3% [28 of 172]; relative risk, 0.41; 95% CI, 0.22–0.79; P=0.005). Hydration speed was much lower than that in the above‐mentioned fast hydration groups, suggesting that these protocols may magnify the benefits of hydration to reduce CI‐AKI.
As mentioned, in the present study, the hydration protocol was conducted according to the recent guidelines, which include a safety evaluation of the LVEF and any clinical signs before the procedure. On the other hand, the RenalGuard system– and LVEDP‐based protocols lack effective safety guidelines. However, a higher hydration volume at the routine speed also increases risk of AHF, which may contribute to the increased risk of death observed in this study. In addition, we speculate that patients with a low hydration volume might drink more water (higher oral hydration volume), which may result in a nearly identical hydration volume between groups without increased risk of AHF.
It has been hypothesized that fast hydration may reduce risk of CI‐AKI by expanding plasma volume, reducing renin activation and loss of nitric oxide, reducing production of reactive oxygen species, and through dilution of the contrast medium within the tubular lumen.25, 26 In addition, hydration decreases urine viscosity, which, in turn, may speed up excretion of the contrast medium.27, 28 Hence, rather than the average‐rate long‐term fluid administration, more‐rapid hydration may help attenuate the increase in urine viscosity attributed to contrast administration, thereby aiding in contrast excretion and shortening the length of time tubular cells are exposed to the contrast medium.
Short‐term periprocedural hydration, such as 4 hours postprocedural hydration, may be the most important method, owing to the short excretion half‐life for most patients. When kidney function is normal, the contrast medium is excreted relatively quickly (within a few hours); however, in patients with chronic kidney disease, the excretion half‐life might be more than 10 hours.29 In addition, excessive hydration may increase risk of heart failure, consequently leading to volume overload or increased preload, lower cardiac output, and inadequate renal perfusion, such as cardiorenal syndrome, which, in turn, may increase venous pressure, leading to kidney congestion, activation of the renin angiotensin aldosterone system, and marked alterations of immune and somatic cell signaling.30, 31 These are likely the reasons that higher hydration volumes at the average speed did not show any benefits in preventing CI‐AKI in the present study. Furthermore, despite significant advances in the identification of high‐risk patients as well as in the therapeutic approaches for risk reduction, CI‐AKI is currently not preventable in high‐risk patients who receive higher hydration volumes. Finally, yet importantly, residents may continue additional hydration in patients who develop CI‐AKI in spite of sufficient hydration.
The current study has several limitations. First, because this retrospective observational study was conducted in a single center, the evidence may not be as strong as that obtained in a randomized, controlled trial; cardiologists may have had some bias in performing intensive hydration with higher volumes for patients with more risk factors, which, in turn, may confound the effect of hydration volume on risk of CI‐AKI. The fourth‐quartile group is associated with the highest risk for CI‐AKI; indeed, all the major predictors of CI‐AKI appear more frequently in this group as compared to the others, although we adjusted all these factors through multivariate analysis. Second, around 50% of patients were discharged 2 days post‐PCI, so the serum creatinine concentrations were not measured on day 2 in these patients. This variation, along with the lack of measurement data, may lead to an underestimation of the true incidence of CI‐AKI in the current study population. Third, the CrCl was computed using the Cockcroft–Gault formula rather than by direct measurement. Fourth, variation in the measurement times may have led to missed peak levels of creatinine postprocedure. Finally, oral water intake data was lacking, and we were therefore unable to investigate whether patients with low hydration volumes might have had higher oral hydration volumes, thus potentially reaching nearly the same hydration volume as those treated with a higher intravenous hydration volume.
In conclusion, overall, in patients with renal insufficiency undergoing PCI, excessive hydration volume with normal saline adjusted by weight at a routine speed showed no potential benefits, and even harm, for prevention of CI‐AKI. However, this result should be considered a hypothesis‐generating, preliminary finding rather than an end conclusion, and further multicenter, randomized, controlled trials are needed to investigate the effects of different hydration volumes on risk of CI‐AKI.
Sources of Funding
This study was supported by grants from the Science and Technology Planning Project of Guangdong Province (Grant No.: 2008A030201002) and Guangdong Cardiovascular Institute and Guangdong Provincial Cardiovascular Clinical Medicine Research Fund (Grant No.: 2009X41). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript; the work was not funded by any industry sponsors.
Disclosures
None.
Supporting information
Table S1. Univariate Analyses and Multivariate Associations Between AHF and HV/W Quartiles
Table S2. Univariate Analyses and multivariate Associations Between AHF and HV/W >25 mL/kg
(J Am Heart Assoc. 2016;5:e003171 doi: 10.1161/JAHA.115.003171)
References
- 1. From AM, Bartholmai BJ, Williams AW, Cha SS, McDonald FS. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc. 2008;83:1095–1100. [DOI] [PubMed] [Google Scholar]
- 2. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, Spertus JA. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath‐PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 4. Authors/Task Force members , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 5. Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non‐ST‐ elevation myocardial infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:1920–1959. [DOI] [PubMed] [Google Scholar]
- 6. Liu Y, Chen JY, Tan N, Zhou YL, Yu DQ, Chen ZJ, He YT, Liu YH, Luo JF, Huang WH, Li G, He PC, Yang JQ, Xie NJ, Liu XQ, Yang DH, Huang SJ, Piao‐Ye , Li HL, Ran P, Duan CY, Chen PY. Safe limits of contrast vary with hydration volume for prevention of contrast‐induced nephropathy after coronary angiography among patients with a relatively low risk of contrast‐induced nephropathy. Circ Cardiovasc Interv. 2015;8:e001859. [DOI] [PubMed] [Google Scholar]
- 7. Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media‐associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–336. [DOI] [PubMed] [Google Scholar]
- 8. Zoungas S, Ninomiya T, Huxley R, Cass A, Jardine M, Gallagher M, Patel A, Vasheghani‐Farahani A, Sadigh G, Perkovic V. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast‐induced nephropathy. Ann Intern Med. 2009;151:631–638. [DOI] [PubMed] [Google Scholar]
- 9. Weisbord SD, Palevsky PM. Prevention of contrast‐induced nephropathy with volume expansion. Clin J Am Soc Nephrol. 2008;3:273–280. [DOI] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 11. Torigoe K, Tamura A, Watanabe T, Kadota J. 20‐Hour preprocedural hydration is not superior to 5‐hour preprocedural hydration in the prevention of contrast‐induced increases in serum creatinine and cystatin C. Int J Cardiol. 2013;167:2200–2203. [DOI] [PubMed] [Google Scholar]
- 12. Koc F, Ozdemir K, Kaya MG, Dogdu O, Vatankulu MA, Ayhan S, Erkorkmaz U, Sonmez O, Aygul MU, Kalay N, Kayrak M, Karabag T, Alihanoglu Y, Gunebakmaz O. Intravenous N‐acetylcysteine plus high‐dose hydration versus high‐dose hydration and standard hydration for the prevention of contrast‐induced nephropathy: CASIS–a multicenter prospective controlled trial. Int J Cardiol. 2012;155:418–423. [DOI] [PubMed] [Google Scholar]
- 13. Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Sonel AF, Fine MJ, Palevsky PM. Prevention, incidence, and outcomes of contrast‐induced acute kidney injury. Arch Intern Med. 2008;168:1325–1332. [DOI] [PubMed] [Google Scholar]
- 14. Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement O, Heinz‐Peer G; Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR) . Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–2541. [DOI] [PubMed] [Google Scholar]
- 15. Aguiar‐Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C. Frequency and predictors of contrast‐induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. 2010;139:68–74. [DOI] [PubMed] [Google Scholar]
- 16. Xun L, Cheng W, Hua T, Chenggang S, Zhujiang C, Zengchun Y, Tanqi L. Assessing glomerular filtration rate (GFR) in elderly Chinese patients with chronic kidney disease (CKD): a comparison of various predictive equations. Arch Gerontol Geriatr. 2010;51:13–20. [DOI] [PubMed] [Google Scholar]
- 17. Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379–386. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available at: http://www.r-project.org/. Accessed July 1, 2015. [Google Scholar]
- 19. Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217. [DOI] [PubMed] [Google Scholar]
- 20. Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. [DOI] [PubMed] [Google Scholar]
- 21. Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, Bellandi F. Sodium bicarbonate versus saline for the prevention of contrast‐induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599–604. [DOI] [PubMed] [Google Scholar]
- 22. Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovasc Interv. 2012;5:90–97. [DOI] [PubMed] [Google Scholar]
- 23. Briguori C, Visconti G, Focaccio A, Airoldi F, Valgimigli M, Sangiorgi GM, Golia B, Ricciardelli B, Condorelli G; REMEDIAL II Investigators . Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II): RenalGuard System in high‐risk patients for contrast‐induced acute kidney injury. Circulation. 2011;124:1260–1269. [DOI] [PubMed] [Google Scholar]
- 24. Brar SS, Aharonian V, Mansukhani P, Moore N, Shen AY, Jorgensen M, Dua A, Short L, Kane K. Haemodynamic‐guided fluid administration for the prevention of contrast‐induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. [DOI] [PubMed] [Google Scholar]
- 25. Seeliger E, Wronski T, Ladwig M, Dobrowolski L, Vogel T, Godes M, Persson PB, Flemming B. The renin‐angiotensin system and the third mechanism of renal blood flow autoregulation. Am J Physiol Renal Physiol. 2009;296:F1334–F1345. [DOI] [PubMed] [Google Scholar]
- 26. Heyman SN, Rosen S, Khamaisi M, Idée JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast‐induced nephropathy. Invest Radiol. 2010;45:188–195. [DOI] [PubMed] [Google Scholar]
- 27. Seeliger E, Becker K, Ladwig M, Wronski T, Persson PB, Flemming B. Up to 50‐fold increase in urine viscosity with iso‐osmolar contrast media in the rat. Radiology. 2010;256:406–414. [DOI] [PubMed] [Google Scholar]
- 28. Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast‐induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015. [DOI] [PubMed] [Google Scholar]
- 29. Nossen JO, Jakobsen JA, Kjaersgaard P, Andrew E, Jacobsen PB, Berg KJ. Elimination of the non‐ionic X‐ray contrast media iodixanol and iohexol in patients with severely impaired renal function. Scand J Clin Lab Invest. 1995;55:341–350. [DOI] [PubMed] [Google Scholar]
- 30. House AA, Anand I, Bellomo R, Cruz D, Bobek I, Anker SD, Aspromonte N, Bagshaw S, Berl T, Daliento L, Davenport A, Haapio M, Hillege H, McCullough P, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P, Ronco C; Acute Dialysis Quality Initiative Consensus Group . Definition and classification of Cardio‐Renal Syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1416–1420. [DOI] [PubMed] [Google Scholar]
- 31. Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Analyses and Multivariate Associations Between AHF and HV/W Quartiles
Table S2. Univariate Analyses and multivariate Associations Between AHF and HV/W >25 mL/kg


