Abstract
Background
Drug‐eluting stents are replacing bare‐metal stents, but in‐stent restenosis (ISR) remains a problem. Reactive hyperemia index (RHI) assessed by peripheral arterial tonometry evaluates endothelial function noninvasively. We prospectively assessed the prognostic value of RHI in predicting ISR after percutaneous coronary intervention.
Methods and Results
RHI was measured before percutaneous coronary intervention and at follow‐up (F/U) angiography (F/U RHI; 6 and 9 months post bare‐metal stents– and drug‐eluting stents– percutaneous coronary intervention, respectively) in 249 consecutive patients. At F/U, ISR (stenosis >50% of diameter) was seen in 68 patients (27.3%). F/U natural logarithm (RHI) was significantly lower in patients with ISR than in those without (0.52±0.23 versus 0.65±0.27, P<0.01); no between‐group difference in initial natural logarithm (RHI) (0.60±0.26 versus 0.62±0.25, P=0.56) was seen. By multivariate logistic regression analysis, even after adjusting for other significant parameters in univariate analysis, F/U natural logarithm (RHI) independently predicted ISR (odds ratio: 0.13; 95% CI: 0.04–0.48; P=0.002). In receiver operating‐characteristic analysis, F/U RHI was the strongest predictor of ISR (area under the curve: 0.67; 95% CI: 0.60–0.75; P<0.01; RHI <1.73 had 67.6% sensitivity, 64.1% specificity); area under the curve significantly improved from 0.62 to 0.70 when RHI was added to traditional ISR risk factors (P=0.02). Net reclassification index was significant after addition of RHI (26.5%, P=0.002).
Conclusions
Impaired RHI at F/U angiography independently correlated with ISR, adding incremental prognostic value to the ISR‐risk stratification following percutaneous coronary intervention.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT02131935.
Keywords: endothelial dysfunction, reactive hyperemia, reactive hyperemia–peripheral arterial tonometry, restenosis, risk factor, stent
Subject Categories: Restenosis, Stent, Endothelium/Vascular Type/Nitric Oxide
Introduction
Although the rate of in‐stent restenosis (ISR) is significantly lower after coronary drug‐eluting stent (DES) implantation than it was during the bare metal stent (BMS) era,1, 2 the total number of ISR cases is increasing as the number of implanted DESs is increasing. In addition, worse outcomes after repeat revascularization are reported for DES restenosis compared with BMS restenosis.3, 4, 5 Patients might be recommended for further angiographic evaluation to detect ISR or other coronary progressive obstruction when chest symptoms occur during the poststent follow‐up period. However, half of patients with ISR remain asymptomatic,6 and might be underdiagnosed without further diagnostic tests. Detection and management of DES‐ISR is an emerging issue that requires careful evaluation of the restenosed lesion; thus, a simple, noninvasive method for identifying ISR‐prone patients might help to determine the need for further angiographic workup.
The vascular endothelium attenuates vascular smooth muscle proliferation via the release of endothelium‐derived nitric oxide,7 an essential pathologic finding of ISR.8 During the BMS era, peripheral endothelial dysfunction assessed by forearm flow‐mediated dilation (FMD) was shown to be a strong predictor of BMS‐ISR.9 However, to our knowledge, no studies evaluated the relationship between endothelial dysfunction and DES‐ISR. Furthermore, results of FMD can vary because of technical problems encountered during measurement (poor reproducibility of FMD in vivo due mainly to physiological factors); thus, FMD is not standardized among institutions.10 However, Kuvin et al developed reactive hyperemia–peripheral arterial tonometry (RH‐PAT), a noninvasive, automated clinical test for digital measurement of hyperemic‐vessel response that evaluates endothelial dysfunction and has low intra‐ and interobserver variability.11 The Framingham Heart Study reported that RH‐PAT indices (RHIs) were inversely correlated with various cardiovascular risk factors,12 indicating the clinical utility and predictive value of RH‐PAT. In this prospective cohort study, we hypothesized that peripheral endothelial dysfunction assessed by RH‐PAT could predict the occurrence of ISR after percutaneous coronary intervention (PCI) in the current DES era.
Methods
Study Population and Protocol
This was a prospective observational study of all consecutive patients with coronary artery disease treated with PCI at Kumamoto University Hospital between January 2010 and September 2012. This study was designed as part of our previous prospective single‐center registry (NCT00737945). The inclusion criterion was treatment with PCI for significant coronary artery disease with documented evidence of myocardial ischemia. Exclusion criteria were balloon angioplasty only without stent deployment (n=15); death during hospitalization (n=11); and comorbidities affecting RH‐PAT results such as hemodialysis (n=40), advanced cancer (n=22), post breast cancer surgery (n=3), dementia (n=23), collagen disease (n=17), and RH‐PAT not performed for unknown reasons (n=14). Five hundred thirteen patients underwent PCI at our institution over the study period, and after evaluation for exclusion criteria, 368 consecutive patients were initially enrolled in the study, and in these patients ISR and follow‐up (F/U) RH‐PAT were evaluated at the time of F/U coronary angiography (CAG) performed 6 and 9 months after PCI with BMS and DES, respectively. We compared RH‐PAT between the ISR and non‐ISR groups (Figure 1).
Figure 1.
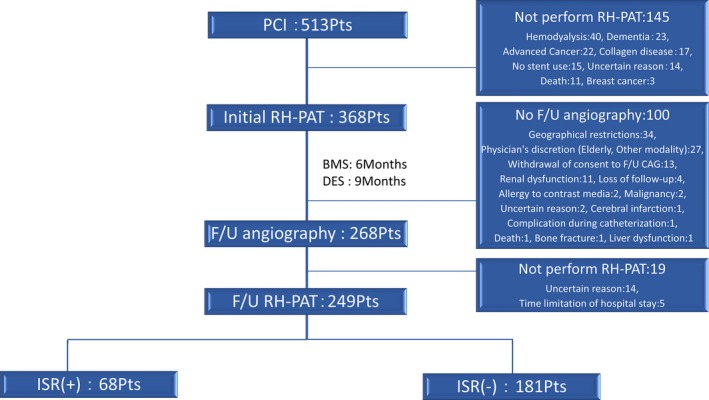
Flowchart of patient enrollment in the present study. During the study F/U period, F/U CAG was not performed in 100 patients, and F/U RH‐PAT was not performed in 19 patients. Excluding these subjects, 249 patients represent the study population in the current study. BMS indicates bare‐metal stent; CAG, coronary angiography; DES, drug‐eluting stent; F/U, follow‐up; ISR, in‐stent restenosis; PCI, percutaneous coronary intervention; RH‐PAT, reactive hyperemia–peripheral arterial tonometry.
Coronary risk factors included diabetes mellitus (diet‐controlled or treated with oral agents or insulin), hypertension (treated medically only), hyperlipidemia (treated medically or measuring >220 mg/dL), current smoking (smoking within 1 year), and family history of coronary artery disease.
The study complied with the Declaration of the Helsinki with respect to investigation in humans, was approved by an institutional review committee, and was conducted in accordance with the guidelines of the ethics committee at Kumamoto University Hospital. Written informed consent was obtained from all patients. This study was registered with Clinicaltrials.gov, number NCT02131935.
Angiographic Analysis
Routine F/U angiography has been integrated into clinical practice to diagnose ISR in Japan. ISR was defined as percent diameter stenosis >50% at F/U angiography assessed by quantitative CAG. Cineangiograms were analyzed with a computer‐assisted automated edge‐detection algorithm (CAAS 5.7.0; Pie Medical Imaging, Maastricht, Netherlands) by 2 experienced investigators who were not aware of the purpose of this study. Lesion length, minimum lumen diameter, reference diameter, and percent diameter stenosis were measured at index procedures and at F/U angiography. Based on these parameters, acute gain and late lumen loss were calculated. When stents were implanted in multiple vessels, the most restenosed segment by quantitative CAG was selected as the representative stented lesion for the current analysis.
Measurement of RHI
RHI was measured using the RH‐PAT system (EndoPAT 2000; Itamar Medical, Caesarea, Israel) before PCI (initial RHI) and at F/U angiography (F/U RHI) using the following method: A blood pressure cuff was placed on 1 upper arm with the contralateral arm serving as a control. A PAT probe was placed on 1 finger of each hand. After a 5‐minute equilibration period, the cuff was inflated to 60 mm Hg above the systolic pressure or to 200 mm Hg for 5 minutes, then deflated to induce reactive hyperemia. RHI reflected the extent of reactive hyperemia and was calculated as the ratio of the average amplitude of the PAT signal over 1 minute, starting 1.5 minutes after cuff deflation (control arm, A; occluded arm, C) divided by the average amplitude of the PAT signal of a 2.5‐minute time period before cuff inflation (control arm, B; occluded arm, D). Thus, RHI=(C/D)/(A/B)×baseline correction. RHI values themselves were not normally distributed both at index PCI procedure and at F/U angiography; therefore, we used a natural logarithmic transformation of RHI. RH‐PAT measurement is largely operator independent, and a computerized algorithm with an online system automatically calculates RHI; thus, there is minimal inter‐ and intraoperator variability. For assessment of day‐to‐day and intraobserver reproducibility of RH‐PAT, based on the Bland‐Altman plot, the 95% CI of the mean difference of 2 measurements included zero (95% CI: −0.16 to 0.11), and the slope of the regression line in the Bland‐Altman plot was not significantly different from zero (r=0.07, P=0.69). For assessment of the interobserver reproducibility of RH‐PAT, the 95% CI of the mean difference of 2 measurements included zero (95% CI: −0.34 to 0.23), and the slope of regression line of the Bland‐Altman plot was not significantly different from zero (r=−0.02, P=1.00). Therefore, both fixed and proportional biases were assumed not to exist.13 RH‐PAT studies were performed when patients were in stable condition after medical therapies and complete revascularization for acute coronary syndrome; in the early morning; with patients in a fasting state, before taking any medications.
Statistical Analysis
A prospective observational study has been planned to evaluate the prognostic impact of RHI on ISR. Based on our preliminary analysis with consecutive patients who underwent PCI and follow‐up CAG and who were recruited from January 2009 to December 2009, ln(RHI) was measured as follows: 0.54 in patients with ISR, 0.64 in patients without ISR, and the SD of this scale was 0.27. Also, the rate of ISR was anticipated to be ≈25%. A sample size of 244 patients who underwent PCI (183 in non‐ISR group, and 61 in ISR group) will be sufficient to detect a difference of ln(RHI) between the ISR and non‐ISR groups at follow‐up CAG, with 80% power and a 5% significance level.
Statistical analysis was performed using SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY). Continuous variables (mean± SD) were compared using unpaired Student t test or the Mann–Whitney U‐test. Categorical variables (frequencies) were compared using χ2 statistics or Fisher's exact test. Logistic regression analysis was employed to determine predictors of ISR. Significant variables (P<0.05 in the univariate analysis) were entered into multivariate analysis. Receiver operating characteristic (ROC) curves were constructed for RHI to predict occurrence of ISR. The area under the curve, sensitivity, and specificity were calculated to predict the ability of RHI to detect patients with ISR, with an area under the curve value of 0.50 indicating no accuracy and a value of 1.00 indicating maximal accuracy. We defined optimal thresholds for RHI by maximizing the sum of sensitivity and specificity.14 The incremental effect of adding RHI to previously reported ISR risk factors (diabetes mellitus, total stent length, minimum stent diameter) to predict future ISR events15, 16, 17 was evaluated by comparing area under the curves between traditional risk factors only and traditional risk factors plus RHI according to the method of DeLong et al.18 Also, the incremental prognostic ability of RHI was evaluated by the net reclassification index.19 A P<0.05 was considered significant.
Results
Study Population
During the study follow‐up period, F/U CAG was not done in 100 patients, and F/U RH‐PAT was not performed in 19 patients. After excluding these subjects, 249 patients represented the study population in the current study (Figure 1). F/U CAG identified 68 patients with ISR (27.3%). As previously noted, ISR was defined as percent diameter stenosis >50% at F/U angiography assessed by quantitative CAG, not defined as clinically ischemia‐driven. A 27.3% restenosis rate was derived from entire cohort including DES‐ISR and BMS‐ISR. In patients treated with DES only, the ISR rate was 19.4%. When defining the ISR as percent diameter stenosis >75% at F/U CAG, the ISR rate was 7.2%. In Table 1, baseline clinical and procedural characteristics of the ISR group (n=68) were compared with those of the non‐ISR group (n=181). There were no significant differences in age, sex, global heart function, and previous myocardial infarction between groups. However, current smoking and clinical presentation of acute coronary syndrome at the index PCI procedure were significantly more common in the ISR group than in the non‐ISR group. Right coronary artery stenting was significantly more common in the ISR group than in the non‐ISR group, and longer and multiple stents and BMS use were significantly associated with ISR.
Table 1.
Baseline Clinical and Procedural Characteristics
| Variables | ISR (+) (n=68) | ISR (−) (n=181) | P Value |
|---|---|---|---|
| Clinical parameters | |||
| Age, y | 71.6±9.3 | 69.7±10.2 | 0.18 |
| Male sex, n (%) | 52 (76) | 116 (64) | 0.06 |
| Coronary risk factors | |||
| Hypertension, n (%) | 48 (71) | 137 (76) | 0.41 |
| Dyslipidemia, n (%) | 53 (78) | 148 (82) | 0.50 |
| Diabetes mellitus, n (%) | 39 (57) | 92 (51) | 0.36 |
| Current smoking, n (%) | 22 (32) | 32 (18) | 0.01 |
| Family history of coronary artery disease, n (%) | 18 (26) | 44 (24) | 0.73 |
| Clinical presentation of acute coronary syndrome, n (%) | 32 (47) | 56 (31) | 0.01 |
| Prior myocardial infarction, n (%) | 14 (21) | 36 (20) | 0.90 |
| Left ventricular ejection fraction, % | 58.9±7.8 | 60.2±9.5 | 0.33 |
| Procedural parameters | |||
| Stented vessel | |||
| Left main trunk, n (%) | 13 (19) | 30 (17) | 0.64 |
| Left anterior descending, n (%) | 44 (65) | 115 (64) | 0.17 |
| Left circumflex, n (%) | 15 (22) | 56 (31) | 0.86 |
| Right, n (%) | 43 (63) | 74 (41) | <0.01 |
| Number of diseased coronary vessels | 0.24 | ||
| One, n (%) | 39 (57) | 124 (69) | |
| Two, n (%) | 23 (34) | 47 (26) | |
| Three, n (%) | 6 (9) | 10 (6) | |
| Chronic total occlusion, n (%) | 14 (21) | 23 (13) | 0.12 |
| Bifurcations with kissing balloon technique, n (%) | 11 (16) | 26 (14) | 0.72 |
| Number of stents, n | 2.7±1.9 | 2.0±1.3 | <0.01 |
| Total stent length, mm | 60.5±44.5 | 43.2±32.3 | <0.01 |
| Minimum stent diameter, mm | 2.8±0.4 | 2.9±0.4 | 0.13 |
| Bare metal stent use, n (%) | 33 (49) | 36 (20) | <0.01 |
Data are presented as mean±SD or number (%). ISR indicates in‐stent restenosis.
Medications and Laboratory Values at Baseline and Follow‐Up
Concomitant medications and laboratory values at baseline and follow‐up that could affect the rate of ISR are presented in Table 2. Clopidogrel at F/U, statins at baseline, and calcium antagonists at F/U were prescribed less often in the ISR group, probably reflecting the higher rate of clinical presentation with acute myocardial infarction with BMS use in the ISR group. High‐density‐lipoprotein cholesterol was significantly lower in the ISR group than in the non‐ISR group at both baseline and F/U.
Table 2.
Concomitant Medications and Blood Examination Parameters
| Baseline | Follow‐Up | |||||
|---|---|---|---|---|---|---|
| ISR (+) (n=68) | ISR (−) (n=181) | P Value | ISR (+) (n=68) | ISR (−) (n=181) | P Value | |
| Concomitant medications | ||||||
| Aspirin | 43 (63%) | 131 (72%) | 0.16 | 67 (99%) | 180 (99%) | 0.47 |
| Clopidogrel | 18 (26%) | 44 (24%) | 0.73 | 51 (75%) | 160 (88%) | 0.01 |
| Statins | 34 (50%) | 124 (69%) | 0.01 | 60 (88%) | 171 (94%) | 0.09 |
| ACE inhibitors | 8 (12%) | 24 (13%) | 0.75 | 26 (38%) | 52 (29%) | 0.15 |
| ARB | 27 (40%) | 68 (38%) | 0.76 | 26 (38%) | 69 (38%) | 0.98 |
| β‐blockers | 26 (38%) | 85 (47%) | 0.22 | 58 (85%) | 137 (76%) | 0.10 |
| Calcium antagonists | 31 (46%) | 96 (53%) | 0.29 | 29 (43%) | 117 (65%) | <0.01 |
| Nitrates | 14 (21%) | 35 (19%) | 0.82 | 7 (10%) | 24 (13%) | 0.53 |
| Antidiabetic agents | 30 (44%) | 73 (40%) | 0.59 | 34 (50%) | 76 (42%) | 0.26 |
| Blood examination parameters | ||||||
| TC, mg/dL | 157±31 | 158±35 | 0.83 | 141±25 | 139±26 | 0.43 |
| LDL‐C, mg/dL | 94±28 | 93±33 | 0.73 | 77±23 | 72±20 | 0.15 |
| HDL‐C, mg/dL | 44±11 | 47±11 | 0.04 | 46±10 | 49±12 | 0.04 |
| Triglycerides, mg/dL | 119±53 | 123±59 | 0.57 | 129±68 | 119±66 | 0.29 |
| HbA1c, % | 7.0±1.2 | 6.9±1.2 | 0.66 | 6.8±1.2 | 6.8±1.0 | 0.83 |
| Creatinine, mg/dL | 0.82±0.27 | 0.84±0.23 | 0.49 | 0.86±0.24 | 0.86±0.24 | 0.85 |
| hs CRP, mg/dL | 0.62±1.28 | 0.51±1.19 | 0.53 | 0.19±0.48 | 0.21±0.99 | 0.86 |
Data are presented as mean±SD or number (%). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II‐receptor blockers; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; hs CRP, high‐sensitivity C‐reactive protein; ISR, in‐stent restenosis; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol.
Quantitative CAG Findings
Table 3 presents summary data for quantitative CAG findings. No significant differences were observed in the target lesions, reference lumen diameter, minimum lumen diameter, and percent diameter stenosis immediately after PCI. Evaluation of the morphology of the original angiographic lesion revealed significantly longer lesion length, smaller reference diameter, and smaller pre‐PCI minimum lumen diameter in the ISR group than in the non‐ISR group. At F/U, minimum lumen diameter was significantly greater, and late lumen loss was significantly smaller, in the non‐ISR group.
Table 3.
Serial Quantitative Coronary Angiography of Most Restenosed Site at Follow‐Up
| Variables | ISR (+) (n=68) | ISR (−) (n=181) | P Value |
|---|---|---|---|
| Baseline quantitative coronary angiography | |||
| Lesion length, mm | 22.6±17.2 | 17.9±12.5 | 0.02 |
| Reference diameter, mm | 2.43±0.68 | 2.63±0.54 | 0.01 |
| Pre‐PCI minimum lumen diameter, mm | 0.51±0.51 | 0.68±0.45 | 0.01 |
| Post‐PCI minimum lumen diameter, mm | 2.32±0.49 | 2.46±0.48 | 0.05 |
| Residual percent diameter stenosis, % | 9.8±8.8 | 8.6±7.3 | 0.31 |
| Acute gain, mm | 1.82±0.58 | 1.78±0.63 | 0.67 |
| Follow‐up quantitative coronary angiography | |||
| Minimum lumen diameter, mm | 0.92±0.52 | 2.50±0.53 | <0.01 |
| Percent diameter stenosis, % | 68.4±16.1 | 14.1±11.0 | <0.01 |
| Late lumen loss, mm | 1.40±0.56 | −0.05±0.48 | <0.01 |
Data are presented as mean±SD. ISR indicates in‐stent restenosis; PCI, percutaneous coronary intervention.
Impact of Endothelial Function on ISR
Figure 2 shows representative records of RH‐PAT signals and CAG for ISR and non‐ISR patients. The ISR group was found to have a significantly lower F/U ln(RHI) than the non‐ISR group, whereas the difference in initial ln(RHI) between groups was not significant (Figure 3). The same results were obtained when using the raw RHI value (Figure S1). In patients without ISR, ln(RHI) at the F/U period significantly improved from initial ln(RHI) (P=0.03), whereas the F/U ln(RHI) in patients with ISR tended to deteriorate compared with the initial ln(RHI) (P=0.08). The serial change in RHI was also associated with ISR. Absolute change in ln(RHI) was −0.15±0.56 in the ISR group and 0.08±0.57 in the non‐ISR group (P=0.004). ISR occurred more frequently in the worsening‐ln(RHI) group than in the improving‐ln(RHI) group (43 of 122 [35%] versus 25 of 127 [20%], P=0.006).
Figure 2.
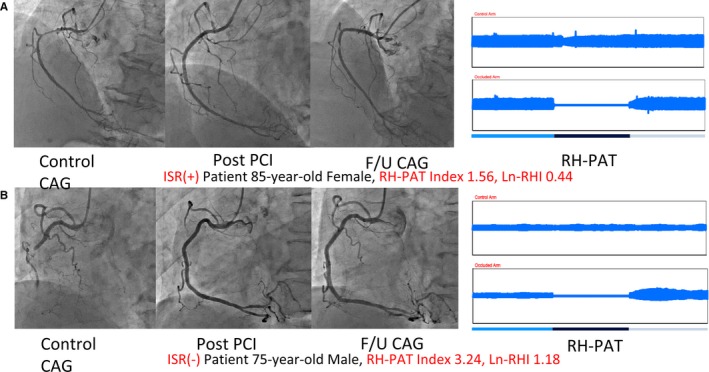
Representative records of RH‐PAT signals and CAG in ISR and non‐ISR patients. A, Typical ISR patient. B, Non‐ISR patient. CAG indicates coronary angiography; F/U, follow‐up; ISR, in‐stent restenosis; ln‐RHI, natural logarithm of reactive hyperemia–peripheral arterial tonometry index; PCI, percutaneous coronary intervention; RH‐PAT, reactive hyperemia–peripheral arterial tonometry.
Figure 3.
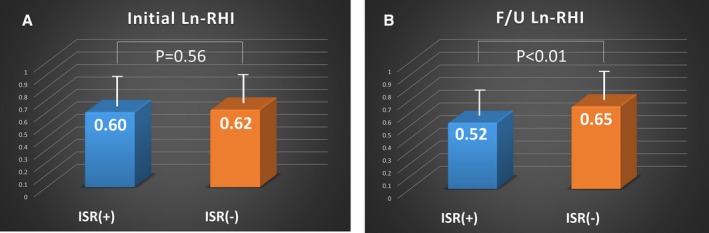
RHI and ISR. ln(RHI) in ISR and non‐ISR patients at (A) index percutaneous coronary intervention and (B) F/U. Bars represent averages of the ln(RHI) in each group; error bars indicate 1 SD. F/U indicates follow‐up; ISR, in‐stent restenosis; ln(RHI), natural logarithm of reactive hyperemia–peripheral arterial tonometry index.
Even after adjustment for other significant parameters in univariate analysis (BMS use, right coronary artery stenting, total stent length, calcium antagonist use, current smoking, post‐PCI minimum lumen diameter, high‐density‐lipoprotein cholesterol, and clinical presentation of acute coronary syndrome), multivariate logistic regression analysis revealed F/U ln(RHI) to be an independent predictor of ISR (odds ratio 0.13; 95% CI: 0.04–0.48, P=0.002) (Table 4). Furthermore, among patients with only DES implanted, RHI was also found to be a significant factor in predicting ISR by multiple logistic regression analysis (odds ratio 0.09; 95% CI: 0.02–0.49; P=0.006) after adjusting for parameters identified to be significant in univariate analysis (total stent length, post‐PCI minimum lumen diameter, and diabetes mellitus).
Table 4.
Multiple Logistic Regression Analysis: Predictors of In‐Stent Restenosis
| Variables | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| ln(RHI) at follow‐up period | 0.13 | 0.04 to 0.48 | 0.002 |
| BMS use | 3.56 | 1.42 to 8.93 | 0.007 |
| Right coronary artery stenting | 2.01 | 0.98 to 4.10 | 0.06 |
| Total stent length, mm | 1.01 | 1.00 to 1.02 | 0.07 |
| Calcium antagonist use | 0.56 | 0.29 to 1.08 | 0.08 |
| Current smoking | 1.84 | 0.89 to 3.83 | 0.10 |
| Post‐PCI MLD, mm | 0.56 | 0.27 to 1.15 | 0.11 |
| HDL‐C, mg/dL | 0.98 | 0.96 to 1.02 | 0.30 |
| Clinical presentation of ACS | 1.15 | 0.47 to 2.82 | 0.77 |
ACS indicates acute coronary syndrome; BMS, bare‐metal stent; HDL‐C, high‐density lipoprotein cholesterol; ln(RHI), natural logarithm of reactive hyperemia–peripheral arterial tonometry index; MLD, minimum lumen diameter; PCI, percutaneous coronary intervention; RHI, reactive hyperemia–peripheral arterial tonometry index.
ROC Analysis for RHI to Predict Occurrence of ISR
ROC curves were constructed to assess the ability of RHI to predict the occurrence of ISR. The area under the curve for detection of ISR was 0.67 (95% CI: 0.60–0.75; P<0.01) of RHI in all patients (Figure 4A) and 0.67 (95% CI: 0.57–0.77; P<0.01) of RHI in DES‐only patients (Figure 4B), and 0.68 (95% CI: 0.55–0.81; P=0.01) of RHI in BMS‐only patients (Figure 4C). Using an RHI cutoff value of 1.73, the sensitivity and specificity for the detection of ISR were 67.6% and 64.1% for all patients and 64.1% and 65.7% for DES patients, respectively. Using an RHI cutoff value of 1.87, the sensitivity and specificity for the detection of ISR were 63.9% and 78.8% for BMS‐only patients.
Figure 4.
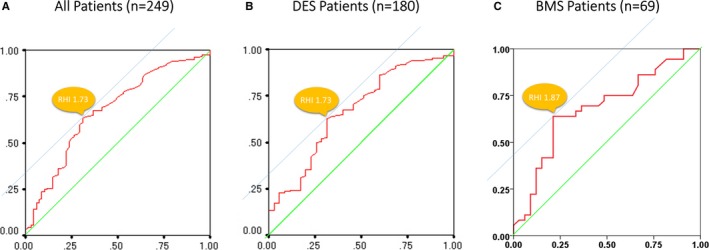
ROC curves to identify ISR. ROC curves for RHI identifying patients with ISR for (A) all patients, (B) patients with DES implantation, and (C) patients with BMS implantation. AUC for detection of ISR was 0.67 (95% CI: 0.60–0.75; P<0.01) of RHI in all patients, 0.67 (95% CI: 0.57–0.77; P<0.01) of RHI in DES‐only patients alone, and 0.68 (95% CI: 0.55–0.81; P=0.01) of RHI in BMS‐only patients. AUC indicates area under the curve; BMS, bare‐metal stent; DES, drug‐eluting stent; ISR, in‐stent restenosis; RHI, reactive hyperemia–peripheral arterial tonometry index; ROC, receiver operating characteristic.
Incremental Prognostic Ability of RHI
To assess the incremental prognostic ability of RHI, ROC analysis was performed for the logistic regression models of known traditional risk factors alone (diabetes mellitus, total stent length, and minimum stent diameter) and traditional risk factors plus RHI, and the area under the curves were compared (Figure 5). ROC analysis indicated that the area under the curve was 0.62 (95% CI: 0.54–0.70) for traditional risk factors alone. However, area under the curve increased to 0.70 (95% CI: 0.63–0.78) and the difference in area under the curve was statistically significant after adding RHI to traditional risk factors (P=0.02, Figure 5A). Moreover, in subgroup analysis of DES‐only patients, area under the curve increased from 0.72 (95% CI: 0.62–0.83) to 0.78 (95% CI: 0.69–0.86) after adding RHI to the traditional risk factors (Figure 5B); in subgroup analysis of BMS‐only patients, area under the curve increased from 0.65 (95% CI: 0.52–0.78) to 0.73 (95% CI: 0.60–0.85) after adding RHI to the traditional risk factors (Figure 5C). We also reclassified the risks of known traditional ISR factors after PCI. The net reclassification index was significant with the inclusion of RHI (17.7% for non‐ISR patients, 8.8% for ISR patients, and 26.5% overall; P=0.002) (Table 5).
Figure 5.
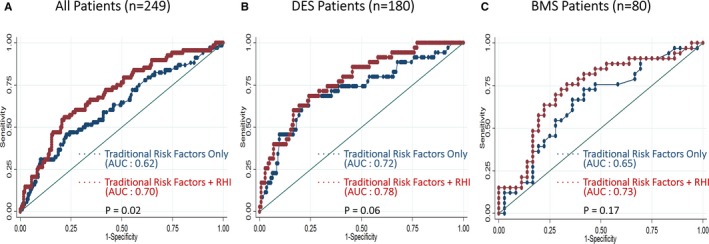
Comparison of ROC curves to identify ISR between traditional risk factors only and traditional risk factors+RHI in all patients, DES patients, and BMS patients. ROC curves for traditional risk factors only and traditional risk factors+RHI to identify ISR in (A) all patients, (B) patients with DES implantation, and (C) patients with BMS implantation. AUC indicates area under the curve; BMS, bare‐metal stent; DES, drug‐eluting stent; ISR, in‐stent restenosis; RHI, reactive hyperemia–peripheral arterial tonometry index; ROC, receiver operating characteristic.
Table 5.
Reclassification by Addition of RHI to Traditional ISR Risk Factorsa
| Original Risk Categoryb (Using Traditional ISR Risk Factor Alone) | New Risk Category (Using Traditional ISR Risk Factor+RHI) | ||
|---|---|---|---|
| Low Risk | Intermediate Risk | High Risk | |
| Patients without ISR (n=181) | |||
| Low risk | 19 | 10 | 0 |
| Intermediate risk | 51 | 76 | 12 |
| High risk | 1 | 2 | 10 |
| Patients with ISR (n=68) | |||
| Low risk | 2 | 6 | 0 |
| Intermediate risk | 9 | 31 | 9 |
| High risk | 0 | 0 | 11 |
Net reclassification index was 17.7% (32 of 181 patients) for patients without ISR, 8.8% (6 of 68 patients) for those with ISR, and 26.5% for all patients (P=0.002) when RHI was used in conjunction with traditional ISR risk factors. ISR indicates in‐stent restenosis; RHI, reactive hyperemia–peripheral arterial tonometry index.
Traditional risk factors: diabetes mellitus, total stent length, and stent diameter.
Low risk: <20%; intermediate risk: 20% to 40%; high risk: >40%.
Discussion
To our knowledge, this is the first report to reveal that peripheral endothelial dysfunction as assessed by RH‐PAT can predict the occurrence of ISR after PCI in the current DES era. In this prospective study, significantly impaired endothelial function at F/U was observed in patients with ISR, and RHI at F/U was significantly correlated with the presence of ISR independently of various comorbidities and known traditional ISR risk factors. Furthermore, the addition of RHI‐derived endothelial dysfunction to the previously described ISR prognostic factors improved risk stratification in patients with ISR. These findings suggest that an additional physiological assessment of endothelial function could be of clinical value in the identification of patients vulnerable to developing ISR.
Endothelial Dysfunction as an Underlying Mechanism of ISR
The development of ISR is a complex and multifactorial process.20 Over the years, many predictive clinical, biological, genetic, lesion‐related, and procedural risk factors for restenosis have been identified (eg, diabetes mellitus, stent length, stent diameter, extent of medial injury during angioplasty, and resistance to the eluting drug). Although the underlying causes are yet to be completely elucidated, ISR represents an abnormal vascular response to, and repair of, injury that results in excessive tissue growth and is most likely another manifestation of the same atherosclerotic disease. The status of the vascular wall, especially the vascular endothelium, is the key component of the response to the vascular injury initiated by coronary stenting. In fact, it has previously been shown that endothelial‐derived nitric oxide suppresses smooth muscle cell proliferation, leading to inhibition of intimal hyperplasia after vascular injury in animal models.7, 21 Recently, it has been reported that traditional coronary risk factors and/or coronary atherosclerosis changes the mediator of flow‐induced vasodilation from endothelial‐derived nitric oxide to hydrogen peroxide in the human coronary circulation22; moreover, although the upregulation of hydrogen peroxide preserves flow‐induced vasodilation, it may produce chronic structural changes in the coronary artery.23 This means coronary endothelial dysfunction plays an extremely important role in the development of ISR. In a prior study by Bonetti et al,24 RH‐PAT was found to be closely correlated with coronary endothelial function assessed using intracoronary injection of acetylcholine during coronary catheterization, which is the “gold standard.” These findings might help explain the significant relationship between ISR and RH‐PAT‐derived endothelial dysfunction observed in the present study.
We recently reported that endothelial dysfunction has been recognized as a surrogate for atherosclerotic‐disease activity and is an independent predictor of cardiovascular events and prognosis.25 Also, our institute has consistently demonstrated that RH‐PAT‐derived endothelial dysfunction can predict future cardiovascular events in a broad range of heart disease: (1) female patients,26 (2) patients with heart failure with preserved ejection fraction,27 (3) high‐risk patients,25 (4) patients with chronic kidney disease,28 and (5) patients with heart failure with reduced ejection fraction.13 Endothelial dysfunction can be defined as reduced bioavailability of nitric oxide, which plays many roles in maintaining vascular health (eg, regulating vasomotion). Hence, endothelial dysfunction is considered an impairment of endothelium‐dependent vasodilation. Lerman and Zeiher defined endothelial dysfunction as the ultimate risk of the risk factors, a summation of the integrated effects of cardiovascular risk factors.29 Risk factors for atherosclerosis, such as elevated serum cholesterol level, higher plasma glucose level, hypertension, smoking, aging, obesity, chronic infection, and inflammation, can all disrupt this balance and lead to endothelial dysfunction. Taken together, we suggest that peripheral microvascular endothelial dysfunction could be associated with, and considered a barometer of, the total burden of cardiovascular risk, or as described by Lerman et al, ultimate risk of the risk factors.29 Another perspective is that endothelial dysfunction, as assessed by RHI, can be explained to a certain extent by clinically evident risk factors,30 which suggests that the majority of pathogenic factors contributing to endothelial dysfunction have not yet been fully clarified. Healthcare professionals must therefore recognize that a clinical assessment of endothelial function could be an integrated parameter reflecting unknown atherogenic factors, including mental stress, environmental and genetic background, and even residual risks. In the current study, the prognostic ability of RHI for ISR was significantly stronger than that of other factors, perhaps because RHI acted as a summation of the integrated effects of cardiovascular risk factors, although other, already known ISR factors were also associated with the occurrence of ISR.
Endothelial Function and ISR in the Literature
In the BMS era, 2 studies using FMD to assess peripheral endothelial dysfunction showed the relationship between endothelial dysfunction at F/U and ISR. Our results are consistent with those observed by Kitta et al,31 who found that impairment of FMD at the time of 6‐month F/U was independently associated with late ISR (defined as >50% diameter stenosis) in native coronary arteries, whereas baseline FMD did not show such an association. In the study by Patti et al,32 impaired FMD assessed 30 days after PCI independently predicted the occurrence of ISR at 6‐month F/U. In their retrospective study, Kitta et al measured FMD before and 6 months after PCI in 141 consecutive patients and showed that impairment of FMD in the brachial artery at the time of F/U was independently and closely associated with late ISR in native coronary arteries. However, this study was retrospective in design and included only patients with BMS placement. Patti et al, in their prospective study, conducted during the BMS era, demonstrated that impaired FMD independently predicted the occurrence of ISR. However, because F/U angiography was performed in the presence of signs or symptoms of myocardial ischemia and only clinical ISR was evaluated, the relationship between overall binary ISR rate and endothelial function was not determined. In both studies, endothelial function was related to late ISR at F/U but not at index PCI. Similar to their findings, RHI at index PCI also showed no relationship to late ISR in the current study. A possible explanation is that initial RHI reflects the history of atherosclerosis up to the point of index PCI but that F/U RHI reflects endothelial function between index PCI and F/U. It seems clear that our patient cohort was treated with current optimal medical therapy, including dual antiplatelet therapy and statins. However, many studies have shown that endothelial function measured by RH‐PAT is reversible using both pharmacological and nonpharmacological treatments,33, 34, 35 which suggests that endothelial dysfunction may be a therapeutic target for reducing residual risk, even in patients successfully treated with coronary stenting and optimal medical therapy. Thus, improving endothelial dysfunction (if present) by pharmacological or nonpharmacological means after PCI may be a promising strategy for reducing residual risk in patients undergoing coronary stent placement.
Advantage of RHI Compared With FMD
Endothelial function determined by brachial artery FMD has not been successfully incorporated into the current comprehensive risk stratification system because of its operator dependency and technical problems.36, 37 Added benefits of FMD to traditional risk factors in cardiovascular risk reclassification have not been established.38, 39 Digital RHI is a reproducible and less operator‐dependent technique for peripheral endothelial function assessment11, 12, 36, 40 that noninvasively reflects coronary endothelial function in a way that is practical and clinically useful.24 Whereas FMD attenuates markedly with advancing age, digital RHI is a good reflection of modifiable metabolic risk factors, including obesity, cholesterolemia, diabetes mellitus, and smoking,41 suggesting the clinical utility of RHI in providing therapeutic guidance. The use of RHI as a noninvasive assessment of endothelial function could represent an important advance in comprehensive clinical cardiovascular risk evaluation even after invasive coronary revascularization with optimal medical treatment.
Study Limitations
Several study limitations should be noted. First, this is a single‐center study with a relatively small number of study patients. However, to the best of our knowledge, the present study is the largest one to investigate the association between endothelial dysfunction and post‐PCI ISR prospectively in the current DES era. Second, we defined ISR as in‐stent angiographic narrowing represented by percent diameter stenosis >50% rather than as clinical ischemia‐driven. However, RHI‐derived endothelial function was significantly impaired in patients with target‐lesion revascularization compared with those without target‐lesion revascularization. Third, patients with an extremely high risk of ISR (eg, those undergoing hemodialysis) were excluded from this study. Fourth, the higher percentage of the patients without F/U CAG and F/U RH‐PAT would create selection bias. Fifth, the F/U periods were different between patients treated with BMS and DES. However, the present study results were consistent among all cohort, BMS cohort, and DES cohort. Therefore, the robust results of the present study might not be affected by the differential F/U duration between the stent types. Furthermore, it would be expected that RHI is truly a useful device to predict ISR precisely because the RHI just prior to F/U angiography can detect the patients at high risk for the occurrence of ISR after PCI in each stent type. Sixth, the most restenosed segment was chosen for analysis in the current study. Generally, the severity of ISR has been most commonly expressed as parameters at the most restenosed segment, since such parameters at the segment were significantly associated with the occurrence of target lesion revascularization, which is one of the major adverse cardiovascular events after coronary stenting. Multicenter studies will be required to confirm our results in a larger patient population.
Conclusions
Impaired RHI at F/U angiography was independently correlated with the occurrence of ISR, adding incremental prognostic significance to ISR risk stratification in patients undergoing PCI.
Sources of Funding
This work was supported by a Grant‐in‐Aid for Young Scientists B (22790713, 24790769) and a Grant‐in‐Aid for Scientific Research C (26461075) from the Ministry of Education, Science, and Culture, Japan (to Tsujita) and Smoking Research Foundation.
Disclosures
None.
Supporting information
Figure S1. RHI and ISR. RHI in ISR and non‐ISR patients at (A) index percutaneous coronary intervention and (B) F/U. Bars represent averages of the RHI in each group; error bars indicate 1 SD. F/U indicates follow‐up; ISR, in‐stent restenosis; RHI, reactive hyperemia–peripheral arterial tonometry index.
Acknowledgments
The authors thank Michiyo Saito, MT; Daisuke Obara, BMET; Taiki Harada, BMET; Chieko Kojo, BMET; and Koichi Ashimura, BMET, for their dedicated assistance with catheterization laboratory data acquisition and data administration. We also thank Yumie Harada, RT; and Shuichi Tochihara, RT for their skillful assistance with quantitative coronary angiographic analysis.
(J Am Heart Assoc. 2016;5:e003202 doi: 10.1161/JAHA.116.003202)
References
- 1. Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R; Lesions RAVEL Study Group . Randomized study with the sirolimus‐coated Bx velocity balloon‐expandable stent in the treatment of patients with de novo native coronary artery lesions. A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. [DOI] [PubMed] [Google Scholar]
- 2. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE; SIRIUS Investigators . Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. [DOI] [PubMed] [Google Scholar]
- 3. Ribamar Costa J, Sousa AG, Moreira A, Alves da Costa R, Cano MN, Maldonado G, Campos Neto C, Jardim C, Pavanello R, Sousa JE. Comparison of the very long term (>1 year) outcomes of drug‐eluting stents for the treatment of bare‐metal and drug‐eluting stent restenosis. EuroIntervention. 2009;5:448–453. [DOI] [PubMed] [Google Scholar]
- 4. Steinberg DH, Gaglia MA Jr, Pinto Slottow TL, Roy P, Bonello L, De Labriolle A, Lemesle G, Torguson R, Kineshige K, Xue Z, Suddath WO, Kent KM, Satler LF, Pichard AD, Lindsay J, Waksman R. Outcome differences with the use of drug‐eluting stents for the treatment of in‐stent restenosis of bare‐metal stents versus drug‐eluting stents. Am J Cardiol. 2009;103:491–495. [DOI] [PubMed] [Google Scholar]
- 5. Whan Lee C, Kim SH, Suh J, Park DW, Lee SH, Kim YH, Hong MK, Kim JJ, Park SW, Park SJ. Long‐term clinical outcomes after sirolimus‐eluting stent implantation for treatment of restenosis within bare‐metal versus drug‐eluting stents. Catheter Cardiovasc Interv. 2008;71:594–598. [DOI] [PubMed] [Google Scholar]
- 6. Giedd KN, Bergmann SR. Myocardial perfusion imaging following percutaneous coronary intervention: the importance of restenosis, disease progression, and directed reintervention. J Am Coll Cardiol. 2004;43:328–336. [DOI] [PubMed] [Google Scholar]
- 7. Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium‐derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999;99:44–52. [DOI] [PubMed] [Google Scholar]
- 9. Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–1932. [DOI] [PubMed] [Google Scholar]
- 10. Hijmering ML, Stroes ES, Pasterkamp G, Sierevogel M, Banga JD, Rabelink TJ. Variability of flow mediated dilation: consequences for clinical application. Atherosclerosis. 2001;157:369–373. [DOI] [PubMed] [Google Scholar]
- 11. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 12. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujisue K, Sugiyama S, Matsuzawa Y, Akiyama E, Sugamura K, Matsubara J, Kurokawa H, Maeda H, Hirata Y, Kusaka H, Yamamoto E, Iwashita S, Sumida H, Sakamoto K, Tsujita K, Kaikita K, Hokimoto S, Matsui K, Ogawa H. Prognostic significance of peripheral microvascular endothelial dysfunction in heart failure with reduced left ventricular ejection fraction. Circ J. 2015;79:2623–2631. [DOI] [PubMed] [Google Scholar]
- 14. Zou KH, O'Malley AJ, Mauri L. Receiver‐operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. [DOI] [PubMed] [Google Scholar]
- 15. Stone GW, Parise H, Witzenbichler B, Kirtane A, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Lansky AJ, Mehran R. Selection criteria for drug‐eluting versus bare‐metal stents and the impact of routine angiographic follow‐up: 2‐year insights from the HORIZONS‐AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2010;56:1597–1604. [DOI] [PubMed] [Google Scholar]
- 16. Tu JV, Bowen J, Chiu M, Ko DT, Austin PC, He Y, Hopkins R, Tarride JE, Blackhouse G, Lazzam C, Cohen EA, Goeree R. Effectiveness and safety of drug‐eluting stents in Ontario. N Engl J Med. 2007;357:1393–1402. [DOI] [PubMed] [Google Scholar]
- 17. Yeh RW, Normand SL, Wolf RE, Jones PG, Ho KK, Cohen DJ, Cutlip DE, Mauri L, Kugelmass AD, Amin AP, Spertus JA. Predicting the restenosis benefit of drug‐eluting versus bare metal stents in percutaneous coronary intervention. Circulation. 2011;124:1557–1564. [DOI] [PubMed] [Google Scholar]
- 18. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 19. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 20. Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2012;9:53–62. [DOI] [PubMed] [Google Scholar]
- 21. Cayatte AJ, Palacino JJ, Horten K, Cohen RA. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb. 1994;14:753–759. [DOI] [PubMed] [Google Scholar]
- 22. Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow‐induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res. 2014;115:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weil BR, Canty JM Jr. Ceramide signaling in the coronary microcirculation: a double‐edged sword? Circ Res. 2014;115:475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 25. Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, Matsubara J, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H, Nagayoshi Y, Yamamuro M, Sakamoto K, Iwashita S, Jinnouchi H, Taguri M, Morita S, Matsui K, Kimura K, Umemura S, Ogawa H. Peripheral endothelial function and cardiovascular events in high‐risk patients. J Am Heart Assoc. 2013;2:e000426 doi: 10.1161/JAHA.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55:1688–1696. [DOI] [PubMed] [Google Scholar]
- 27. Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–1786. [DOI] [PubMed] [Google Scholar]
- 28. Hirata Y, Sugiyama S, Yamamoto E, Matsuzawa Y, Akiyama E, Kusaka H, Fujisue K, Kurokawa H, Matsubara J, Sugamura K, Maeda H, Iwashita S, Jinnouchi H, Matsui K, Ogawa H. Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol. 2014;173:481–486. [DOI] [PubMed] [Google Scholar]
- 29. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. [DOI] [PubMed] [Google Scholar]
- 30. Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T. Noninvasive vascular function measurement in the community: cross‐sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4:371–380. [DOI] [PubMed] [Google Scholar]
- 31. Kitta Y, Nakamura T, Kodama Y, Takano H, Umetani K, Fujioka D, Saito Y, Kawabata K, Obata JE, Ichigi Y, Mende A, Kugiyama K. Endothelial vasomotor dysfunction in the brachial artery is associated with late in‐stent coronary restenosis. J Am Coll Cardiol. 2005;46:648–655. [DOI] [PubMed] [Google Scholar]
- 32. Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D'Ambrosio A, Montesanti R, Di Sciascio G. Impaired flow‐mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75. [DOI] [PubMed] [Google Scholar]
- 33. Cornelissen VA, Onkelinx S, Goetschalckx K, Thomaes T, Janssens S, Fagard R, Verhamme P, Vanhees L. Exercise‐based cardiac rehabilitation improves endothelial function assessed by flow‐mediated dilation but not by pulse amplitude tonometry. Eur J Prev Cardiol. 2014;21:39–48. [DOI] [PubMed] [Google Scholar]
- 34. Matsue Y, Matsumura A, Suzuki M, Hashimoto Y, Yoshida M. Differences in action of atorvastatin and ezetimibe in lowering low‐density lipoprotein cholesterol and effect on endothelial function: randomized controlled trial. Circ J. 2013;77:1791–1798. [DOI] [PubMed] [Google Scholar]
- 35. Philpott AC, Hubacek J, Sun YC, Hillard D, Anderson TJ. Niacin improves lipid profile but not endothelial function in patients with coronary artery disease on high dose statin therapy. Atherosclerosis. 2013;226:453–458. [DOI] [PubMed] [Google Scholar]
- 36. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vlachopoulos C. Progress towards identifying biomarkers of vascular aging for total cardiovascular risk prediction. J Hypertens. 2012;30(suppl):S19–S26. [DOI] [PubMed] [Google Scholar]
- 38. Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 39. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. [DOI] [PubMed] [Google Scholar]
- 41. Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham Heart Study. Hypertension. 2011;57:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. RHI and ISR. RHI in ISR and non‐ISR patients at (A) index percutaneous coronary intervention and (B) F/U. Bars represent averages of the RHI in each group; error bars indicate 1 SD. F/U indicates follow‐up; ISR, in‐stent restenosis; RHI, reactive hyperemia–peripheral arterial tonometry index.


