Abstract
Background
Alirocumab undergoes target‐mediated clearance via binding of proprotein convertase subtilisin/kexin type 9 (PCSK9). Statins increase PCSK9 levels; the effects of nonstatin lipid‐lowering therapies are unclear. Every‐4‐weeks dosing of alirocumab may be appropriate for some patients in absence of background statin but is not yet approved.
Methods and Results
Low‐density lipoprotein cholesterol (LDL‐C), PCSK9, and alirocumab levels were assessed in subjects (LDL‐C >130 mg/dL, n=24/group) after a 4‐week run‐in taking oral ezetimibe, fenofibrate, or ezetimibe placebo, when alirocumab 150 mg every 4 weeks (days 1, 29, and 57) was added. Maximal mean LDL‐C reductions from day −1 baseline (prealirocumab) occurred on day 71 in all groups: alirocumab plus placebo, 47.4%; alirocumab plus ezetimibe, 56.6%; and alirocumab plus fenofibrate, 54.3%. LDL‐C reductions were sustained through day 85 with alirocumab plus placebo (47.0%); the duration of effect was slightly diminished at day 85 versus day 71 with ezetimibe (49.6%) or fenofibrate combinations (43.2%). Free PCSK9 concentrations were lowest at day 71 in all groups, then increased over time; by day 85, free PCSK9 concentrations were higher, and alirocumab levels lower, with alirocumab plus fenofibrate, and to a lesser extent alirocumab plus ezetimibe, versus alirocumab plus placebo.
Conclusions
Alirocumab 150 mg every 4 weeks produced maximal LDL‐C reductions of 47% in combination with placebo and 54% to 57% in combination with ezetimibe or fenofibrate. The oral lipid‐lowering therapies appear to increase PCSK9 levels, leading to increased alirocumab clearance. Although the duration of effect was modestly diminished with alirocumab plus ezetimibe/fenofibrate versus placebo, the effect was less than observed in trials with background statins, and it would not preclude the use of alirocumab every 4 weeks in patients taking these nonstatin lipid‐lowering therapies concomitantly.
Clinical Trial Registration
URL: http://www.Clinicaltrials.gov. Unique identifier: NCT01723735.
Keywords: cholesterol, hypercholesterolemia, lipids, pharmacokinetics
Subject Categories: Lipids and Cholesterol
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds the low‐density lipoprotein receptor (LDLR) on hepatocytes.1 Monoclonal antibodies to PCSK9 block its effect on the LDLR and reduce low‐density lipoprotein cholesterol (LDL‐C) levels. Such antibodies undergo target‐mediated clearance by binding to PCSK9. Although statins reduce LDL‐C, they also increase PCSK9 levels.2 This may limit the extent of LDL‐C reduction possible with statins as well as affecting clearance of PCSK9 antibodies when added to statins.
Addition of the PCSK9 antibody alirocumab to statin therapy produces sustained LDL‐C reductions when dosed every 2 weeks (Q2W).3, 4, 5, 6 However, when alirocumab was dosed every 4 weeks (Q4W) in combination with statin, reductions were not fully sustained over the dosing interval.4, 6 This may be because of a statin‐induced increase in PCSK9 levels and target‐mediated clearance of alirocumab. A single 150‐mg dose of alirocumab without background statin appeared to give a more stable reduction over 4 weeks.7 However, multiple dosings of alirocumab Q4W without background statins have not been investigated. Moreover, little is known about potential effects on LDL‐C and PCSK9 from combining alirocumab with nonstatin lipid‐lowering therapies (LLTs) such as ezetimibe and fenofibrate.
Ezetimibe inhibits the digestive absorption of cholesterol and related plant sterols and is primarily used for reducing LDL‐C as an adjunct to statin.8 Fenofibrate, an activator of peroxisome proliferator‐activated receptor α, increases lipolysis and clearance of triglyceride‐rich lipoproteins; although fenofibrate reduces LDL‐C (in the context of lower baseline triglycerides), it also has a marked effect on triglycerides.9 The effect of ezetimibe and fenofibrate on PCSK9 is unclear. Animal studies suggest that ezetimibe increases PCSK9 levels,10, 11 but no effect was seen in humans.12 The change in PCSK9 levels with fibrates varies widely among reports.13
This study assessed the pharmacokinetic and pharmacodynamic profile and circulating PCSK9 levels when alirocumab 150 mg is administered Q4W with placebo or in combination with ezetimibe or fenofibrate.
Materials and Methods
This randomized partial‐blinded study (clinicaltrials.gov identifier: NCT01723735) was conducted in 2 centers in France in 3 parallel groups. The study protocol was approved by the appropriate institutional review boards and was performed in accordance with the ethical principles of the Declaration of Helsinki and in compliance with good clinical practice. All subjects provided written informed consent.
Subjects
Male or female subjects were eligible if they were aged 18 to 65 years with a body mass index between 18.0 and 30.0 kg/m2 and had LDL‐C levels >130 mg/dL at screening. Subjects were certified as healthy on the basis of a comprehensive clinical assessment (detailed medical history and complete physical examination) and were not taking any LLTs at the time of enrolment. Any previous LLT had been discontinued at least 4 weeks before screening. Subjects were excluded if they had any clinically significant medical history or if they had taken any medication within the 2 weeks before the study start (with the exception of hormonal contraception or menopausal hormone replacement therapy).
Study Design
After the screening, subjects were randomized into 3 groups, entered a 28‐day initial therapy run‐in period, and received once‐daily oral doses of either ezetimibe placebo, ezetimibe 10 mg, or fenofibrate 160 mg (Figure 1). Subjects with LDL‐C levels ≥100 mg/dL at the end of the run‐in period then entered the alirocumab treatment period, during which they received alirocumab 150 mg Q4W subcutaneously on days 1, 29, and 57, in addition to continuing initial oral therapy. Subjects were instructed to keep to their usual diet throughout the study. The oral ezetimibe placebo matched the ezetimibe tablets; fenofibrate treatment was unblinded because placebo fenofibrate was not available for use in the study. The run‐in period was included to allow steady state of both LLT compounds to be reached and full lipid‐lowering effect to be achieved,14 as well as to assess their effect on PCSK9 concentrations before alirocumab was administered.
Figure 1.
Study design. *Placebo for ezetimibe. Red arrows indicate time of alirocumab injections. At the bottom left of the figure, PK refers to free PCSK9 and total alirocumab. D indicates study day; EZE, ezetimibe; FENO, fenofibrate; PCSK9, proprotein convertase subtilisin/kexin type 9; PO, oral administration; Q4W, every 4 weeks; QD, every day; R, randomization; SC, subcutaneous administration.
End Points
The primary end point was the mean percent change in LDL‐C from day −29 (the day before the initial therapy run‐in). However, because this study was intended to specifically assess the effect of alirocumab, an additional baseline of day −1 (the day before first administration of alirocumab) was considered at the time of the statistical analysis plan. This additional baseline therefore accounted for changes in lipid profiles during the initial therapy run‐in period with ezetimibe and fenofibrate alone and allowed assessment of the specific effect of alirocumab on LDL‐C in combination with placebo (ie, as monotherapy) or in combination with the LLTs. The primary analysis was considered to be percent change from baseline to day 71. Two time points were used as baseline as shown in Figure 1.
Secondary end points included the percent change in secondary lipid parameters, including total cholesterol, non–high‐density lipoprotein cholesterol (non–HDL‐C), apolipoprotein B, lipoprotein(a), triglycerides, HDL‐C, and apolipoprotein A1 and changes in free and total PCSK9 levels and total alirocumab levels.
Pharmacokinetic parameters calculated for alirocumab included maximum serum concentration (Cmax) following the third injection and the area under the serum concentration–versus–time curve from day 57 to day 85 (AUCD57–85; ie, the 28‐day period after the last alirocumab dose).
Safety, including adverse events (AEs), vital signs, electrocardiogram, and laboratory assessments, was also evaluated.
Laboratory Assessments
Blood samples were taken in the morning after a 10‐hour overnight fast and before study drug administration. Sampling times are shown on Figure 1; note that no measurements were taken after the second alirocumab injection (indicated by a dotted line on the results figures). Total cholesterol, HDL‐C, and triglycerides were directly measured at local laboratories; apolipoprotein B, apolipoprotein A1, and lipoprotein(a) were directly measured at a central laboratory. LDL‐C was calculated by using the Friedewald formula (LDL‐C=total cholesterol−HDL‐C−[triglycerides/5]).
Free and total PCSK9 levels and total alirocumab levels in serum were determined by using specific validated enzyme‐linked immunosorbent assays (Regeneron Pharmaceuticals). The lower limits of detection were 31.2 ng/mL for free PCSK9, 156 ng/mL for total PCSK9, and 78 ng/mL for alirocumab; values below these levels are set to 0 for presentation of results.
Statistical Methods
Enrollment of 72 subjects into the alirocumab treatment phase was planned to ensure ≥60 subjects (20 per group) could be included in the primary analysis. This sample size was calculated to give a maximal imprecision of ≤18.7% in terms of 95% CIs, for the relative change of LDL‐C means between alirocumab plus ezetimibe or alirocumab plus fenofibrate versus alirocumab plus placebo, with 90% assurance, assuming an SD of 25% based on previous trial results.7 The study was not powered for statistical comparisons of changes in secondary end points.
The percent change from baseline in LDL‐C was analyzed by using a linear mixed‐effects model. This model had fixed terms for treatment (alirocumab 150 mg Q4W plus ezetimibe, alirocumab 150 mg Q4W plus fenofibrate, or alirocumab 150 mg Q4W plus placebo), sex, study site, day, and treatment × day interaction term and with “subject (treatment×sex)” as a random effect. A variance component structure was used for the variance–covariance matrix of the random effects. The variance component structure was defined as:
Other parameters such as age were not included in the model, because differences between groups were not significant. Estimates and 95% CIs were determined at each time point for the difference between means of alirocumab plus ezetimibe or fenofibrate combinations versus alirocumab plus placebo. This analysis was conducted by using day −29 as baseline and was repeated with day −1 as baseline. Secondary lipid parameters were assessed in a similar way, and P‐values are given for descriptive purposes only. Because all analyses were considered as fully exploratory, multiplicity was not considered.
Changes in free and total PCSK9 levels were summarized by using descriptive statistics. Estimates (and 90% CIs) of geometric mean ratios of alirocumab plus ezetimibe versus alirocumab plus placebo, or alirocumab plus fenofibrate versus alirocumab plus placebo, were calculated by using a linear fixed‐effects model.
Pharmacokinetic parameters were summarized by using descriptive statistics. The effect on alirocumab serum levels of coadministering alirocumab with ezetimibe or with fenofibrate versus alirocumab plus placebo was assessed by using a linear fixed‐effects model with fixed terms for treatment, sex, and study site and with log of weight as covariate. Estimates and 90% CIs for the ratios of geometric means of Cmax and AUC values between each treatment group were determined.
Safety data were summarized by using descriptive statistics. Statistical analyses were conducted with use of SAS Proc Mixed (SAS Institute).
Results
One hundred sixty‐seven subjects were screened, and 79 subjects were randomized and entered the run‐in period. Of these 79 subjects, 72 (24 per group) qualified to enter the alirocumab treatment period and 7 were excluded: 4 because their LDL‐C was <100 mg/dL by the end of the run‐in period, 2 because of AEs (increased alanine transaminase levels while receiving fenofibrate), and 1 withdrew for personal reasons. Baseline characteristics for the 72 subjects who commenced alirocumab treatment and were included in the primary analysis are shown in Table 1.
Table 1.
Subject Characteristics at Main Baseline (Day −29)
| Treatment Group | Alirocumab 150 mg Q4W+Placebo (n=24) | Alirocumab 150 mg Q4W+Ezetimibe (n=24) | Alirocumab 150 mg Q4W+Fenofibrate (n=24) |
|---|---|---|---|
| Age, y | 48.5 (12.8) | 49.5 (10.7) | 54.6 (7.6) |
| Male, n (%) | 11 (45.8) | 11 (45.8) | 10 (41.7) |
| Race, n (%) | |||
| Caucasian/white | 23 (95.8) | 21 (87.5) | 24 (100) |
| Black | 1 (4.2) | 3 (12.5) | 0 |
| Body mass index, kg/m2 | 23.9 (2.0) | 25.5 (2.7) | 24.7 (2.5) |
| Calculated LDL‐C, mg/dL | 183.3 (38.7) | 181.7 (37.1) | 180.6 (31.3) |
| Total cholesterol, mg/dL | 264.5 (43.7) | 260.6 (40.6) | 263.7 (40.6) |
| Apolipoprotein B, g/L | 1.28 (0.21) | 1.28 (0.22) | 1.28 (0.17) |
| Non–HDL‐C, mg/dL | 198.4 (40.6) | 200.3 (39.8) | 199.5 (31.7) |
| HDL‐C, mg/dL | 65.7 (12.4) | 60.3 (13.1) | 64.2 (15.5) |
| Apolipoprotein A1, g/L | 1.58 (0.16) | 1.55 (0.21) | 1.57 (0.20) |
| Triglycerides (mg/dL), median (range) | 78.8 (44.3–177.1) | 95.7 (35.4–168.3) | 94.8 (53.1–194.9) |
| Lipoprotein(a) (g/L), median (range) | 0.27 (0.0–1.6) | 0.33 (0.0–1.6) | 0.17 (0.0–1.5) |
| Free PCSK9, ng/mL | 146.5 (54.3) | 150.7 (48.5) | 152.1 (54.1) |
Values are mean (SD) unless otherwise stated. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; Q4W, every 4 weeks.
Effects on LDL‐C
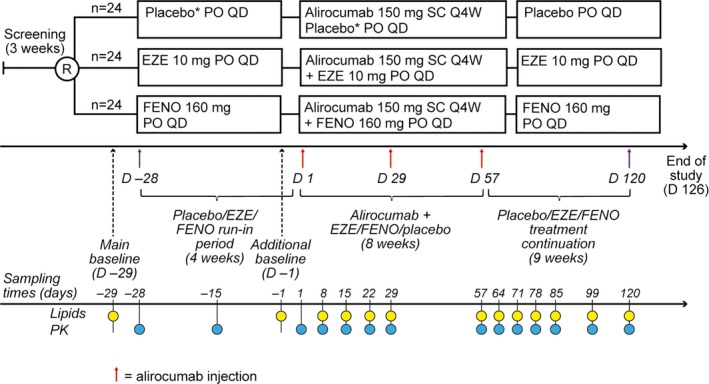
During the course of the 28‐day initial therapy run‐in, LDL‐C levels were reduced by a mean (SEM) of 19.8% (2.1%) with ezetimibe and 25.9% (3.2%) with fenofibrate from day −29 baseline values; mean change in the placebo group was +1.6% (3.0%) (Figure 2A).
Figure 2.
Time‐courses of mean LDL‐C percent change from main (day −29) baseline (A) and additional (day −1) baseline (B). Dotted lines between days 29 and 57 indicate no measurement taken for this period. Triangles below the x‐axis indicate timing of alirocumab injections. EZE indicates ezetimibe; FENO, fenofibrate; LDL‐C, low‐density lipoprotein cholesterol; Q4W, every 4 weeks. A, Differences in mean LDL‐C percentage reductions from pre–run‐in baseline values (day −29) were statistically significant between alirocumab+ezetimibe vs alirocumab+placebo at all time points, and between alirocumab+fenofibrate vs alirocumab+placebo at all time points except day 99 (P<0.05). B, Differences in mean LDL‐C percent reductions from prealirocumab baseline values (day −1) were statistically significant between alirocumab+ezetimibe vs alirocumab+placebo on days 8, 15, 22, 64, 71, and 120, and between alirocumab+fenofibrate vs alirocumab+placebo on days 8, 15, and 29 (P<0.05).
After the initiation of alirocumab Q4W, the greatest mean percent reduction in LDL‐C levels was observed in all groups on day 71 (Figure 2; Table 2). Coadministration of alirocumab with ezetimibe or fenofibrate produced significantly greater mean LDL‐C reductions from the main (day −29) baseline (ie, pre–run‐in) versus alirocumab plus placebo at almost every time point (P<0.05; primary analysis; Figure 2A). These data represent the combined effect of alirocumab and the concomitant lipid therapy from the original (pre–run‐in) LDL‐C baseline level.
Table 2.
Percentage Change in Lipid Parameters From the Main Baseline (Day −29) and the Additional Baseline (Day −1) to Day 71 (14 Days After the Third Alirocumab Dose)
| Treatment Group | % Change From Day −29 to 71 (Only Placebo, Ezetimibe or Fenofibrate Were Given From Days −29 to −1) | % Change From Day −1 to Day 71 (First Alirocumab Injection Administered on Day 1; Placebo/Ezetimibe/Fenofibrate Treatment Continued) | ||||
|---|---|---|---|---|---|---|
| Alirocumab 150 mg Q4W+Placebo (n=24) | Alirocumab 150 mg Q4W+Ezetimibe (n=24) | Alirocumab 150 mg Q4W+Fenofibrate (n=24) | Alirocumab 150 mg Q4W+Placebo (n=24) | Alirocumab 150 mg Q4W+Ezetimibe (n=24) | Alirocumab 150 mg Q4W+Fenofibrate (n=24) | |
| LDL‐C | −48.2 (2.3) | −65.3 (2.0)† | −66.8 (2.7)† | −47.4 (3.2) | −56.6 (2.5)* | −54.3 (3.5) |
| Total cholesterol | −31.6 (1.4) | −45.7 (1.5)† | −46.1 (1.9)† | −31.5 (2.6) | −36.5 (1.4) | −32.4 (2.2) |
| Non–HDL‐C | −43.0 (1.7) | −60.6 (1.9)† | −64.4 (2.5)† | −43.0 (2.7) | −51.9 (2.1)* | −50.5 (3.2) |
| Apo B | −39.1 (1.5) | −53.5 (1.8)† | −58.3 (2.1)† | −38.4 (2.4) | −44.9 (2.0) | −44.6 (2.5) |
| HDL‐C | 3.3 (3.4) | 5.4 (3.2) | 12.3 (3.1)* | 3.6 (2.9) | 6.4 (3.1) | 8.7 (3.0) |
| ApoA1 | 1.9 (6.3) | 1.2 (9.0) | 3.4 (7.3) | 1.4 (10.1) | 1.0 (6.9) | 2.9 (8.4) |
| Triglycerides, median (range) | 5.7 (−48.5 to 266.7) | −13.8 (−53.4 to 53.5)* | −36.0 (−57.9 to 11.3)† | −3.9 (−41.3 to 77.6) | −16.5 (−37.2 to 24.2) | −3.5 (−58.0 to 74.1) |
| Lp(a), median (range) | −20.3 (−63.2 to 33.3) | −27.0 (−71.4 to 35.8) | −19.9 (−57.6 to 38.3) | −11.7 (−58.8 to 160.9) | −9.2 (−67.0 to 66.7) | −20.4 (−56.8 to 17.7)* |
Values are mean (SEM) unless otherwise stated. Apo indicates apolipoprotein; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a); Q4W, every 4 weeks.
P‐values are linked to tests of the means. *P<0.05, † P<0.0001 vs alirocumab+placebo.
The analysis with use of the day −1 baseline (ie, prealirocumab) allows for examination of the specific effects of alirocumab. Mean (SEM) percentage LDL‐C reductions with alirocumab plus placebo showed little change during the 28‐day period after the third dose: day 57, 44.4% (2.8%); day 71, 47.4% (3.2%); and day 85, 47.0% (2.7%) (Figure 2B), suggesting a sustained effect over the Q4W dosing interval. Mean (SEM) LDL‐C reductions from day −1 to day 71 were greater with alirocumab plus ezetimibe versus with alirocumab plus placebo (56.6% [2.5%] versus 47.4% [3.2%], P<0.05); corresponding reductions with alirocumab plus fenofibrate (54.3% [3.5%]) were not significantly different from those with alirocumab plus placebo (P=0.11; Figure 2B, Table 2). In comparison with alirocumab plus placebo, for the alirocumab–LLT combinations, the LDL‐C reduction from day −1 to day 85 was smaller than the reduction to day 71 (49.6% [1.6%] versus 56.6% [2.5%] with ezetimibe and 43.2% [3.8%] versus 54.3% [3.5%] with fenofibrate). This suggests a modest attenuation of the LDL‐C–lowering effect with the alirocumab–LLT combinations during the Q4W dosing interval compared with the placebo combination (Figure 2B).
Effects on PCSK9 and Relationship With LDL‐C
PCSK9 is described here as either free PCSK9, representing unbound PCSK9 available to interact with the LDLR, or total PCSK9, representing all circulating PCSK9 including inactive alirocumab‐bound PCSK9.
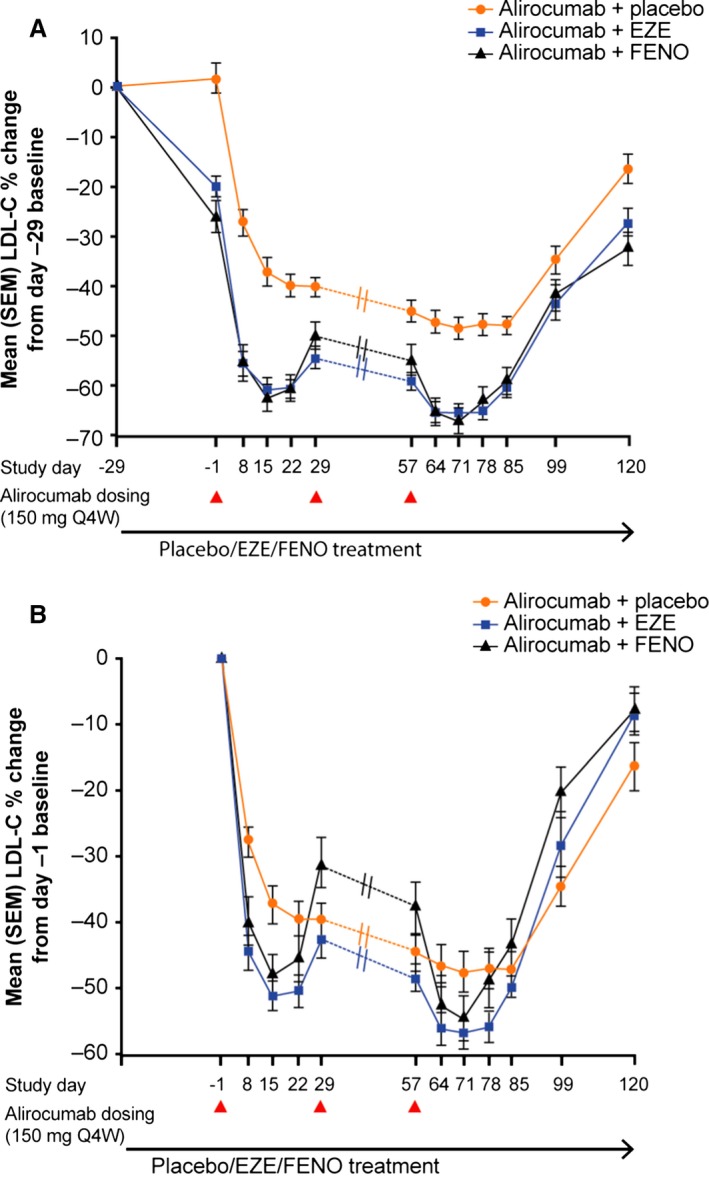
Mean baseline free PCSK9 concentrations were similar across all groups at the start of the initial therapy run‐in period (day −29; 147–152 ng/mL). By the end of the run‐in and before the first alirocumab dose (day 1), mean free PCSK9 concentrations were 119 ng/mL with placebo, 142 ng/mL with ezetimibe (24% greater than placebo; 90% CI 4–47%), and 217 ng/mL with fenofibrate (92% greater than placebo; 90% CI 62–128%; Figure 3A).
Figure 3.
Mean free PCSK9 levels in all 3 treatment groups (A), and free PCSK9 levels compared with percentage changes in LDL‐C from the main baseline (day −29) for alirocumab plus placebo (B), plus ezetimibe (C), and plus fenofibrate (D). Dotted lines between days 29 and 57 indicate no measurement taken for this period. EZE indicates ezetimibe; FENO, fenofibrate; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin 9; Q4W, every 4 weeks.
Free PCSK9 was reduced to undetectable or very low concentrations (0–5 ng/mL) by day 71 in all groups; then concentrations gradually increased over days 71 to 85 (Figure 3A). In the group receiving alirocumab plus placebo, mean free PCSK9 remained low (17 ng/mL) at day 85, consistent with the relatively stable LDL‐C reductions in this group (Figure 3B). In comparison, mean free PCSK9 concentrations were higher at day 85 with ezetimibe (60 ng/mL) and fenofibrate (102 ng/mL), corresponding with the modest attenuation of the LDL‐C–lowering effect observed when alirocumab was administered with the LLTs (Figure 3C and 3D).
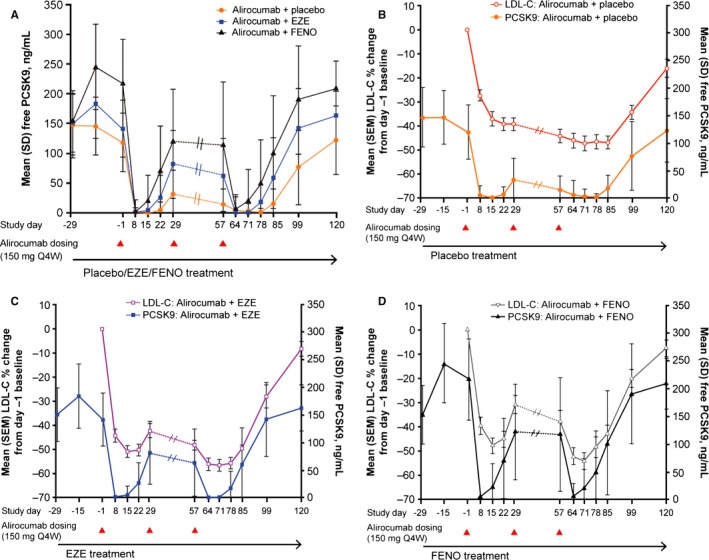
Total PCSK9 concentrations increased from baseline in all groups after the first alirocumab dose (Figure 4). Overall, mean total PCSK9 concentrations were highest in the group receiving fenofibrate (Figure 4).
Figure 4.
Total PCSK9 concentrations and LDL‐C percentage changes from the additional baseline (day −1). The continuous line indicates total PCSK9, dashed line indicates LDL‐C. Dotted lines between days 29 and 57 indicate no measurement taken for this period. Triangles below the x‐axis indicate timing of alirocumab injections. EOS indicates end of study; EZE, ezetimibe; FENO, fenofibrate; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin 9.
Effects on Alirocumab (Pharmacokinetics)
When comparing alirocumab plus ezetimibe versus alirocumab plus placebo, total alirocumab Cmax values did not differ statistically (mean [SD] Cmax, 21.9 [8.9] versus 24.3 [8.6] mg/L; point estimate: 0.92 [90% CI 0.78–1.09]), with a nonsignificant trend toward lower AUCD57–85 in the alirocumab plus ezetimibe treatment group (364 [143] versus 445 [189] mg·day/L; point estimate: 0.85 [0.70–1.03]), suggesting slightly faster clearance of alirocumab in this group.
Both alirocumab Cmax and AUCD57–85 were reduced with alirocumab plus fenofibrate versus alirocumab plus placebo (Cmax, 17.1 [6.7] versus 24.3 [8.6] mg/L; AUCD57–85, 292 [138] versus 445 [189] mg·day/L; point estimates: 0.71 [0.60–0.84] and 0.64 [0.53–0.77], respectively) indicating a greater rate of clearance for alirocumab when combined with fenofibrate.
Relationship Between LDL‐C, PCSK9, and Alirocumab Concentrations
Figure 5 compares LDL‐C reductions and concentrations of free and total PCSK9 and of alirocumab for days 57 to 85 (the 4‐week period after the third alirocumab dose). Maximal LDL‐C reductions for the LLT combinations were greater than those for alirocumab plus placebo, although PCSK9 levels and the alirocumab clearance rate were increased with the LLTs, and the duration of efficacy was slightly reduced with the LLTs (Figure 5). Comparison of mean free PCSK9 concentrations and total alirocumab levels suggests that if free PCSK9 remains lower than 40 to 50 ng/mL and alirocumab levels remain above 9 to 10 mg/L, then the LDL‐C–lowering effect of alirocumab is stable.
Figure 5.
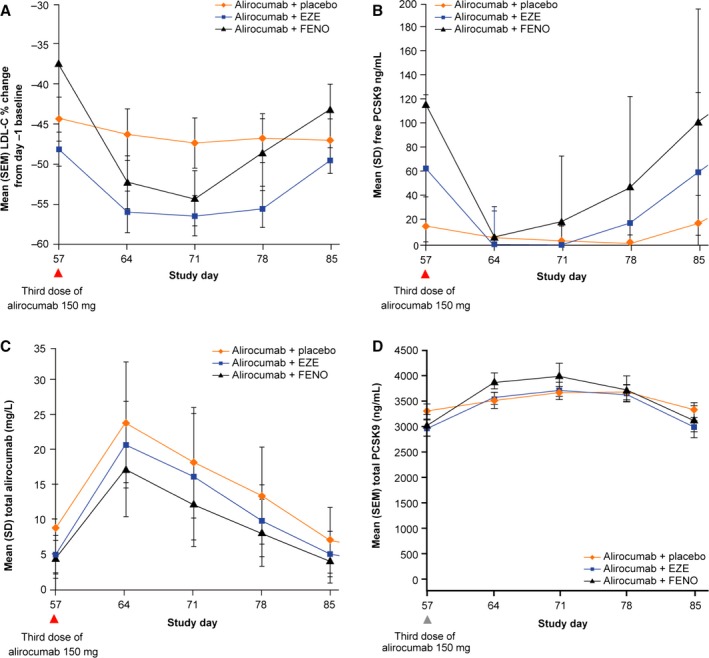
Mean percentage change in LDL‐C from the additional baseline (day −1) (A) and concentrations of free PCSK9 (B), total PCSK9 (C), and total alirocumab (D) for days 57 to 85. EZE indicates ezetimibe; FENO, fenofibrate; LDL‐C, low‐density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin 9.
The temporal relationships between free and total PCSK9 concentrations, alirocumab levels, and LDL‐C are plotted in Figure 6. A lag (hysteresis effect) was observed between the time when free PCSK9 concentrations reached the lowest point and when maximal reductions in LDL‐C levels were observed (Figure 6B).
Figure 6.
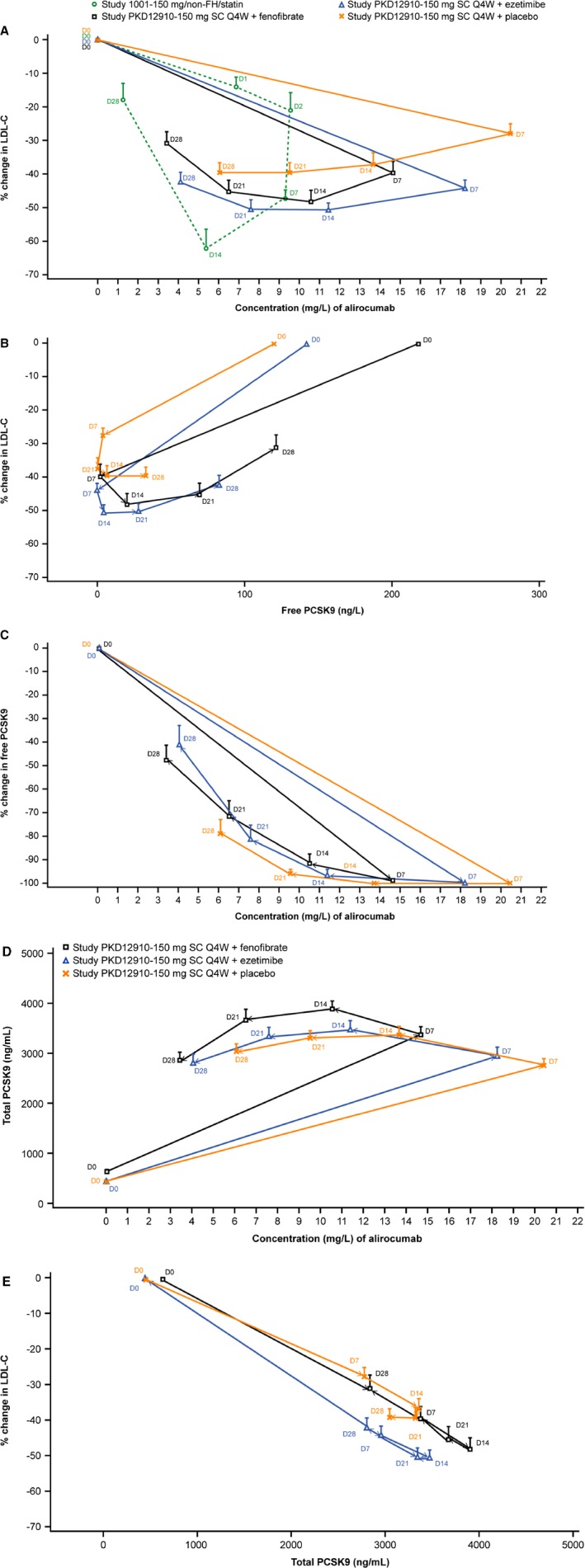
Hysteresis plots comparing changes over time in LDL‐C vs alirocumab (A) and vs free PCSK9 (B), free PCSK9 vs alirocumab (C), total PCSK9 vs alirocumab (D) and LDL‐C vs total PCSK9 (E). Study variables are plotted on x and y axes with data points representing sample time points (day 0–28 following administration of alirocumab). In the key in (A) “PKD12910” refers to the current study; (A) also includes data from alirocumab Phase I study 1001 for comparison. D indicates study day relative to administration of alirocumab; LDL‐C, low‐density lipoprotein cholesterol; non‐FH, non‐familial hypercholesterolemia; PCSK9, proprotein convertase subtilisin/kexin 9; Q4W, every 4 weeks; SC, subcutaneous.
Effects on Other Lipids and Lipoproteins
Effects on other lipids and lipoproteins are summarized in Table 2. Similar to the effects on LDL‐C, reductions in total cholesterol, apolipoprotein B, non–HDL‐C, and triglycerides (from day −29 to day 71) were greater when alirocumab was coadministered with the LLTs compared with placebo. The fenofibrate combination, but not the ezetimibe combination, resulted in larger increases in HDL‐C compared with alirocumab plus placebo, reflecting the independent effects of fenofibrate on raising HDL‐C that are not seen with ezetimibe.
Safety
Treatment‐emergent AEs are summarized in Table 3. There were no serious AEs and no subjects discontinued because of AEs. No AEs were considered by the investigator to be related to the study treatments. There were no clinically significant changes in vital signs or electrocardiograms or in hematologic or biochemical parameters.
Table 3.
Safety Summary From the Main Baseline (Day −29) to End of Study at Day 120 (All Randomized Patients)
| Treatment Group | Alirocumab 150 mg Q4W+Placebo (n=24) | Alirocumab 150 mg Q4W+Ezetimibe (n=24) | Alirocumab 150 mg Q4W+Fenofibrate (n=24) |
|---|---|---|---|
| Subjects with any TEAEs, n (%) | 12 (50.0) | 14 (58.3) | 12 (50.0) |
| Most frequent TEAEs (recorded in ≥2 subjects in any group), n (%) | |||
| Headache | 3 (12.5) | 5 (20.8) | 2 (8.3) |
| Nasopharyngitis | 3 (12.5) | 4 (16.7) | 4 (16.7) |
| Influenza | 2 (8.3) | 0 (0) | 1 (4.2) |
| Gastroenteritis viral | 0 (0) | 0 (0) | 2 (8.3) |
| Influenza‐like illness | 1 (4.2) | 3 (12.5) | 1 (4.2) |
| Abdominal pain | 2 (8.3) | 1 (4.2) | 1 (4.2) |
Q4W indicates every 4 weeks; TEAE, treatment‐emergent adverse event.
Discussion
This study was designed to explore changes in LDL‐C, PCSK9, and alirocumab concentrations when alirocumab was administered Q4W without background statins, either with placebo or in combination with nonstatin LLTs (ezetimibe and fenofibrate). LDL‐C reductions were maintained duringthe 4‐week dosing interval with alirocumab 150 mg Q4W plus placebo. Maximal LDL‐C reductions for the LLT combinations (65.3% and 66.8% with ezetimibe and fenofibrate, respectively) were greater those than for alirocumab plus placebo (48.2% versus day −29). In contrast, PCSK9 levels and the alirocumab clearance rate were increased and the duration of efficacy was slightly reduced with the LLTs. These findings are summarized in Figure 7. This contrasts with results from previous studies in which alirocumab was dosed Q4W on background statins, showing a notably reduced duration of effect compared with Q2W dosing.4, 6
Figure 7.
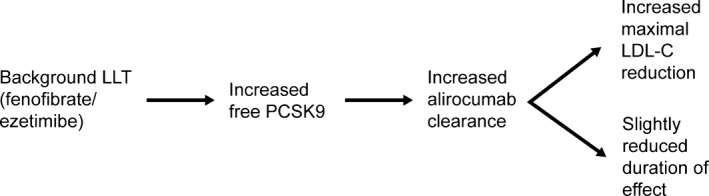
Relationship between LDL‐C, free PCSK9, and alirocumab levels when alirocumab 150 mg Q4W is administered in combination with ezetimibe or fenofibrate without background statin. LDL‐C indicates low‐density lipoprotein cholesterol; LLT, lipid‐lowering therapy; PCSK9, proprotein convertase subtilisin/kexin 9.
Treatment with ezetimibe or fenofibrate during the course of a 28‐day run‐in resulted in reductions in LDL‐C of 19.8% and 25.9%, respectively, and caused increases in free PCSK9 levels of 24% and 92% (versus placebo). Although free PCSK9 concentrations before the third alirocumab dose were higher with fenofibrate cotherapy (versus ezetimibe or placebo co‐therapy), at day 71 they were reduced to similar levels in all groups. At the same time, total PCSK9 concentrations were highest with fenofibrate cotherapy. By comparing free and total PSCK9 concentrations, we speculate that total PCSK9 mainly represents inactivated PCSK9 complexed to alirocumab. Following the last alirocumab dose and up to end of study, a more rapid rate of return of free PCSK9 was observed with fenofibrate versus either ezetimibe or placebo.
We hypothesize that the increases in PCSK9 levels with the LLTs are the result of increased PCSK9 production, with fenofibrate resulting in a greater increase in PCSK9 production compared with ezetimibe. Further, the actual increase in PCSK9 production would be underestimated by simply measuring free PCSK9 levels, because some are eliminated from the system as a result of the interaction of PCSK9 with the LDLR. Because alirocumab can be thought of as a trap for newly produced PCSK9, we propose that the difference in peak total PCSK9 values after alirocumab administration may serve as a better indicator of the effect on PCSK9 production. It has been proposed that the effect of fenofibrate on PCSK9 is indirect,13, 15 rather than a direct effect on gene transcription as is observed with statins.2 There is some evidence from animal studies that ezetimibe upregulates the PCSK9 and LDLR genes11; however, a previous study in humans did not find an effect of ezetimibe on PCSK9.12 Increases in PCSK9 concentrations of 13% to 41% (versus placebo) have been reported after treatment with simvastatin or atorvastatin.15 These increases will, however, underestimate the actual increased PCSK9 production for reasons noted earlier, because statins also induce an increase in LDLR levels.
Monoclonal antibodies that bind PCSK9 undergo target‐mediated clearance.16 Therefore, we hypothesize that the increased rate of clearance of total alirocumab with the LLT combinations versus the placebo combination resulted from the increased PCSK9 concentrations (and thus increased target‐mediated clearance). Measurement of total alirocumab concentrations will underestimate clearance of the biologically active drug (“free” alirocumab—ie, that which is not bound to PCSK9). Based on the binding stoichiometry of PCSK9 to alirocumab (2:1) in the target‐mediated phase and on the total and free PCSK9 concentrations, we estimate that the proportion of total alirocumab present as free alirocumab becomes negligible toward the end of the dosing interval when free PCSK9 is again detectable and begins to increase. However, this will need to be confirmed in future studies.
The modest attenuation of LDL‐C–lowering efficacy during the Q4W dosing interval with the alirocumab–LLT combinations versus alirocumab plus placebo can be explained by the increased PCSK9 concentrations and clearance of alirocumab with these combinations. Results from the current study suggest that if free PCSK9 concentrations remain lower than 40 to 50 ng/mL and alirocumab levels remain above 9 to 10 mg/L, then the LDL‐C–lowering effect of alirocumab is stable.
Based on the results from the current study and previous work,4, 6 we hypothesize that there is a gradient in terms of the magnitude of peak LDL‐C reductions and duration of effect after alirocumab administration, from alirocumab added to statins, to coadministration with fenofibrate and ezetimibe, and finally as monotherapy (ie, alirocumab plus placebo in the current study). This gradient reflects the extent of PCSK9 induction by each LLT that is coadministered with alirocumab. In a reciprocal manner, we also hypothesize that by raising PCSK9 levels, the LLTs (statin, ezetimibe, or fenofibrate) may exert a self‐imposed limit on the maximal LDL‐C reduction possible. Adding alirocumab to the LLT and thus bringing down PCSK9 levels may blunt the limitation in LDL‐C reduction, leading to an additive LDL‐C–lowering effect.
With regard to safety, alirocumab in combination with placebo, ezetimibe, or fenofibrate was generally well tolerated, with no serious AEs throughout the study. There was a similar incidence of AEs reported in each group, and none of the reported AEs were considered by the investigator to be related to the study drugs. However, this was a small study and not powered for safety. Safety findings will be extended with data from the large cardiovascular outcomes study.17
Observational studies suggest that a large proportion of patients do not tolerate statins.18 Ezetimibe and fenofibrate are often used as alternative drugs for these patients. The present study indicates that alirocumab 150 mg Q4W—representing a single, once‐monthly injection—could be an option for such patients not currently receiving a statin. The results from the present study informed the designs of the phase 3 alirocumab trials ODYSSEY CHOICE I and CHOICE II (clinicaltrials.gov identifiers: NCT01926782 and NCT02023879, respectively). In CHOICE I, alirocumab 300 mg Q4W is administered either with or without a concomitant statin; in CHOICE II, alirocumab 150 mg Q4W is administered without a statin. Both studies allow for a dose regimen change to 150 mg every 2 weeks if LDL‐C targets are not reached after 8 weeks of treatment.
Conclusions
Alirocumab 150 mg Q4W produced robust LDL‐C reductions when administered either with placebo or in combination with ezetimibe or fenofibrate. Maximal LDL‐C reductions were greater when in combination with the LLTs. The oral LLTs appear to increase free PCSK9 levels, leading to increased clearance of alirocumab; thus, LDL‐C reductions with the LLT combinations were slightly less sustained between injections versus the placebo combination. However, the slight loss of duration of efficacy was not as pronounced as seen in trials performed with Q4W dosing with background statin treatment. Therefore, alirocumab 150 mg Q4W may provide an additional treatment option when used as monotherapy or when administered on a background of ezetimibe or fenofibrate therapy in patients in need of LDL‐C lowering. The ODYSSEY CHOICE studies are investigating the efficacy and safety of alirocumab Q4W dosing.
Sources of Funding
This work was funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Disclosures
Jacques Rey and Jean‐Louis Pinquier were both employees of and stockholders in Sanofi during conduct of this study. Franck Poitiers, Tobias Paehler, Aurélie Brunet, Howard Surks, and Corinne Hanotin are all employees of and stockholders in Sanofi. A. Thomas DiCioccio and William Sasiela are employees of and stockholders in Regeneron Pharmaceuticals. Christopher Cannon has received grants from Accumetrics, Arisaph, AstraZeneca, Boehringer‐Ingelheim, CSL Behring, Essentialis, GlaxoSmithKline, Janssen, Merck, Regeneron, Sanofi, and Takeda and consultant/advisory board fees from Bristol‐Myers Squibb, Lipimedix, and Pfizer.
Acknowledgments
The study was conducted at 2 centers: Biotrial, Rennes, France, under the direction of Dr H. Charfi, MD (coordinating principal investigator) (laboratory: centre Eugène Marquis, Rennes), and Biotrial, Rueil‐Malmaison, France, under the direction of Dr P. Kousignian, MD (principal investigator) (laboratory: SELARL Biosmose IDF, Rueil). The clinical study director was Jacques Rey, PhD. The medical officer was Dr Jean‐Louis Pinquier, MD. Franck Poitiers, MSc, was the biostatistician. Tobias Paehler, PhD, and Aurélie Brunet, PharmD, were the pharmacokineticists. All authors were involved in interpretation of the data, critically revising the manuscript, and approving the final manuscript. Medical writing support was provided by Rob Campbell, PhD, of Prime Medica Ltd (Knutsford, Cheshire, UK), funded by Sanofi and Regeneron.
(J Am Heart Assoc. 2016;5:e003323 doi: 10.1161/JAHA.116.003323)
References
- 1. Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–1036. [DOI] [PubMed] [Google Scholar]
- 2. Guo YL, Zhang W, Li JJ. PCSK9 and lipid lowering drugs. Clin Chim Acta. 2014;437:66–71. [DOI] [PubMed] [Google Scholar]
- 3. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM; ODYSSEY COMBO II Investigators . Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. [DOI] [PubMed] [Google Scholar]
- 5. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; Investigators OLT . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 6. Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low‐density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. [DOI] [PubMed] [Google Scholar]
- 7. Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. [DOI] [PubMed] [Google Scholar]
- 8. Merck Sharp & Dohme Limited . Ezetrol 10 mg tablets. Available at: https://www.medicines.org.uk/emc/medicine/12091/SPC/Ezetrol+10mg+Tablets/. Accessed January 4, 2016.
- 9. Genus Pharmaceuticals . Fenofibrate 160 mg tablets. Available at: https://www.medicines.org.uk/emc/medicine/28612. Accessed January 4, 2016.
- 10. Ason B, Tep S, Davis HR Jr, Xu Y, Tetzloff G, Galinski B, Soriano F, Dubinina N, Zhu L, Stefanni A, Wong KK, Tadin‐Strapps M, Bartz SR, Hubbard B, Ranalletta M, Sachs AB, Flanagan WM, Strack A, Kuklin NA. Improved efficacy for ezetimibe and rosuvastatin by attenuating the induction of PCSK9. J Lipid Res. 2011;52:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Liu J, Li S, Xu RX, Sun J, Li JJ. Impact of currently prescribed lipid‐lowering drugs on plasma PCSK9 concentration: single or in combination study in rats. Lipids Health Dis. 2014;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berthold HK, Seidah NG, Benjannet S, Gouni‐Berthold I. Evidence from a randomized trial that simvastatin, but not ezetimibe, upregulates circulating PCSK9 levels. PLoS One. 2013;8:e60095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahebkar A. Circulating levels of proprotein convertase subtilisin kexin type 9 are elevated by fibrate therapy: a systematic review and meta‐analysis of clinical trials. Cardiol Rev. 2014;22:306–312. [DOI] [PubMed] [Google Scholar]
- 14. Gustavson LE, Schweitzer SM, Burt DA, Achari R, Rieser MJ, Edeki T, Chira T, Yannicelli HD, Kelly MT. Evaluation of the potential for pharmacokinetic interaction between fenofibrate and ezetimibe: a phase I, open‐label, multiple‐dose, three‐period crossover study in healthy subjects. Clin Ther. 2006;28:373–387. [DOI] [PubMed] [Google Scholar]
- 15. Khera AV, Qamar A, Reilly MP, Dunbar RL, Rader DJ. Effects of niacin, statin, and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 levels in patients with dyslipidemia. Am J Cardiol. 2015;115:178–182. [DOI] [PubMed] [Google Scholar]
- 16. Chaparro‐Riggers J, Liang H, DeVay RM, Bai L, Sutton JE, Chen W, Geng T, Lindquist K, Casas MG, Boustany LM, Brown CL, Chabot J, Gomes B, Garzone P, Rossi A, Strop P, Shelton D, Pons J, Rajpal A. Increasing serum half‐life and extending cholesterol lowering in vivo by engineering antibody with pH‐sensitive binding to PCSK9. J Biol Chem. 2012;287:11090–11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long‐term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–689. [DOI] [PubMed] [Google Scholar]
- 18. Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high‐dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. [DOI] [PubMed] [Google Scholar]


