Abstract
Background
The adoption of the transradial (TR) approach over the traditional transfemoral (TF) approach has been hampered by concerns of increased radiation exposure—a subject of considerable debate within the field. We performed a patient‐level, multi‐center analysis to definitively address the impact of TR access on radiation exposure.
Methods and Results
Overall, 10 centers were included from 6 countries—Canada (2 centers), United Kingdom (2), Germany (2), Sweden (2), Hungary (1), and The Netherlands (1). We compared the radiation exposure of TR versus TF access using measured dose‐area product (DAP). To account for local variations in equipment and exposure, standardized TR:TF DAP ratios were constructed per center with procedures separated by coronary angiography (CA) and percutaneous coronary intervention (PCI). Among 57 326 procedures, we demonstrated increased radiation exposure with the TR versus TF approach, particularly in the CA cohort across all centers (weighted‐average ratios: CA, 1.15; PCI, 1.05). However, this was mitigated by increasing TR experience in the PCI cohort across all centers (r=−0.8; P=0.005). Over time, as a center transitioned to increasing TR experience (r=0.9; P=0.001), a concomitant decrease in radiation exposure occurred (r=−0.8; P=0.006). Ultimately, when a center's balance of TR to TF procedures approaches 50%, the resultant radiation exposure was equivalent.
Conclusions
The TR approach is associated with a modest increase in patient radiation exposure. However, this increase is eliminated when the TR and TF approaches are used with equal frequency—a guiding principle for centers adopting the TR approach.
Keywords: coronary angiography, dose‐area product, percutaneous coronary intervention, radial artery catheterization, radiation, radiation dosing, transfemoral, transradial
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Percutaneous Coronary Intervention, Revascularization, Stent
Introduction
Recent evidence supports that the transradial (TR) approach diminishes the risk of bleeding and vascular complications when compared to the traditional transfemoral (TF) approach.1, 2 Anecdotally, a TR approach usually provides a patient with increased comfort. Given these advantages, the TR approach is being progressively employed by centers around the world.3
Rising frequency of both noninvasive and invasive cardiac investigations increasingly expose patients to ionizing radiation.4, 5 Reported associations between cardiac testing and subsequent risk of cancer further substantiates the need for concern for both patients and operators.5, 6, 7 Accordingly, minimizing both patient and the catheterization staffs’ radiation exposure remains a priority. The TR approach is assumed to result in a higher radiation exposure than its TF equivalent, but this continues to be the subject of considerable debate.8, 9, 10 As the TR approach becomes more commonly adopted, so too do concerns over a subsequent risk of increased radiation exposure.
To this end, a number of studies have attempted to quantify the differences in radiation exposure between access sites with increasing TR experience, with conflicting results.9, 10, 11, 12, 13, 14, 15, 16, 17 Given this, we performed a patient‐level, multi‐center, international, collaborative analysis to determine differences in patient radiation exposure in the context of TR access. Specifically, we explored the impact of TR experience at a center level and examined a center's transition from being a predominately TF center to increasing adoption of the TR approach.
Methods
Population and Outcomes
Anonymized patient‐level data from 10 centers were pooled in a common database, yielding 57 326 cases from January 2000 to March 2013. Inclusion required reporting of patient procedural data, absorbed doses, access site (TR or TF), and procedural type (coronary angiography [CA] or percutaneous coronary intervention [PCI]). For the purpose of the current study, left and right radials were grouped. Right heart catheterization studies and structural heart disease cases were excluded from the study. This protocol was approved by the Ottawa Hospital Research Ethics Board and deemed not to require patient consent. To specifically account for ad‐hoc PCI cases, the corresponding CA data were summed with the matching ad‐hoc PCI data, when available. Otherwise, ad‐hoc PCI and staged PCI were included in the overall PCI cohort, reflecting “real‐world” practice. For each center, TR access experience was expressed as a ratio generated by dividing the total number of cases performed there by the TR approach by those by the TF approach. Patient radiation exposure was quantified as the median dose‐area product (DAP) or as a ratio. Radiation exposure ratios were generated by dividing the median DAP of the TR cohort by the median DAP of the TF cohort at each center. In this way, we adjusted for intracenter DAP variability attributed to reporting and equipment differences between sites. To further delineate the influence of a center's TR experience affecting radiation exposure, we performed a separate analysis with a cohort of cases from the center with the largest number of contributing cases (N=34 647) from October 2007 to March 2013. Within this period, consecutive 6‐month intervals were assessed, commencing once the operators had sufficient initial TR experience (defined as 5 or more of the center's 30 operators performing more than 10 TR cases within a 6‐month block)—yielding 25 747 cases spanning from 2009 to 2013. Over this time, we assessed the transition from a predominantly TF center to increasing adoption of the TR approach, in order to evaluate the resultant change in radiation exposure. For each 6‐month block, we generated the median DAP of both the TR cases and TF cases performed. We then similarly generated a radiation ratio by dividing the TR median DAP by the TF median DAP for each 6‐month block.
Statistical Analysis
The ratio of the median DAPs in the radial versus femoral cohorts was calculated for each center. Spearman rank‐order correlations were employed to assess the association between the median DAP ratios and the center experience ratios, and a sensitivity analysis removing outlying centers was also conducted. Based on patient‐level data from the largest center, a regression model using the logarithmic transformation of DAPs as the outcome was constructed comparing the TR and TF cohorts as the center transitions from predominately TF to TR, while controlling for the variables of staff operator and type of procedure (ie, CA or PCI) performed. All P values were 2‐tailed with an accepted significance level of 0.05. All analyses were performed using SAS software (version 9.3; SAS Institute Inc., Cary, NC).
Results
Data Description
Overall, 10 centers were included from 6 countries—Canada (2 centers), United Kingdom (2 centers), Germany (2 centers), Sweden (2 centers), Hungary (1 center), and The Netherlands (1 center) with procedure dates spanning from October 2000 to March 2013. In total, 37 306 CA cases (11 516 TR and 25 790 TF) and 20 020 PCI cases (6860 TR and 13 160 TF) composed the final database for analysis, for a total of 57 326 patients (Table).
Table 1.
Center's Locations and Case Numbers
| Center Location | Angiography Cases (N=37 306) | PCI Cases (N=20 020) | Center Totals | ||
|---|---|---|---|---|---|
| Radial | Femoral | Radial | Femoral | ||
| Ottawa, Canada | 7068 | 19 104 | 2436 | 6039 | 34 647 |
| Hamilton, Canada | 1956 | 5304 | 947 | 3214 | 11 421 |
| Linköping, Sweden | 36 | 40 | 24 | 42 | 142 |
| Budapest, Hungary | 58 | 14 | 389 | 91 | 552 |
| Frankfurt, Germany | 503 | 470 | 302 | 373 | 1648 |
| Örebro, Sweden | — | — | 55 | 114 | 169 |
| Middlesbrough, United Kingdom | — | — | 520 | 480 | 1000 |
| Kettering, United Kingdom | 1650 | 441 | 955 | 478 | 3524 |
| Bremen, Germany | 107 | 103 | 20 | 20 | 250 |
| Amsterdam, The Netherlands | 138 | 314 | 1212 | 2309 | 3973 |
| Cumulative | 11 516 | 25 790 | 6860 | 13 160 | 57 326 |
PCI indicates percutaneous coronary intervention.
Impact of TR Approach on Radiation in All Centers
Eight centers had CA procedure data available (Figure 1A) with all centers demonstrating respective TR:TF DAP ratios >1, indicating higher radiation exposure with the TR approach. Weighted averages (WA‐DAP ratio) based on center size demonstrated a 15% higher DAP associated with the TR approach. PCI data, which were available for all 10 centers, yielded more variability in distribution, with 4 centers demonstrating higher radiation exposure by the TR approach (ie, TR:TF ratio >1) and the remainder with less radiation by the TR approach (Figure 1B). Accounting for center size with weighted averages, there was an overall 5% higher DAP in the TR cohort among PCI cases, which remained unchanged after removal of outliers.
Figure 1.
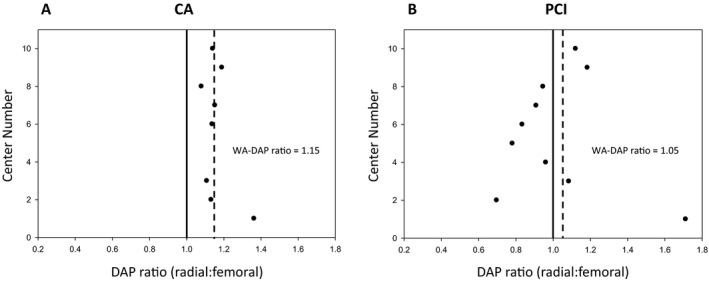
Radiation exposure across all centers. Ratios of dose‐area products (DAPs) in the transradial (TR) vs transfemoral (TF) cases are expressed for each center (center numbers 1–10, circles) for both coronary angiography (CA; 8 centers) (A) and percutaneous coronary intervention (PCI; 10 centers) (B) cohorts across all centers. The overall weighted average TR:TF DAP ratio (WA‐DAP; dashed lines) for all centers was generated and depicted as 1.15 for all CA procedures and 1.05 for all PCI procedures. Unity depicted as solid vertical lines.
Impact of Intercenter Experience on Radiation Exposure
On a center level, we further analyzed the TR:TF DAP ratio as a function of each center's respective TR experience, based on the center's TR:TF case ratio. In the CA cohort, no trend was demonstrated between increasing TR experience and TR radiation exposure (Figure 2A). However, the PCI cohort did yield a trend toward diminishing TR radiation exposure as the proportion of TR cases increased (r=−0.8; P=0.005; Figure 2B). Significantly, this trend was maintained after sensitivity analyses by removing noted outliers both individually and collectively.
Figure 2.
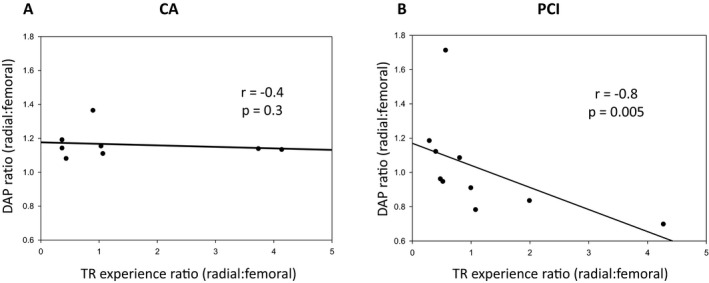
Impact of intercenter experience on radiation exposure. Dose‐area product (DAP) ratios represent the ratio of DAPs in transradial (TR) vs transfemoral (TF) cohorts within each individual center (denoted as circles). TR experience ratios generated by dividing the number of TR by TF cases performed at each center. DAP ratio are then expressed as a function of the TR experience ratio in both the coronary angiography (CA) (A) and percutaneous coronary intervention (PCI) (B) cohorts across all centers.
Radiation Exposure During a Center's Transition to the TR Approach
Given this trend, we performed an analysis on 25 747 of 34 647 cases recruited from the largest center for which data were available, during the transition from a predominately TF center to increasing TR use. Using a regression model with a logarithmic transformation of the DAP values as the outcome, a significant interaction between approach and time was observed (P<0.0001). Importantly, because operator data was available, including number of TR cases completed chronologically by each operator, we were able to incorporate this into the regression analysis to account for individual operator TR experience during the transition phase of the center, as well as procedure type (ie, CA or PCI). Using median DAPs for consecutive 6‐month intervals during this transition period, the trends over time in both approach groups are depicted in Figure 3. Additionally, models incorporating the DAP ratios and percentage of TR cases over the same time period were also considered (Figure 4). Over the transition time, there was an increasing proportion of cases performed by the TR approach (r=0.9; P=0.001), coupled with a concomitant diminishing radiation exposure ratio (r=−0.8; P=0.006). A significant observation, the point at which the radiation exposure became equivalent between the TR and TF approach (ie, ratio of 1) coincided with the point at which the TR and TF experience became equal (ie, 50% TR cases).
Figure 3.
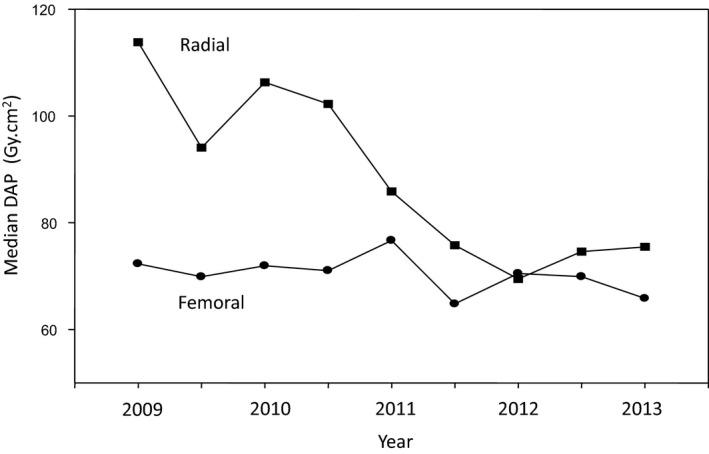
Radiation exposure during a center's transition to the transradial approach. Median dose‐area products (DAP) for both transfemoral (TF; circles) and transradial (TR; squares) cohorts are depicted for consecutive 6‐month time periods and fitted accordingly. Using a regression model with a logarithmic transformation of the DAP values as the outcome, a significant interaction between the approach and time was observed (P<0.0001).
Figure 4.
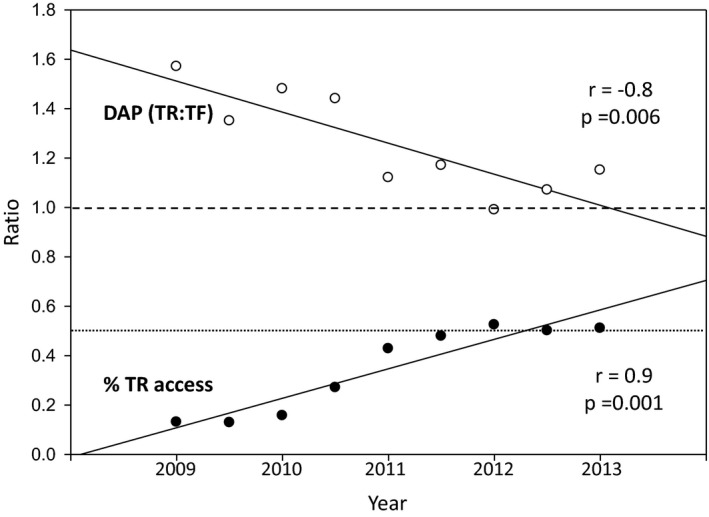
Equivalent radiation exposures with equal access site experiences. Median dose‐area products (DAPs) for consecutive 6‐month intervals are expressed as a ratio of median DAP in transradial (TR) divided by the transfemoral (TF) cohorts for each 6‐month period (white circles). TR experience is depicted as the percentage of total cases performed by the TR approach per 6‐month period (% TR access, black circles). Spearman correlations displayed for nonparametric data. The value of 1 (dashed line) denotes the equivalency point for TR:TF radiation exposure (DAP TR:TF) where the radiation exposure is equal in both cohorts. Similarly, the value of 0.5 (dotted line) demonstrates the point at which the TR:TF experience is equivalent (% TR access=50%), in that the number of TR and TF cases for each 6‐month period are equal.
Discussion
Advancement of the radial approach has been hampered by concerns of increasing procedural complexity and resultant augmentation of radiation exposure.9 Accordingly, we conducted a real‐world all‐comer study consisting of over 50 000 patients from 10 centers worldwide, which supports modestly increased radiation exposure in the TR versus TF approach. Of note, this risk appears mitigated by increasing TR experience, more prominently in PCI cases. Moreover, we report that differences in radiation exposure are effectively eliminated as a center achieves equivalent experience in both the TR and TF approaches—a so‐called “equivalency threshold.”
The impact of the TR or TF approach on patient radiation exposure remains challenging because of numerous confounding factors. The randomized RadIal Vs femorAL access for coronary intervention (RIVAL) trial examined all comers planned for PCI and randomized to a TR versus TF approach among experienced operators. Though not specifically examining radiation doses, the trial demonstrated prolonged fluoroscopy times with the TR approach, with no difference in procedural times or contrast volumes—in keeping with past large reports based on National Cardiovascular Data Registry (NCDR) registry data.1, 2, 3 However, fluoroscopy time has been demonstrated to be a poor surrogate marker of true patient‐level radiation exposure.18, 19 This is evident in a recent NCDR‐based analysis,3 which had used fluoroscopy and contrast use as surrogates of patient‐level radiation. Indeed, the previous study3 demonstrated greater fluoroscopy time with the TR approach, but paradoxically reported greater contrast volume with the TF approach, thus raising the question of these parameters as surrogates of patient‐level radiation. Furthermore, the predictors of radiation exposure remain far more complex than simply the access site alone. Indeed, recent analyses have suggested that increasing body mass index, history of coronary artery bypass grafting, increasing number of treated lesions, and chronic‐total occlusions were associated with increased radiation exposure.8, 10 Others invoke patient sex, lesion complexity, lesion location, and the performing physician as also contributing to subsequent radiation exposure.20, 21 Conversely, some report that the TR and TF approaches have similar radiation exposures, even after controlling for case complexity.13
In contrast to previous studies,1, 2, 3 we employ patient‐level data with DAP measurements to better reflect true patient radiation exposure compared to fluoroscopy time alone.18, 19 Our data support a modest increase in radiation exposure for a TR approach, which was more prominent in CA cases. This likely reflects potential case selection among the PCI cohort given that previous diagnostic angiography may have provided information on case complexity and thus dictated selection of a TF versus TR approach. Our study also observed considerable variability in radiation exposure between centers in the PCI cohort. This variability is likely multifactorial. First, it is possible that centers vary in their operator's TR experience, in that, at certain centers, only PCI operators may perform radial cases. Second, some centers may have selectively chosen the TR approach based on the complexity assessed in the preceding angiogram, thereby introducing a bias that less‐complex PCI at a center may be predominantly TR. It is also possible that the centers may differ in their experience and approach in TR cases, or perhaps even alter practice given a greater awareness of radiation exposure in TR cases. Therefore, the radiation exposure between a TR and TF approach may be in flux during a transition period where increasing TR approaches are undertaken. Accounting for these possible explanations, the amount of radiation exposure is likely influenced by more than the access site alone.
Experience at an operator level is thought to be a determinant of radiation exposure. Indeed, a previous study comparing expert TR and TF operators performing CA and controlling for confounding procedural factors yielded no difference in radiation exposure, though differences between trainee and expert operators were present, regardless of approach.22 Interestingly, previous work demonstrated higher radiation exposure with inexperienced TF operators compared to experienced TR operators—suggesting that experience and not the access site itself dictate the observed radiation differences.23 Accordingly, at an operator level, reported differences in radiation between either approach may actually reflect the adoption of the newer TR approach by inexperienced TR operators rather than an independent difference between either approaches. Indeed, subgroup analysis from the RIVAL trial investigating radiation exposure examined the impact of center experience based upon each center's respective median operator TR PCI volume. In this way, only low‐volume centers demonstrated a difference between TF and TR access, with procedural volume being the greatest independent predictor of radiation exposure.24 Similarly, we observed an association between increasing intercenter TR experience and reduced TR radiation exposure, more so in the PCI than the CA cohort (Figure 2). This discrepancy likely reflects that PCI has greater procedural complexity and hence a greater learning curve over its CA counterpart. Hence, we report improved radiation exposure with increasing TR experience at a center level.
Given this trend, we then moved to specifically analyze one center's transition from a predominately TF center to increasing use of the TR approach. Our data support that as a center's ratio of TR to TF procedures increases, the subsequent radiation exposure similarly diminished overall. Moreover, our study is the first to demonstrate the concept of an “equivalency threshold,” whereby when TR is utilized as frequently as the TF approach, the radiation exposure between approaches become equivalent. In fact, all centers in our study that performed PCI with greater frequency by the TR approach had lower radiation in the TR cohort. This concept is integral to centers that are in evolution or considering adoption of the TR approach. Furthermore, it suggests a critical ratio of TR to TF cases in order to attain proficiency and minimize radiation exposure. Interestingly, our data support that these changes are independent of specific operators, suggesting that center‐level factors may indeed influence radiation exposure.
Regardless of the approach used, minimizing radiation exposure in the cardiac catheterization laboratory is of importance. Specifically at a center level, studies have demonstrated up to a 40% reduction in patient radiation dose after implementation of several clinical practice changes, including routine intraprocedural radiation dose announcements, reporting of procedures with excess radiation (especially prolonged complex procedures), including radiation dose in each clinical report, and implementing mandatory safety training for fellows in addition to numerous technical changes.25, 26 Indeed, center‐level differences in radiation levels are likely also related to the differing radiation protection practices used by each center. Moreover, multiple professional associations have released position statements with recommendations on best‐practice protocols for minimizing radiation exposure in interventional procedures—particularly addressing the TR approach.5, 27, 28 All of these measures support that an evolution to increasing TR use is best managed at a center level with adoption of novel practices to minimize radiation exposure while transitioning.
There are some limitations in our study. First, our data is retrospective and hence is subject to innate limitations when compared to a randomized study. For example, selection bias, whereby more‐complex cases may be preferentially performed by a TF approach, may result in relatively increased radiation doses in the TF cohort. Second, as in any observational multicenter study, there is variability in the data reporting, particularly with respect to radiation doses at each individual center likely attributed to equipment differences—an effect we attempt to standardize across all centers by implementation of a ratio model. Third, the varying retrospective data sets among all centers limit our ability to adjust for baseline patient and procedural characteristics across the centers. Specifically, we are unable to control for operator heterogeneity and pattern of practice across all centers. Nonetheless, the current report is the largest multicenter comparison of TR and TF approaches in a real‐life setting for diagnostic and PCI procedures and, as such, is inclusive of varying demographics and procedural scenarios.
Conclusion
The TR approach for CA and intervention is associated with a modest increase in patient radiation exposure. However, this increase is eliminated when TR and TF approaches are used with equal frequency. The implication is that centers may benefit from the improved vascular and bleeding profile the TR approach affords without increased radiation exposure, provided they maintain equivalent experience. Hence, the concept of an “equivalency threshold” is integral to guiding centers transitioning to the TR approach.
Disclosures
Benjamin Chow received research support from General Electric (GE) Healthcare and educational support from TeraRecon Inc. and holds the Saul and Edna Goldfarb Research Chair in Cardiac Imaging.
Acknowledgments
We thank Carol Mitchell and Gary Heddon for their invaluable assistance.
(J Am Heart Assoc. 2016;5:e003333 doi: 10.1161/JAHA.116.003333)
References
- 1. Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicenter trial. Lancet. 2011;377:1409–1420. [DOI] [PubMed] [Google Scholar]
- 2. Rao SV, Ou F‐S, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. [DOI] [PubMed] [Google Scholar]
- 3. Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, Minutello RM, Messenger JC, Moussa I, Garratt KN, Piana RN, Hillegass WB, Cohen MG, Gilchrist IC, Rao SV. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the National Cardiovascular Data Registry (2007–2012). Circulation. 2013;127:2295–2306. [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Einstein AJ, Fazel R, Krumholz HM, Wang Y, Ross JS, Ting HH, Shah ND, Nasir K, Nallamothu BK. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population‐based analysis. J Am Coll Cardiol. 2010;56:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Natarajan MK, Paul N, Mercuri M, Waller EJ, Leipsic J, Traboulsi M, Banijamali HS, Benson L, Sheth TN, Simpson CS, Brydie A, Love MP, Gallo R. Canadian Cardiovascular Society position statement on radiation exposure from cardiac imaging and interventional procedures. Can J Cardiol. 2013;29:1361–1368. [DOI] [PubMed] [Google Scholar]
- 6. Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64‐slice computed tomography coronary angiography. J Am Med Assoc. 2007;298:317–323. [DOI] [PubMed] [Google Scholar]
- 7. Eisenberg MJ, Afilalo J, Lawler PR, Abrahamowicz M, Richard H, Pilote LMMP. Cancer risk related to low‐dose ionizing radiation from cardiac imaging in patients after acute myocardial infarction. CMAJ. 2011;183:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delewi R, Hoebers LP, Råmunddal T, Henriques JPS, Angerås O, Stewart J, Robertsson L, Wahlin M, Petursson P, Piek JJ, Albertsson P, Matejka G, Omerovic E. Clinical and procedural characteristics associated with higher radiation exposure during percutaneous coronary interventions and coronary angiography. Circ Cardiovasc Interv. 2013;6:501–506. [DOI] [PubMed] [Google Scholar]
- 9. Hibbert B, Simard T, Wilson KR, Hawken S, Wells GA, Ramirez FD, Le May MR, So DY, Glover CA, Froeschl M, Marquis J‐F, Labinaz M, Dick A, O'Brien ER. Transradial versus transfemoral artery approach for coronary angiography and percutaneous coronary intervention in the extremely obese. JACC Cardiovasc Interv. 2012;5:819–826. [DOI] [PubMed] [Google Scholar]
- 10. Mercuri M, Mehta S, Xie C, Valettas N, Velianou JL, Natarajan MK. Radial artery access as a predictor of increased radiation exposure during a diagnostic cardiac catheterization procedure. JACC Cardiovasc Interv. 2011;4:347–352. [DOI] [PubMed] [Google Scholar]
- 11. Geijer H, Persliden J. Radiation exposure and patient experience during percutaneous coronary intervention using radial and femoral artery access. Eur Radiol. 2004;14:1674–1680. [DOI] [PubMed] [Google Scholar]
- 12. Hetherington SL, Adam Z, Morley R, De Belder MA, Hall JA, Muir DF, Sutton AGC, Swanson N, Wright RA. Primary percutaneous coronary intervention for acute ST‐segment elevation myocardial infarction: changing patterns of vascular access, radial versus femoral artery. Heart. 2009;95:1612–1618. [DOI] [PubMed] [Google Scholar]
- 13. Kuipers G, Delewi R, Velders XL, Vis MM, Van Der Schaaf RJ, Koch KT, Henriques JPS, De Winter RJ, Baan J Jr, Tijssen JGP, Piek JJ. Radiation exposure during percutaneous coronary interventions and coronary angiograms performed by the radial compared with the femoral route. JACC Cardiovasc Interv. 2012;5:752–757. [DOI] [PubMed] [Google Scholar]
- 14. Lange HW, Von Boetticher H. Reduction of operator radiation dose by a pelvic lead shield during cardiac catheterization by radial access: comparison with femoral access. JACC Cardiovasc Interv. 2012;5:445–449. [DOI] [PubMed] [Google Scholar]
- 15. Lehmann R, Ehrlich JR, Weber V, De Rosa S, Gotarda MNB, Schächinger V, Zeiher AM, Fichtlscherer S. Implementation of the transradial approach for coronary procedures is not associated with an elevated complication rate and elevated radiation patient exposure. J Interv Cardiol. 2011;24:56–64. [DOI] [PubMed] [Google Scholar]
- 16. Ruzsa Z, Ungi I, Horváth T, Sepp R, Zimmermann Z, Thury A, Jambrik Z, Sasi V, Tóth G, Forster T, Nemes A. Five‐year experience with transradial coronary angioplasty in ST‐segment‐elevation myocardial infarction. Cardiovasc Revasc Med. 2009;10:73–79. [DOI] [PubMed] [Google Scholar]
- 17. Sandborg M, Fransson SG, Peterson H. Evaluation of patient‐absorbed doses during coronary angiography and intervention by femoral and radial artery access. Eur Radiol. 2004;14:653–658. [DOI] [PubMed] [Google Scholar]
- 18. Chambers CE. Radiation dose in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:344–346. [DOI] [PubMed] [Google Scholar]
- 19. Chida K, Saito H, Otani H, Kohzuki M, Takahashi S, Yamada S, Shirato K, Zuguchi M. Relationship between fluoroscopic time, dose area product, body weight, and maximum radiation skin dose in cardiac interventional procedures. AJR Am J Roentgenol. 2006;186:774–778. [DOI] [PubMed] [Google Scholar]
- 20. Fetterly KA, Lennon RJ, Bell MR, Holmes DR Jr, Rihal CS. Clinical determinants of radiation dose in percutaneous coronary interventional procedures: influence of patient size, procedure complexity, and performing physician. JACC Cardiovasc Interv. 2011;4:336–343. [DOI] [PubMed] [Google Scholar]
- 21. Mercuri M, Xie C, Levy M, Valettas N, Natarajan MK. Predictors of increased radiation dose during percutaneous coronary intervention. Am J Cardiol. 2009;104:1241–1244. [DOI] [PubMed] [Google Scholar]
- 22. Lo TS, Ratib K, Chong AY, Bhatia G, Gunning M, Nolan J. Impact of access site selection and operator expertise on radiation exposure; a controlled prospective study. Am Heart J. 2012;164:455–461. [DOI] [PubMed] [Google Scholar]
- 23. Ratib K, Mamas MA, Fraser DG, Routledge H, Stables R, Nolan J. Operator experience and radiation exposure during transradial and transfemoral procedures. JACC Cardiovasc Interv. 2011;4:936–937. [DOI] [PubMed] [Google Scholar]
- 24. Jolly SS, Cairns J, Niemela K, Steg PG, Natarajan MK, Cheema AN, Rao SV, Cantor WJ, Džavík V, Budaj A, Sheth T, Valentin V, Fung A, Widimsky P, Ferrari E, Gao P, Jedrzejowski B, Mehta SR. Effect of radial versus femoral access on radiation dose and the importance of procedural volume: a substudy of the multicenter randomized RIVAL trial. JACC Cardiovasc Interv. 2013;6:258–266. [DOI] [PubMed] [Google Scholar]
- 25. Fetterly KA, Mathew V, Lennon R, Bell MR, Holmes J, Rihal CS. Radiation dose reduction in the invasive cardiovascular laboratory: implementing a culture and philosophy of radiation safety. JACC Cardiovasc Interv. 2012;5:866–873. [DOI] [PubMed] [Google Scholar]
- 26. Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13–20. [DOI] [PubMed] [Google Scholar]
- 27. Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M, Sabate M, Mauri‐Ferré J, Huber K, Niemelä K, Haude M, Wijns W, Dudek D, Fajadet J, Kiemeneij F, Barbeau G, Saïto S, Jolly S, Louvard Y, Patel T, Rao SV, Reifart N, Steg PG, Valsecchi O, Yang Y. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care and Thrombosis of the European Society of Cardiology. EuroIntervention. 2013;8:1242–1251. [DOI] [PubMed] [Google Scholar]
- 28. Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff AR, Crisco V, Woody W, Zoghbi G, Duffy PL, Sanghvi K, Krucoff MW, Pyne CT, Skelding KA, Patel T, Pancholy SB. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention's transradial working group. Catheter Cardiovasc Interv. 2014;83:228–236. [DOI] [PubMed] [Google Scholar]


