Abstract
Background
The clinical implications of ankle‐brachial index (ABI) cutpoints are not well defined in patients with chronic kidney disease (CKD) despite increased prevalence of high ABI attributed to arterial stiffness. We examined the relationship of ABI with cardiovascular disease (CVD) and all‐cause mortality among CKD patients.
Methods and Results
Three thousand six hundred twenty‐seven participants without clinical peripheral artery disease (PAD) at baseline from the Chronic Renal Insufficiency Cohort Study were included. ABI was obtained per standard protocol and CVD events were confirmed by medical record adjudication. A U‐shaped association of ABI with PAD, myocardial infarction (MI), composite CVD, and all‐cause mortality was observed. Individuals with an ABI between 1.0 and <1.4 had the lowest risk of outcomes. Compared to participants with an ABI between 1.0 and <1.4, multiple‐adjusted hazard ratios (95% confidence intervals) for those with an ABI of <0.9, 0.9 to <1.0, and ≥1.4 were 5.78 (3.57, 9.35), 2.76 (1.56, 4.88), and 4.85 (2.05, 11.50) for PAD; 1.67 (1.23, 2.29), 1.85 (1.33, 2.57), and 2.08 (1.10, 3.93) for MI; 1.51 (1.27, 1.79), 1.39 (1.15, 1.68), and 1.23 (0.82, 1.84) for composite CVD; and 1.55 (1.28, 1.89), 1.36 (1.10, 1.69), and 1.00 (0.62, 1.62) for all‐cause mortality, respectively.
Conclusions
This study indicates that ABI <1.0 was related to risk of PAD, MI, composite CVD, and all‐cause mortality whereas ABI ≥1.4 was related to clinical PAD. These findings suggest that ABI cutpoints of <1.0 or ≥1.4 for diagnosing PAD and ABI <1.0 for CVD risk stratification should be further evaluated among CKD patients.
Keywords: ankle brachial index, cardiovascular disease, chronic kidney disease, heart failure, mortality, myocardial infarction, peripheral arterial disease
Subject Categories: Cardiovascular Disease, Epidemiology
Introduction
Patients with chronic kidney disease (CKD) have a higher prevalence of peripheral artery disease (PAD) compared to the general population.1, 2, 3 Data from the National Health and Nutrition Examination Survey indicate that 24% of persons with creatinine clearance <60 mL/min per 1.73 m2 have prevalent PAD, defined as ankle brachial index (ABI) <0.9, compared with only 3.7% of persons with creatinine clearance ≥60 mL/min per 1.73 m2.1 In the Cardiovascular Health Study, Ix et al. reported that CKD was associated with a 2‐fold increased risk for low ABI (<0.9) and 60% increased risk for high ABI (>1.4) in older people and that the association of CKD with a high ABI was not explained by traditional cardiovascular disease (CVD) risk factors.4
Both low and high ABI have been associated with increased CVD morbidity and mortality in the general population.5, 6, 7 In addition, Adragao et al. reported that both low (<0.9) and high (>1.3) ABI were independently associated with all‐cause and CVD mortality in 219 hemodialysis patients.8 Large meta‐analysis data suggested that ABI of 0.9 to 1.0 was associated with increased risk of major coronary events, CVD, and total mortality in the general population.6 However, there are no published prospective studies evaluating the association of the spectrum of ABI with PAD and other CVD outcomes, as well as all‐cause mortality in persons with CKD preceding kidney failure.
We examined the association of baseline ABI with subsequent risk of PAD, myocardial infarction (MI), heart failure (HF), CVD, and all‐cause mortality among participants from the Chronic Renal Insufficiency Cohort (CRIC) Study, a large prospective cohort study designed to investigate risk factors for progression of CKD and development of CVD in patients with CKD.9
Methods
Study Participants
The design and baseline characteristics of the CRIC Study participants have been previously described.9, 10 Briefly, the CRIC Study enrolled a racially and ethnically diverse group of men and women ages 21 to 74 years with CKD as determined by an age‐based estimated glomerular filtration rate (eGFR) of 20 to 70 mL/min per 1.73 m2.9 A total of 3939 participants were recruited between May 2003 and August 2008 from 7 clinical centers in the United States. Patients with cirrhosis, human immunodeficiency virus infection, polycystic kidney disease, or renal cell carcinoma, those on dialysis or recipients of a kidney transplant, and those taking immunosuppressant drugs were excluded. We excluded 312 participants who reported a history of lower‐extremity revascularization or amputation at baseline. The CRIC Study protocol was approved by the institutional review boards at each of the participating sites. Written informed consent was obtained from all participants.
Data Collection
At the baseline examination, medical history, demographic information, and lifestyle factors were collected by trained research staff using standard questionnaires. Self‐reported history of clinical PAD, including claudication, amputation, or angioplasty and procedures to open up blood vessels in the arms or legs, was acquired. Current smokers were defined as participants who currently smoked and had smoked >100 cigarettes in their lifetimes. Alcohol drinkers were defined as participants who consumed >1 beverage containing alcohol each week over the previous year. Physical activity was estimated by total metabolic equivalent of task (MET)/week.
ABI measurements were obtained per standard protocol.11 After the participant rested supine for 5 minutes, systolic blood pressure (BP) was measured in both arms with the appropriate‐sized arm cuff. For each leg, systolic BP in each posterior tibial and dorsalis pedis artery was measured. All pressures were detected with a continuous‐wave Doppler ultrasound probe. Leg‐specific ABI was calculated by dividing the higher systolic BP in the posterior tibial or dorsalis pedis by the higher of the right or left brachial systolic BPs.
Body weight and height were each measured twice at baseline, and the mean was used to calculate body mass index (BMI) as weight in kilograms divided by height in meters squared. Waist circumference was measured at the uppermost lateral border of the iliac crest with a Gulick II tape and repeated until 2 measures agreed within 1 cm. Three seated BP measurements were obtained by trained and certified staff members after >5 minutes of quiet rest and were averaged for analysis. These measurements were performed according to a standard protocol using an aneroid sphygmomanometer.12 Hypertension was defined as systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg and/or current use of antihypertensive medication.
Blood glucose, cholesterol, and triglycerides were measured using standard laboratory methods. Diabetes was defined as a fasting glucose ≥126 mg/dL, or a random glucose ≥200 mg/dL, and/or use of insulin or other antidiabetic medication. Serum high‐sensitivity C‐reactive protein (hsCRP) and cystatin C were measured using a particle‐enhanced immunonephelometric method. Urinary albumin was measured by radioimmunoassay. The eGFR was calculated using the re‐expressed Modification of Diet in Renal Disease equation after calibrating serum creatinine measurements to isotope dilution mass‐spectrometry–traceable values.13 All laboratory analyses were conducted at the CRIC Study Central Laboratory at the University of Pennsylvania (Philadelphia, PA) with stringent quality control.
Follow‐up and Outcomes
Study participants were followed up with annual clinical visits and 6‐month telephone interviews. Clinical information on incident CVD was extracted from hospital records by a trained research nurse. Clinical diagnoses of CVD were adjudicated by at least 2 physician reviewers from the outcome assessment committee using standard criteria. Incident clinical PAD was defined as a history of amputation attributed to PAD, peripheral surgical or percutaneous revascularization procedures, any arterial angioplasty, or any artery‐artery bypass graft. Deaths were confirmed by death certificate and the National Death Master File. We defined a composite CVD outcome of incident MI, stroke, and total mortality. Median duration of follow‐up was 7.5 years for this analysis, and 197 participants were lost to follow‐up.
Statistical Analysis
Baseline characteristics among the study participants in the different categories of ABI were described using mean (SD) for continuous variables and count (%) for categorical variables. Differences were compared with the use of ANOVA for continuous variables and the chi‐square test for categorical variables. We used restricted‐cubic‐spline plots to explore the shape of the association between ABI measurements and each clinical outcome. On the basis of our restricted‐cubic‐spline plots for PAD, CVD, and all‐cause mortality and the results of previous studies,6, 7, 14 we selected a level of ABI of 1.0 to 1.4 as the reference category. Furthermore, ABI values were categorized into 4 groups (<0.9, 0.9 to <1.0, 1.0 to <1.4, and ≥1.4) in our analysis.
Cumulative event rates of incident PAD, MI, HF, and CVD, and all‐cause mortality were calculated according to ABI groups using the Kaplan–Meier method and compared using the log‐rank test.15 We also reported the number of events and calculated the incidence rate for each ABI group using Poisson regression. Hazards ratios for the associations of ABI with PAD, MI, HF, CVD, and all‐cause mortality were estimated using Cox proportional hazards models.16 Age, race, sex, clinic site, history of CVD, diabetes, hypertension, cigarette smoking, alcohol consumption, high school education, physical activity (total METs/week), systolic BP, BMI, low‐density lipoprotein (LDL)‐cholesterol, high‐density lipoprotein (HDL)‐cholesterol, glucose, hsCRP, 24‐hour albuminuria, eGFR, and use of medications (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, beta blockers, aspirin, and statins) were adjusted in multivariable models. Hazard ratios and 95% confidence intervals of the clinical CVD events and mortality were calculated for each category of ABI using ABI of 1.0 to 1.4 as the reference group. The assumption of proportional hazards was tested using interaction terms with ABI groups by time for each outcome variable and covariate. No substantial deviations from proportionality were observed (P>0.08). All analyses were conducted using SAS software (version 9.1; SAS Institute Inc., Cary, NC). All P values were 2‐sided, and statistical significance was defined as P<0.05.
Results
Baseline characteristics according to ABI categories are shown in Table 1. Participants with an ABI <0.9 were significantly older, more likely to be female, African American, and current smokers, but less likely to be high school graduates, physically active, and alcohol drinkers than those with ABI of 1.0 to <1.4. In addition, they had significantly higher BMI, waist circumference, systolic BP, plasma glucose, hsCRP, cystatin C, and albuminuria and lower HDL‐ and LDL‐cholesterol and eGFR. Proportions of participants who reported a history of clinical CVD, hypertension, and diabetes and use of antihypertensive medications, aspirin, and statins were significantly higher among patients with ABI <0.9. Compared to those with ABI of 1.0 to <1.4, patients with an ABI >1.4 were older and less likely to be female, physically active, and alcohol drinkers. Persons with an ABI >1.4 also had significantly higher BMI, waist circumference, systolic BP, hsCRP, cystatin C, and albuminuria, lower HDL‐ and LDL‐cholesterol and eGFR, and were more likely to have a history of clinical CVD, hypertension, and diabetes and use antihypertensive medications and aspirin. Baseline characteristics were comparable between those lost to follow‐up and those not, except systolic BP (132.1±26.3 vs 127.7±21.8 mm Hg; P=0.01) and albuminuria (0.9±1.7 vs 0.6±1.5 g/24 hours; P=0.04).
Table 1.
Baseline Characteristics of Study Participants According to ABI
| Characteristic | Ankle Brachial Index | P Value for Differences | |||
|---|---|---|---|---|---|
| <0.9 (n=542) | 0.9 to <1.0 (n=571) | 1.0 to <1.4 (n=2430) | ≥1.4 (n=84) | ||
| Age, y | 62.6 (9.1) | 58.2 (10.9) | 57.0 (11.3) | 58.4 (10.1) | <0.001 |
| Female, n (%) | 276 (50.9) | 331 (58.0) | 1033 (42.5) | 28 (33.3) | <0.001 |
| Race/ethnicity, n (%) | |||||
| White | 177 (32.7) | 202 (35.4) | 1110 (45.7) | 35 (41.7) | |
| African American | 291 (53.7) | 283 (49.6) | 926 (38.1) | 27 (32.1) | <0.001 |
| Other | 74 (13.7) | 86 (15.1) | 394 (16.2) | 22 (26.2) | |
| High school graduates, n (%) | 382 (70.5) | 447 (78.3) | 2007 (82.6) | 65 (77.4) | <0.001 |
| Physical activity, MET/week | 172.0 (126.1) | 199.3 (161.5) | 210.1 (147.9) | 176.8 (140.2) | <0.001 |
| Current smoking, n (%) | 109 (20.1) | 96 (16.8) | 258 (10.6) | 8 (9.5) | <0.001 |
| Alcohol drinking, n (%) | 278 (51.3) | 354 (62.0) | 1629 (67.0) | 50 (59.5) | <0.001 |
| Body mass index, kg/m2 | 32.7 (8.3) | 32.8 (8.0) | 31.5 (7.5) | 33.9 (7.5) | <0.001 |
| Waist circumference, cm | 108.0 (18.0) | 106.7 (18.2) | 104.6 (17.1) | 111.2 (15.7) | <0.001 |
| Systolic blood pressure, mm Hg | 133.9 (23.8) | 127.6 (22.2) | 126.6 (21.5) | 131.8 (22.7) | <0.001 |
| Plasma glucose, mg/dL | 119.3 (49.4) | 115.8 (53.5) | 111.8 (48.6) | 112.9 (47.0) | <0.001 |
| HDL‐cholesterol, mg/dL | 45.5 (13.4) | 48.1 (15.3) | 48.2 (15.9) | 45.0 (15.7) | 0.001 |
| LDL‐cholesterol, mg/dL | 100.9 (33.8) | 104.4 (36.0) | 103.7 (35.1) | 91.6 (37.4) | 0.005 |
| hsCRP, mg/L | 7.43 (13.34) | 6.74 (13.44) | 4.85 (7.76) | 5.47 (7.09) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 38.4 (12.0) | 42.3 (13.6) | 44.6 (13.6) | 40.4 (13.4) | <0.001 |
| Cystatin C, mg/L | 1.72 (0.55) | 1.53 (0.57) | 1.43 (0.51) | 1.73 (0.58) | <0.001 |
| Albuminuria, g/24 hours | 0.76 (1.79) | 0.51 (1.39) | 0.62 (1.46) | 1.08 (2.22) | 0.004 |
| History of clinical CVD, n (%) | 289 (53.3) | 173 (30.3) | 603 (24.8) | 32 (38.1) | <0.001 |
| Hypertension, n (%) | 508 (93.7) | 500 (87.6) | 2024 (83.3) | 73 (86.9) | <0.001 |
| Diabetes, n (%) | 341 (62.9) | 273 (47.8) | 998 (41.1) | 59 (70.2) | <0.001 |
| Use of RAAS blockers, n (%) | 409 (75.9) | 378 (66.8) | 1617 (67.0) | 60 (71.4) | 0.001 |
| Use of β‐blockers, n (%) | 328 (60.9) | 275 (48.6) | 1085 (44.9) | 49 (58.3) | <0.001 |
| Use of aspirin, n (%) | 300 (55.7) | 236 (41.7) | 917 (38.0) | 41 (48.8) | <0.001 |
| Use of statins, n (%) | 373 (69.2) | 295 (52.1) | 1221 (50.6) | 44 (52.4) | <0.001 |
All values reported as mean (SD) or n (%). SI conversion factors: to convert glucose from mg/dL to mmol/L, multiply by 0.0555; LDL and HDL from mg/dL to mmol/L, multiply by 0.0259; and hsCRP from mg/dL to nmol/L, multiply by 9.524. ABI indicates ankle brachial index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; MET, metabolic equivalent of task; RAAS, renin‐angiotensin‐aldosterone system.
Multivariate spline regressions indicated a U‐shaped association of ABI with PAD, MI, CVD, and all‐cause mortality (Figure 1). Individuals with an ABI between 1.0 and <1.4 had the lowest risk of developing clinical outcomes.
Figure 1.
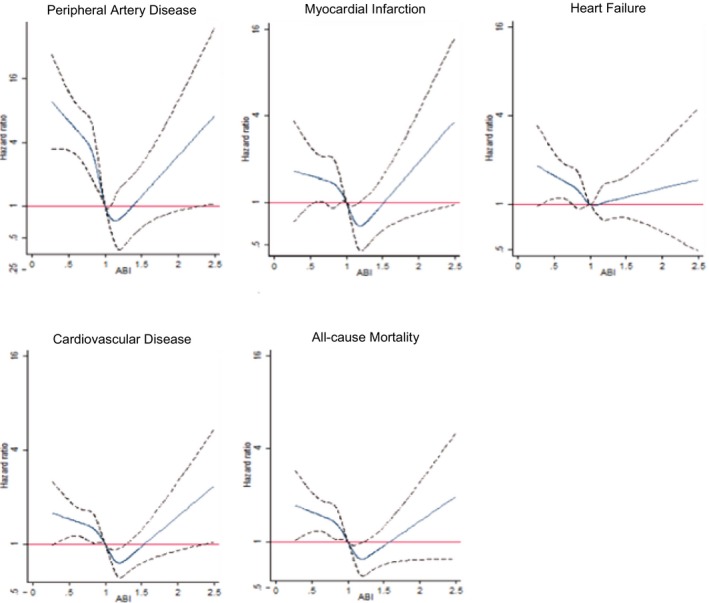
Spline plots of multiple‐adjusted hazard ratios and 95% confidence intervals of peripheral artery disease, myocardial infarction, heart failure, composite cardiovascular disease, and all‐cause mortality associated with baseline ankle‐brachial index. In each plot, the solid blue line represents the point estimate and the dotted black lines represent 95% confidence intervals. ABI indicates ankle‐brachial index.
Kaplan–Meier plots showed that individuals with an ABI between 1.0 and <1.4 had the lowest cumulative incidences whereas those with an ABI <0.9 has the highest cumulative incidences of PAD, MI, HF, CVD, and all‐cause mortality (Figure 2). Persons with an ABI ≥1.4 or ABI of 0.9 to <1.0 also had an increased cumulative incidences of CVD events and all‐cause mortality compared to those with ABI of 1.0 to <1.4.
Figure 2.
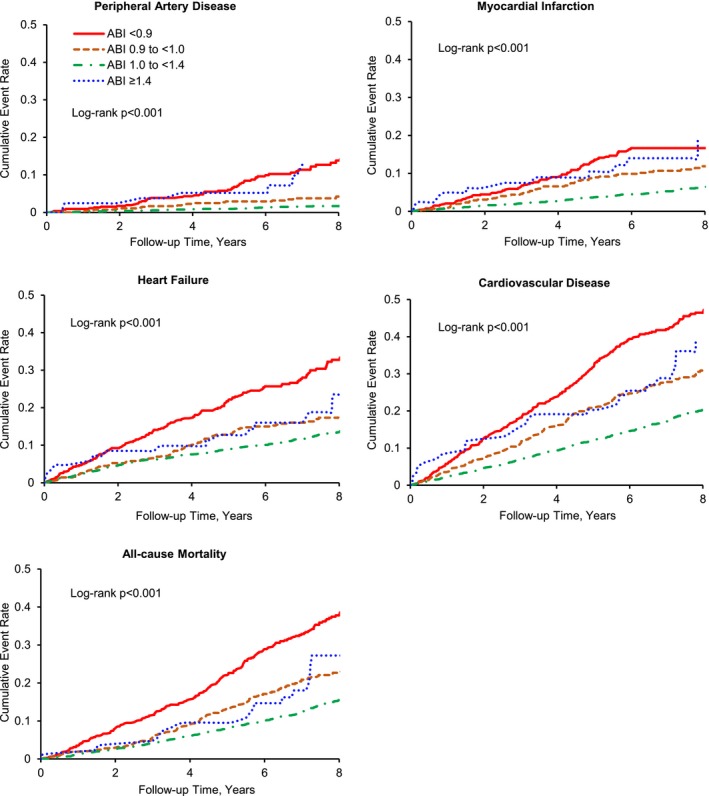
Kaplan–Meier estimates of cumulative incidence of peripheral artery disease, myocardial infarction, heart failure, composite cardiovascular disease, and all‐cause mortality. ABI indicates ankle‐brachial index.
Table 2 shows numbers of events and event rates as well as multiple‐adjusted hazard ratios of cardiovascular diseases and deaths associated with ABI categories. Compared to the reference group (ABI 1.0 to <1.4) persons with an ABI <0.9 had a 5.8‐fold increased risk of PAD whereas those with ABI of 0.9 to <1.0 had a 2.8‐fold increased risk of PAD after adjustment for multiple important CVD risk factors. Likewise, individuals with an ABI ≥1.4 had a 4.9‐fold increased risk of PAD. The relationship of other traditional risk factors with the CVD outcomes and mortality are presented in Table S1; all significant traditional risk factors were adjusted in the final Cox proportional hazards models (Table 2).
Table 2.
Multivariable Adjusted Hazard Ratios of Cardiovascular Diseases and Mortality Associated With ABI
| Outcome | No. of Events | Incidence, per 1000 | Age, Sex, Race, and Clinic Site‐Adjusted | Multivariable‐Adjusteda | ||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |||
| PAD | ||||||
| <0.9 | 53 | 1.67 | 8.04 (5.17, 12.50) | <0.001 | 5.78 (3.57, 9.35) | <0.001 |
| 0.9 to <1.0 | 21 | 0.56 | 2.98 (1.72, 5.15) | <0.001 | 2.76 (1.56, 4.88) | <0.001 |
| 1.0 to <1.4 | 37 | 0.22 | Ref | Ref | ||
| ≥1.4 | 7 | 1.41 | 5.03 (2.21, 11.46) | <0.001 | 4.85 (2.05, 11.5) | <0.001 |
| P value for nonlinear trend | <0.001 | <0.001 | ||||
| MI | ||||||
| <0.9 | 79 | 2.55 | 2.70 (2.02, 3.61) | <0.001 | 1.67 (1.23, 2.29) | 0.001 |
| 0.9 to <1.0 | 57 | 1.58 | 2.03 (1.47, 2.79) | <0.001 | 1.85 (1.33, 2.57) | <0.001 |
| 1.0 to <1.4 | 129 | 0.79 | Ref | Ref | ||
| ≥1.4 | 11 | 2.33 | 2.59 (1.39, 4.82) | 0.003 | 2.08 (1.10, 3.93) | 0.024 |
| P value for nonlinear trend | <0.001 | <0.001 | ||||
| HF | ||||||
| <0.9 | 142 | 4.89 | 2.10 (1.70, 2.59) | <0.001 | 1.27 (1.01, 1.58) | 0.039 |
| 0.9 to <1.0 | 86 | 2.43 | 1.22 (0.96, 1.56) | 0.14 | 1.10 (0.85, 1.42) | 0.48 |
| 1.0 to <1.4 | 289 | 1.84 | Ref | Ref | ||
| ≥1.4 | 15 | 3.12 | 1.37 (0.81, 2.31) | 0.24 | 0.89 (0.52, 1.51) | 0.66 |
| P value for nonlinear trend | <0.001 | 0.19 | ||||
| CVD (MI, stroke, and total mortality) | ||||||
| <0.9 | 247 | 7.89 | 2.25 (1.88, 2.69) | <0.001 | 1.39 (1.15, 1.68) | <0.001 |
| 0.9 to <1.0 | 169 | 4.53 | 1.51 (1.23, 1.85) | <0.001 | 1.39 (1.15, 1.68) | <0.001 |
| 1.0 to <1.4 | 479 | 2.84 | Ref | Ref | ||
| ≥1.4 | 27 | .27 | 1.26 (0.79, 2.01) | 0.32 | 1.23 (0.82, 1.84) | 0.32 |
| P value for nonlinear trend | <0.001 | <0.001 | ||||
| All‐cause mortality | ||||||
| <0.9 | 200 | 5.85 | 2.21 (1.89, 2.60) | <0.001 | 1.55 (1.28, 1.89) | <0.001 |
| 0.9 to <1.0 | 129 | 3.23 | 1.54 (1.29, 1.85) | <0.001 | 1.36 (1.08, 1.69) | 0.005 |
| 1.0 to <1.4 | 364 | 2.09 | Ref | Ref | ||
| ≥1.4 | 19 | 3.33 | 1.54 (1.04, 2.27) | 0.031 | 1.00 (0.62, 1.62) | 1.00 |
| P value for nonlinear trend | <0.001 | <0.001 | ||||
Adjusted for age, race, sex, clinic site, history of cardiovascular disease, diabetes, hypertension, current smoking, alcohol use, high school education, physical activity, systolic blood pressure, body mass index, LDL‐ and HDL‐cholesterol, plasma glucose, high‐sensitivity C‐reactive protein, 24‐hour excretion of albuminuria, estimated glomerular filtration rate, and use of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, beta‐blockers, aspirin, or statins. ABI indicates ankle brachial index; CVD, cardiovascular disease; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease.
Compared to the reference group, individuals with an ABI <0.9 and ABI of 0.9 to <1.0 had a 1.7‐ and 1.9‐fold increased risk of MI, respectively, after multiple adjustment. Individuals with an ABI <0.9 also had a 27% increased risk of HF. Furthermore, individuals with an ABI <0.9 and ABI of 0.9 to <1.0 had a 51% and 39% increased risk of composite CVD, respectively. However, an ABI >1.4 was not significantly associated with risk of CVD or all‐cause mortality (Table 2).
Compared to ABI 1.0 to <1.4, ABI <0.9 and ABI 0.9 to <1.0 were significantly associated with a 56% and 34% increase in all‐cause mortality after adjustment for multiple risk factors whereas ABI >1.4 was not significantly associated with all‐cause mortality after multiple adjustment.
Discussion
Our study indicated that an ABI of <1.0 was strongly and significantly associated with an increased risk of clinical PAD, MI, composite CVD, and all‐cause mortality among patients with CKD. In addition, an ABI ≥1.4 was strongly and significantly associated with risk of developing clinical PAD. These associations were independent of albuminuria and eGFR in addition to other established CVD risk factors and current treatment.
These findings have important clinical and public health implications, because patients with CKD are at an increased risk of developing PAD.1, 2 In addition, CKD patients with PAD have a very high risk of CVD and all‐cause mortality.8, 17 Proper detection and intervention are the key to prevent adverse CVD outcomes associated with PAD among patients with CKD. ABI is a simple, inexpensive, and noninvasive measure of subclinical PAD.3 Traditionally, an ABI cutpoint of <0.9 was considered subclinical peripheral arterial atherosclerosis. However, we observed a 2.8‐ or 4.9‐fold increase in risk of clinical PAD among participants with ABI of 0.9 to <1.0 or ABI ≥1.4, respectively. These results suggest that ABI cutpoints for the clinical diagnosis of PAD, as well as CVD risk stratification among patients with CKD, may need to be further evaluated.
Our study is the first to report that an ABI ≥1.4 is significantly related to future clinical PAD among predialysis CKD patients after adjusting for established CVD risk factors. In a previous cross‐sectional study, ABI >1.4 was shown to be associated with leg ulcers in the general population.5 ABI ≥1.4 was associated with vascular calcification in peripheral and distal arteries among dialysis patients.8 Vascular calcification is highly prevalent in CKD patients.18, 19 Medial arterial calcification is common in CKD patients and causes arterial stiffness, a decrease in perfusion, and impairment of collateral circulation formation,20, 21, 22, 23 which may contribute to PAD. Our study suggests that ABI ≥1.4 is not significantly associated with MI, HF, composite CVD, and all‐cause mortality in patients with CKD. A previous meta‐analysis suggested that ABI >1.4 was associated with total mortality, but not major coronary events, in the general population.6 Adragao et al. reported that ABI >1.3 was associated with increased all‐cause and CVD mortality among 219 dialysis patients.8 The inconsistent findings may be partially attributed to the small sample size in the ABI ≥1.4 group in our study. Future studies are warranted to confirm the association of ABI ≥1.4 with PAD, MI, CVD, and mortality in a large cohort of CKD patients.
Our study found that ABI of 0.9 to <1.0 was significantly related with future clinical PAD, MI, composite CVD, as well as all‐cause mortality among predialysis CKD patients after adjusting for established CVD risk factors. Previous studies in non‐CKD patients suggested that ABI of 0.9 to <1.0 predicted CVD and total mortality.6, 24 The American Heart Association (AHA) suggested that ABI should be interpreted according to the a priori probability of PAD, and values between 0.91 and 1.00 should be considered borderline.25 However, the sensitivity and specificity of ABI 0.91 to 1.00 as a cutpoint to detect PAD compared to an angiographic finding of ≥50% stenosis in patients with PAD were varied, and its predictive value for future PAD events remains unknown.25 Our study reported that ABI of 0.9 to <1.0 was associated with a 2.8‐fold higher risk of future PAD, suggesting that ABI of 0.9 to <1.0 might have clinical significance for the CKD population without history of clinical PAD. Further studies are needed to confirm the association between ABI of 0.9 to <1.0 and incident clinical PAD and to evaluate ABI of <1.0 as a cutpoint for diagnosis of PAD among CKD patients. In addition, ABI of 0.9 to <1.0 was considered borderline in terms of cardiovascular risk in the general population per the AHA Scientific Statement,25 whereas our findings provide additional evidence that an ABI of 0.9 to <1.0 represents significantly high risk for further MI, CVD, and all‐cause mortality among CKD patients, who may benefit from earlier intervention or intensive treatment.
There are several strengths of our study. This is the first large prospective cohort study to examine ABI cutpoints with risk of PAD, other CVD, and mortality among patients with CKD. Numerous important confounding factors were collected and adjusted in the multivariable models. Therefore, our study should provide a valid and reliable assessment of ABI cutpoints with the outcomes. Several limitations of our study should be noted. First, the number of patients with an ABI >1.4 was small in our study. Future larger prospective cohort studies are needed to more precisely estimate the risk associated with ABI >1.4 among patients with CKD. Second, a single measurement of ABI at baseline was used instead of the mean of multiple measurements. However, ABIs were measured by trained and certified study staff. Third, we did not include patients with symptoms, such as rest pain or vascular ulceration, alone as clinical PAD because those symptoms are nonspecific and are not clearly described in detail in the study questionnaire. This could potentially result in lower outcome rates and reduced statistical power, but decreases the chance of misclassification. In addition, a recent study has suggested that an alternative ABI method utilizing the ratio of the lower of dorsalis pedis and posterior tibial pressures to the higher of the right or left brachial systolic pressures may provide better prediction of CVD mortality compared to the traditional method.26 However, the alternative method is not validated for clinical use, particularly in the CKD population. Finally, time‐dependent covariates, such as medication use during follow‐up, were not adjusted in this analysis. In future research, more‐sophisticated statistical methods, such as marginal structural models, could be used to study potential causal relationships between change in ABI and the clinical outcomes adjusting for time‐dependent covariates (ie, medication use).27, 28
In conclusion, our study indicates that ABI <1.0 and ≥1.4 are significantly associated with future clinical PAD among CKD patients. In addition, ABI <1.0 is significantly associated with increased risk of MI, CVD, and all‐cause mortality among CKD patients. These findings indicate that the ABI cutpoints for the diagnosis of PAD may need to be further evaluated in patients with CKD, and confirmatory tests to diagnose PAD may be beneficial among CKD patients with ABI of 0.9 to <1.0. Furthermore, ABI <1.0 may be useful for risk stratification of CVD and all‐cause mortality among patients with CKD.
Appendix
The CRIC Investigators are Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, Mahboob Rahman, MD, and Raymond R. Townsend, MD.
Sources of Funding
Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this study was supported, in part, by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award from the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences UL1TR000003, Johns Hopkins University UL1 TR‐000424, University of Maryland General Clinical Research Center M01 RR‐16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Awards UL1RR029879, Tulane Centers of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, and Kaiser Permanente, University of California, San Francisco–Clinical and Translational Science Institute (funded by NIH/The National Center for Research Resources) UL1 RR‐024131.
Disclosures
None.
Supporting information
Table S1. Hazard Ratios of Clinical Outcomes Associated With Traditional Risk Factors
(J Am Heart Assoc. 2016;5:e003339 doi: 10.1161/JAHA.116.003339)
References
- 1. O'Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;109:320–323. [DOI] [PubMed] [Google Scholar]
- 2. Chen J, Mohler ER III, Xie D, Shlipak MG, Townsend RR, Appel LJ, Raj DS, Ojo AO, Schreiber MJ, Strauss LF, Zhang X, Wang X, He J, Hamm LL. Risk factors for peripheral arterial disease among patients with chronic kidney disease. Am J Cardiol. 2012;110:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. 2011 Writing Group Members; 2005 Writing Committee Members; ACCF/AHA Task Force Members . 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–2045. [DOI] [PubMed] [Google Scholar]
- 4. Ix JH, Katz R, De Boer IH, Kestenbaum BR, Allison MA, Siscovick DS, Newman AB, Sarnak MJ, Shlipak MG, Criqui MH. Association of chronic kidney disease with the spectrum of ankle brachial index the CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2009;54:1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle‐brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51:1292–1298. [DOI] [PubMed] [Google Scholar]
- 6. Ankle Brachial Index Collaboration . Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality. A meta‐analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle‐brachial index and incident cardiovascular events in the MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56:1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adragao T, Pires A, Branco P, Castro R, Oliveira A, Nogueira C, Bordalo J, Curto JD, Prata MM. Ankle‐brachial index, vascular calcifications and mortality in dialysis patients. Nephrol Dial Transplant. 2012;27:318–325. [DOI] [PubMed] [Google Scholar]
- 9. Feldman HI, Appel LJ, Chertow GM, Li SL, Swiderski P, Putta S, Jonnalagadda M, Kato M, Natarajan R; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . The chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. [DOI] [PubMed] [Google Scholar]
- 10. Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group . Chronic renal insufficiency cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. [DOI] [PubMed] [Google Scholar]
- 12. Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 14. Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all‐cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16. Cox DR. Regression models and life tables (with discussion). J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 17. Liew YP, Bartholomew JR, Demirjian S, Michaels J, Schreiber MJ Jr. Combined effect of chronic kidney disease and peripheral arterial disease on all‐cause mortality in a high‐risk population. Clin J Am Soc Nephrol. 2008;3:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS. Incidence and progression of coronary calcification in chronic kidney disease: the Multi‐Ethnic Study of Atherosclerosis. Kidney Int. 2009;76:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He J, Reilly M, Yang W, Chen J, Go AS, Lash JP, Rahman M, DeFilippi C, Gadegbeku C, Kanthety R, Tao K, Hamm LL, Ojo A, Townsend R, Budoff M. Risk factors for coronary artery calcium among patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am J Cardiol. 2012;110:1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proudfoot D, Shanahan CM. Biology of calcification in vascular cells: intima versus media. Herz. 2001;26:245–251. [DOI] [PubMed] [Google Scholar]
- 21. Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age‐related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–317. [DOI] [PubMed] [Google Scholar]
- 23. Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle‐arm index spectrum: results from the Cardiovascular Health Study. Circulation. 2006;113:388–393. [DOI] [PubMed] [Google Scholar]
- 25. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat‐Jacobson D; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. [DOI] [PubMed] [Google Scholar]
- 26. Nead KT, Cooke JP, Olin JW, Leeper NJ. Alternative ankle‐brachial index method identifies additional at‐risk individuals. J Am Coll Cardiol. 2013;62:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER, Rahman M, Steigerwalt S, Weir M, Wright JT, Feldman HI. Time‐updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med. 2015;162:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hazard Ratios of Clinical Outcomes Associated With Traditional Risk Factors


