Abstract
Background
The introduction of non–vitamin K antagonist oral anticoagulants has been a major advance for stroke prevention in atrial fibrillation; however, outcomes achieved in clinical trials may not translate to routine practice. We aimed to evaluate the effectiveness and safety of dabigatran, rivaroxaban, and apixaban by comparing each agent with warfarin.
Methods and Results
Using a large US insurance database, we identified privately insured and Medicare Advantage patients with nonvalvular atrial fibrillation who were users of apixaban, dabigatran, rivaroxaban, or warfarin between October 1, 2010, and June 30, 2015. We created 3 matched cohorts using 1:1 propensity score matching: apixaban versus warfarin (n=15 390), dabigatran versus warfarin (n=28 614), and rivaroxaban versus warfarin (n=32 350). Using Cox proportional hazards regression, we found that for stroke or systemic embolism, apixaban was associated with lower risk (hazard ratio [HR] 0.67, 95% CI 0.46–0.98, P=0.04), but dabigatran and rivaroxaban were associated with a similar risk (dabigatran: HR 0.98, 95% CI 0.76–1.26, P=0.98; rivaroxaban: HR 0.93, 95% CI 0.72–1.19, P=0.56). For major bleeding, apixaban and dabigatran were associated with lower risk (apixaban: HR 0.45, 95% CI 0.34–0.59, P<0.001; dabigatran: HR 0.79, 95% CI 0.67–0.94, P<0.01), and rivaroxaban was associated with a similar risk (HR 1.04, 95% CI 0.90–1.20], P=0.60). All non–vitamin K antagonist oral anticoagulants were associated with a lower risk of intracranial bleeding.
Conclusions
In patients with nonvalvular atrial fibrillation, apixaban was associated with lower risks of both stroke and major bleeding, dabigatran was associated with similar risk of stroke but lower risk of major bleeding, and rivaroxaban was associated with similar risks of both stroke and major bleeding in comparison to warfarin.
Keywords: atrial fibrillation, bleeding, non–vitamin K antagonist oral anticoagulants, stroke, warfarin
Subject Categories: Atrial Fibrillation, Secondary Prevention, Transplantation, Anticoagulants, Cerebrovascular Disease/Stroke
Introduction
Atrial fibrillation (AF) is common, with a 1‐in‐4 lifetime risk after age 40 years,1 and is associated with a 3‐ to 5‐fold increased risk of stroke.2, 3 Treatment with warfarin can reduce the risk of stroke by 60% to 70%,4 but its use can be cumbersome because of numerous food and drug interactions and the need for ongoing laboratory testing and dose adjustment.5 Non–vitamin K antagonist oral anticoagulants (NOACs) provide more convenient therapeutic options and have demonstrated at least equivalent efficacy in comparison to warfarin in large phase III clinical trials.6, 7, 8, 9
The efficacy and safety achieved in the idealized clinical trial settings may not necessarily translate to routine practice because of the differences in the patient populations, the intensity of follow‐up, and the variations in care that patients receive. Extrapolating findings from trials to general practice is especially challenging for anticoagulation therapies. Because anticoagulants are long‐term preventive medications that address no ongoing symptoms, adherence is substantially lower in observational studies than in clinical trials.10, 11, 12, 13 Furthermore, appropriate dosing may be hard to achieve in clinical practice because of the complexity of real‐world settings.14
As these medications are more broadly adopted,15, 16 ongoing evaluation of their effectiveness and safety is important. Until observational studies confirm the generalizability of the clinical trials, some clinicians may remain skeptical and withhold NOACs from patients who stand to benefit from them.17, 18 Several observational studies have compared dabigatran or rivaroxaban with warfarin,19, 20, 21, 22, 23, 24, 25 but very few studies have examined apixaban. In addition, because these medications have been available longer, there is an opportunity for greater follow‐up and better powered analyses. Using a large patient population from a wide variety of health care settings, we evaluated stroke and bleeding outcomes associated with dabigatran, rivaroxaban, and apixaban use by comparing each agent with warfarin.
Methods
Data Source and Study Population
We conducted a retrospective analysis using administrative claims data from OptumLabs Data Warehouse (OLDW), which contains >100 million privately insured and Medicare Advantage enrollees over the past 20 years throughout the United States.26, 27 We identified adult patients (aged ≥18 years) with nonvalvular AF who were users of apixaban, dabigatran, rivaroxaban, and warfarin between October 1, 2010, and June 30, 2015. A cohort creation flow chart is shown in Figure 1.
Figure 1.
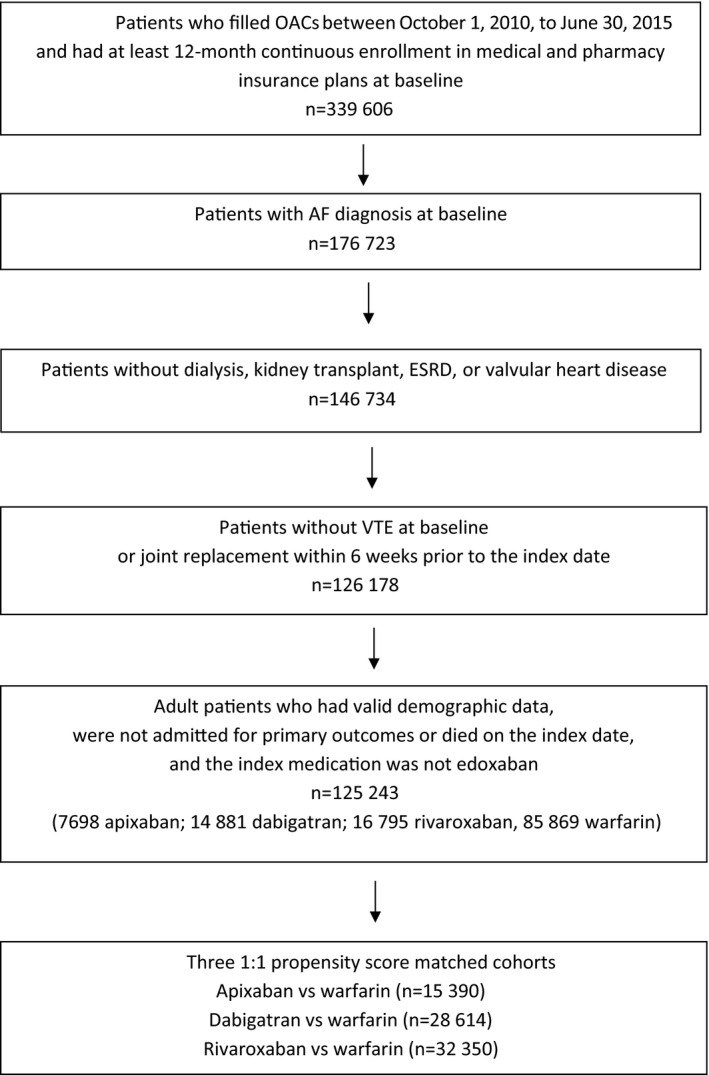
Cohort creation flowchart. AF indicates atrial fibrillation; ESRD, end‐stage renal disease; OAC, oral anticoagulants; VTE, venous thromboembolism.
If a patient ever used NOACs during the study period, the first fill of NOACs was defined as the index medication. Patients were required to have at least 12 months of continuous enrollment in both medical and pharmacy insurance plans prior to the index date, defined as the baseline period. For patients who only filled warfarin and never filled NOACs, the index medication was defined as the first warfarin fill after enrolling in health plans for at least 12 months; therefore, both warfarin and NOACs cohorts included patients who had previous warfarin exposure but none had previous NOACs exposure.
We included patients with prior warfarin exposure because in all pivotal trials and routine clinical practice, the majority of patients initiating NOACs have previously used warfarin.24 This is partly due to the higher costs of NOACs in comparison to warfarin. In the United States, many insurance plans require prior authorization for NOAC prescriptions, and some require patients to have documented trial of warfarin before using NOACs. Consequently, selecting only warfarin‐naïve NOACs users would bias toward including patients with more generous insurance benefits, which often correlate with higher socioeconomic status. Similar methods were used in previous comparative effectiveness studies of NOACs.24 Subgroup analyses were conducted to determine whether the effectiveness and safety of NOACs in comparison to warfarin differ in patients with and without prior warfarin exposure.
All patients were required to have at least 1 inpatient or outpatient AF diagnosis at either primary or secondary positions (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9] diagnosis 427.31) on the index date or at baseline (ie, 12 months before the index date). ICD‐9 diagnosis code 427.31 performed relatively well in previous validation studies, with a median positive predictive value of 89%.28
Patients who had valvular heart disease, end‐stage chronic kidney disease, kidney transplant, or dialysis at any time were excluded. Valvular heart disease was defined as rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair, based on the definition of “nonvalvular” AF in the 2014 American College of Cardiology, American Heart Association, and Heart Rhythm Society guideline.5 We also excluded patients who underwent hip or knee replacement surgery within 6 weeks prior to the index date and who had a diagnosis of deep vein thrombosis or pulmonary embolism at baseline.
Our study was exempt by the institutional review board for approval because we used only preexisting deidentified data.
Study End Points
The primary effectiveness outcome was stroke or systemic embolism, including ischemic stroke, hemorrhagic stroke, and systemic embolism. The primary safety outcome was major bleeding, including gastrointestinal bleeding, intracranial bleeding, and bleeding from other sites. We included outcomes that occurred on treatment, defined as the time after the first eligible prescription fill until the end of enrollment in health plans, the end of the study period (June 30, 2015), discontinuation of treatment, or switching to another oral anticoagulant.
The outcomes were identified using ICD‐9 codes in the primary or secondary diagnosis positions of inpatient claims (Table 1). These codes performed relatively well in previous validation studies. The positive predictive value in general ranged from 85% to 95%.29, 30, 31, 32, 33 Transient ischemic attack was not included in the main effectiveness end point because of the difficulty in validating transient ischemic attack and its’ use as a diagnosis for diffuse symptoms or dizziness.34 We censored patients when they had an inpatient admission for transient ischemic attack caused by increased thromboembolic risk following a transient ischemic attack event. A sensitivity test was conducted to include transient ischemic attack in the effectiveness end point, and the results did not differ from the main analysis.
Table 1.
ICD 9‐CM Codes Used to Define Study Outcomes
| Outcomes | ICD‐9‐CM Codes |
|---|---|
| Major bleeding | |
| Intracranial bleeding | 430, 431, 432.x, 852.x, 853.x |
| Gastrointestinal bleeding | 456.0, 456.20, 530.21, 530.7, 530.82, 531.0x, 531.2x, 531.4x, 531.6x, 532.0x, 532.2x, 532.4x, 532.6x, 533.0x, 533.2x, 533.4x, 533.6x, 534.0x, 534.2x, 534.4x, 534.6x, 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 535.71, 537.83, 537.84, 562.02, 562.03, 562.12, 562.13, 568.81, 569.3, 569.85, 578.x |
| Bleeding from other sites | 423.0, 459.0, 596.7, 599.71, 719.1x, 784.8, 786.3 |
| Stroke or systemic embolism | |
| Ischemic stroke | 433.x1, 434.x1, or 436 |
| Hemorrhagic stroke | 430, 431 |
| Systemic embolism | 444.x |
| TIA | 435.x |
Outcomes were identified using primary or secondary diagnosis on inpatient claims. When assessing stroke or systemic embolism, we excluded the events that had a primary discharge diagnosis of rehabilitation (ICD‐9‐CM code V57) or any additional diagnoses of trauma (ICD‐9‐CM codes 800–804 and 850–854). When assessing major bleeding, we excluded the events that had a primary discharge diagnosis of rehabilitation (ICD‐9‐CM code V57). ICD‐9‐CM indicates International Classification of Disease, 9th Revision, Clinical Modification; TIA, transient ischemic attack.
We used the fill dates and days supplied per prescription to determine patients’ treatment episodes, defined as the period from the fill date to the date when there were no residual days of supply. A gap of a maximum of 30 days between treatment episodes was allowed; patients were considered to be continuing on treatment as long as they had another medication fill within 30 days of the end of the last treatment episode. The allowable gap in treatment varied in previous observational NOAC comparison studies, ranging from 3 to 60 days.19, 20, 35, 36 We chose 30 days instead of a longer gap (eg, 60 days) because oral anticoagulants, especially NOACs, have a short half‐life. A shorter allowable gap increased the likelihood that patients were indeed on treatment. We did not choose a shorter gap such as 3 days because, in reality, a short gap in treatment often reflects imperfect adherence to treatment rather than full discontinuation during the gap. Patients may miss a few pills from time to time, and some patients may intentionally miss or split pills as a cost‐saving strategy37; however, when patients had a 1‐month gap, their adherence since the last prescription fill (measured by proportion of days covered) would fall to 50% to 75%, depending on whether the last fill was a 30‐ or 90‐day supply. Consequently, we chose 1 month as the allowable gap. We conducted additional sensitivity tests to change the allowable gap to 7 days, and the findings remained largely the same.
Statistical Analysis
We created 3 matched cohorts (dabigatran versus warfarin, rivaroxaban versus warfarin, and apixaban versus warfarin) using 1:1 propensity score matching without replacement and with a caliper of 0.01. Propensity scores for NOAC treatment were estimated using logistic regression, which included information on 48 sociodemographic and clinical characteristics: age, sex, race, residence region, baseline medication use, Charlson‐Deyo comorbidity index,38 CHA2DS2‐VASc score,39 HAS‐BLED score,40 SAMe‐TT2R2 score,41 and individual risk factors for these scores. The International Normalized Ratio (INR) was available in only some of the patients with prior warfarin treatment; therefore, a modified HAS‐BLED score was calculated with a range of 0 to 8.
Baseline characteristics were presented descriptively, and standardized difference was used to assess the balance of covariates after matching. A standardized difference <10% was considered acceptable.42 When conducting subgroup analyses, we also checked the balance of baseline characteristics within each subgroup. When imbalance of a baseline characteristic was detected, this variable was included in the Cox proportional hazards regression.
Cox proportional hazards regression was used to compare outcomes in each of the propensity score–matched cohorts, with robust sandwich estimates to account for the clustering within matched sets.43 Because all baseline characteristics were balanced after propensity score matching, the regression included only treatment (a NOAC or warfarin) as the independent variable. The proportional hazards assumption was tested on the basis of Schoenfeld residuals44 and was valid for all outcomes.
Subgroup analyses were performed based on patients’ baseline risk of stroke (assessed by CHA2DS2‐VASc score), baseline risk of bleeding (assessed by HAS‐BLED score), previous warfarin exposure, and whether patients received reduced‐dose NOAC.
Sensitivity Analysis
First, we compared the risk of stroke or systemic embolism including all events that occurred between the index date and the end of enrollment or study period (an intent‐to‐treat analytic approach). This analysis was performed to assess whether primary findings using on‐treatment analytic approach would be affected by differential censoring between treatment groups; however, this method has its own limitations of increasing treatment misclassification with longer follow‐up.
Second, we limited the study population to patients initiating NOACs from January 1, 2013, to June 30, 2015. This analysis excluded early users of NOACs, who may be different from those who started NOACs later (eg, eagerness to adopt new treatments or abnormal baseline risk). This was also the time period in which all the 3 NOACs were available in the United States. Moreover, because of the long study period, there could be some unmeasured trends over time. The negative publicity of dabigatran in the earlier years may have led to higher nonadherence, discontinuation, and switch among dabigatran users. Limiting analysis to the second half of the study period may have helped address these concerns.
Third, because apixaban became available in the United States in December 2012, apixaban users had shorter follow‐up time compared with other agents. We conducted sensitivity analyses to censor patients at 6 months, so all drugs have similar follow‐up time.
Fourth, some patients—especially those with low risk of stroke at baseline—may have received oral anticoagulation for catheter ablation or cardioversion procedures rather than for long‐term stroke prevention. Anticoagulation is recommended for at least 3 weeks before and 4 weeks after cardioversion and for at least 2 months after catheter ablation.5 We excluded patients who had catheter ablation within 2 months prior to the index medication and those who had cardioversion 1 month before and 1 month after the index medication.
Last, we conducted subgroup analyses based on baseline time in therapeutic range (TTR) in patients with prior warfarin experience and based on follow‐up TTR. We calculated TTR using Rosendaal's method, which uses linear interpolation to assign an INR value to each day between successive observed INR values. Gaps of 56 days between INR values were not interpolated. After interpolation, the percentage of time during which the interpolated INR values lay between 2.0 and 3.0 (from 0% to 100%) was calculated.45 The follow‐up TTRs of NOAC‐treated patients were assigned based on the TTRs of their matched warfarin controls. Labile INR was defined as TTR <60%.
All analyses were conducted using SAS 9.4 (SAS Institute Inc) and Stata 14.1 (Stata Corp).
Results
Baseline Characteristics
We created 3 matched cohorts using 1:1 propensity score matching: apixaban versus warfarin (n=15 390), dabigatran versus warfarin (n=28 614), and rivaroxaban versus warfarin (n=32 350). Based on the assessment of standardized difference, patients were all balanced on 48 dimensions. The logistic regressions for the propensity score models achieved C‐statistics of 0.78, 0.74, and 0.77 for apixaban, dabigatran and rivaroxaban, respectively. The baseline characteristics are shown in Table 2. Dabigatran patients were younger than apixaban and rivaroxaban patients (median age 73, 70, and 72 years in the apixaban, dabigatran and rivaroxaban patients, respectively), had lower risk of stroke or bleeding at baseline, and included a larger percentage of warfarin‐naïve patients. On average, patients were followed for 0.5±0.6, 0.7±0.8, and 0.6±0.7 year in the apixaban‐, dabigatran‐, and rivaroxaban‐matched cohorts, respectively.
Table 2.
Baseline Characteristics in Propensity Score–Matched NOAC or Warfarin Users
| Apixaban (n=7695) | Warfarin (n=7695) | Dabigatran (n=14 307) | Warfarin (n=14 307) | Rivaroxaban (n=16 175) | Warfarin (n=16 175) | |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| Median (IQR) | 73 (66–81) | 73 (66–81) | 70 (62–78) | 70 (61–78) | 72 (64–79) | 72 (64–80) |
| 18–64 | 22.7 | 23.0 | 34.1 | 35.0 | 25.3 | 25.8 |
| 65–74 | 30.9 | 30.9 | 31.5 | 30.4 | 32.9 | 32.8 |
| ≥75 | 46.4 | 46.1 | 34.4 | 34.6 | 41.8 | 41.4 |
| Female | 46.9 | 46.8 | 39.7 | 40.4 | 43.2 | 43.7 |
| Nonwhite race | 20.2 | 20.4 | 18.9 | 19.3 | 19.9 | 20.4 |
| Medical history | ||||||
| Congestive heart failure | 31.4 | 31.9 | 27.2 | 27.3 | 28.9 | 29.5 |
| Hypertension | 87.5 | 87.5 | 85.2 | 84.9 | 85.7 | 85.9 |
| Diabetes mellitus | 35.0 | 34.3 | 34.0 | 34.0 | 34.6 | 35.1 |
| Stroke/TIA/SE | 15.1 | 15.5 | 13.8 | 14.2 | 14.0 | 14.4 |
| Vascular disease | 28.3 | 28.4 | 23.1 | 23.4 | 26.9 | 27.5 |
| Abnormal renal function | 10.1 | 10.1 | 5.6 | 5.6 | 7.4 | 7.3 |
| Abnormal liver function | 4.0 | 4.1 | 3.5 | 3.6 | 3.7 | 3.8 |
| Bleeding history or predisposition | 31.4 | 31.8 | 29.4 | 30.1 | 30.7 | 31.5 |
| Alcoholism | 2.8 | 2.7 | 2.6 | 2.6 | 2.9 | 3.1 |
| Pulmonary disease | 33.1 | 33.7 | 28.2 | 28.4 | 31.2 | 32.1 |
| Obesity | 19.6 | 19.9 | 17.6 | 17.3 | 18.3 | 18.9 |
| Smoking | 19.8 | 20.0 | 16.1 | 16.0 | 18.5 | 19.4 |
| Medication use | ||||||
| Antiplatelets/NSAID | 12.1 | 12.5 | 10.3 | 10.2 | 11.6 | 11.6 |
| Amiodarone | 9.6 | 10.1 | 8.4 | 8.4 | 8.3 | 8.8 |
| Dronedarone | 2.8 | 2.6 | 3.7 | 4.2 | 2.4 | 2.6 |
| Other antiarrhythmic drugs | 11.1 | 10.7 | 12.8 | 12.9 | 11.0 | 11.2 |
| Digoxin | 8.9 | 9.1 | 13.6 | 13.6 | 10.8 | 11.1 |
| Diltiazem | 16.9 | 17.0 | 17.5 | 17.3 | 17.5 | 17.9 |
| Verapamil | 1.3 | 1.3 | 1.9 | 1.9 | 1.7 | 1.7 |
| Other calcium channel blockers | 16.6 | 16.3 | 13.3 | 13.4 | 14.9 | 14.7 |
| Statin | 45.6 | 46.7 | 41.5 | 41.2 | 43.0 | 43.9 |
| Other cholesterol reducers | 5.9 | 5.9 | 7.3 | 7.6 | 5.7 | 5.7 |
| β‐Blockers | 47.5 | 47.8 | 44.6 | 44.5 | 45.6 | 45.0 |
| Renin angiotensin system antagonists | 47.1 | 47.2 | 45.4 | 45.0 | 45.5 | 46.0 |
| Diuretics | 32.3 | 31.8 | 28.5 | 28.5 | 29.6 | 29.6 |
| Metformin | 11.1 | 10.7 | 10.2 | 9.9 | 10.6 | 11.0 |
| Sulfonylureas | 6.0 | 6.0 | 6.0 | 5.9 | 6.0 | 5.9 |
| Thiazolidinedione | 0.8 | 0.8 | 1.5 | 1.3 | 0.9 | 0.9 |
| Insulin | 7.3 | 7.3 | 6.8 | 7.1 | 7.1 | 7.5 |
| Other diabetes drugs | 3.1 | 2.9 | 2.8 | 2.9 | 2.7 | 2.9 |
| Antiulcer agents | 21.9 | 21.4 | 18.4 | 18.4 | 20.3 | 21.2 |
| Antidepressant | 16.2 | 16.1 | 14.5 | 15.0 | 15.3 | 15.6 |
| CHA2DS2‐VASc | ||||||
| Median (IQR) | 4 (3–5) | 4 (3–5) | 3 (2–5) | 3 (2–5) | 4 (2–5) | 4 (2–5) |
| 0–1 | 9.9 | 10.0 | 15.9 | 16.6 | 12.2 | 12.1 |
| 2–3 | 33.2 | 33.0 | 38.2 | 36.9 | 35.6 | 35.6 |
| ≥4 | 56.8 | 57.0 | 45.9 | 46.5 | 52.2 | 52.3 |
| HAS‐BLED | ||||||
| Median (IQR) | 2 (2–3) | 2 (2–3) | 2 (1–3) | 2 (1–3) | 2 (2–3) | 2 (2–3) |
| ≥3 | 41.5 | 41.9 | 33.7 | 33.9 | 38.6 | 39.1 |
| Charlson index | ||||||
| Median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 2 (1–4) |
| 0–1 | 37.7 | 37.9 | 45.5 | 45.3 | 41.3 | 40.6 |
| 2–3 | 32.0 | 32.1 | 30.4 | 30.4 | 30.8 | 30.5 |
| ≥4 | 30.3 | 30.0 | 24.1 | 24.3 | 27.9 | 28.9 |
| SAMe‐TT2R2 | ||||||
| Median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| ≥3 | 30.7 | 31.1 | 26.1 | 26.4 | 28.8 | 30.5 |
| Warfarin experienced | 20.2 | 20.4 | 37.8 | 38.6 | 24.4 | 25.0 |
| Reduced‐dose NOAC | 18.1 | NA | 8.8 | NA | 21.5 | NA |
Data are shown as percentages except as noted. IQR indicates interquartile range; NA, not available; NOAC, Non‐vitamin K antagonist oral anticoagulant; NSAID, nonsteroidal anti‐inflammatory drug; SE, systemic embolism; TIA, transient ischemic attack.
Effectiveness Outcomes
Apixaban was associated with reduced risk of stroke or systemic embolism compared with warfarin (hazard ratio [HR] 0.67, 95% CI 0.46–0.98, P=0.04). The reduction was driven mainly by the lower risk of hemorrhagic stroke (HR 0.35, 95% CI 0.14–0.88, P=0.03).
Dabigatran was associated with similar risk of stroke or systemic embolism compared with warfarin (HR 0.98, 95% CI 0.76–1.26, P=0.98). No significant differences were found in the risk of ischemic stroke or hemorrhagic stroke, but the risk of hemorrhagic stroke was numerically lower in dabigatran patients (HR 0.56, 95% CI 0.30–1.04, P=0.07).
Rivaroxaban was associated with similar risk of stroke or systemic embolism compared with warfarin (HR 0.93, 95% CI 0.72–1.19, P=0.56). No significant differences were found in the risk of ischemic stroke or hemorrhagic stroke, but the risk of hemorrhagic stroke was also numerically lower in rivaroxaban patients compared with warfarin (HR 0.61, 95% CI 0.35–1.07, P=0.08) (Figure 2).
Figure 2.
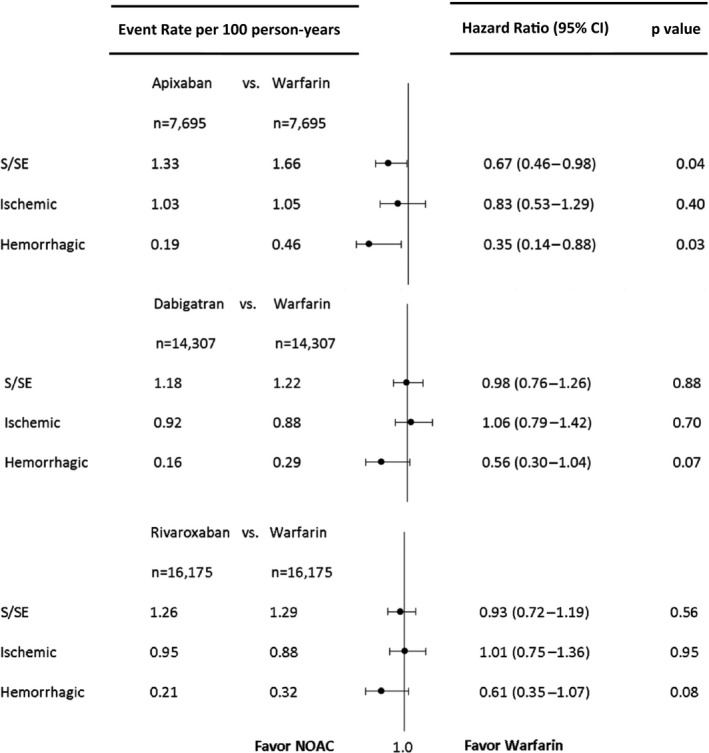
Forest plot depicting the hazard ratio for each pairwise propensity‐matched medication comparison (dabigatran, rivaroxaban, and apixaban each vs warfarin) for stroke and systemic embolism (S/SE), ischemic stroke, and hemorrhagic stroke. NOAC, non–vitamin K oral anticoagulant.
Safety Outcomes
Apixaban was associated with lower risks of major bleeding (HR 0.45, 95% CI 0.34–0.59, P<0.001), intracranial bleeding (HR 0.24, 95% CI 0.12–0.50, P<0.001), and gastrointestinal bleeding (HR 0.51, 95% CI 0.37–0.70, P<0.001) compared with warfarin.
Dabigatran was associated with lower risks of major bleeding (HR 0.79, 95% CI 0.67–0.94, P<0.01) and intracranial bleeding (HR 0.36, 95% CI 0.23–0.56, P<0.001) than warfarin use. There was no significant difference in the risk of gastrointestinal bleeding (HR 1.03, 95% CI 0.84–1.26, P=0.78) between dabigatran and warfarin users.
Rivaroxaban was associated with similar risk of major bleeding (HR 1.04, 95% CI 0.90–1.20, P=0.60) compared with warfarin but lower risk of intracranial bleeding (HR 0.51, 95% CI 0.35–0.75, P<0.001) and higher risk of gastrointestinal bleeding (HR 1.21, 95% CI 1.02–1.43, P=0.03) (Figure 3).
Figure 3.
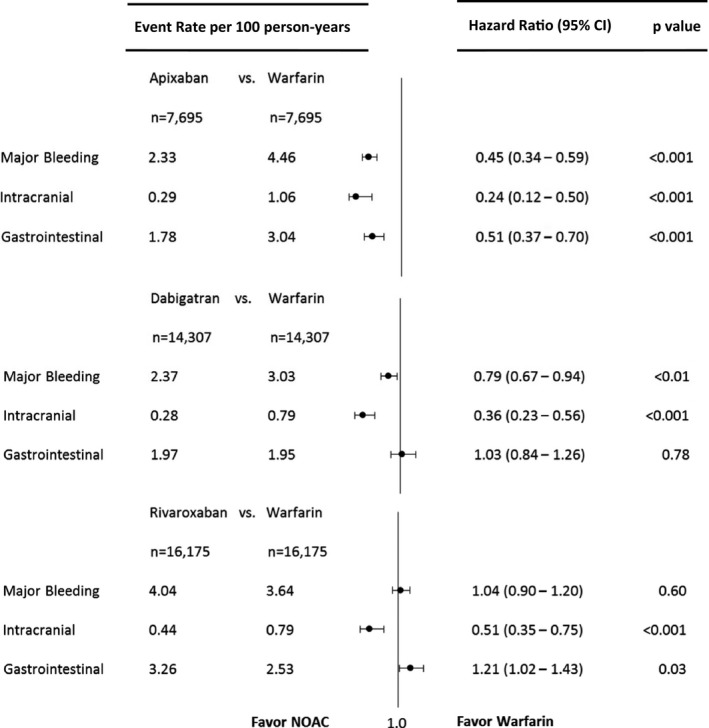
Forest plot depicting the hazard ratio for each pairwise propensity‐matched medication comparison (dabigatran, rivaroxaban, and apixaban each vs warfarin) for major, intracranial, and gastrointestinal bleeding. NOAC, non–vitamin K oral anticoagulant.
Subgroup Analyses
In the comparison of apixaban and warfarin, the main findings were broadly consistent in all subgroup analyses. The only significant interaction found was for dose used in the major bleeding end point (P=0.04). Regular‐dose apixaban was associated with lower risk of major bleeding compared with warfarin, whereas reduced‐dose apixaban was associated with similar risk of major bleeding (Table 3).
Table 3.
Subgroup Analysis in Propensity Score–Matched Apixaban Versus Warfarin Users
| Apixaban (n=7695) | Warfarin (n=7695) | Apixaban vs Warfarin (n=15 390) | ||
|---|---|---|---|---|
| Event Ratec | Event Ratec | HR (95% CI) | P Value | |
| Stroke or systemic embolism | ||||
| CHA2DS2‐VASc | 0.96 | |||
| 0–1 | 0.00 | 0.23 | NA | |
| 2–3 | 0.93 | 1.15 | 0.70 (0.33–1.50) | |
| ≥4 | 1.80 | 2.16 | 0.68 (0.44–1.06) | |
| HAS‐BLED | 0.45 | |||
| 0–2 | 1.08 | 1.17 | 0.79 (0.45–1.38) | |
| ≥3 | 1.69 | 2.35 | 0.59a (0.35–0.99) | |
| Warfarin experienced | 0.28 | |||
| No | 1.13 | 1.72 | 0.59a (0.38–0.93) | |
| Yes | 2.00 | 1.47 | 0.94 (0.46–1.93) | |
| Dose | 0.84 | |||
| Reduced | 2.16 | 2.09 | 0.71 (0.34–1.50) | |
| Regular | 1.14 | 1.56 | 0.65 (0.42–1.01) | |
| Major bleeding | ||||
| CHA2DS2‐VASc | 0.21 | |||
| 0–1 | 0.66 | 1.62 | 0.36 (0.07–1.72) | |
| 2–3 | 1.03 | 3.22 | 0.28b (0.14–0.54) | |
| ≥4 | 3.43 | 5.62 | 0.53b (0.39–0.71) | |
| HAS‐BLED | 0.99 | |||
| 0–2 | 1.40 | 2.65 | 0.46b (0.29–0.72) | |
| ≥3 | 3.71 | 7.07 | 0.46b (0.33–0.64) | |
| Warfarin experienced | 0.13 | |||
| No | 2.09 | 4.88 | 0.41b (0.30–0.56) | |
| Yes | 3.15 | 3.28 | 0.65 (0.39–1.09) | |
| Dose | 0.04 | |||
| Reduced | 4.53 | 3.95 | 0.74 (0.44–1.25) | |
| Regular | 1.85 | 4.58 | 0.38b (0.28–0.53) | |
P value in the table is for interaction. HR indicates hazard ratio.
P<0.05.
P<0.001.
Event rate is expressed per 100 person‐years.
In the comparison of dabigatran and warfarin, 2 significant interactions were found for major bleeding outcomes: CHA2DS2‐VASc score (P<0.001) and previous warfarin experience (P<0.01). Dabigatran was associated with lower risk of major bleeding in patients with CHA2DS2‐VASc 2 or 3 but similar risk in patients with CHA2DS2‐VASc ≥4. Dabigatran was also associated with lower risk of major bleeding in warfarin‐naïve patients but had similar risk for warfarin‐experienced patients (Table 4).
Table 4.
Subgroup Analysis in Propensity Score–Matched Dabigatran Versus Warfarin Users
| Dabigatran (n=14 307) | Warfarin (n=14 307) | Dabigatran vs Warfarin (n=28 614) | ||
|---|---|---|---|---|
| Event Rated | Event Rated | HR (95% CI) | P Value | |
| Stroke or systemic embolism | ||||
| CHA2DS2‐VASc | 0.20 | |||
| 0–1 | 0.44 | 0.13 | 3.18 (0.64–15.74) | |
| 2–3 | 0.87 | 0.75 | 1.19 (0.72–1.98) | |
| ≥4 | 1.66 | 1.94 | 0.87 (0.64–1.18) | |
| HAS‐BLED | 0.67 | |||
| 0–2 | 0.83 | 0.79 | 1.04 (0.71–1.52) | |
| ≥3 | 1.88 | 2.09 | 0.93 (0.66–1.31) | |
| Warfarin experienced | 0.41 | |||
| No | 1.27 | 1.56 | 0.92 (0.66–1.26) | |
| Yes | 1.07 | 0.86 | 1.14 (0.75–1.73) | |
| Dose | 0.15 | |||
| Reduced | 1.58 | 1.74 | 0.59 (0.28–1.24) | |
| Regular | 1.15 | 1.17 | 1.07 (0.81–1.40) | |
| Major bleeding | ||||
| CHA2DS2‐VASc | <0.001 | |||
| 0–1 | 0.44 | 1.07 | 0.40 (0.16–1.03) | |
| 2–3 | 1.12 | 2.50 | 0.46c (0.32–0.65) | |
| ≥4 | 4.01 | 4.07 | 1.00 (0.82–1.22) | |
| HAS‐BLED | 0.90 | |||
| 0–2 | 1.52 | 1.90 | 0.80 (0.62–1.04) | |
| ≥3 | 4.05 | 5.31 | 0.78a (0.63–0.98) | |
| Warfarin experienced | <0.01 | |||
| No | 2.24 | 3.89 | 0.63c (0.50–0.79) | |
| Yes | 2.52 | 2.09 | 1.11 (0.85–1.45) | |
| Dose | 0.56 | |||
| Reduced | 5.29 | 3.82 | 0.89 (0.56–1.39) | |
| Regular | 2.11 | 2.95 | 0.76b (0.63–0.92) | |
P value in the table is for interaction. HR indicates hazard ratio.
P<0.05.
P<0.01.
P<0.001.
Event rate is expressed per 100 person‐years.
In the comparison of rivaroxaban and warfarin, significant interactions were found for previous warfarin experience for both effectiveness and safety end points (both P<0.01). In warfarin‐naïve patients, rivaroxaban was associated with similar risk of both stroke or systemic embolism and major bleeding; however, in warfarin‐experienced patients, rivaroxaban was associated with elevated risk of both outcomes (Table 5).
Table 5.
Subgroup Analysis in Propensity Score–Matched Rivaroxaban Versus Warfarin Users
| Rivaroxaban (n=16 175) | Warfarin (n=16 175) | Rivaroxaban vs Warfarin (n=32 350) | ||
|---|---|---|---|---|
| Event Ratec | Event Ratec | HR (95% CI) | P Value | |
| Stroke or systemic embolism | ||||
| CHA2DS2‐VASc | 0.37 | |||
| 0–1 | 0.33 | 0.18 | 1.72 (0.29–10.21) | |
| 2–3 | 0.48 | 0.70 | 0.65 (0.35–1.21) | |
| ≥4 | 2.00 | 1.91 | 0.99 (0.75–1.31) | |
| HAS‐BLED | 0.76 | |||
| 0–2 | 0.78 | 0.74 | 0.98 (0.65–1.48) | |
| ≥3 | 2.06 | 2.18 | 0.90 (0.66–1.24) | |
| Warfarin experienced | <0.01 | |||
| No | 1.09 | 1.55 | 0.77 (0.57–1.04) | |
| Yes | 1.70 | 0.77 | 1.63a (1.01–2.62) | |
| Dose | 0.20 | |||
| Reduced | 2.21 | 1.31 | 1.21 (0.76–1.92) | |
| Regular | 1.03 | 1.29 | 0.84 (0.62–1.14) | |
| Major bleeding | ||||
| CHA2DS2‐VASc | 0.17 | |||
| 0–1 | 0.89 | 1.26 | 0.65 (0.27–1.55) | |
| 2–3 | 2.35 | 2.53 | 0.86 (0.64–1.16) | |
| ≥4 | 5.85 | 4.86 | 1.13 (0.95–1.34) | |
| HAS‐BLED | 0.10 | |||
| 0–2 | 2.66 | 2.03 | 1.22 (0.96–1.54) | |
| ≥3 | 6.32 | 6.28 | 0.95 (0.79–1.14) | |
| Warfarin experienced | <0.01 | |||
| No | 3.80 | 4.23 | 0.94 (0.79–1.12) | |
| Yes | 4.66 | 2.45 | 1.48b (1.12–1.95) | |
| Dose | 0.45 | |||
| Reduced | 6.42 | 3.98 | 1.15 (0.87–1.51) | |
| Regular | 3.46 | 3.54 | 1.01 (0.85–1.21) | |
P value in the table is for interaction. HR indicates hazard ratio.
P<0.05.
P<0.01.
Event rate is expressed per 100 person‐years.
Sensitivity Analyses
The first 4 sensitivity analyses showed results similar to the main analysis (Tables 6, 7, 8 through 9). In the TTR analyses, 4634 patients, including 912 patients who initiated NOACs on the index date, had baseline TTR data. The median baseline TTR was 56% (interquartile range 34–76%). Patients who stayed on warfarin in general had better baseline warfarin control than those who switched to NOACs (Table 10). Overall, 7163 patients had TTR data during follow‐up, including 714, 1367, and 1569 patients included in the apixaban‐, dabigatran‐, and rivaroxaban‐matched cohorts, respectively. The median TTR during follow‐up was 55% (interquartile range 29–78%) (Table 11). The event rates and HRs by baseline and follow‐up TTR are presented in Tables 12 and 13, but none of the HRs were statistically significant.
Table 6.
Sensitivity Test Based on Intent‐to‐Treat Approach
| Apixaban vs Warfarin (n=15 390) | Dabigatran vs Warfarin (n=28 614) | Rivaroxaban vs Warfarin (n=32 350) | |
|---|---|---|---|
| Stroke or systemic embolism | 0.72a (0.55–0.95) | 1.01 (0.87–1.16) | 1.04 (0.89–1.21) |
| Ischemic stroke | 0.73a (0.53–1.00) | 1.10 (0.94–1.30) | 1.14 (0.95–1.37) |
| Hemorrhagic stroke | 0.69 (0.36–1.32) | 0.61b (0.42–0.87) | 0.63a (0.43–0.91) |
P<0.05.
P<0.01.
Table 7.
Sensitivity Analysis Limited to January 1, 2013, to June 30, 2015
| Apixaban vs Warfarin (n=14 926) | Dabigatran vs Warfarin (n=7552) | Rivaroxaban vs Warfarin (n=24 504) | |
|---|---|---|---|
| Stroke or systemic embolism | 0.75 (0.51–1.10) | 0.84 (0.47–1.50) | 1.00 (0.75–1.34) |
| Major bleeding | 0.46b (0.35–0.61) | 0.64a (0.45–0.92) | 1.07 (0.90–1.26) |
P<0.05.
P<0.001.
Table 8.
Sensitivity Analysis Censoring Patients at the End of 6 Months
| Apixaban vs Warfarin (n=15 390) | Dabigatran vs Warfarin (n=28 614) | Rivaroxaban vs Warfarin (n=32 350) | |
|---|---|---|---|
| Stroke or systemic embolism | 0.70 (0.46–1.08) | 1.01 (0.73–1.41) | 0.93 (0.69–1.26) |
| Major bleeding | 0.40b (0.30–0.55) | 0.80a (0.65–1.00) | 1.05 (0.88–1.24) |
P<0.05.
P<0.001.
Table 9.
Sensitivity Analysis Excluding Patients Undergoing Ablation or Cardioversion
| Apixaban vs Warfarin (n=13 190) | Dabigatran vs Warfarin (n=24 660) | Rivaroxaban vs Warfarin (n=27 964) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Stroke or systemic embolism | 0.67 (0.44–1.01) | 0.06 | 0.99 (0.75–1.30) | 0.93 | 0.95 (0.73–1.23) | 0.70 |
| CHA2DS2‐VASc | 0.93 | 0.36 | 0.09 | |||
| 0–1 | NA | 2.04 (0.37–11.12) | 1.24 (0.18–8.73) | |||
| 2–3 | 0.66 (0.25–1.75) | 1.29 (0.75–2.24) | 0.52a (0.28–0.97) | |||
| ≥4 | 0.69 (0.44–1.10) | 0.89 (0.65–1.22) | 1.12 (0.84–1.50) | |||
| Major bleeding | 0.59 (0.44–0.81) | <0.001 | 0.84 (0.70–1.00) | 0.05 | 1.07 (0.92–1.24) | 0.41 |
| CHA2DS2‐VASc | 0.52 | <0.01 | 0.32 | |||
| 0–1 | 0.63 (0.11–3.41) | 0.36 (0.13–1.00) | 0.55 (0.23–1.36) | |||
| 2–3 | 0.43a (0.22–0.84) | 0.54b (0.36–0.80) | 1.06 (0.77–1.46) | |||
| ≥4 | 0.66a (0.47–0.94) | 1.01 (0.82–1.25) | 1.12 (0.94–1.34) | |||
Patients who underwent catheter ablation during the 2 months prior to the index date and who underwent cardioversion during the 1 month before or 1 month after cardioversion were excluded; apixaban patients did not have any stroke or systemic embolism of CHA2DS2‐VASc 0 or 1. P value in the table is for interaction HR indicates hazard ratio.
P<0.05.
P<0.01.
Table 10.
Baseline TTR in Propensity Score–Matched NOACs or Warfarin Users With Previous Warfarin Experience
| Apixaban (n=128) | Warfarin (n=108) | Dabigatran (n=394) | Warfarin (n=415) | Rivaroxaban (n=390) | Warfarin (n=289) | |
|---|---|---|---|---|---|---|
| Age, y | 77 (68–85) | 75.5 (65–82.5) | 70 (63–79) | 69 (61–79) | 74 (67–81) | 74 (66–82) |
| TTR, % | 46.6 (20.5–65.6) | 59.6 (35.9–80.5) | 48.0 (22.7–69.8) | 58.3 (37.5–78.6) | 44.1 (22.2–67.0) | 61.6 (36.6–79.2) |
| bTTR, % | 33.3 (14.0–58.9) | 18.2 (0.0–47.5) | 26.8 (11.0–54.2) | 21.1 (3.9–42.9) | 28.3 (7.9–60.0) | 19.8 (4.0–40.4) |
| aTTR, % | 8.7 (0.0–26.5) | 8.0 (0.0–18.1) | 8.2 (0.0–23.1) | 6.8 (0.0–22.5) | 8.6 (0.0–27.6) | 7.7 (0.0–21.7) |
| Labile INR,a % | 65.6 | 50.0 | 62.7 | 52.3 | 65.4 | 49.5 |
Data are shown as median (IQR). aTTR indicates time above therapeutic range; bTTR, time below therapeutic range; INR, International Normalized Ratio; IQR, interquartile range; TTR, time in therapeutic range.
Labile INR was defined as TTR <60%.
Table 11.
Follow‐Up TTR in Warfarin‐Treated Patients
| Apixaban‐Matched Warfarin Control (n=714) | Dabigatran‐Matched Warfarin Control (n=1367) | Rivaroxaban‐Matched Warfarin Control (n=1569) | |
|---|---|---|---|
| Age, y | 73 (64–81) | 69 (61–78) | 72 (64–80) |
| TTR, % | 48.2 (22.2–69.9) | 50.0 (23.8–74.4) | 48.8 (22.6–71.5) |
| bTTR, % | 27.5 (7.0–58.8) | 24.4 (3.8–57.7) | 28.3 (5.9–57.9) |
| aTTR, % | 4.3 (0.0–25.0) | 3.1 (0.0–25.0) | 2.9 (0.0–23.9) |
| Labile INR,a % | 63.6 | 59.5 | 62.7 |
Data are shown as median (IQR). aTTR indicates time above therapeutic range; bTTR, time below therapeutic range; INR, International Normalized Ratio; IQR, interquartile range; TTR, time in therapeutic range.
Labile INR was defined as TTR <60%.
Table 12.
Subgroup Analysis by Baseline TTR
| Event Rate | Event Rate | HR (95% CI) | P Value | |
|---|---|---|---|---|
| Apixaban | Warfarin | Apixaban vs Warfarin | ||
| Stroke or systemic embolism | NA | |||
| TTR <60% | 2.09 | 2.96 | 0.57 (0.04–8.98) | |
| TTR ≥60% | 0.00 | 4.69 | NA | |
| Major bleeding | ||||
| TTR <60% | 4.29 | 5.85 | 0.57 (0.10–3.32) | |
| TTR ≥60% | 0.00 | 2.31 | NA | |
| Dabigatran | Warfarin | Dabigatran vs Warfarin | ||
|---|---|---|---|---|
| Stroke or systemic embolism | 0.97 | |||
| TTR <60% | 1.49 | 2.45 | 0.56 (0.12–2.54) | |
| TTR ≥60% | 0.73 | 1.31 | 0.53 (0.05–5.15) | |
| Major bleeding | 0.95 | |||
| TTR <60% | 2.94 | 2.45 | 1.06 (0.30–3.82) | |
| TTR ≥60% | 1.48 | 1.30 | 1.14 (0.17–7.73) | |
| Rivaroxaban | Warfarin | Rivaroxaban vs Warfarin | ||
|---|---|---|---|---|
| Stroke or systemic embolism | NA | |||
| TTR <60% | 1.91 | 0.00 | NA | |
| TTR ≥60% | 0.00 | 0.00 | NA | |
| Major bleeding | 0.91 | |||
| TTR <60% | 3.78 | 2.16 | 1.91 (0.38–9.56) | |
| TTR ≥60% | 1.94 | 0.83 | 2.25 (0.21–24.32) | |
P value in the table is for interaction; event rate is expressed per 100 person‐years. HR indicates hazard ratio; NA, not applicable because of no event; TTR, time in therapeutic range.
Table 13.
Subgroup Analysis by Follow‐up TTR
| Event Rate | Event Rate | HR (95% CI) | P Value | |
|---|---|---|---|---|
| Apixaban (n=714) | Warfarin (n=714) | Apixaban vs Warfarin (n=1428) | ||
| Stroke or systemic embolism | NA | |||
| TTR <60% | 1.72 | 1.58 | 1.16 (0.28–4.83) | |
| TTR ≥60% | 0.00 | 0.36 | NA | |
| Major bleeding | 0.28 | |||
| TTR <60% | 4.00 | 5.05 | 0.79 (0.31–2.00) | |
| TTR ≥60% | 3.42 | 1.47 | 1.92 (0.52–7.11) | |
| Dabigatran (n=1367) | Warfarin (n=1367) | Dabigatran vs Warfarin (n=2734) | ||
|---|---|---|---|---|
| Stroke or systemic embolism | 0.18 | |||
| TTR <60% | 0.71 | 1.04 | 0.72 (0.22–2.36) | |
| TTR ≥60% | 0.81 | 0.17 | 4.14 (0.42–41.15) | |
| Major bleeding | 0.17 | |||
| TTR <60% | 3.58 | 3.92 | 1.03 (0.57–1.84) | |
| TTR ≥60% | 3.53 | 1.67 | 2.05 (0.90–4.65) | |
| Rivaroxaban (n=1569) | Warfarin (n=1569) | Rivaroxaban vs Warfarin (n=3192) | ||
|---|---|---|---|---|
| Stroke or systemic embolism | 0.77 | |||
| TTR <60% | 1.06 | 0.80 | 1.36 (0.44–4.14) | |
| TTR ≥60% | 0.89 | 0.49 | 1.83 (0.34–9.70) | |
| Major bleeding | 0.20 | |||
| TTR <60% | 3.20 | 5.66 | 0.60 (0.34–1.05) | |
| TTR ≥60% | 2.98 | 2.47 | 1.13 (0.51–2.50) | |
P value in the table is for interaction; event rate is expressed per 100 person‐years. HR indicates hazard ratio; NA, not applicable because of no event; TTR, time in therapeutic range.
Discussion
In this large cohort of patients with nonvalvular AF, we assessed the real‐world effectiveness and safety of dabigatran, rivaroxaban, and apixaban, comparing each agent with warfarin. Apixaban was associated with better effectiveness and safety, dabigatran was associated with similar effectiveness but better safety, and rivaroxaban was associated with similar outcomes for both effectiveness and safety in comparison to warfarin.
Our study is the largest contemporary evaluation comparing NOACs and warfarin and the first to report outcomes of apixaban in practice. Prior studies either reported on a single NOAC19, 20, 22, 23, 24 or had smaller samples21, 23 or shorter follow‐up.19, 21, 23 Our findings provide an estimate of the anticipated outcomes of the various oral anticoagulants used in everyday practice and may help clinicians and patients choose from among NOACs and warfarin.
Dabigatran patients were younger and had lower risks at baseline than rivaroxaban and apixaban patients, and that finding is consistent with previous observation.15 This could be due to the concerns regarding dabigatran‐related bleeding. Analyses of RE‐LY data suggested a lower risk of major bleeding in patients aged <75 years but a trend toward higher risk in patients aged ≥75 years.46 Moreover, physicians tended to prescribe medications to patients who were similar to the trial population; therefore, rivaroxaban may be prescribed more commonly in for elderly patients because ROCKET‐AF included a mostly elderly high‐risk population. Among the 3 studied NOACs, dabigatran 150 mg (110 mg was not approved in the United States) and rivaroxaban were both related to a higher risk of gastrointestinal bleeding, whereas apixaban was related to a nonsignificant numerically lower risk of gastrointestinal bleeding.6, 7, 8 The lower bleeding risk of apixaban may explain why it has been prescribed for many elderly patients. A larger percentage of dabigatran patients had used warfarin previously. This is likely because dabigatran was the first NOAC to the market. Many insurance plans require patients to have a documented trial of warfarin before using NOACs, and this requirement may be less prevalent in the latter part of the study period.
The results of apixaban and rivaroxaban were consistent with the pivotal clinical trials. In the ARISTOTLE trial comparing apixaban and warfarin, apixaban was superior to warfarin in preventing stroke or systemic embolism and caused less bleeding.8 In the ROCKET‐AF trial comparing rivaroxaban and warfarin, rivaroxaban was noninferior to warfarin for both primary effectiveness and safety end points.7 There have been concerns regarding the validity of ROCKET‐AF. The device used to monitor INR has been subject to a recall because it could deliver clinically significantly lower values than a laboratory INR method.47 The anticoagulation control in the warfarin‐treated arm was also suboptimal. The mean TTR was only 55% compared with 64% in the dabigatran trial and 62% in the apixaban trial.6, 7, 8 Moreover, lack of low‐risk patients in the ROCKET‐AF raised a question regarding its effectiveness and safety in lower risk patients. Our study, consistent with a previous French study comparing rivaroxaban and warfarin21 and observations from registries of rivaroxaban patients,48, 49 supports the effectiveness and safety of rivaroxaban in patients with various baseline risks of stroke and bleeding.
The comparative effectiveness and safety of dabigatran versus warfarin appear to be somewhat attenuated in routine clinical practice. In the RE‐LY trial comparing dabigatran and warfarin, dabigatran 150 mg reduced the risk of stroke or systemic embolism with a similar risk of major bleeding. In our study, we found a similar risk of stroke or systemic embolism but lower risk of major bleeding in comparison to warfarin. The similar effectiveness comparing dabigatran to vitamin K antagonists has been reported consistently in many observational studies,21, 23, 24, 35, 50, 51 but the results regarding major bleeding risk are less clear. Most studies found a similar risk of major bleeding between dabigatran and warfarin,20, 21, 22, 23 and the event rates were numerically lower in dabigatran cohorts.52 Several studies found a significantly lower risk associated with dabigatran in comparison with vitamin K antagonists.35, 51, 52 One study reported higher bleeding risks associated with dabigatran.36 The inconsistencies in the results could be related to differences in the study populations, time frames, and numbers of patients included in the study. Both RE‐LY and an observational study suggested that dabigatran was associated with a lower risk of major bleeding in patients aged <75 years but a risk similar to warfarin in patients aged ≥75 years24, 46; therefore studies using elderly Medicare patients are more likely to report a higher relative risk of dabigatran compared with warfarin.
Although inappropriate dose reduction can be a tempting explanation for the reduced comparative effectiveness and improved safety of dabigatran in our study, only a small percentage of patients (<10%) received the 75‐mg dose, an observation consistent with what was previously reported from the ORBIT‐AF registry.14 In the subgroup analyses, we also failed to find any differential effects among those receiving regular or reduced doses. Another possibility is higher nonadherence among patients managed in routine clinical practice than in clinical trials; however, we censored patients at the time of treatment discontinuation, and the adherence was good, with a mean proportion of days covered of ≈95%. It is possible that some patients filled the prescription but did not actually take the medication, leading to lower adherence than what we measured using pharmacy claims data.
Unexpected interactions were found between treatment and previous warfarin experience for both primary outcomes in the comparison of rivaroxaban and warfarin and for major bleeding in the comparison of dabigatran and warfarin. Subgroup analyses from RE‐LY found no such interaction.53 Nevertheless, our result was consistent with a previous study using Danish registries and suggesting that dabigatran 150 mg was associated with lower major bleeding risk in vitamin K antagonist–naïve patients but had risk similar to warfarin in vitamin K antagonist–experienced patients.54 Because it is generally considered reasonable to continue warfarin treatment in patients with high TTR (eg, >70%),55 physicians may tend to switch patients with poor warfarin control to NOACs, making the switch from warfarin to NOACs a marker of high‐risk status. We matched patients based on clinical and sociodemographic characteristics that can potentially predict their subsequent warfarin control, such as the SAMe‐TT2R2 score, and no such interaction was found in the comparisons between apixaban and warfarin users or between dabigatran and warfarin for stroke or systemic embolism.
We conducted subgroup analyses by TTR in patients with sufficient INR values. Anticoagulation control was suboptimal in most patients (median ≈55%), and that is consistent with a recent national assessment that found a mean TTR of 53.7%.56 Our results provided some insights regarding whether to switch patients from warfarin to NOACs; however, the results should be interpreted with caution because the analyses were based on a small number of patients with sufficient INR values to calculate TTR, and none of the HRs or interactions were statistically significant. Because of the small number of events, the results can just be a play of chance and should be viewed as hypothesis generating rather than hypothesis testing.
Another interesting finding is that we found dabigatran was associated with lower risk of major bleeding in patients with low or intermediate risk of stroke at baseline but had risk similar to warfarin in patients with elevated risk of stroke at baseline. Similar results were observed in the RE‐LY trial, although the interaction was not statistically significant in the trial (P=0.14).57
Consistent with the trials, all 3 NOACs were associated with lower intracranial bleeding than warfarin. Intracranial bleeding is the most fearful and deadly complication for patients on oral anticoagulation.58 Considering the at least similar or lower risks of the primary effectiveness and safety end points, NOACs may have higher net clinical benefits than warfarin for many patients.
Our study has some limitations. First, despite careful adjustment using propensity score matching, there is always a possibility of residual unmeasured confounding caused by lack of randomized treatment allocation of observational studies, thus we were not able to draw any causal inferences. The goal of this study, however, was not to reevaluate the efficacy of the medications, which has been well established in clinical trials, but rather to assess how these drugs performed under usual clinical conditions in real‐world populations. This question can be answered only by observational studies.
Second, our study relied on billing codes to define baseline comorbidities and outcomes. In addition to the potential for misclassification, we were not able to adjudicate events based on more precise clinical criteria, such as International Society of Thrombosis and Haemostasis major bleeding criteria. Nevertheless, the algorithms used to define our outcomes of interest and important covariates are commonly used and demonstrated good performance in validation studies.31, 32, 33, 59, 60, 61 We anticipate that any existing residual bias associated with coding is nondifferential among exposure groups and thus should not meaningfully influence the assessment of our outcomes.
Third, certain clinical and health behavior parameters, such as the type of AF and left ventricular ejection fraction, are not available in the claims database. Observational studies using registries and single‐center electronic medical records may have more clinical details; however, using administrative claims data, we were able to examine a larger and less selective patient population with longer follow‐up that may have better external validity. Prescriptions of antiplatelet and nonsteroidal anti‐inflammatory drugs were considered in the HAS‐BLED score, but over‐the‐counter aspirin and nonsteroidal anti‐inflammatory drugs are not available in the claims database. Such information may not be available in any form in the US medical record or pharmacy data.
Fourth, we are unable to accurately assess mortality. This limitation is common in studies using US commercial insurance claims data. Before November 2011, mortality data were available for patients whose social security numbers were available to OptumLabs Data Warehouse (≈70–80% of the patients); however, effective November 1, 2011, section 205(r) of the Social Security Act prohibits the Social Security Administration from disclosing state death records that it receives through its contracts with the states, except in limited circumstances. Consequently, if the Social Security Administration knows of a death only from the state data and not from any of its other sources of death information, which happens roughly one‐third of the time, those death data will not appear on the Death Master File.62 We censored patients at insurance plan disenrollment (of which death is a cause), but we were unable to assess mortality on its own. Nevertheless, the inability to assess mortality should not jeopardize our findings on the primary outcomes of stroke and major bleeding. In patients with SSN, only ≈0.25% of the patients died while they were on treatment and before a stroke or major bleeding occurred. We conducted survival regression using the method of Fine and Gray and considering death as a competing risk.63 The findings remained unchanged. We acknowledged that if a fatal event happened out of the hospital or in the hospital before inpatient admission, this event could be missing. We did a sensitivity test examining the potential missing fatal events that happened in hospital, defined as a primary diagnosis of stroke or bleeding on any emergency room or inpatient claims during the 2 days prior to death. We found potentially 0.3% of events that could be fatal and that were not captured in the main analyses; therefore, missing fatal events that happened in hospital before an inpatient admission were rare. For out‐of‐hospital death, the cause can be variable and uncertain without autopsy confirmation and difficult to assess in any studies. It is relatively unlikely that autopsies would be performed on patients with AF because they are more likely to die of cardiovascular disease. There may be a small underestimation of stroke or bleeding, but this should be nondifferential between NOACs and warfarin patients and should not jeopardize our comparative effectiveness and safety findings.
Fifth, we were not able to obtain INR values for every patient treated with warfarin. We have laboratory data for ≈40% of patients. The availability of laboratory data depends mainly on the contracts between laboratories and OLDW rather than on individual patient characteristics. Among patients treated with warfarin, <10% had at least 2 INR results within a reasonable time range to calculate TTR. This is not only because of the availability of laboratory data but also because of the large systematic variation of anticoagulation monitoring across facilities and patients’ nonadherence to INR monitoring.64, 65 Moreover, we only have INR tested in a traditional way, namely, blood drawn and then sent to a laboratory. We do not have tests done using a point‐of‐care device either in a physician office or at patients’ homes. Although we did not match patients with previous warfarin experience on baseline TTR, we included the SAMe‐TT2R2 score and other comorbidities related to the risk of labile INR during follow‐up; therefore, lack of baseline TTR should not substantially affect our matching.
Last, there are well‐known limitations in subgroup analyses, namely, false positives caused by a large amount of comparisons and false negatives caused by inadequate power.66, 67, 68 The larger numbers of dabigatran and rivaroxaban patients in our study provided more statistical power than the subgroup analyses of the RE‐LY and ROCKET‐AF trials; however, because of the lack of prespecified hypotheses and the multiplicity issues, the heterogeneity in treatment effects found in our study is at best hypothesis generating and needs to be confirmed by other studies.
In summary, large‐scale observational studies such as ours constitute a crucial ongoing assessment of outcomes achieved in strictly controlled clinical trial settings. Using a large cohort of patients treated with NOACs or warfarin for stroke prevention in nonvalvular AF, we demonstrated that in comparison to warfarin, apixaban was associated with lower risks of both stroke and major bleeding, dabigatran was associated with similar risk of stroke but lower risk of major bleeding, and rivaroxaban was associated with similar risks of both stroke and major bleeding. Our findings provide some reassurance of the effectiveness and safety of NOAC use in everyday practice and may facilitate clinical decision making. Nevertheless, the choice between NOACs and warfarin will ultimately depend on individual patient risk and preference.
Sources of Funding
This study was funded by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003725 doi: 10.1161/JAHA.116.003725)
References
- 1. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA. Lifetime risk for development of atrial fibrillation the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 4. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 6. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 7. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 8. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 9. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 10. Gorst‐Rasmussen A, Skjøth F, Larsen TB, Rasmussen LH, Lip GY, Lane DA. Dabigatran adherence in atrial fibrillation patients during the first year after diagnosis: a nationwide cohort study. J Thromb Haemost. 2015;13:495–504. [DOI] [PubMed] [Google Scholar]
- 11. Zalesak M, Siu K, Francis K, Yu C, Alvrtsyan H, Rao Y, Walker D, Sander S, Miyasato G, Matchar D. Higher persistence in newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran versus warfarin. Circ Cardiovasc Qual Outcomes. 2013;6:567–574. [DOI] [PubMed] [Google Scholar]
- 12. Cutler TW, Chuang A, Huynh TD, Witt RG, Branch J, Pon T, White R. A retrospective descriptive analysis of patient adherence to dabigatran at a large academic medical center. J Manag Care Pharm. 2014;20:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. [DOI] [PubMed] [Google Scholar]
- 14. Steinberg BA, Holmes DN, Piccini JP, Ansell J, Chang P, Fonarow GC, Gersh B, Mahaffey KW, Kowey PR, Ezekowitz MD. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2:e000535 doi: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olesen JB, Sørensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP, Køber L, Gislason GH, Torp‐Pedersen C, Fosbøl EL. Non‐vitamin K antagonist oral anticoagulation agents in anticoagulant naïve atrial fibrillation patients: Danish nationwide descriptive data 2011–2013. Europace. 2015;17:187–193 doi: 10.1093/europace/euu225. [DOI] [PubMed] [Google Scholar]
- 16. Patel PA, Zhao X, Fonarow GC, Lytle BL, Smith EE, Xian Y, Bhatt DL, Peterson ED, Schwamm LH, Hernandez AF. Novel oral anticoagulant use among patients with atrial fibrillation hospitalized with ischemic stroke or transient ischemic attack. Circ Cardiovasc Qual Outcomes. 2015;8:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sennesael A‐L, Dogné J‐M, Spinewine A. Optimizing the safe use of direct oral anticoagulants in older patients: a teachable moment. JAMA Intern Med. 2015;175:1608–1609. [DOI] [PubMed] [Google Scholar]
- 18. Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol. 2015;115:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu T‐C, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. [DOI] [PubMed] [Google Scholar]
- 20. Villines T, Schnee J, Fraeman K, Siu K, Reynolds M, Collins J, Schwartzman E. A comparison of the safety and effectiveness of dabigatran and warfarin in non‐valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost. 2015;114:1290–1298. [DOI] [PubMed] [Google Scholar]
- 21. Maura G, Blotière P‐O, Bouillon K, Billionnet C, Ricordeau P, Alla F, Zureik M. Comparison of the short‐term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity‐matched cohort study. Circulation. 2015;132:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Effectiveness and safety of dabigatran and warfarin in real‐world US patients with non‐valvular atrial fibrillation: a retrospective cohort study. J Am Heart Assoc. 2015;4:e001798 doi: 10.1161/JAHA.115.001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsen TB, Rasmussen LH, Skjøth F, Due KM, Callréus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in “real‐world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–2273. [DOI] [PubMed] [Google Scholar]
- 24. Avgil‐Tsadok M, Jackevicius C, Essebag V, Eisenberg M, Rahme E, Behlouli H, Pilote L. Dabigatran use in elderly patients with atrial fibrillation. Thromb Haemost. 2016;115:152–160. [DOI] [PubMed] [Google Scholar]
- 25. Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, Shah ND. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857 doi: 10.1136/bmj.h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff. 2014;33:1187–1194. [DOI] [PubMed] [Google Scholar]
- 27. Optum . Core US data assets. 2014. Available at: https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. Accessed October 23, 2015.
- 28. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. Identifying atrial fibrillation from electronic medical data: a systematic review. Pharmacoepidemiol Drug Saf. 2012;21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. [DOI] [PubMed] [Google Scholar]
- 30. Thigpen JL, Dillon C, Forster KB, Henault L, Quinn EK, Tripodis Y, Berger PB, Hylek EM, Limdi NA. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tirschwell DL, Longstreth W. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 32. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arnason T, Wells P, Van Walraven C, Forster A. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. [DOI] [PubMed] [Google Scholar]
- 34. Lavallée PC, Meseguer E, Abboud H, Cabrejo L, Olivot J‐M, Simon O, Mazighi M, Nifle C, Niclot P, Lapergue B. A transient ischaemic attack clinic with round‐the‐clock access (SOS‐TIA): feasibility and effects. Lancet Neurol. 2007;6:953–960. [DOI] [PubMed] [Google Scholar]
- 35. Seeger J, Bykov K, Bartels D, Huybrechts K, Zint K, Schneeweiss S. Safety and effectiveness of dabigatran and warfarin in routine care of patients with atrial fibrillation. Thromb Haemost. 2015;114:1277–1289. [DOI] [PubMed] [Google Scholar]
- 36. Hernandez I, Baik SH, Piñera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Musich S, Cheng Y, Wang SS, Hommer CE, Hawkins K, Yeh CS. Pharmaceutical cost‐saving strategies and their association with medication adherence in a Medicare supplement population. J Gen Intern Med. 2015;30:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 39. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 40. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest J. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 41. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe‐TT2R2 score. Chest J. 2013;144:1555–1563. [DOI] [PubMed] [Google Scholar]
- 42. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gayat E, Resche‐Rigon M, Mary JY, Porcher R. Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat. 2012;11:222–229. [DOI] [PubMed] [Google Scholar]
- 44. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 45. Rosendaal F, Cannegieter S, Van der Meer F, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 46. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123:2363–2372. [DOI] [PubMed] [Google Scholar]
- 47. Cohen D. Data on trial of anticoagulant is to be reanalyzed after discovery that investigators used faulty device. BMJ. 2015;351:h6431 doi: 10.1136/bmj.h6431. [DOI] [PubMed] [Google Scholar]
- 48. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, van Eickels M, Turpie AG. XANTUS: a real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beyer‐Westendorf J, Förster K, Pannach S, Ebertz F, Gelbricht V, Thieme C, Michalski F, Köhler C, Werth S, Sahin K. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood. 2014;124:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Romanelli RJ, Nolting L, Dolginsky M, Kym E, Orrico KB. Dabigatran versus warfarin for atrial fibrillation in real‐world clinical practice a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2016;9:126–134 doi: 10.1161/CIRCOUTCOMES.115.002369. [DOI] [PubMed] [Google Scholar]
- 51. Korenstra J, Wijtvliet EPJ, Veeger NJ, Geluk CA, Bartels GL, Posma JL, Piersma‐Wichers M, Van Gelder IC, Rienstra M, Tieleman RG. Effectiveness and safety of dabigatran versus acenocoumarol in ‘real‐world'patients with atrial fibrillation. Europace. 2016;euv397 doi: 10.1093/europace/euv397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Potpara TS. Dabigatran in ‘real‐world’ clinical practice for stroke prevention in patients with non‐valvular atrial fibrillation. Thromb Haemost. 2015;114:1093–1098. [DOI] [PubMed] [Google Scholar]
- 53. Ezekowitz MD, Wallentin L, Connolly SJ, Parekh A, Chernick MR, Pogue J, Aikens TH, Yang S, Reilly PA, Lip GY. Dabigatran and warfarin in vitamin K antagonist–naive and–experienced cohorts with atrial fibrillation. Circulation. 2010;122:2246–2253. [DOI] [PubMed] [Google Scholar]
- 54. Larsen TB, Gorst‐Rasmussen A, Rasmussen LH, Skjøth F, Rosenzweig M, Lip GY. Bleeding events among new starters and switchers to dabigatran compared with warfarin in atrial fibrillation. Am J Med. 2014;127:650–656. [DOI] [PubMed] [Google Scholar]
- 55. Diener H‐C, Aisenberg J, Ansell J, Atar D, Breithardt G, Eikelboom J, Ezekowitz MD, Granger CB, Halperin JL, Hohnloser SH. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non‐valvular atrial fibrillation: part 1. Eur Heart J. 2016;ehv643 doi: 10.1093/eurheartj/ehv643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, Hylek EM. A national assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014;129:1407–1414 doi: 10.1161/CIRCULATIONAHA.113.002601. [DOI] [PubMed] [Google Scholar]
- 57. Oldgren J, Alings M, Darius H, Diener H‐C, Eikelboom J, Ezekowitz MD, Kamensky G, Reilly PA, Yang S, Yusuf S. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE‐LY trial. Ann Intern Med. 2011;155:660–667. [DOI] [PubMed] [Google Scholar]
- 58. Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, Singer DE. Death and disability from warfarin‐associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long‐term risk of ischemic stroke. JAMA. 2014;312:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Noseworthy PA, Yao X, Deshmukh AJ, Van Houten H, Sangaralingham LR, Siontis KC, Piccini JP, Asirvatham SJ, Friedman PA, Packer DL. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc. 2015;4:e002597 doi: 10.1161/JAHA.115.002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Noseworthy PA, Kapa S, Deshmukh AJ, Madhavan M, Van Houten H, Haas LR, Mulpuru SK, McLeod CJ, Asirvatham SJ, Friedman PA. Risk of stroke after catheter ablation versus cardioversion for atrial fibrillation: a propensity‐matched study of 24,244 patients. Heart Rhythm. 2015;12:1154–1161. [DOI] [PubMed] [Google Scholar]
- 62. da Graca B, Filardo G, Nicewander D. Consequences for healthcare quality and research of the exclusion of records from the Death Master File. Circ Cardiovasc Qual Outcomes. 2013;6:124–128. [DOI] [PubMed] [Google Scholar]
- 63. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 64. Beyer‐Westendorf J, Gelbricht V, Förster K, Ebertz F, Röllig D, Schreier T, Tittl L, Thieme C, Hänsel U, Köhler C. Safety of switching from vitamin K antagonists to dabigatran or rivaroxaban in daily care—results from the Dresden NOAC registry. Br J Clin Pharmacol. 2014;78:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rose AJ, Miller DR, Ozonoff A, Berlowitz DR, Ash AS, Zhao S, Reisman JI, Hylek EM. Gaps in monitoring during oral anticoagulation: insights into care transitions, monitoring barriers, and medication nonadherence. Chest J. 2013;143:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA. 1991;266:93–98. [PubMed] [Google Scholar]
- 67. Pocock S, Calvo G, Marrugat J, Prasad K, Tavazzi L, Wallentin L, Zannad F, Garcia AA. International differences in treatment effect: do they really exist and why? Eur Heart J. 2013;34:1846–1852 doi: 10.1093/eurheartj/eht071. [DOI] [PubMed] [Google Scholar]
- 68. Burke JF, Sussman JB, Kent DM, Hayward RA. Three simple rules to ensure reasonably credible subgroup analyses. BMJ. 2015;351:h5651 doi: 10.1136/bmj.h5651. [DOI] [PMC free article] [PubMed] [Google Scholar]


