Abstract
Background
In 2015, the Minnesota Resuscitation Consortium (MRC) implemented an advanced perfusion and reperfusion life support strategy designed to improve outcome for patients with out‐of‐hospital refractory ventricular fibrillation/ventricular tachycardia (VF/VT). We report the outcomes of the initial 3‐month period of operations.
Methods and Results
Three emergency medical services systems serving the Minneapolis–St. Paul metro area participated in the protocol. Inclusion criteria included age 18 to 75 years, body habitus accommodating automated Lund University Cardiac Arrest System (LUCAS) cardiopulmonary resuscitation (CPR), and estimated transfer time from the scene to the cardiac catheterization laboratory of ≤30 minutes. Exclusion criteria included known terminal illness, Do Not Resuscitate/Do Not Intubate status, traumatic arrest, and significant bleeding. Refractory VF/VT arrest was defined as failure to achieve sustained return of spontaneous circulation after treatment with 3 direct current shocks and administration of 300 mg of intravenous/intraosseous amiodarone. Patients were transported to the University of Minnesota, where emergent advanced perfusion strategies (extracorporeal membrane oxygenation; ECMO), followed by coronary angiography and primary coronary intervention (PCI), were performed, when appropriate. Over the first 3 months of the protocol, 27 patients were transported with ongoing mechanical CPR. Of these, 18 patients met the inclusion and exclusion criteria. ECMO was placed in 83%. Seventy‐eight percent of patients had significant coronary artery disease with a high degree of complexity and 67% received PCI. Seventy‐eight percent of patients survived to hospital admission and 55% (10 of 18) survived to hospital discharge, with 50% (9 of 18) achieving good neurological function (cerebral performance categories 1 and 2). No significant ECMO‐related complications were encountered.
Conclusions
The MRC refractory VF/VT protocol is feasible and led to a high functionally favorable survival rate with few complications.
Keywords: Extra‐corporeal membrane oxygenation, emergent extracorporeal membrane oxygenation, perfusion, refractory ventricular fibrillation/ventricular tachycardia, resuscitation, ventricular fibrillation
Subject Categories: Ventricular Fibrillation, Sudden Cardiac Death, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Introduction
Approximately one third of patients who suffer out‐of‐hospital cardiac arrest (OHCA) present to emergency medical services (EMS) with a shockable rhythm (ventricular fibrillation/pulseless ventricular tachycardia [VF/VT]).1, 2 Despite being the minority of all cardiac arrests, >80% of survivors come from this group, making it the presenting cardiac arrest rhythm to be targeted for improvements in treatment and outcome.1, 2
The current recommendations, according to the 2015 American Heart Association OHCA Advanced Cardiac Life Support guidelines, is to treat VF/VT patients in the field until they have return of spontaneous circulation (ROSC) or are declared dead. In Minnesota, the efforts for VF patients are typically discontinued if ROSC is not achieved after 45 minutes.3
Patients resuscitated from VF/VT cardiac arrest have a high prevalence of coronary artery disease (CAD) and are likely to have an underlying reversible cause for their cardiac arrest.4 When taken to the cardiac catheterization laboratory, more than half receive revascularization with primary coronary intervention (PCI) or coronary artery bypass grafting (CABG) regardless of the presence or absence of ST elevation in the postresuscitation electrocardiogram (ECG).5 We hypothesized that patients with VF/VT cardiac arrest, refractory to initial resuscitation efforts, would have an even higher prevalence of significant CAD and that their disease would be more complicated.
Recently developed capabilities now make it feasible to potentially reverse causative coronary artery ischemia in patients with refractory VF/VT. Manual cardiopulmonary resuscitation (CPR) precludes patient transport because of risk to unrestrained EMS personnel providing necessary care in the back of a rapidly moving ambulance. However, mechanical CPR alleviates this barrier to patient transport, while providing excellent cardiac and cerebral perfusion and allowing provision of other ongoing care.6 Another advance has been the demonstrated feasibility of performing PCI with ongoing mechanical CPR or, alternatively, placement of emergent extracorporeal membrane oxygenation (ECMO), rapidly establishing perfusion, followed by PCI.7, 8 Applying these advances in a coordinated and effective way, for the first time, provides the potential for a reasonable expectation of achieving a higher rate of functionally favorable survival in a patient population with a very high mortality.
The purpose of this article is to describe the first protocol in the United States using advanced perfusion and reperfusion life support strategies designed to improve outcomes for patients with out‐of‐hospital refractory VF/VT, evaluate initial 3‐months results, and determine the feasibility of this approach.
Methods
Study Design
This is an analysis of 27 consecutive patients that were enrolled in the Minnesota Resuscitation Consortium (MRC) refractory VT/VF protocol at the University of Minnesota (Minneapolis, MN) from December 1, 2015 through February 29, 2016. The clinical protocol was implemented as part of our clinical practice, and data collection was part of the Quality Improvement Project in this population. The Institutional Review Board (IRB) of the University of Minnesota approved the anonymous data analysis and chart review extraction for this publication. Informed consent was waived.
Setting
The protocol was implemented with an agreement between the University of Minnesota Interventional Cardiology Service, North Memorial, St. Paul Fire, and Ridgeview EMS systems. During the 3‐month period, patients presumed to meet inclusion criteria were transported directly from the out‐of‐hospital setting to the University of Minnesota Medical Center with facilitated transfer to the cardiac catheterization laboratory. A total of 27 patients were transferred with ongoing mechanical CPR during this period.
Outcomes
The primary outcome was functionally favorable survival to hospital discharge (cerebral performance categories [CPCs] 1 and 2). Secondary outcomes were 1‐month survival, 1‐month neurological function (CPC 1 or 2), and protocol‐based complications.
Historical Context and Protocol
Four years ago, the MRC established a protocol to provide early cardiac catheterization laboratory (CCL) activation and intervention (within 4 hours) to all OHCA VF/VT patients that achieved ROSC. Fifty‐two percent underwent PCI and 7% underwent CABG regardless of the presence or absence of ST elevation on 12‐lead ECG.5 Given the high prevalence of significant CAD in this population, the MRC hypothesized that the burden of CAD in patients with refractory VF/VT would be higher. Furthermore, early out‐of‐hospital transport using advanced perfusion/circulatory support with mechanical CPR followed by ECMO might be beneficial by stabilizing perfusion, allowing coronary angiography and PCI to reverse causative coronary artery ischemia and improve functionally favorable survival. Accordingly, Dr Yannopoulos (interventional cardiologist) and Drs Conterato, Sipprell, and Frascone (EMS directors) collaboratively developed the MRC refractory VF/VT protocol, trained all paramedic units (North Memorial and Ridgeview EMS) and fire fighter/paramedics units (St. Paul Fire EMS), and widely disseminated this information to the medical community. The first patient arrived at the University of Minnesota CCL on December 2, 2015.
Protocol Description
MRC refractory VF/VT inclusion criteria (must have all)
OHCA with presumed cardiac etiology cardiac arrest.
First presenting rhythm was shockable (VF or VT).
Age 18 to 75 years.
Received at least 3 direct current (DC) shocks without sustained ROSC.
Received amiodarone 300 mg.
Body could accommodate a Lund University Cardiac Arrest System (LUCAS) automated CPR device.
Transfer time from the scene to the CCL of <30 minutes.
Exclusion criteria for early mobilization (presence of 1 would exclude the patient)
ROSC before 3 shocks were delivered (transferred to nearest hospital).
Nursing home residents.
Do Not Resuscitate/Do Not Intubate.
Known terminal illness, (eg, cancer, end‐stage liver, kidney, or heart disease)
Traumatic arrest
Pulseless electrical activity and asystole
Significant bleeding
Manual CPR as the only option.
Mobilization Process and Protocol Activation
The process for activation of the refractory VF/VT protocol was simplified to a single phone call from the dispatch center to a 24/7 on‐call interventional cardiologist (D.Y., G.R., and J.B.). The cardiologist then activates the on‐call CCL perfusion teams and intensive care unit charge nurse by a central paging system and directly calls the emergency department. During regular working hours, patients bypass the emergency department for direct admission to the CCL. During off hours, patients are held in the emergency department where resuscitation efforts are continued until arrival of the catheterization laboratory team.
Refractory VF/VT
The definition of refractory VF/VT (failure to achieve sustained ROSC after treatment with 3 DC shocks and administration of 300 mg of intravenous/intraosseous [IV/IO] amiodarone) represents the timing of initiation of EMS transport and is fundamental to the success of the protocol. The definition of refractory VF/VT was chosen as a cutpoint along a theoretical continuum of failed response to standard care. If the decision to transport occurs too late along this continuum, early CCL activation and treatment may not offer benefit. If transport occurs too early, selection of prompt responders to treatment will occur, resulting in unnecessary mobilization of resources. For these reasons, the MRC chose a cutpoint that provides multiple defibrillation attempts, epinephrine, administration of intravenous antiarrythmics, followed by defibrillation with no response. Retrospective MRC data indicated that the probability of functionally favorable survival at this point is low (≈8.2%). Thus, the definition of refractory VT/VF provides identification of a cardiac arrest patient population with a low likelihood for survival at an early enough time during resuscitation efforts to potentially benefit from the protocol intervention.
Cardiac Catheterization Process, ECMO, and Angiography/PCI Protocol
On arrival in the CCL, ongoing LUCAS plus impedance threshold device (ITD) CPR continues. Emergency cannulation of the femoral vessels with a 15 to 19 French (Fr) arterial cannula and a 25 Fr venous cannula is performed with percutaneous technique and ultrasound guidance. For a detailed procedural guide, see Figure 1. An initial descending aortic angiogram is obtained to assess the ability of the femoral vessels to accommodate the size of the ECMO cannulas. In cases where the femoral vasculature cannot accommodate the ECMO cannulas, additional hemodynamic support is achieved by placement of an intra‐aortic balloon pump (IABP) triggered by the aortic pressure generated by the LUCAS device. ECMO candidates are connected to the preprimed Cardiohelp circuit consisting of a centrifugal pump (Maquet Rotaflo; Maquet Cardiovascular, Wayne, NJ). Interventional cardiologists place all devices. Once hemodynamic/perfusion support is achieved, coronary angiography is performed and revascularization accomplished based on the clinical judgment of the interventional cardiologist. The CCL circulatory support decision‐making protocol is seen in Figure 1.
Figure 1.
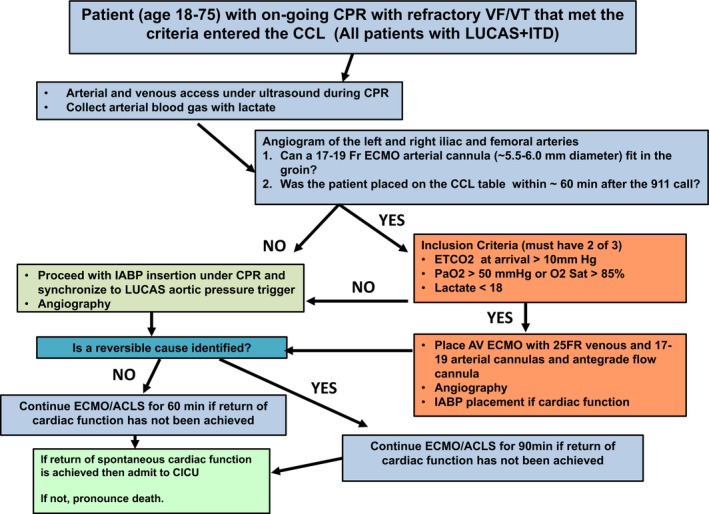
This figure shows the cardiac catheterization laboratory decision‐making process tree from arrival to either admission to the hospital or death. Timely delivery of the patient to the CCL, evidence of adequate CPR‐generated perfusion, and identification of reversible causes for the arrest were the 3 important pillars of the protocol. ACLS indicates advanced cardiac life support; CCL, cardiac catheterization laboratory; CICU, cardiac intensive care unit; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ETCO2, end‐tidal CO2; IABP, intra‐aortic balloon pump; ITD, impedance threshold device; VF/VT, ventricular fibrillation/ventricular tachycardia.
Postresuscitation Protocol
Patients received therapeutic hypothermia (TH) by standard protocol. All patients that arrived to the CCL with ongoing CPR were effectively at target temperature of TH with the majority being 34°C. The temperature was increased to 35°C if they developed bleeding complications and then maintained at that temperature with ECMO thermoregulation for 24 hours. All patients received a baseline head computerized tomography (CT) radiograph and admission to the cardiac intensive care unit (CICU) under the care of interventional cardiology. A multidisciplinary team of heart failure, critical care, and neurocritical care physicians as well as social and religious services also provided continuous expertise to patients and families. To achieve continuity of care, one attending (interventional cardiology) made all final decisions.
Data Collection
Current protocol
Following IRB approval, data were placed into a REDCap database. Individual chart reviews of all the laboratory and imaging studies were imported and analyzed as mean and SDs. Source of information for out‐of‐hospital data included immediate verbal report from the paramedics, review of out‐of‐hospital records, or through the CARES database.
Historical controls
In order to provide historical perspective for comparison, we collected data from January 1, 2013 through December 31, 2014 from the same 3 participating EMS in the identical cardiac arrest population. All data are documented, recorded, and kept in a single CARES database at the University of Minnesota MRC office. Data included used the same criteria as the MRC refractory VF/VT protocol:
OHCA with presumed cardiac etiology.
First presenting rhythm of VF/VT.
18 to 75 years of age.
Did not have ROSC before IV/IO amiodarone 300 mg.
We included all patients that had achieved ROSC anytime after amiodarone was given and their arrival to the emergency department as well as everyone that never had ROSC at any point. We identified 170 patients that met these criteria. Survival to hospital discharge with CPC 1 or 2 were recorded and were used as historical perspective.
Database Management and Statistical Analysis
All relevant information was entered in a REDCap database and exported to an Excel format for biostatistical analysis. For comparisons of characteristics between patients that survived and those that died, a t test and Fisher's exact test were used. A single, not adjusted comparison was performed between our cohort and historical controls with a Fisher's exact test.
Results
Patient characteristics are shown in Table 1. The majority were white males with a mean age of 56 years. Prearrest comorbidities were known in a small percentage of patients and are shown in Table 1.
Table 1.
Demographic Characteristics of the 18 Patients With Refractory VF/VT That Arrived in the CCL of the University of Minnesota
| Patient Characteristics of Refractory VF Patients (18) | N | % |
|---|---|---|
| Sex | ||
| Male | 14 | 78 |
| Female | 3 | 16.6 |
| Age, y | ||
| <40 | 1 | 5.5 |
| 40 to 60 | 8 | 53 |
| >60 to 75 | 9 | 50 |
| Ethnicity | ||
| White | 14 | 77.7 |
| Black | 3 | 16.6 |
| Other | 1 | 5.5 |
| Known comorbidities | ||
| Diabetes mellitus | 4 | 22 |
| CAD | 4 | 22. |
| HTN | 5 | 27.7 |
| Smoking | 6 | 33 |
| Alcoholism | 3 | 16.6 |
| Hyperlipidemia | 6 | 33.3 |
| CABG | 3 | 16.6 |
| Congestive heart disease | 3 | 16.6 |
CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; CCL, cardiac catheterization laboratory; HTN, hypertension; VF/VT, ventricular fibrillation/ventricular tachycardia.
Cardiac arrest and CCL characteristics are shown in Table 2. The cardiac arrest location was most frequently a public place. All patients presented with VF/VT and 66% received bystander CPR. Average time from 911 call to CCL arrival was ≈1 hour. The mean time required to initiate ECMO was 6 minutes from their arrival to the CCL (Table 2).
Table 2.
Cardiac Arrest and CCL Characteristics of the 18 Patients That Met All the Criteria for Advanced Perfusion and Reperfusion Therapies in the Cardiac Catheterization Laboratory
| Cardiac Arrest Characteristics (18 Patients) | N (%) |
|---|---|
| Arrest location | |
| Home | 7 (39) |
| Public place | 11 (61) |
| Initial cardiac rhythm | |
| VF/VT | 18 (100) |
| Other | 0 |
| Bystander CPR | |
| Yes | 12 (66) |
| No | 6 (33) |
| Times, min | |
| 911 to first responder | 5.8±3.1 |
| 911 to CCL arrival | 60.1±11 |
| CCL arrival—on ECMO | 6.3±2 |
CCL indicates cardiac catheterization laboratory; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; VF/VT, ventricular fibrillation/ventricular tachycardia.
Survival to Hospital Discharge With CPC 1 and 2
The patient flow diagram is shown in Figure 2. Of 27 transported patients, 9 did not meet inclusion criteria. Five were initially in nonshockable rhythms and were misclassified by paramedics at the scene. An additional 2 received manual CPR only, 1 arrived in the CCL >90 minutes following initial transport, and 1 had terminal cancer (Figure 2). Of the 18 patients that met inclusion criteria, 15 were supported with ECMO and 3 had PCI performed with ongoing LUCAS plus ITD CPR and IABP hemodynamic support. ROSC was achieved in 14 of 18 (78%) patients who were admitted to the CICU. Overall, survival to hospital discharge occurred in 10 of 18 (53%) and survival to hospital discharge with CPC 1 or 2 occurred in 9 of 18 (50%). Following hospital discharge, all surviving patients required admission to a rehabilitation facility. All 10 patients were alive 1 month after discharge. Of these, 9 of 10 (90%) had CPC 1 or 2 at 1 month. One patient discharged with CPC 3 was CPC 3 at 1 month (Figure 2).
Figure 2.
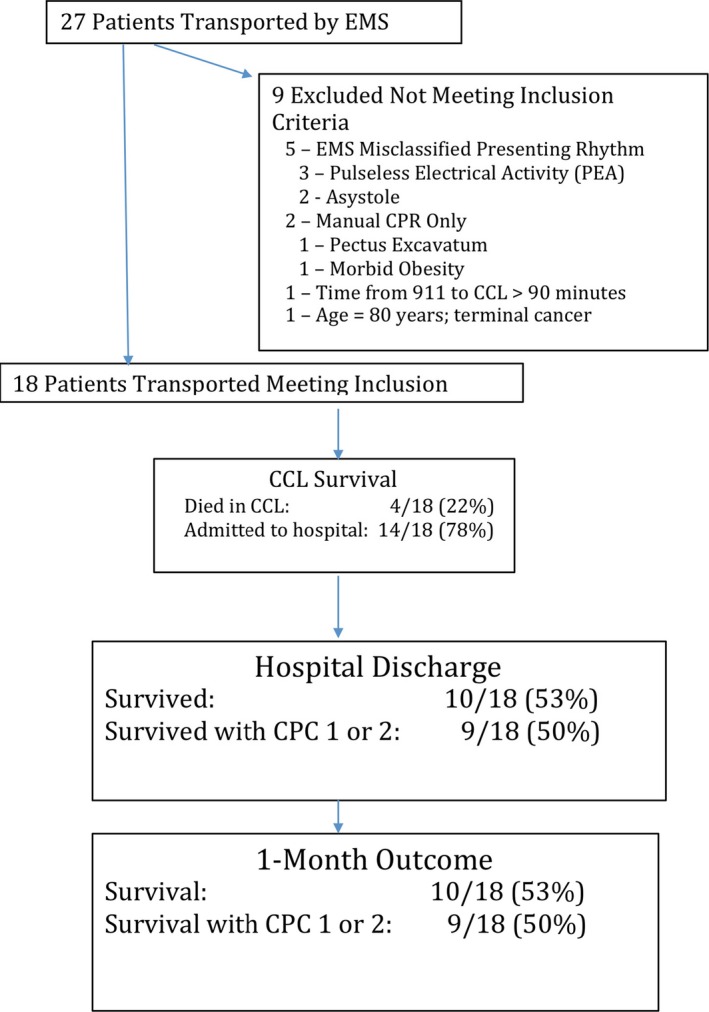
Patient flow diagram, hospital, and 1‐month outcome. CCL indicates cardiac catheterization laboratory; CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; EMS, emergency medical services.
ECMO Characteristics
In the 15 patients that were hemodynamically supported with ECMO, the average time from 911 call to ECMO support was 66.4±9 minutes (range, 38–76). ECMO support was established within 6±1.2 minutes of arrival to the CCL. Of the patients on ECMO admitted to the CICU who survived, average time to decannulation was 52±21 hours. All but 3 patients had ongoing CPR upon arrival to the CCL. Two of the 3 patients with pulses were hypotensive and in cardiogenic shock requiring ECMO initiation. The third patient had placement of IABP for hemodynamic support. The cardiovascular surgery team performed all ECMO decannulations.
Coronary Anatomy
Fourteen of the 18 patients had significant CAD and PCI was performed in 12 patients. Mean Syntax Score was 36±11. Single‐vessel disease was present in 4 of 18 (22%) patients and 2 or more vessel diseases were present in 10 of 18 (56%). Of the 12 patients receiving PCI, the mean number of stents placed per patient was 4±2.
Cardiac Function and Troponin I Levels After ROSC
Patients had a daily echocardiogram. When on ECMO, left ventricular function assessment was performed with turndown of the ECMO support to the minimum tolerated hemodynamically. The temporal evolution of left ventricular function from the first day until discharge can be seen in Figure 3.
Figure 3.
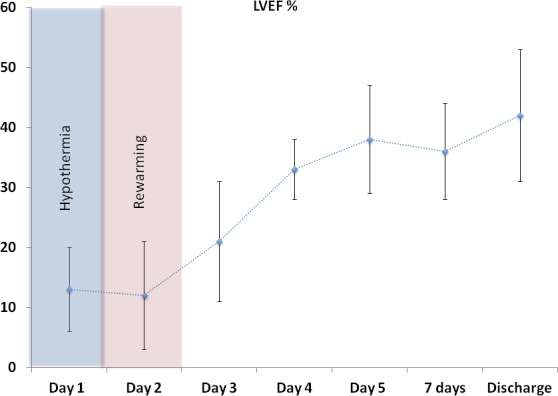
Left ventricular ejection fraction (LVEF) temporal evolution in patients that were admitted after refractory ventricular fibrillation/ventricular tachycardia arrest. A 2‐day period of severe left ventricular depression was evident in the whole cohort. Recovery was observed after 3 days. Values are shown as mean±SD.
Cardiac troponins were not detectable at presentation in the majority of cases. Troponin I peak over 100 U/mL was noticed within 16 hours in the majority of cases (Figure 4), highlighting the role of myocardial ischemia/injury in the pathogenesis of OHCA.
Figure 4.
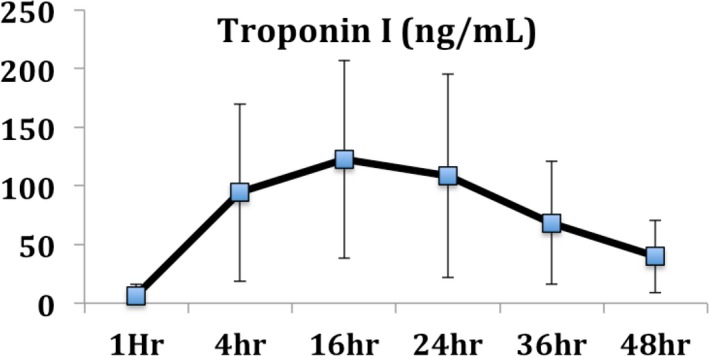
Troponin I evolution in patients that were admitted after refractory ventricular fibrillation/ventricular tachycardia arrest that had evidence of coronary artery disease. Troponin levels of >100 ng/mL were very common within the first 24 hours, suggesting significant myocardial injury. Values are shown as mean±SD.
ECMO‐Related Complications
None of the following ECMO‐related complications were observed: intracranial bleeding, stroke, leg ischemia, or infectious complications from the indwelling lines. We recorded no vascular complications from ECMO placement.
There were 2 patients that had moderate bleeding around the arterial cannula, in part attributed to the presence of anticoagulation (heparin), dual antiplatelet therapy and Eptifibatide infusion due to high coronary thrombus burden. Patients were managed by decreasing the activated clotting time goal to <180 seconds.
Because of the high prevalence of infections in postcardiac arrest patients, all patients were treated with a 5‐day course of broad‐spectrum antibiotics (vancomycin and piperacillin/tazobactam) beginning at CICU admission.9, 10 Despite that, 10 patients developed fever and were diagnosed with pneumonia. All were successfully treated with intravenous and oral antibiotics. Two patients developed deep venous thrombosis, 1 on the left arm and 1 on the opposite leg from the ECMO cannulation site. They were successfully treated with anticoagulants.
Neurological Dysfunction and Brain Death
Three patients had imaging evidence of severe anoxic brain injury on the admission CT. One of these patients developed central herniation within 6 hours despite medical therapy. The second patient had a neurological exam consistent with brain death and a cerebral perfusion scan showing no uptake by the brain leading to discontinuation of supportive therapies. The third patient suffered catastrophic bowel infarct late in his hospitalization as a complication from heparin‐induced thrombocytopenia with thrombosis. Two of the 3 patients became organ donors. One of the patients survived with neurological dysfunction (CPC=3). The patient had receptive aphasia and is still recovering.
Survivor Characteristics
Patients that survived had significant differences in their resuscitation process and cause of the arrest. The following factors were significantly associated with survival to hospital discharge: (1) the presence of bystander CPR; (2) earlier arrival of first responders after the 911 call; (3) earlier arrival to the CCL; (4) lower lactic acid on initial blood gases in the CCL; and (5) the presence of CAD as a reversible cause. The absence of identification of an obvious reversible cause, such as CAD or pulmonary embolism, was associated with bad outcome. Only 1 patient without an identifiable reversible cause had return of spontaneous cardiac function and was admitted to the CICU. That patient survived with moderate neurological dysfunction (CPC=3). Characteristics associated with better outcomes could be summarized as: (1) rapid EMS response time and shorter time from 911 call to delivery to the CCL; (2) bystander CPR; and (3) evidence of reversible CAD (Table 3).
Table 3.
Resuscitation Related Characteristics of Survivors With Favorable Neurological Function and Patients That Died or Survived With Poor Neurological Function
| Refractory VF/VT Patients | Survivors With CPC 1&2 (9) | Deaths and Survivors With CPC >2 (9) | P Value |
|---|---|---|---|
| Age, y | 57±11 | 56±9 | 0.2 |
| 911 call to first response arrival | 3.8±2.5 min | 8±3 min | 0.004a |
| Bystander CPR | 8/9 | 4/9 | 0.13 |
| 911 call to CCL entry | 54±7.6 | 66±10.5 | 0.019 |
| CCL entry—on ECMO | 6±2 | 5.4±4 | 0.2 |
| ETCO2 on arrival | 32±12 | 35±8 | 0.5 |
| pH on ECMO opening ABG | 7.05±0.1 | 7.07±0.3 | 0.4 |
| Lactate at CCL arrival | 9.9±2.8 | 14.6±5.5 | 0.041a |
| Presence of CAD | 9/9 | 4/9 | 0.029a |
| Witnessed arrest | 5/9 | 6/9 | 0.6 |
| Intermittent ROSC before ECMO | 6/9 | 1/9 | 0.049a |
Better outcomes were associated with three main characteristics: (1) rapid EMS response time and shorter time from 911 call to delivery to the CCL; (2) bystander CPR; and (3) evidence of reversible coronary artery disease. ABG indicates arterial blood gas; CCL, cardiac catheterization laboratory; CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ETCO2, end‐tidal CO2; ROSC, return of spontaneous circulation; VF/VT, ventricular fibrillation/ventricular tachycardia.
Statistically significant difference, P<0.05.
Historical Perspective
We identified 170 patients with the same criteria as our refractory VF/VT protocol population treated by the same EMS systems. Average age was 56±7 years, 74.6% were males, and 77% were white. Of those, 63% of the arrests happened in a public place and 61% had bystander CPR. Survival to hospital discharge with CPC 1 or 2 was found to be 14 of 170 (8.2% compared to 9 of 18 [50%] from the MRC refractory VF/VT protocol; P<0.0001).
Discussion
Our initial 3‐month experience with the MRC refractory VF/VT protocol shows, for the first time, that an organized approach to treat refractory out‐of‐hospital VF/VT, capitalizing on advanced perfusion and reperfusion strategies, is feasible and can result in a significant rate of functionally favorable survival to hospital discharge. This preliminary experience reports an overall rate of 53% survival to hospital discharge and a 50% rate of functionally favorable survival to hospital discharge.
With 3 months of experience, our cohort is the largest refractory VF/VT series that has been reported on in the United States. The SAVE‐J trial, performed in Japan, was a prospective, observational study comparing 454 patients with VT/VF arrest admitted to 46 hospitals over 3 years.11 Of these, 234 patients were provided extracorporeal CPR (ECPR). Patients received ECPR if they were admitted to an ECPR‐capable center whereas patients admitted to non‐ECPR centers received standard therapy. Patients receiving ECPR demonstrated a significant improvement in neurologically intact survival (CPC 1 and 2) at 1 month with 13.7% in the per‐protocol group compared to 1.9% in the non‐ECPR group. Overall 1‐month survival was also improved in the ECPR group with 29% versus 6% in the non‐ECPR group. Johnson et al. reported 26 cases over a 7‐year period with only 42% of those patients presenting with VF/VT.12 Wang et al. described a very large cohort of 220 patients receiving ECPR over a period of 5 years, of whom only 31 where OHCA patients. Of those, only 15 had VF/VT as the presenting rhythm.13 Stub et al., in the CHEER trial, enrolled 11 patients with OHCA and all presented with VF. Of the 9 patients that were placed on ECMO, 3 survived (33% survival rate).14
In all studies12, 13, 14 previously reported, ECMO cannulation was performed in the emergency department. Vascular and bleeding complication rates were significantly higher than the present study, although the SAVE‐J did not report complication rates. In those studies, vascular complications ranged from 45% to 65% and bleeding was in the same range in all three cohorts.12, 13, 14 We reported no ECMO‐related vascular complications, no life‐threatening EMCO complications, and lower bleeding complications rates. We believe that one of the fundamental differences in our protocol is that only 3 experienced interventional cardiologists and high‐volume operators performed all the procedures after extensive training with pre‐established protocols for vascular access, including ultrasound guidance and precannulation angiography of the iliofemoral vessels.
An important finding in our cohort of patients was that the left ventricle was severely depressed immediately after the prolonged resuscitation period. Cardiac function returned soon after ECMO was established in the majority of the patients, but 3 to 4 hours later left ventricular (LV) ejection fraction (LVEF) was again severely compromised. Cardiac function was severely depressed for an average of 3 days, at which point a progressive recovery was observed in the majority of patients continuing until the day of discharge (Figure 3). This phenomenon is well described in animal models of cardiac arrest and resuscitation. In those studies, animals that had achieved ROSC had a significant decrease in LVEF to less than 35% within the first 4 hours and recovered slightly within 24 hours.15, 16 The same has been reported for resuscitated patients with prehospital ROSC.17, 18 Our data are a significant addition to the literature because of the severity of LV dysfunction being present in this population that, without circulatory support, would be extremely difficult to be successfully treated in the coronary care unit. The level of troponin elevation in this population further reflects the severity of myocardial injury. The absence of early troponin elevation in the majority of patients suggests that troponin may not be a useful diagnostic tool to emergently diagnose CAD as the cause of cardiac arrest in this patient population.
The characteristics of our MRC protocol are unique in that they evolved as a result of strong, multidisciplinary, community‐wide collaboration with input from interventional cardiology, emergency medicine, and out‐of‐hospital EMS providers. There are 4 critical and unique components to our protocol: (1) early mobilization of patients who, after at least 3 attempted defibrillations and amiodarone, still have not achieved ROSC; (2) simplification of the hospital activation process through a single telephone call from EMS dispatch; (3) a core group of interventional cardiologists for placement of preprimed percutaneous ECMO to achieve consistency of vascular access and limitation of complications; and (4) a multidisciplinary hospital care team with broad cross‐specialty interactions to comprehensively address the medical, psychosocial, and emotional issues for both patients and families. We believe that steps 1 and 2 are critical to identify patients most likely to benefit and then expedite access to the CCL to implement these potentially life‐saving technologies as rapidly as possible. These are further reinforced by the characteristics we found associated with functionally favorable survivors: (1) rapid EMS response time and shorter time from 911 call to delivery to the CCL; (2) bystander CPR; and (3) evidence of reversible CAD.
The presence of CAD was very prevalent in this cohort. The majority of cases had high disease burden with multivessel disease and complicated lesions. Interventions therefore were not easy to perform with ongoing CPR. Circulatory support provided by ECMO gave the operator the freedom to intervene in much more severe disease than would otherwise have been possible.
Thus, selection of patients most likely to benefit, provision of advanced hemodynamic support with mechanical LUCAS plus ITD CPR, early and rapid transport to the CCL, hemodynamic support with rapid placement of ECMO, and identification and intervention in causative, complex CAD appear to have formed the basis for the success of this initial experience.
Limitations
We report functionally favorable survival rates in patients with refractory VF/VT cardiac arrest from a single, high‐volume resuscitation center that may be difficult to replicate in other centers. By definition, this initial 3‐month experience reports on a small cohort of patients. Functionally favorable survival rates may increase or decrease with broader experience. As with any clinical protocol registry, selection bias cannot be excluded. Nonetheless, no patient meeting criteria for the protocol was missed from inclusion during the reported period. EMS providers initially “overtransported” patients with 100% sensitivity in case selection, but only 65% specificity. This is being addressed in ongoing system‐wide EMS quality improvement processes. One patient (6%) survived to hospital discharge with CPC >2. Investigators recognize the issue of increasing survival with poor neurological outcome as an important issue with this approach. Nonetheless, survival with poor neurological outcome also occurs with standard resuscitation practice. A larger experience/patient cohort, comparison with outcome from standard resuscitation practice, and weight in relation to any improvement in functionally favorable survival requires careful monitoring as greater experience is realized with this approach. Only a randomized, clinical trial could eliminate potential selection bias and provide comparative outcome data with standard resuscitation practice to definitively determine the benefits and risks of this approach.
Conclusions
The MRC refractory VF/VT protocol is feasible and led to a high functionally favorable survival rate with few complications.
Disclosures
Dr Yannopoulos is the principal investigator (PI) and co‐PI for the following NIH (NHLBI) grants: R01 HL123227, 1R01HL126092‐01, R01HL1223231, R43HL123194‐01. Dr Yannopoulos also received funds for the Minnesota Resuscitation Consortium from the Medtronic Foundation. Dr Garcia is a recipient of a career development award (1IK2CX000699‐01) from the VA Office of Research and Development. Dr Garcia is a consultant for Surmodics. Dr Aufderheide has received multiple grants from the NHLBI and NINDS for research in emergency cardiovascular care.
(J Am Heart Assoc. 2016;5:e003732 doi: 10.1161/JAHA.116.003732)
References
- 1. Aufderheide TP, Frascone RJ, Wayne MA, Mahoney BD, Swor RA, Domeier RM, Olinger ML, Holcomb RG, Tupper DE, Yannopoulos D, Lurie KG. Standard cardiopulmonary resuscitation versus active compression‐decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out‐of‐hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aufderheide TP, Nichol G, Rea TD, Brown SP, Leroux BG, Pepe PE, Kudenchuk PJ, Christenson J, Daya MR, Dorian P, Callaway CW, Idris AH, Andrusiek D, Stephens SW, Hostler D, Davis DP, Dunford JV, Pirrallo RG, Stiell IG, Clement CM, Craig A, Van Ottingham L, Schmidt TA, Wang HE, Weisfeldt ML, Ornato JP, Sopko G; Resuscitation Outcomes Consortium I . A trial of an impedance threshold device in out‐of‐hospital cardiac arrest. N Engl J Med. 2011;365:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O'Neil BJ, Paxton JH, Silvers SM, White RD, Yannopoulos D, Donnino MW. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S444–S464. [DOI] [PubMed] [Google Scholar]
- 4. Spaulding CM, Joly LM, Rosenberg A, Monchi M, Weber SN, Dhainaut JF, Carli P. Immediate coronary angiography in survivors of out‐of‐hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. [DOI] [PubMed] [Google Scholar]
- 5. Garcia S, Drexel T, Bekwelem W, Raveendran G, Caldwell E, Hodgson L, Wang Q, Adabag S, Mahoney B, Frascone R, Helmer G, Lick C, Conterato M, Baran K, Bart B, Bachour F, Roh S, Panetta C, Stark R, Haugland M, Mooney M, Wesley K, Yannopoulos D. Early access to the cardiac catheterization laboratory for patients resuscitated from cardiac arrest due to a shockable rhythm: the Minnesota Resuscitation Consortium Twin Cities Unified Protocol. J Am Heart Assoc. 2016;5:e002670 doi: 10.1161/JAHA.115.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubertsson S, Karlsten R. Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation. 2005;65:357–363. [DOI] [PubMed] [Google Scholar]
- 7. Larsen AI, Hjornevik A, Bonarjee V, Barvik S, Melberg T, Nilsen DW. Coronary blood flow and perfusion pressure during coronary angiography in patients with ongoing mechanical chest compression: a report on 6 cases. Resuscitation. 2010;81:493–497. [DOI] [PubMed] [Google Scholar]
- 8. Wagner H, Terkelsen CJ, Friberg H, Harnek J, Kern K, Lassen JF, Olivecrona GK. Cardiac arrest in the catheterisation laboratory: a 5‐year experience of using mechanical chest compressions to facilitate PCI during prolonged resuscitation efforts. Resuscitation. 2010;81:383–387. [DOI] [PubMed] [Google Scholar]
- 9. Sun HY, Ko WJ, Tsai PR, Sun CC, Chang YY, Lee CW, Chen YC. Infections occurring during extracorporeal membrane oxygenation use in adult patients. J Thorac Cardiovasc Surg. 2010;140:1125–1132.e1122. [DOI] [PubMed] [Google Scholar]
- 10. Mongardon N, Perbet S, Lemiale V, Dumas F, Poupet H, Charpentier J, Pene F, Chiche JD, Mira JP, Cariou A. Infectious complications in out‐of‐hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med. 2011;39:1359–1364. [DOI] [PubMed] [Google Scholar]
- 11. Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, Hase M, Tahara Y, Atsumi T; Group S‐JS . Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out‐of‐hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. [DOI] [PubMed] [Google Scholar]
- 12. Johnson NJ, Acker M, Hsu CH, Desai N, Vallabhajosyula P, Lazar S, Horak J, Wald J, McCarthy F, Rame E, Gray K, Perman SM, Becker L, Cowie D, Grossestreuer A, Smith T, Gaieski DF. Extracorporeal life support as rescue strategy for out‐of‐hospital and emergency department cardiac arrest. Resuscitation. 2014;85:1527–1532. [DOI] [PubMed] [Google Scholar]
- 13. Wang CH, Chou NK, Becker LB, Lin JW, Yu HY, Chi NH, Hunag SC, Ko WJ, Wang SS, Tseng LJ, Lin MH, Wu IH, Ma MH, Chen YS. Improved outcome of extracorporeal cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest—a comparison with that for extracorporeal rescue for in‐hospital cardiac arrest. Resuscitation. 2014;85:1219–1224. [DOI] [PubMed] [Google Scholar]
- 14. Stub D, Bernard S, Pellegrino V, Smith K, Walker T, Sheldrake J, Hockings L, Shaw J, Duffy SJ, Burrell A, Cameron P, Smit de V, Kaye DM. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation. 2015;86:88–94. [DOI] [PubMed] [Google Scholar]
- 15. Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. [DOI] [PubMed] [Google Scholar]
- 16. Schultz J, Segal N, Kolbeck J, Caldwell E, Thorsgard M, McKnite S, Aufderheide TP, Lurie KG, Yannopoulos D. Sodium nitroprusside enhanced cardiopulmonary resuscitation prevents post‐resuscitation left ventricular dysfunction and improves 24‐hour survival and neurological function in a porcine model of prolonged untreated ventricular fibrillation. Resuscitation. 2011;82(suppl 2):S35–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out‐of‐hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. [DOI] [PubMed] [Google Scholar]
- 18. Conlon TW, Falkensammer CB, Hammond RS, Nadkarni VM, Berg RA, Topjian AA. Association of left ventricular systolic function and vasopressor support with survival following pediatric out‐of‐hospital cardiac arrest. Pediatr Crit Care Med. 2015;16:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]


