Contrast‐induced acute kidney injury (CI‐AKI), previously referred to as contrast‐induced nephropathy, remains a vexing problem despite extensive research on the topic and recognition of the condition >50 years ago.1 CI‐AKI generally describes a decrease in renal function occurring in temporal association with intravenous or intra‐arterial iodinated contrast exposure. The typical timeline for a decline in renal function is 2 to 5 days after iodinated contrast exposure. Common definitions for CI‐AKI include an absolute rise in serum creatinine ≥0.5 mg/dL or a relative increase of ≥25% at 48 to 72 hours after contrast exposure.
CI‐AKI is fairly common. CI‐AKI was demonstrated to be the third most common cause of AKI among hospitalized patients.2 The incidence of CI‐AKI among patients undergoing percutaneous coronary intervention (PCI) varies from 2% to 25%, depending on the characteristics of the population.3, 4 In a large contemporary cohort study using the National Cardiovascular Data Registry (NCDR) CathPCI Registry, the incidence of CI‐AKI was 7.1%, and the incidence of CI‐AKI requiring hemodialysis was 0.3%.5 In this NCDR CathPCI Registry study, multiple factors were associated with CI‐AKI, including older age, worse baseline renal function, prior heart failure or heart failure on presentation, prior PCI, mode of presentation (nonacute coronary syndrome, non–ST‐segment elevation myocardial infarction, or ST‐segment elevation myocardial infarction), diabetes mellitus, chronic lung disease, hypertension, cardiac arrest, anemia, balloon pump use, and cardiogenic shock. Among these factors, presentation of ST‐segment elevation myocardial infarction, cardiogenic shock, and severe baseline chronic kidney disease were the strongest predictors of AKI.5 Alternatively, in the Mehran risk score, a similar set of factors predict the risk of CI‐AKI: baseline creatinine, hypotension, intra‐aortic balloon pump, congestive heart failure, chronic kidney disease, diabetes mellitus, age >75 years, anemia, and volume of contrast.3 It is evident from either score that sicker patients with more severe comorbidities and more hemodynamic disarray are at a greater risk of CI‐AKI after PCI.
Many potential therapies have been tested for prevention of CI‐AKI; however, very few successful treatments have been clearly demonstrated to prevent CI‐AKI. Multiple trials have assessed acetylcysteine, sodium bicarbonate containing intravenous fluid, vitamin C, vitamin E, calcium channel blockers, dopamine, remote ischemic preconditioning, statins, and other treatments for the prevention of CI‐AKI. In fact, the literature in this field is an excellent demonstration of publication bias, with initial strong, positive results published in high‐impact journals followed by negative studies in lower profile journals. Acetylcysteine initially demonstrated very strong prevention of CI‐AKI,6, 7 but now, after many years of study and countless trials and meta‐analyses, we are less certain about its protective effect.8 Sodium bicarbonate fluids were thought to be superior to sodium chloride,9 but that finding did not appear to hold up to further scrutiny.10 Antioxidant vitamins have been tested at multiple junctures, but the results are inconclusive.11 Candidate interventions that have been tested and demonstrated to be either inconclusively protective or unsuccessful for the prevention of contrast nephropathy are almost innumerable.
The main mechanisms thought to underlie CI‐AKI included renal vasoconstriction, tubule toxicity, generation of reactive oxygen species, and medullary hypoxia.12, 13 Contrast viscosity and osmolality also determine the likelihood of CI‐AKI. Interventions that decrease the concentration of iodinated contrast within the kidney, and within the renal tubules more specifically, appear to reduce the risk of CI‐AKI; therefore, emphasis is on pre‐ and postprocedural hydration to prevent CI‐AKI.
In this issue of the Journal of the American Heart Association, Liu et al report their findings on hydration volume and incidence of CI‐AKI from a single‐center retrospective cohort study of participants undergoing PCI.14 They found that participants with a high ratio of hydration volume to weight (HV/W) actually had higher rates of CI‐AKI and worse outcomes overall. This finding seems counterintuitive because the bedrock for prevention of CI‐AKI is adequate hydration, typically with intravenous saline before and after contrast exposure. How can we reconcile these findings with long‐established practice and a large evidence base supporting intravenous hydration as a preventive strategy to minimize CI‐AKI?
As with any observational study, this study is limited by measured, unmeasured, and residual confounding. Importantly, almost every factor that has been previously associated with CI‐AKI was present at higher frequency in participants with high HV/W ratios. Participants in quartile 4 (Q4) for HV/W ratio were more likely to be older and to have congestive heart failure, intra‐aortic balloon pump use, and diabetes mellitus. In addition, participants in HV/W ratio Q4 had higher baseline creatinine and lower estimated glomerular filtration rates and creatinine clearance. Many of the variables discussed earlier that are associated with risk of CI‐AKI from both the Mehran score and the NCDR CathPCI score were also present in participants in Q4 for HV/W ratio; therefore, it is likely that physicians had identified patients at risk for CI‐AKI and instituted a liberal pre‐ and postprocedure hydration strategy. The finding that high HV/W ratio is associated with CI‐AKI may be related to an adverse effect of the excessive hydration itself or to residual confounding from the measured and unmeasured factors that were more frequently present in the group with high HV/W. It is interesting that the comparison of HV/W ratio Q1 and Q4 demonstrates an unadjusted odds ratio for CI‐AKI of 3.95. After multivariate adjustment, the odds ratio was 1.87; therefore, significant positive confounding is present among the characteristics of participants in HV/W ratio Q4 and the primary outcome of CI‐AKI. In other words, participants in Q4 for HV/W ratio are different from those in Q1 (generally having many baseline variables associated with increased risk of CI‐AKI), and no amount of multivariate adjustment can perfectly capture these differences. Moreover, by normalizing the hydration volumes to body weight, the investigators may have introduced unnecessary bias. Low body weight is associated with CI‐AKI risk, and the participants in Q4 for HV/W ratio had lower body weights. Multiple formulas for predicting a maximal acceptable contrast dose use body weight or creatinine clearance (which incorporates body weight).15 Simply put, smaller people tolerate lower volumes of contrast than do larger people.
Recent publications shed light on alternative approaches to intravenous fluid administration for prevention of CI‐AKI. The POSEIDON study and a similar study by Qian et al both found that a hemodynamically guided strategy with high‐volume saline infusion for low intravascular volume status was protective against CI‐AKI. In the POSEIDON study, left ventricular end‐diastolic pressure (LVEDP) was measured at the beginning of the coronary procedure, and then intravenous fluid was administered on a sliding scale depending on the baseline LVEDP.16 Participants with high LVEDP received less intravenous fluids, and participants with low LVEDP received high‐volume rapid delivery of intravenous fluids. Qian et al followed a similar study design but used right atrial pressure rather than LVEDP.17 In both studies, participants receiving higher fluid volumes had protection against CI‐AKI. In fact, in the study by Qian et al, higher volumes of intravenous saline were administered among participants in the central venous pressure–guided group, and these higher volumes of hydration were associated with reduced risk of CI‐AKI. Similarly, in the POSEIDON study, participants with LVEDP‐guided hydration received more than twice the total intravenous fluid volume than participants in the control group, and participants in the LVEDP‐guided group had a markedly reduced rate of CI‐AKI.
Furthermore, studies of the RenalGuard device, which uses furosemide diuresis with matched intravenous fluid hydration to increase urine output, have demonstrated protection from CI‐AKI.18, 19 The principle of the RenalGuard device is to achieve maximal intra‐ and postprocedural urine flow rates while avoiding dehydration or excessive hydration. Intraprocedural urine‐output rates of >450 mL/h were associated with prevention of CI‐AKI.20 With these high urine‐output rates and with matched intravenous hydration, participants treated with RenalGuard receive high rates of intravenous fluid hydration, albeit in manner that maintains euvolemic volume status.
How can we reconcile the findings of the current article by Liu et al with the findings from POSEIDON and Qian et al, who found that high volumes of intravenous fluids, administered under hemodynamic guidance, were protective against CI‐AKI? As addressed earlier, it is possible that the current findings are largely attributable to residual confounding because participants in Q4 for HV/W ratio had many baseline variables associated with CI‐AKI risk. Beyond this important limitation, perhaps the message from the current study is that too much hydration increases the risk of CI‐AKI in some people; it is well known that congestive heart failure is associated with CI‐AKI risk, and renal venous hypertension (or congestion) may contribute to the pathogenesis of AKI. Perhaps we have refinement of the message regarding intravenous fluids (Figure). It is not that intravenous fluid hydration is automatically good but rather that the right amount of fluid for each patient is needed to optimize outcomes and reduce the risk of CI‐AKI.
Figure 1.
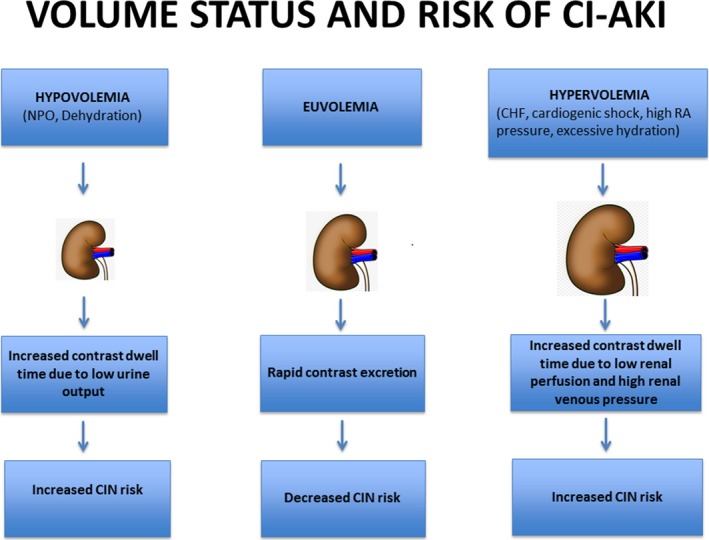
An integrated model of intravascular volume status and risk of CI‐AKI. CHF indicates congestive heart failure; CI‐AKI, contrast‐induced acute kidney injury; CIN, contrast‐induced nephropathy; NPO, nothing by mouth; RA, right atrial.
Disclosures
None.
J Am Heart Assoc. 2016;5:e003777 doi: 10.1161/JAHA.116.003777.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Berlyne N, Berlyne GM. Acute renal failure following intravenous pyelography with hypaque. Acta Med Scand. 1962;171:39–41. [DOI] [PubMed] [Google Scholar]
- 2. Nash K, Hafeez A, Hou S. Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. [DOI] [PubMed] [Google Scholar]
- 3. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 4. Solomon R, Dauerman HL. Contrast‐induced acute kidney injury. Circulation. 2010;122:2451–2455. [DOI] [PubMed] [Google Scholar]
- 5. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Weintraub WS, Curtis JP, Messenger JC, Rumsfeld JS, Spertus JA. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath‐PCI Registry. J Am Heart Assoc. 2014;3:e001380 doi: 10.1161/JAHA.114.001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic‐contrast‐agent‐induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. [DOI] [PubMed] [Google Scholar]
- 7. Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, Veglia F, Bartorelli AL. N‐acetylcysteine and contrast‐induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. [DOI] [PubMed] [Google Scholar]
- 8. Investigators ACT . Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast‐induced nephropathy Trial (ACT). Circulation. 2011;124:1250–1259. [DOI] [PubMed] [Google Scholar]
- 9. Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA III, Rittase RA, Norton HJ, Kennedy TP. Prevention of contrast‐induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–2334. [DOI] [PubMed] [Google Scholar]
- 10. Solomon R, Gordon P, Manoukian SV, Abbott JD, Kereiakes DJ, Jeremias A, Kim M, Dauerman HL; BOSS Trial Investigators . Randomized trial of bicarbonate or saline study for the prevention of contrast‐induced nephropathy in patients with CKD. Clin J Am Soc Nephrol. 2015;10:1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A. Renal insufficiency following contrast media administration trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–1217. [DOI] [PubMed] [Google Scholar]
- 12. Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, Condorelli G. Contrast agents and renal cell apoptosis. Eur Heart J. 2008;29:2569–2576. [DOI] [PubMed] [Google Scholar]
- 13. Meyer M, LeWinter MM, Bell SP, Chen Z, Selby DE, Singla DK, Dauerman HL. N‐acetylcysteine‐enhanced contrast provides cardiorenal protection. JACC Cardiovasc Interv. 2009;2:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Li H, Chen D, Chen J, Tan N, Zhou Z, Liu Y, Ye P, Ran P, Duan C, Chen P. Excessively high hydration volume may not be associated with decreased risk of contrast‐induced acute kidney injury after percutaneous coronary intervention in patients with renal insufficiency. J Am Heart Assoc. 2016;5:e003171 doi: 10.1161/JAHA.115.003171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gurm HS, Dixon SR, Smith DE, Share D, Lalonde T, Greenbaum A, Moscucci M; BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) Registry . Renal function‐based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2011;58:907–914. [DOI] [PubMed] [Google Scholar]
- 16. Brar SS, Aharonian V, Mansukhani P, Moore N, Shen AY, Jorgensen M, Dua A, Short L, Kane K. Haemodynamic‐guided fluid administration for the prevention of contrast‐induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. [DOI] [PubMed] [Google Scholar]
- 17. Qian G, Fu Z, Guo J, Cao F, Chen Y. Prevention of contrast‐induced nephropathy by central venous pressure‐guided fluid administration in chronic kidney disease and congestive heart failure patients. JACC Cardiovasc Interv. 2016;9:89–96. [DOI] [PubMed] [Google Scholar]
- 18. Briguori C, Visconti G, Focaccio A, Airoldi F, Valgimigli M, Sangiorgi GM, Golia B, Ricciardelli B, Condorelli G; REMEDIAL II Investigators . Renal insufficiency after contrast media administration trial II (REMEDIAL II): RenalGuard system in high‐risk patients for contrast‐induced acute kidney injury. Circulation. 2011;124:1260–1269. [DOI] [PubMed] [Google Scholar]
- 19. Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (induced diuresis with matched hydration compared to standard hydration for contrast induced nephropathy prevention) trial. JACC Cardiovasc Interv. 2012;5:90–97. [DOI] [PubMed] [Google Scholar]
- 20. Briguori C, Visconti G, Donahue M, De Micco F, Focaccio A, Golia B, Signoriello G, Ciardiello C, Donnarumma E, Condorelli G. RenalGuard system in high‐risk patients for contrast‐induced acute kidney injury. Am Heart J. 2016;173:67–76. [DOI] [PubMed] [Google Scholar]


