Abstract
Arabidopsis ESD7 locus encodes the catalytic subunit of the DNA Pol ϵ involved in the synthesis of the DNA leading strand and is essential for embryo viability. The hypomorphic allele esd7-1 is viable but displays a number of pleiotropic phenotypic alterations including an acceleration of flowering time. Furthermore, Pol ϵ is involved in the epigenetic silencing of the floral integrator genes FT and SOC1, but the molecular nature of the transcriptional gene silencing mechanisms involved remains elusive. Here we reveal that ESD7 interacts with components of the PRC2 such as CLF, EMF2 and MSI1, and that mutations in ESD7 cause a decrease in the levels of the H3K27me3 mark present in the chromatin of FT and SOC1. We also demonstrate that a domain of the C-terminal region of ESD7 mediates the binding to the different PRC2 components and this interaction is necessary for the proper recruitment of PRC2 to FT and SOC1 chromatin. We unveil the existence of interplay between the DNA replication machinery and the PcG complexes in epigenetic transcriptional silencing. These observations provide an insight into the mechanisms ensuring that the epigenetic code at pivotal loci in developmental control is faithfully transmitted to the progeny of eukaryotic cells.
INTRODUCTION
In eukaryotic organisms chromatin is duplicated during cell division to ensure faithful transmission of both genetic and epigenetic information, maintaining in the daughter cells the memory of the chromatin status of their progenitors (1). Mitotic inheritance of cellular identity is crucial for proper development in multicellular organisms and is maintained through cell division by DNA replication, and chromatin assembly and remodeling (2). Furthermore, chromatin structure is closely linked to these processes which in turn maintain the stability and function of the genome. However, despite its relevance for proper function of eukaryotic organisms, the molecular bases for the inheritance of epigenetic states through cell division remain to be elucidated.
In plants, organogenesis occurs post-embryonically from a group of undifferentiated stem cells that generate all plant organs through reiterative cell divisions and subsequent differentiation processes that drive developmental transitions. For that reason, besides accurate DNA replication, the preservation of the epigenetic information in developmental genes is especially important along the post-embryonic growth of plants to maintain cell identity (3). Inheritance of gene expression states is also essential for cells to recall past events, such as environmental or developmental cues (4).
Different DNA Polymerases (Pol) replicate and maintain eukaryotic genomes, but only three of them (Pol α, Pol ϵ and Pol δ) are mainly responsible for nuclear DNA replication (5). Among them, DNA polymerase epsilon (ϵ) was proposed to synthesize the nascent leading strand (6), but questions regarding which polymerases are responsible for copying the leading and lagging strand templates have been recently raised (7). In Arabidopsis, EARLY IN SHORT DAYS 7 (ESD7)/POL2a/TILTED1(TIL1)/ABA OVERLY SENSITIVE 4 (ABO4) encodes the catalytic subunit of the DNA Pol ϵ, an enzyme that is essential for viability of the embryo (8–11). However, the partial loss of function displayed by different hypomorphic alleles of this gene causes several pleiotropic phenotypic alterations including abnormal development and acceleration of flowering time, which is totally dependent on the expression of the floral integrator genes FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) (8,10,11). In Arabidopsis, the flowering signals from environmental and internal cues converge eventually on the transcriptional regulation of the FT and SOC1 genes, which in turn, activate the floral meristem identity genes to initiate the floral transition (12). On the other hand, INCURVATA2 (ICU2) encodes the catalytic subunit of DNA Pol alpha (α) (13–15), which synthesizes together with DNA Pol delta (δ) the nascent lagging strand as a series of ∼200-bp Okazaki fragments (6). The hypomorphic icu2-1 allele also displayed a number of developmental phenotypic alterations with a conspicuous early flowering phenotype (13). Both ESD7 and ICU2 negatively regulate FT and SOC1 expression through the same genetic pathway (11) but the molecular nature of the transcriptional gene silencing mechanisms involved remains elusive. Recently, viable thermosensitive mutations in Arabidopsis Pol δ have been described (16). These mutants display ectopic expression of SEPALLATA3 (SEP3) leading to early flowering and curly leaf phenotypes.
Following replication, the newly synthesized DNA daughter strands are properly assembled into chromatin by the Chromatin Assembly Factor 1 (CAF-1), that deposits histones H3/H4 onto DNA in a replication-dependent manner (17). In plants, CAF-1 is a heterotrimeric complex that includes FASCIATA 1 and 2 (FAS1, FAS2) and MULTICOPY SUPPRESSOR OF IRA 1 (MSI1) proteins (18). Loss of function of these components results in activation of particular cell cycle and DNA repair genes (19). This derepression of typically silenced genes is similar to that reported in different mutant alleles of ESD7 or ICU2 (10,11,14). Interestingly, a role for the DNA replication machinery in the maintenance of epigenetic memory has been proposed; ICU2 seems essential to ensure the stable maintenance of repressive histone modifications (4). In contrast, DNA Pol δ is required for the proper establishment of transcriptionally active epigenetic marks at particular loci and its failure might alter developmental processes by affecting the epigenetic control of master genes (16). Additional evidence supports a role for DNA Pols in these processes. For instance, the deregulation of FT and SOC1 expression in esd7-1 is dependent on the CAF-1 activity since the early flowering phenotype of esd7-1 is suppressed by mutations in FAS2 (11). Furthermore, ESD7 and ICU2 interact synergistically with FAS genes (11,13), indicating a functional link between these DNA Pols and the CAF-1 complex. Besides, the p150 subunit related to FAS1 in mammals is also known to interact with HETEROCHROMATIN PROTEIN 1 (HP1) (20,21), revealing another point of connection between nucleosome assembly and epigenetic regulation processes. Remarkably, the early flowering phenotype of esd7-1 and icu2-1 mutants is similar to that displayed by loss of function mutants affected in TERMINAL FLOWER 2 /LIKE HETEROCHROMATIN PROTEIN 1 (TFL2/LHP1) gene. This gene encodes the Arabidopsis POLYCOMB REPRESSIVE COMPLEX 1 (PRC1) component homolog to HP1 (22) that plays a Polycomb-linked role in H3K27me3 binding (23,24). TFL2 is able to interact in vitro with ESD7 (11) and participates in the regulation of flowering time being necessary for FT repression and for maintaining the vernalization-mediated silencing of the floral repressor FLOWERING LOCUS C (FLC) after exposure to cold temperatures (25–28).
In addition to being part of the CAF1 complex, MSI1 is also present in PRC2 and participates in the regulation of the floral transition through multiple pathways that repress flowering linked to the function of this Polycomb complex (29). The PRC2 mediates the trimethylation of H3K27 (H3K27me3), a repressive histone mark recognized by the PRC1 (30,31). In Arabidopsis, PcG proteins can repress target genes or indirectly promote gene expression repressing microRNA genes (32). Around 10-15% of all Arabidopsis genes are covered by H3K27me3 (32). MSI1 together with additional PRC2 components like EMBRYONIC FLOWER 2 (EMF2) or VERNALIZATION 2 (VRN2), represses the expression of flowering activators like FT and SOC1 (33–35) and also the floral repressor FLC after vernalization (36). Similarly to ESD7, MSI1 was also found to physically interact with TFL2/LHP1 facilitating the recruitment of PRC2 to target loci, which indicates that MSI1 is a PRC2 complex subunit that links PRC2 to PRC1 activity necessary for transcriptional gene silencing (29).
Epigenetic inheritance during DNA replication is crucial to maintain cellular identity following cell division (37). Despite the recent progress made in understanding the role of PRC1 and PRC2 complexes in the temporal regulation of gene repression involved in different developmental processes, how these complexes may interact with the DNA replication machinery to contribute to the mitotic inheritance of cellular identity in the daughter cells remains unknown. In this study, we take advantage of viable PRC2 and Pol mutations in Arabidopsis to address the mechanism underlying the maintenance of the epigenetic states during replication. Sound evidence presented here supports a role for the catalytic subunit of Pol ϵ in preserving high H3K27me3 levels at floral target loci. Using flowering time control as a subject, we examine the function of the Pol ϵ in epigenetic transcriptional silencing and demonstrate the genetic and molecular interaction of ESD7 with PRC2 components such as CURLY LEAF (CLF), the catalytic subunit of the complex, EMF2 and MSI1. Moreover, we show that the constitutive overexpression of a C-terminal fragment of Pol ϵ subunit, (POLA5) (9), causes pleiotropic alterations in vegetative and reproductive traits, including early flowering, small size, curly leaves, loss of apical dominance, floral organs alterations and reduced fertility. In addition, these POLA5 overexpressor plants showed altered expression of key floral integrator genes. These phenotypic and molecular alterations are also conspicuous in hypomorphic esd7 alleles (11) and some mutant alleles deficient in PcG components (38), suggesting that the overexpression of POLA5 might interfere with the activity of PcG complexes in vivo. Finally, we present evidence indicating that ESD7 binds the chromatin of at least a subset of its target loci in Arabidopsis and is necessary for the proper binding of PRC2 complexes to them. Altogether, these data suggest the existence of interplay between the DNA replication machinery and the PcG complexes in the control of different plant developmental processes, including flowering time, through a mechanism involving epigenetic transcriptional gene silencing and might help to explain how these complexes preserve chromatin modification states during DNA replication.
MATERIALS AND METHODS
Genetic stocks and growth conditions
Mutant seed stocks used were in the Ler and Col genetic backgrounds. The esd7-1 (11) or the icu2-1 (13) alleles in Col and Ler backgrounds respectively, were obtained after three backcrosses to WT genotypes. The monogenic tfl2-1 (26), icu2-1 (13) emf2-2 (39) and clf-16/pif-2 (40) mutants were obtained from personal donations. The pMSI1::GFP-MSI1 msi1-1 line was described in (41); the pMSI1::MSI1-TAP msi1-1 mutant by (42) and the 35S::GFP-CLF transgenic plants in (43).
Controlled environmental conditions were provided by walk-in growth chambers at 22°C and 65% relative humidity. For the in vitro experiments seedlings were cultured on agar-solidified MS medium. Plants were illuminated with cool-white fluorescent lights (120 μmoles/m2/s); LD conditions consisted of 16 h light/8 h dark and; SD conditions were 8 h light/16 h dark.
Phenotypic and genetic analyses
Flowering time was scored as the total leaf number, including rosette and cauline leaves, just before the first flower opened. Double mutants were generated by crossing the monogenic esd7-1 mutant with lines carrying the mutations tfl2-1, emf2-2 and clf-16, and were isolated from F2 progeny showing esd7 phenotype and segregating the second mutation. The pMSI1::MSI1-TAP msi1-1 mutant was crossed with esd7-1 in Col background. The msi1-1 esd7-1 double mutant was selected on MS-glucose hygromycin-containing media plates. The 35S::GFP-CLF transgenic plants were crossed with clf-16 esd7-1 and double mutants expressing GFP-tagged CLF were selected on MS-glucose kanamycin-containing media plates.
Plant transformation
To generate gene constructs with the C-terminal half of the catalytic subunit of DNA Pol ϵ, POLA5 (9), tagged with either GFP or HA epitopes, the corresponding cDNA fragments were cloned into pGWB6 or pEarleyGate201, respectively. The resulting clones were transformed in Col plants by Agrobacterium tumefaciens-mediated transformation using the floral-dip method (44). The Agrobacterium strain used was C58C1. The EMF2 cDNA was introduced in emf2-2/EMF2 heterozygous plants by floral-dip method and thus derived emf2-2 homozygous plants that expressed GFP-tagged EMF2 were selected on GM-glucose hygromycin-containing media plates. At least 10 independent transformants were evaluated for each construct. POLA5-HA lines were crossed with both 35S::GFP-CLFclf-50 and 35S::GFP-EMF2 emf2-2 plants.
Expression analysis
RNA samples were obtained from 9-day-old Col or 7 day-old Ler seedlings grown under LD conditions on MS media plates using E.Z.N.A.® RNA purification system (OMEGA BIOTEK). Total RNA samples were processed with TURBO™ DNase (Ambion, Life Technologies) treatment to remove traces of DNA. Q-PCR analyses were performed using LightCycler 480 SYBR Green I Master mix real-time polymerase chain reaction (PCR) system (Roche Applied Science) in LightCycler® 480 System device (Roche). Data acquisition and analyses were performed using the Roche Light Cycler software. Samples were normalized using the expression of UBIQUITIN 10 (UBQ10) and UBIQUITIN-CONJUGATING ENZYME (UBC) 21 housekeeping genes. Oligonucleotide sequences used in this work can be found in S1 Appendix.
Yeast two-hybrid analysis
Yeast two-hybrid interaction analyses were performed in the PJ694α yeast strain with the MatchMaker two-hybrid system using the vectors pGBT9 and pGADT7 (Clontech) that express protein fusions to the GAL4 BD or AD domains, respectively. The pGADT7 constructs containing either POLA3 or POLA5 fragments were described by (9); and the pGBT9-CLF and pGBT9 EMF2 were previously described by (38). Selection was performed on synthetic complete (SC) minimal medium without Leu, Trp, His and Ade supplemented with different concentrations of 3-amino-1,2,4-triazole (3-AT).
Protein expression, purification and pull-down assays
POLA5 and POLA3 were pGADT7-derived (9), which allows the generation of fusion proteins from a T7 promoter. In vitro-translated 35S-Met-labeled GAD were produced with the TNT Quick Coupled Transcription/Translation system (Promega) in the presence of 35S-Met. The N-terminal CLF fragment including the C5 domain (amino acids 1-331), and the 4AD and 5AD EMF2 fragments containing the VEFS domain were described by (38). POLA5 cDNA fragment was cloned into the pGEX Gateway system. CLF, EMF2-VEFS and POLA5 constructs were introduced into the BL21 Escherichia coli strain to express the recombinant proteins under standard conditions. The amount of recombinant protein bound to beads was quantified by resolving the proteins in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The recombinant CLF and POLA5 fused to GST proteins were bound to glutathione sepharose beads (Upstate) and washed extensively with PBS-0.1% Tween 20, whereas the HIS6-EMF2-VEFS proteins were purified using a Ni-NTA His-Bind resin (QUIAGEN) followed by imidazole elution. For pull-down assays, 1 μg of GST or recombinant protein bound to beads was incubated in 200 μl of binding buffer [20 mM 2-amino-2-(hydroxymethyl)-1,3-propanediol (TRIS)-HCl, pH 7.0, 100 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 10% glycerol, 0.01% Nonidet P-40] with 25 μl of the TNT reactions or 1 μg HIS6-EMF2-VEFS and rinsed with binding buffer supplemented with 500 mM NaCl. Samples were boiled with Laemmli buffer and resolved on an SDS-PAGE/8% Bis-acrylamide gel. Labelled ESD7 35S-products were detected by autoradiography and the EMF2 fragments were revealed by Western blots with αHIS-HRP antibody (SIGMA).
Protein nuclei extraction, histone western blotting and co-immunoprecipitation assays
Crude nuclei extracts were produced by mixing 10 g of ground fresh plant material in 100 ml of nuclei ground buffer (10 mM PIPES-KOH pH 7, 1 M hexylene glycol, 10 mM MgCl2, 0.2% Triton-X100, 5 mM β-mercaptoethanol and 0.8 mM PMSF) at 4°C. The homogenate was filtered successively through Miracloth (Calbiochem), 100 and 50 µm filters. This extract was centrifuged at 2000 g for 10 min at 4°C and the resulting pellet was washed tree times (at 3000 g for each wash for 5 min) with nuclei washed buffer (10 mM PIPES-KOH pH 7, 0.5 M hexylene glycol, 10 mM MgCl2, 0.2% Triton-X100, 5 mM β-mercaptoethanol and 0.8 mM PMSF) at 4°C. For histone western blotting assays the isolated nuclei were dissolved in lysis buffer (10 mM HEPES pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM Dithiothreitol (DTT)) with EDTA-free protease inhibitor cocktail (Roche). Identical amount of proteins in Laemmli buffer from each of the nuclei protein extract samples were resolved on an SDS-PAGE/8% bis-acrylamide gel. We used αH3K9me2 (Millipore CS200550), αH3K27me3 (Millipore 07-449) and αH3 (Abcam 1791) antibodies to detect histones. For co-IP assays the isolated nuclei were dissolved by soft sonication in extraction buffer (50 mM Tris/HCl pH 7, 150 mM NaCl, 10% Glycerol, 5 mM β-mercaptoethanol, 0.5 mM DTT, 1% triton, 0.5% CHAPS) with EDTA-free protease inhibitor cocktail (Roche). Identical amounts of proteins for each sample were incubated with antibodies in interaction buffer (20 mM Tris/HCl pH 7, 50 mM NaCl, 1.5 mM MgCl2, 10% Glycerol, 0.3% Triton, 0.15% CHAPS) for 2 hours at 4°C and then were incubated for 30 min with Protein G-coupled magnetic beads (Dynabeads, Life Technologies). We used αHA (high affinity 3F10 clone, Roche 11867423001), αGFP (Roche 11814460001) and αGFP-HRP conjugate (Miltenyi Biotec 120-002-105) antibodies. αESD7 serum was first incubated with Protein A-coupled magnetic beads (Dynabeads, Life Technologies) for 30 min and then it was incubated with identical amounts of protein for each sample for 1 h at 4°C. This experiment was performed adding 50 μM of Proteasome and Cathepsin K inhibitor (Peptide Institute, INC) and 2 μM Z-VAD-FMK caspase inhibitor (Apex Bio) to the lysis buffer.
Proteomic assays
Nuclei protein extracts from 9-day-old POLA5-HA and GFP-POLA5 transgenic seedlings were pulled-down respectively using αHA (high affinity 3F10 clone, Roche 11867423001) and αGFP (Roche 11814460001) antibodies coupled to Protein G-magnetic beads. Specific POLA5 interactors were identified comparing the results obtained using POLA5-tagged lines and control Arabidopsis plants without the tags. The pulled-down samples were reduced, alkylated, digested with trypsin and desalted on C18 reverse phase micro-columns. Analyses were performed on a nano‐HPLC system coupled with a hybrid ion trap-orbitrap mass spectrometer (Orbitrap Elite, Thermo Fisher Scientific). For LC analysis, each sample was loaded in Buffer A (0.1% formic acid) and on-line desalted on a 2 cm packed pre-column (Thermo Acclaim PepMap 100). Analytical separation was performed over a 50 cm column (Thermo Acclaim PepMap 100, 75 μm ID × 500 mm) at 200 nl/min with a 180 min gradient from 8 to 31% of Buffer B (0.1% formic acid/90% acetonitrile) using an EASY-nLC 1000 HPLC (Thermo Fisher Scientific).
For peptide identification, all spectra were analyzed with Proteome Discoverer (version 1.4.0.29, Thermo Fisher Scientific) using SEQUEST-HT (Thermo Fisher Scientific). For database searching at the Uniprot database containing all sequences from Arabidopsis thaliana and crap contaminants (28 February 2013; 31 930 sequences), parameters were selected as follows: trypsin digestion with two maximum missed cleavage sites, precursor and fragment mass tolerances of 2 Da and 0.02 Da, respectively, carbamidomethyl cysteine as fixed modification and methionine oxidation as dynamic modifications. Peptide identification was validated using the probability ratio method (45) with an additional filtering for precursor mass tolerance of 12 ppm. False discovery rate (FDR ≤ 0.05) was calculated using inverted databases and the refined method (46) was used to filter peptides for quantitation, as previously described (47).
Chromatin immunoprecipitation experiments
Chromatin immunoprecipitation (ChIP) was performed as described (36) with minor modifications. About 0.5 g of seedling tissue was incubated for 10 min under constant vacuum at room temperature in extraction buffer plus 1% formaldehyde to fix the chromatin, and then the tissue was ground in liquid nitrogen to obtain powder. We used αH3K27me3 (Millipore 17-662), αH3 (Abcam 1791) and αESD7 antibodies to perform the immunoprecipitation reactions. All ChIP experiments were quantified by Q-PCR analyses. The oligonucleotide sequences used for the amplification reactions can be found in S1 Appendix.
Preparation and purification of αESD7 antibody
Polyclonal antibodies were produced by Covalab (France), using ESD7-specific peptides coupled to a carrier protein. Amino-acids 3-17 and 2139-2153 corresponding to the extreme N- and C-terminal peptides of ESD7 were selected for immunization. Two rabbits were immunized for 88 days. Antibodies were next purified by affinity using the synthetic peptides coupled to sepharose beads.
RESULTS
ESD7 genetically interacts with the PRC2 genes CLF and EMF2 to control flowering time in Arabidopsis
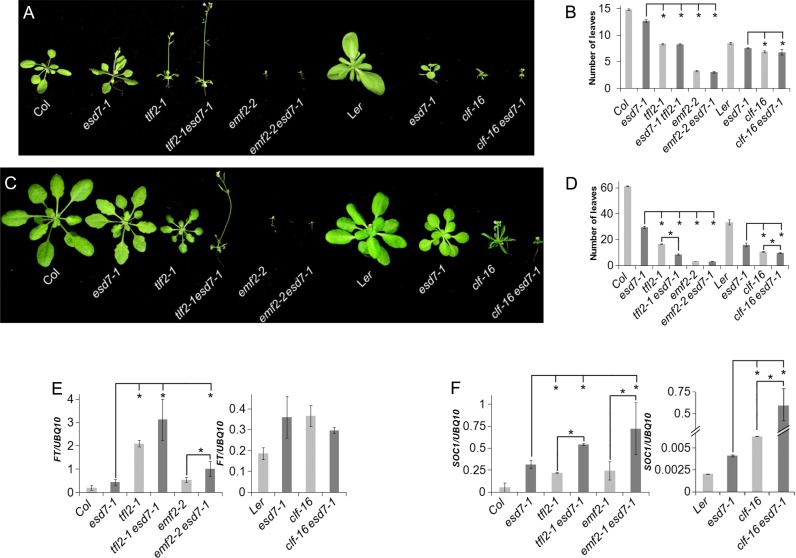
Loss of function of AtPol2a causes embryonic lethality (9) and severe alleles display sterility (8). However, the isolation of the esd7-1 mutant, a hypomorphic allele of this locus (11), has allowed us to investigate the unanticipated role of this gene in the regulation of different developmental processes and transcriptional control. As previously described, the esd7-1 mutation causes an acceleration of flowering time under long day (LD) and short day (SD) conditions (Figure 1A). Furthermore, the phenotypic alterations displayed by mutant alleles of ESD7 resemble those shown by severe alleles affected in the catalytic subunit of PRC2, CLF and also weak mutant alleles of EMF2, another PRC2 component (38). This similarity is consistent with the notion that these PcG genes might act together with ESD7 in a common genetic pathway. In fact, TFL2 and ESD7 interact genetically to control flowering time ((11), Figure 1). To assess the possible existence of genetic interactions between ESD7 and genes encoding components of PRC2, we combined the clf-16 (40) and emf2-2 (39) mutations with esd7-1. These double mutants displayed more extreme phenotypic alterations than any of the parental single mutants, generating extremely tiny plants (Figure 1A–D). In contrast, the clf-16 esd7-1 and emf2-2 esd7-1 double mutants flowered at the same time as the corresponding PRC2 parental mutant (Figure 1B and D). This is similar to previous observations with esd7 tfl2 double mutants (11) and suggests that in the absence of PRC2 function, esd7 mutations cannot further accelerate flowering consistently with CLF and EMF2 acting in a common genetic pathway with ESD7 to regulate flowering time.
Figure 1.
ESD7 genetically interacts with the PRC1 component TFL2 and the PRC2 components CLF and EMF2. (A and C) Flowering phenotype of Col, esd7-1 introgressed in the Col background, tf2-1, esd7-1 tf2-1, emf2-2, emf2-2 esd7-1, Ler, esd7-1, clf-16 and clf-16 esd7-1 mutant plants grown under LD (A) or SD conditions (C). (B and D) Flowering time of these plants under LD (B) and SD conditions (D). (E and F) Analysis of the expression of FT and SOC1 floral integrator genes in WT and different mutants under LD. Total RNA was extracted from pool samples of 9-day-old (WT and mutants in Col background) and 6-day old (WT and mutants in Ler background) seedlings grown under LDs collected 4 h after dawn. All the differences were statistically significant (P > 0.05) according to Student's t-tests in the comparison of mutants with WT. Asterisks indicate statistically significant differences (P < 0.05) between mutants.
The early flowering phenotype of the esd7-1 mutant requires functional FT and SOC1 genes (11). To further investigate a possible overlapping function of ESD7 with PcG genes in the control of flowering time, we analyzed the expression of these floral integrator genes under LD photoperiods in the double mutants generated. As previously described for tfl2-1 esd7-1 plants (11), the expression levels of FT observed in clf-16 esd7-1 and emf2-2 esd7-1 double mutants were comparable to those in any of the single parental mutants (Figure 1E and F). These expression data are consistent with the flowering time displayed by these double mutants indicating that esd7 mutations do not cause increased expression of the floral integrator FT in plants deficient in PRC2 function. Similar results were obtained when the expression of SOC1 was analyzed in the double mutant emf2-2 esd7-1. However, the double mutant clf-16 esd7-1 displayed higher levels of expression of this master gene of flowering as compared with the single mutant lines, although this increase was not mirrored in an additional acceleration of flowering time (Figure 1F).
The DNA Pol ϵ is required for high H3K27me3 levels on the chromatin of FT and SOC1
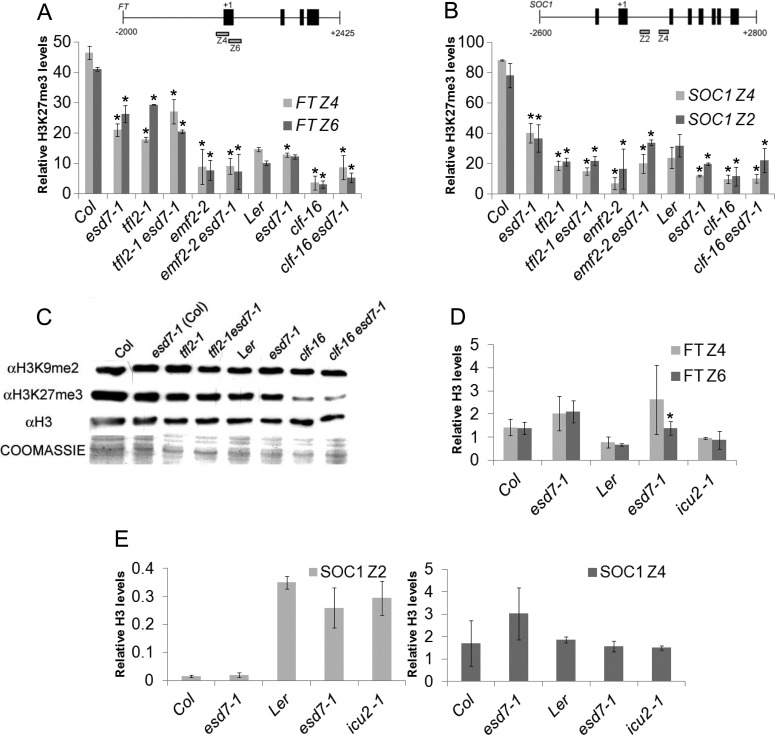
The genetic interactions described above between ESD7 and the PcG genes CLF and EMF2 in the control of flowering time and the expression of the master genes of flowering FT and SOC1 suggest a common role for ESD7 and PRC2 components in the epigenetic repression of these floral integrator genes during vegetative growth. In order to analyze whether ESD7 might also be required to modulate H3K27me3 levels in these loci, we performed ChIP analysis in different genetic backgrounds. Chromatin from LD-grown wild-type (WT), esd7, tfl2, emf2 and clf seedlings and from double mutant combinations with esd7 mutant was immunoprecipitated with αH3K27me3 antibodies. Real time quantitative PCR approaches (Q-PCR) were used to assess the levels of H3K27me3 in genomic fragments spanning discrete regions of FT and SOC1 loci (Figure 2A and B), known to be enriched in this histone mark (48,49). As expected, our results show that the levels of H3K27m3 in the promoter and first exon regions of FT locus (Z4 and Z6 respectively in our analysis), are lower in PRC2 mutants affected in the deposition or the maintenance of this repressive mark (Figure 2A). Interestingly, both regions of the FT chromatin were depleted in this mark in the esd7-1 mutant as compared to WT seedlings (Figure 2A), suggesting that the DNA Pol ϵ is necessary for high levels of H3K27me3 in this master gene of flowering. On the other hand, FT H3K27me3 levels were similar in emf2-2 esd7-1 and clf-16 esd7-1 double mutants when compared with the corresponding Polycomb single mutants (no statistically significant differences (P < 0.05) according to Student's t-tests) (Figure 2A). This observation suggests that ESD7 does not cause any additional loss of this repressive mark in the absence of PRC2 function. Although TFL2 is not part of the canonical PRC2, loss of function of this H3K27me3 binding protein also causes a decrease in the level of this histone modification in the chromatin of FT, suggesting that TFL2 may also be required for either the deposition and/or the maintenance of this repressive mark in this locus, similarly to ESD7 (Figure 2A). As observed in double mutants combining esd7-1 with PRC2 mutants, comparable levels of H3K27me3 were found in tlf2-1 and tfl2-1 esd7-1 mutants (Figure 2A). These observations are in agreement with the flowering time and FT expression data shown in Figure 1.
Figure 2.
ESD7 is required to maintain high levels of the repressive mark H3K27me3 in discrete regions of the FT and SOC1 chromatin. (A and B) Levels of H3K27me3 on FT and SOC1 chromatin were examined after ChIP assays. Amplified regions are depicted below each loci; black boxes represent exons and black lines intron regions. Chromatin was extracted from pool samples of 9-day-old (WT and mutants in Col background) and 6-day old (WT and mutants in Ler background) seedlings grown under LD collected 4 h after dawn. ChIP was performed with αH3K27me3 antibodies. DNA fragments after chromatin immunoprecipitation (ChIP) were quantified by Q-PCR and subsequently normalized to ACT2 as internal control. Values shown are relative to input and are mean ± SD (n = 3). Asterisks indicate statistically significant differences (P < 0.05) according to Student's t-tests comparing mutants with WT. All comparisons were no significantly different except for FT Z6 region in esd7-1 in Ler background. (C) Western blotting from nuclei extracts showing that esd7 mutation does not alter the global levels of either H3K9me2 or H3K27me3 histone marks. (D and E) Levels of histone H3 present on FT and SOC1 chromatin in DNA pol mutants. Chromatin from WT, esd7-1 and icu2-1 mutant seedlings was immunoprecipitated against αH3 antibodies and the same regions of FT and SOC1 chromatin analyzed in panels 2A and B were amplified; the values were subsequently normalized to ACT2 as internal control. Levels shown are relative to input and are mean ± SD (n = 3). Asterisks indicate statistically significant differences (P < 0.05) according to Student's t-tests comparing mutants with WT.
ChIP assays were extended to explore whether esd7 mutation affects the levels of H3K27me3 in the discrete regions analyzed of SOC1 chromatin. The levels of this histone mark on Z2 and Z4 regions were consistently lower in the chromatin immunoprecipitated from esd7 mutant than in Col or Ler WT plants (Figure 2B), indicating that ESD7 is also required to maintain proper levels of this repressive mark in these regions of the SOC1 chromatin.
ESD7 is also necessary to establish correct levels of FLC and AG expression and it is known that histone H3 post-translational modifications such as H3 acetylation, H3K4me3 or H3K27me3 along the body of these genes are altered in the esd7 background (10,11). In order to assess whether the esd7 mutation affects the global deposition of H3K27me3, as it occurs with some PcG mutants, we isolated histones from nuclei of WT and tfl2-1, tfl2-1 esd7-1, clf-16 and clf-16 esd7-1 mutant seedlings. Western blot studies showed that the global levels of H3K9me2 and H3K27me3 are not disrupted by the esd7 mutation (Figure 2C), whereas clf mutations clearly decreased the levels of H3K27me3 but not those of H3K9me2. These data suggest that reduced function of Pol ϵ is affecting the levels of H3K27me3 present in some particular developmental genes like the floral integrator genes, but not globally. Furthermore, the increased FT and SOC1 expression in esd7 mutants compared with WT seedlings is consistent with the reduced levels of the repressive H3K27me3 mark observed in the chromatin regions analyzed.
In parallel, we performed ChIP analyses using αH3 antibodies to determine if the levels of histone H3 may be altered by esd7 and icu2 mutations in the previously analyzed regions of the chromatin of FT and SOC1 loci. As shown in Figure 2 D and E, there were no significant alterations in the levels of H3 on these chromatin regions in the DNA Pol mutants examined. These findings suggest that the loss of function of ESD7 and ICU2 reduce H3K27me3 levels on the target genes analyzed without substantially affecting H3 deposition (Supplementary Figure S1), revealing functional roles for these proteins in the maintenance of high levels of this repressive mark.
Plants overexpressing the C-terminal region of Arabidopsis DNA Pol ϵ catalytic subunit display pleiotropic phenotypic alterations resembling some features of esd7 and PcG deficient mutants
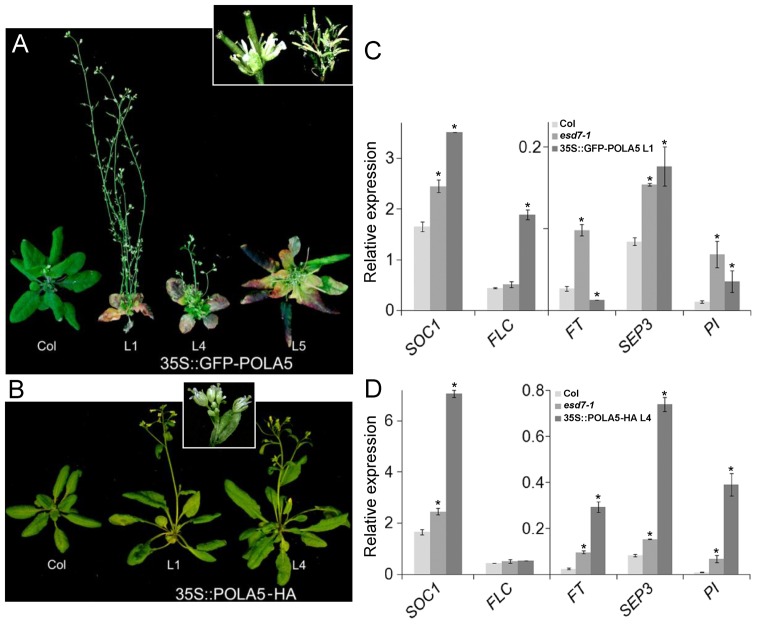
TFL2 can interact with a C-terminal fragment of ESD7 protein (11) and also with ICU2 (13). ESD7, ICU2 and other catalytic subunits of DNA Pols as well as chromatin assembly factors have a MOD1-INTERACTING REGION (MIR) domain (20) that is essential for the binding to TFL2 homologs in eukaryotes (50). Intriguingly, the ICU2 C-terminal domain containing the putative MIR region might also be important for the epigenetic function carried out by the DNA Pol α since plants overexpressing this domain resemble the PcG mutant plants (4). To assess whether the ESD7 MIR-containing domain plays a similar role, we generated different 35S::GFP-POLA5 and 35S::POLA5-HA Arabidopsis transgenic plants. As expected, the GFP fluorescence was detected in root cell nuclei of Arabidopsis 35S::GFP-POLA5 plants (Supplementary Figure S2C), and also in tobacco leaves expressing transiently the same construct (Supplementary Figure S2A), indicating that the fusion GFP-POLA5 protein was imported as expected into the cell nucleus, similarly to EMF2 PRC2 component (Supplementary Figure S2B,D). When analyzed phenotypically the 35S::GFP-POLA5 lines showed several pleiotropic alterations in vegetative and reproductive traits including early flowering phenotype, smaller size than WT plants, curly leaves, loss of apical dominance, altered floral organs and infertility (Figure 3A). The 35S::POLA5-HA transgenic lines also displayed pleiotropic phenotypic alterations but milder than those observed in 35S::GFP-POLA5, including early flowering phenotype and conspicuous homeotic alterations in around 20% of the flowers (Figure 3A and B). The acceleration of flowering and the altered floral organs observed in the 35S::GFP-POLA5 and 35S::POLA5-HA transgenic lines resemble the phenotypic alterations displayed by hypomorphic esd7 alleles (11) and by some mutants deficient in PcG components (51,52). For that reason, we decided to analyze in these transgenic lines the expression of several key genes involved in the control of flowering initiation and floral development. Representative transgenic lines showed an altered expression of key flowering time genes like FLC, FT andSOC1, and of floral homeotic genes such as SEP3 andPISTILLATA (PI), most of which are also misregulated in esd7 mutants (Figure 3C and D). The expression of these genes is also regulated by PcG complexes (34,53,54), suggesting that the overexpression of POLA5 might interfere with Polycomb gene repression activity in vivo.
Figure 3.
Plants overexpressing POLA5 display pleiotropic phenotypic alterations. (A) 35S::GFP-POLA5 transgenic lines showing strong phenotypic alterations such as early flowering with small curly leaves (L1, L4) and loss of apical dominance (L4, L5). On top, close-up showing the phenotype of flowers from 35S::GFP-POLA5 with two pistils (left) and mature inflorescences carrying these flowers (right). (B) 35S::POLA5-HA transgenic lines displaying early flowering phenotype. The close-up shows that the inflorescences of these plants display a number of flowers with homeotic alterations. (C and D) Expression analysis of FT, SOC1, SEP3, PI and FLC genes relative to UBCin WT, esd7-1 (Col background) and transgenic lines L1 (C) and L4 (D) grown under LD conditions. Total RNA was extracted from pools of 9-day-old seedlings collected 4 h. Data shown are mean ± SD (n = 3). Asterisks indicate statistically significant differences (P < 0.05) according to Student's t-tests comparing expression levels of these genes in mutants or transgenic plants with the corresponding WT.
Arabidopsis DNA Pol ϵ catalytic subunit physically interacts with PRC2 components
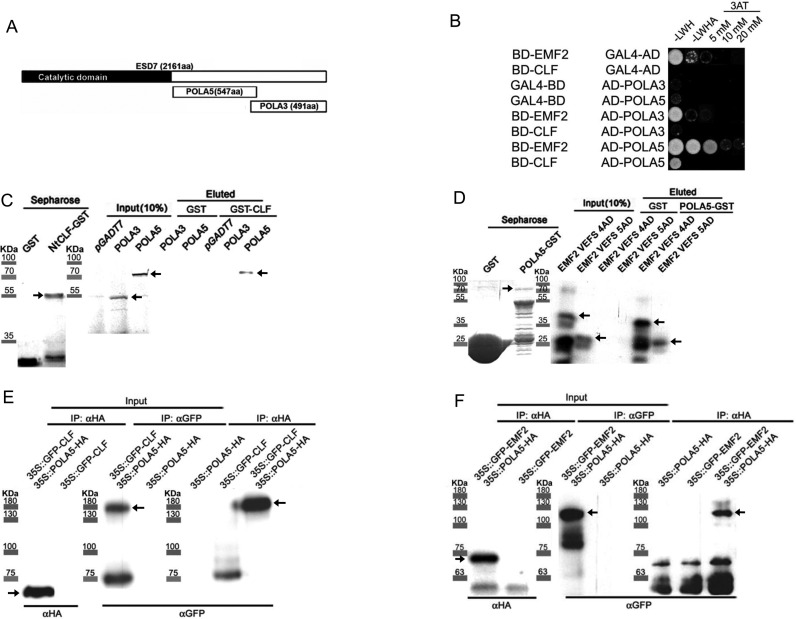
TFL2/LHP1 collaborates in the recruitment of PRC2 to restore H3K27me3 levels in target loci (29). Given the defects in the maintenance of high H3K27me3 levels observed in esd7 mutants (Figure 2A,B) and that TFL2 binds ESD7, it is tempting to propose that ESD7 may also interact with PRC2 components and thus participate in the re-establishment of full H3K27me3 levels after replication at particular loci. To assess whether two different C-terminal fragments of the ESD7 protein named as POLA5 and POLA3 respectively (29) (Figure 4A) might interact with PRC2 components, we carried out yeast two-hybrid analyses involving these ESD7 fragments and PRC2 proteins such as CLF and EMF2. For this, we expressed the full-length EMF2 and CLF proteins fused to the GAL4 DNA-binding domain (BD) and analyzed their possible interaction with POLA5 and POLA3 fragments fused to the GAL4 transcriptional activation domain (AD). As shown in Figure 4B, these assays revealed a physical interaction between the full-length PRC2 proteins and the POLA5 fragment of ESD7 but not with POLA3. The interaction between EMF2 and POLA5 appears to be stronger than that observed with CLF (Figure 4B). We confirmed this interaction with in vitro pull down assays involving a truncated CLF protein containing the SET domain fused to GST and the radiolabeled POLA5 ESD7 fragment. The expressed fragment of CLF contains both the central C5 domain known to interact with EMF2, FERTILIZATION INDEPENDENT SEED 2 (FIS2), MSI1 and VRN2 PcG proteins (38) and also the N-terminal domain that binds the F-box protein UPWARD CURLY LEAF1 (UCL1) (55). This CLF-GST protein fusion, but not GST alone, was able to specifically interact with POLA5 fragment (Figure 4C). On the same manner, we also observed by pull-down assays a physical interaction between POLA5 with C-terminal fragments of EMF2, named as VEFS4 and VEFS5, and containing the VEFS domain, a conserved motif that binds CLF (38) (Figure 4D). These observations support a physical interaction between the POLA5 fragment of ESD7 with a CLF protein fragment harboring the SET domain, and also with two different EMF2 protein fragments spanning the VEFS region.
Figure 4.
Interaction of ESD7 with PRC2 components. (A) Diagrams showing ESD7 protein and the fragments used in these analyses, POLA5 and POLA3. The N-terminal catalytic domain is indicated by the black box and the C-terminal domain (white box) includes the POLA5 fragment carrying a MIR region and the POLA3 fragment carrying a zinc finger domain. (B) Yeast two-hybrid experiment showing interaction of ESD7 fragments with EMF2 and CLF. One out of three independent co-transformants is shown in the picture growing onto -LWH, -LWHA and -LWHA media containing different 3AT concentrations. (C) In vitro binding assay of a fragment of CLF containing the SET domain fused to GST with POLA3 and POLA5 ESD7 radiolabelled fragments. Protein molecular weights are indicated. Arrows indicate the expected size for the corresponding fusion proteins. (D) In vitro binding assays of the fusion proteins GST-POLA5 and His6-EMF2-VEFS containing different fragments of the VEFS domain (4AD and 5AD). The EMF2-derived proteins were revealed by western blot with an αHis antibody. Protein molecular weights are indicated. The lower bands in the input lanes correspond to His6-EMF2 VEFS degradation products. Arrows indicate the expected size for the corresponding fusion proteins. (E) In vivo co-IP of POLA5 and CLF proteins. Nuclei proteins were extracted from plants overexpressing functional GFP-CLF protein in clf-16 mutant background, POLA5-HA (L4) and from 35S::GFP-CLF 35S::POLA5-HA lines in clf-16 mutant background. An αHA antibody was used to pull-down the protein complexes and CLF protein was detected using an αGFP antibody. Protein molecular weights are indicated. Arrows indicate the expected size for the corresponding fusion proteins. (F) In vivo co-IP of POLA5 and EMF2 proteins. Nuclei proteins were extracted from plants overexpressing functional GFP-EMF2 protein in emf2-2 mutant background, POLA5-HA and from 35S::GFP-EMF2 35S::POLA5-HA lines in emf2-2 mutant background. An αHA antibody was used to pull-down the protein complexes and EMF2 protein was detected using an αGFP antibody. Protein molecular weights are indicated. Arrows indicate the expected size for the corresponding fusion proteins.
To further confirm in vivo the observed interactions, we performed genetic crosses between POLA5-HA overexpressor lines and plants overexpressing functional tagged CLF or EMF2 proteins in clf-16 or emf2-2 mutant background, respectively. These double transgenic combinations were the basis to perform co-IP experiments, using nuclei protein extracts from the single and double transgenic plants. When we used the αHA antibody for co-IP experiments, we were able to co-immunoprecipitate the GFP-CLF protein from the 35S::GFP-CLF 35S::POLA5-HA plants but not from 35S::GFP-CLF lines (Figure 4E). Similar co-IP assays involving nuclei extracts from 35S::GFP-EMF2 35S::POLA5-HA plants revealed that GFP-EMF2 protein was co-immunoprecipitated with POLA5-HA (Figure 4F). These results reveal that both CLF and EMF2 proteins showed an in vivo nuclear interaction with the POLA5 fragment of ESD7 in Arabidopsis transgenic plants, corroborating the previously observed interactions in vitro, and are consistent with the existence of interplay between DNA pol ϵ and the repressive PcG complexes in the transcriptional silencing of gene expression.
Proteomic approaches reveal MSI1 and other chromatin remodelers as novel interactors of Arabidopsis catalytic subunit of DNA Pol ϵ
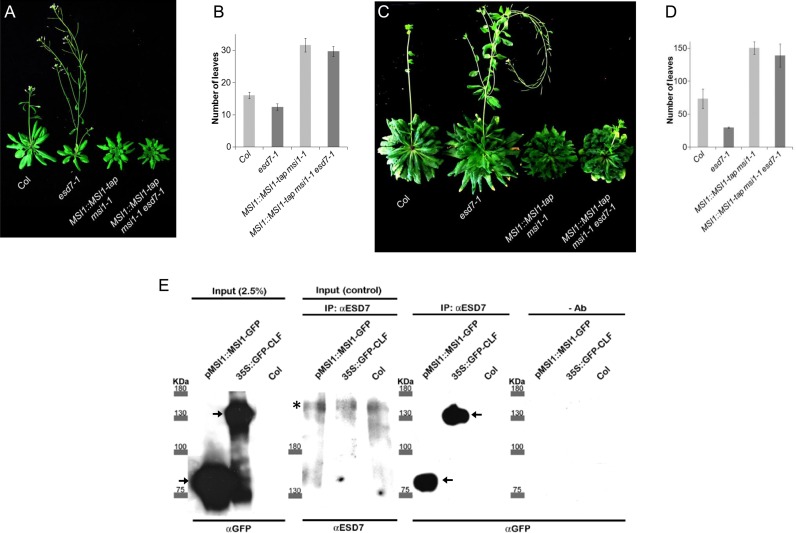
The pleiotropic phenotypic alterations displayed by the POLA5 overexpressor plants (Figure 3A and B) resemble some of those observed in various mutant alleles of PcG (52), suggesting an interference of the truncated ESD7 protein in the recruitment of PRC components to target loci in these transgenic lines. In consequence, we decided to carry out proteomic analysis with these POLA5 transgenic plants to determine the possible existence of PcG proteins as interactors of ESD7 in vivo. Interestingly, in the different replica experiments we found clear evidence of the specific interaction of PRC2 and CAF-1 complexes component MSI1, among other proteins (Table 1 and Supplementary Table S1). This interaction supports a crosstalk between DNA replication and chromatin assembly. In fact, the epistatic relationship found between FAS2 and ESD7 indicates that the CAF-1 chromatin assembly complex acts downstream of ESD7, facilitating the formation of nucleosomes on newly replicated DNA (11). Similarly to fas2 mutations, plants partially complemented with MSI1 fusion proteins (msi1-1 pMSI1::MSI1-TAP) show a late flowering phenotype (35,42) whereas homozygous msi1 null mutants are lethal (56). The partial loss of function displayed by msi1-1 pMSI1::MSI1-TAP plants is enough to suppress the early flowering phenotype of esd7-1 both in LD and SD conditions, suggesting that MSI1 is required for the premature flowering displayed by esd7 mutants (Figure 5 A-D). To assess a possible interaction of MSI1 with the ESD7 full length protein we raised an antibody against ESD7. When it was used to immunoprecipitate nuclear protein extracts, this polyclonal antibody showed specific immune-reactivity against a 250 kDa band that corresponds to the expected size for the Arabidopsis catalytic subunit of DNA pol ϵ protein (Figure 5E, second panel). Next, we were able to co-immunoprecipitate in vivo both the recombinant GFP-CLF protein, and also the MSI1-GFP fusion with the αESD7 antibody (Figure 5E, third and fourth panels, respectively). These data confirm that both CLF and MSI1, central components of Arabidopsis PRC2 complex, interact with the catalytic subunit of DNA pol ϵ, supporting the previously revealed interactions with the POLA5 fragment (Figure 4).
Table 1. Identified POLA5-interacting proteins related to chromatin remodeling and gene expression regulation processes.
| POLA5-HA | GFP-POLA5 | |||||
|---|---|---|---|---|---|---|
| AGI locus | Protein | Description | R1 | R2 | R3 | R4 |
| At1G09770 | ATCDC5 | Cell division Cycle 5 protein | 8 | 2 | 10 | 19 |
| At5G03740 | HD2C | Histone deacetylase 2C protein | 7 | 2 | 10 | 10 |
| At5G15020 | SLN2 | SIN3-like 2 protein | 7 | 2 | 10 | 10 |
| At5G58230 | MSI1 | Multicopy suppressor of Ira 1 protein | 1 | 2 | 7 | 18 |
| At4G29830 | VIP3 | Transducin/WD40 repeat-like superfamily protein | 1 | 8 | 6 | 15 |
| At2G46020 | CHR2/BRM | SWI/SNF chromatin remodeling complex BRAHMA protein | 1 | 5 | 6 | 10 |
| At1G80670 | RAE1 | Transducin/WD40 repeat-like superfamily protein | 1 | 1 | 4 | 5 |
| At3G18165 | MOS4 | Modifier of snc1,4 (MOS4) protein | 2 | 12 | 2 | 4 |
| At1G79730 | ELF7 | Hydroxyproline-rich glycoprotein family protein/ EARLY FLOWERING 7 | 1 | 7 | 1 | 2 |
| At4G15900 | PRL1 | Pleiotropic regulatory locus 1 protein | 3 | 1 | 1 | 1 |
| At4G38130 | HDA19 | Histone deacetylase 19 | 2 | 8 | 3 | 2 |
| At4G26630 | DEK3 | DEK domain-containing chromatin associated protein | 2 | 6 | 1 | 1 |
| At2G45640 | SAP18 | SIN3 associated polypeptide P18 | 1 | 4 | 1 | 2 |
| At2G37190 | RPL12A | Ribosomal protein L11 family protein | 2 | 1 | 1 | 1 |
A selection of proteins identified as interactors of POLA5 C-terminal fragment of the catalytic subunit of DNA Pol ϵ related to chromatin remodeling and gene expression regulation processes. These chromatin remodeling proteins, transcriptional and translational regulators were pull-down by POLA5 and detected by mass spectrometry analyses in four independent experiments. No peptides from these proteins were identified in the corresponding negative control of the pull-down assays with the exception of one peptide in the sample R1 for HDA19.
Figure 5.
The catalytic subunit of DNA Pol ϵ interacts physical and genetically with MSI1. (A and C) Flowering phenotype of Col, esd7-1 introgressed in Col background, MSI1::MSI1-tap msi1-1 and MSI1::MSI1-tap msi1-1 esd7-1 plants grown under LD (A) or under SD photoperiodic conditions (C). (B and D) Flowering time of these plants under LD (B) and SD conditions (D). The differences were not statistically significant (P < 0.05) according to Student's t-tests when comparing MSI1::MSI1-tap msi1-1 and MSI1::MSI1-tap msi1-1 esd7-1 plants. (E) Coimmunoprecipitation of MSI1 and CLF fusion proteins with ESD7. Nuclei proteins were extracted from plants overexpressing the functional GFP-CLF fusion protein in clf-16 mutant background and from pMSI1::GFP-MSI1 in msi1-1 mutant background. Protein molecular weights are indicated. Arrows indicate the expected size for the corresponding fusion proteins. An αESD7 antibody was used to pull-down the protein complexes; asterisk corresponds to the expected mobility size for native ESD7; CLF and MSI1 protein were detected using an αGFP antibody.
During replication, DNA Pols associate with a large number of auxiliary factors that assist these enzymes to remodel chromatin structure and to move along DNA. Accordingly, and in addition to MSI1, POLA5 recruits a large array of gene expression regulators (Table 1, Supplementary Table S1), including components of PAF1 complex, that plays critical roles in RNA polymerase II transcription elongation and in the regulation of histone modifications, such as VERNALIZATION INDEPENDENCE 3 (VIP3) (57). Several nuclear pore proteins and components of the MOS4-CDC5-associated complex (MAC), involved in pre-mRNA splicing and spliceosome assembly (58) were also identified as POLA5 interactors. We also detected peptides from BRAHMA/CHROMATIN REMODELING 2 (BRM/CHR2) (59) and proteins associated to this complex, as well as Histone deacetylase 19 (HDA19) (60),HDA complex interactors such as DEK-DOMAIN CONTAINING PROTEIN 3 (DEK3) (61) and SIN3 complex components including SIN3-ASSOCIATED POLYPEPTIDE 18 (62), SIN3-like 2 protein (63) and HD2C (64). These proteomic results reinforce the existence of a physical link between DNA Pol ϵ and PRC2 complexes in the control of epigenetic states and unveil that ESD7 may participate in the regulation of gene expression recruiting several chromatin remodelers.
ESD7 binds the chromatin of FT and SOC1 loci and is necessary for the proper recruitment of PRC2 complexes to these target genes
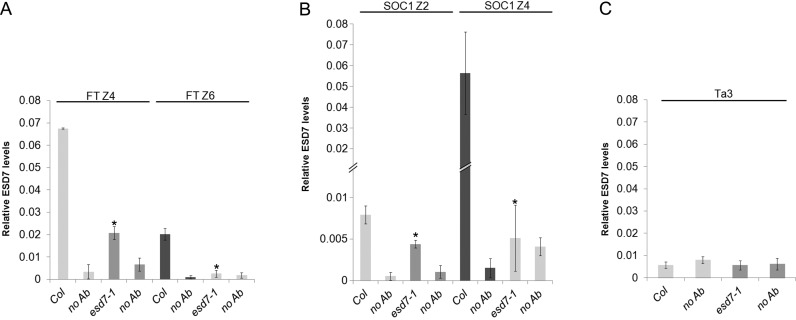
PRC2 binds directly the chromatin of key floral integrator genes (34). Given the revealed interaction of PRC2 components with the DNA Pol ϵ catalytic subunit, and the altered levels of H3K27me3 observed in FT and SOC1 genes in the esd7-1 mutant, we wondered whether ESD7 could interact directly with these floral integrator genes. To address this point, chromatin from LD-grown WT and esd7 seedlings was immunoprecipitated with an αESD7 antibody, and Q-PCR analyses were performed to assess the possible binding of ESD7 to distinct regions enriched in H3K27me3 of the FT (Z4 and Z6) and SOC1 (Z2 and Z4) loci. As shown in Figure 6 A and B, ESD7 protein was able to interact with these chromatin regions and the esd7-1 mutation clearly decreased the binding of Pol ϵ catalytic subunit to their floral target loci. No binding of ESD7 to the Ta3 transposable element chromatin was detected (Figure 6C) (65). Thus, ESD7 might contribute to the proper function of the repressive PRC2 complex in the deposition of H3K27me3 in the target genes (66) through anchoring to the chromatin of FT and SOC1 loci (Figure 6), and also by interacting with PRC2 proteins (Figures 4 and 5).
Figure 6.
ESD7 binds the chromatin of FT and SOC1 loci. Relative levels of ESD7 on FT (A), SOC1 (B) and Ta3 (C) chromatin were examined after ChIP assays using an αESD7 antibody. Chromatin was prepared from pool samples of 9-day-old WT and esd7-1 mutant seedlings grown under LD; the samples were collected 4h after dawn. DNA fragments amplified after ChIP were quantified by Q-PCR and subsequently normalized to ACT2 as internal control. Levels shown are relative to input and are mean ± SD (n = 3). Amplified FT and SOC1 discrete chromatin regions were similar to those analyzed in Figure 2. Asterisks indicate statistically significant differences (P < 0.05) according to Student's t-tests comparing mutants with WT using an αESD7 antibody for the ChIP assay.
Next, we examined whether the association of the PRC2 components at their target loci was affected by esd7 mutations. For this, we performed genetic crosses between esd7-1 and transgenic plants overexpressing functional GFP-tagged CLF or EMF2 proteins in clf-16 or emf2-2 mutant background, respectively. We carried out ChIP assays with 35S::GFP-CLF lines in clf-16 and clf-16 esd7-1 double mutant background grown under LD conditions. ChIP assays done on WT seedlings with αGFP antibodies were used as a control. Subsequently, qPCR analyses were performed to amplify DNA fragments spanning regions Z4 and Z6 (Figure 7A and C) and Z2 and Z4 (Figure 7B and D) from FT and SOC1, respectively. The results demonstrated that the deficiency of DNA Pol ϵ function abolished GFP-CLF binding at most of the analyzed regions of FT and SOC1 chromatin (Figure 7A and B). Similarly, we observed lower amounts of GFP-EMF2 protein in the analyzed regions of these loci in 35S::GFP-EMF2emf2-2 esd7-1 seedlings in comparison to the levels of GFP-EMF2 detected in the fully complemented 35S::GFP-EMF2emf2-2 mutant line (Figure 7C and D). Analysis involving a FT promoter region, upstream of Z4, spanning the −1159 to −959 region from the translation initiation codonof this locus, demonstrated no PRC2 occupancy in this genomic location and thus it can be considered as a negative control for these ChIP assays. (Supplementary Figure S3). We could rule out alterations caused by the esd7 mutation either in the subcellular localization of CLF or EMF2 proteins (Supplementary Figure S4A and B), or in their stability (Supplementary Figure S4C), that may account for the lower presence of these PRC2 proteins in FT and SOC1 chromatin in esd7 mutants. Taken together, these data suggest that Pol ϵ contributes to the binding and/or retention of PRC2 complexes at their flowering target loci in Arabidopsis, revealing a functional link between Pol ϵ with the PRC2 repressive machinery in the epigenetic silencing of these floral integrator genes.
Figure 7.
ESD7 is necessary for the proper recruitment of PRC2 complexes to FT and SOC1 chromatin. Relative levels of CLF and EMF2 PRC2 components on FT (A and C) and SOC1 (B and D) chromatin were examined after ChIP assays using αGFP antibodies. Chromatin was prepared from pools of 9-day-old WT seedlings or expressing GFP-CLF in clf-16 or clf-16 esd7-1 mutant background (A and B) and expressing GFP-EMF2 in emf2-2 or emf2-2 esd7-1 mutant background (C and D) grown under LD; the samples were collected 4 h after dawn. DNA fragments after ChIP were quantified by Q-PCR and subsequently normalized against the total amount of DNA (ng/ml DNA). Amplified FT and SOC1 chromatin regions were similar to those analyzed in Figure 2. The fold enrichment of each PRC2 component in comparison to the corresponding control (WT seedlings without the transgene) is indicated as mean ± SD (n = 3) of at least three independent experiments.
DISCUSSION
The heritability of cell-specific gene regulation patterns argues for the faithful propagation of chromatin states across cell generations (67,68). During DNA replication, the chromatin epigenetic status must be maintained in order to preserve cell identity (37,69). The establishment and propagation of chromatin structures is thought to determine stable gene expression patterns, including those maintained by PcG complexes, which provide an essential strategy for repressing gene expression in higher eukaryotes (70,71). Histones modified by PRC2 can be inherited but how these complexes maintain the epigenetic configuration during DNA replication remains unclear. In this study, using Arabidopsis as a model we provide novel insights into the mechanism mediating epigenetic maintenance of H3K27me3 histone marks during replication in eukaryotic organisms. We have revealed the existence of interplay between the catalytic subunit of DNA Pol ϵ and several PRC2 complex components, shedding new light on the role of the replication machinery in epigenetic transcriptional silencing. Notably, we have demonstrated an essential role for Pol ϵ in the proper recruitment of PcG complexes to particular target loci to maintain their epigenetic-repressed state in close collaboration with PRC2 components.
ESD7 genetically interacts with PRC1 and PRC2 components to mediate the repression of FT and SOC1 expression during Arabidopsis vegetative development
The genetic interactions observed between ESD7 and PcG genes suggest that both DNA replication and PcG-mediated gene repression share common pathways regulating the epigenetic transcriptional silencing of flowering master regulatory genes. Our previous results demonstrated a physical interaction between TFL2 and Pol ϵ, indicating that the DNA replication machinery was involved in Polycomb-mediated regulation of gene expression (11). ESD7 acts as a repressor of flowering time that negatively regulates the floral integrator genes FT and SOC1 together with the floral organ identity genes AG, SEP2, SEP3, SHATTERPROOF1 (SHP1) and PI (11), and that also regulates upstream flowering repressor genes such as FLC (10). Similarly, both CLF and EMF2 regulate target genes with opposite roles in flowering, either promoting (FT, AG, SEP1,2, and3, PI, SHP1 and 2) or repressing (FLC) the floral transition (34,53,54). Furthermore, we have observed that both CLF and EMF2 regulate FT and SOC1 expression (Figure 1).
In contrast to the tfl2-1 esd7-1 double mutant, which flowers earlier than any of the single parental mutants, there was no variation in the flowering time phenotype under SD between clf-16 or emf2-2 single mutants and clf-16 esd7-1 or emf2-2 esd7-1 double mutants, respectively (Figure 1). These results reveal that CLF and EMF2 act in a common genetic pathway with ESD7 to regulate flowering time. Besides, the expression levels of FT in clf-16 and esd7-1 single mutants were comparable to clf-16 esd7-1 (Figure 1), indicating that ESD7 might regulate the expression of the flowering integrator gene FT together with CLF. Given that the expression levels of FT were significantly higher in emf2-2 esd7-1 double mutant than in single parental mutants (P < 0.05) (Figure 1E), we cannot rule out that ESD7 may also work in parallel with PcG proteins in controlling the expression of FT. Similarly, the expression levels of SOC1 in clf-16 esd7-1 and emf2-2 esd7-1 double mutants were higher than the observed in esd7-1, clf-16 or emf2-2 single mutants, consistent with a role for ESD7 regulating SOC1 expression independently of TFL2, EMF2 and CLF function. Different proteins related with histone acetylation status revealed in the proteomic analyses with POLA5 (Table 1 and Supplementary Table S1) may be linked to chromatin remodelers such as MSI1 (42) and SHL (72) in this regulation.
Arabidopsis PRC2 function requires the replication machinery to maintain high H3K27me3 levels at particular loci
PRCs silence key developmental regulators and are centrally integrated in the transcriptional circuitry of stem cells (73). Inheritance of stable gene expression states is essential for plant development and to keep memory of past cell events (4,74). In Arabidopsis, transcriptional gene silencing is affected by mutations in genes encoding core replication machinery components such as ESD7, ICU2, POL δ, REPLICATION FACTOR C1 (RFC1) and REPLICATION PROTEIN A2 (RPA2) (11,13,16,75). However, to date DNA replication-mediated transcriptional silencing has not been conclusively linked with epigenetic inheritance of the H3K27me3 repressive mark to preserve cellular memory. Compelling evidence indicates that Arabidopsis PcG complexes might require both Pol α and Pol ϵ activities to maintain H3K27me3 repressive mark at target loci ((4) and this work). ICU2 function is necessary to recruit CLF to the FLC locus and for the binding of TFL2 to FT and AG loci (4). Similarly, we have demonstrated that both CLF and EMF2 are recruited to FT and SOC1 chromatin by Pol ϵ to maintain proper levels of H3K27me3 on these loci (Figure 7A–D). Interestingly, we have demonstrated that Pol ϵ is able to bind the chromatin of its floral target loci and that the esd7-1 protein is affected in the binding to FT and SOC1 chromatin (Figure 6), showing that the mutated protein may recruit less PRC2 to these target loci.
We have observed that in addition to the catalytic subunit of Pol ϵ, TFL2 is also required for the deposition and/or maintenance of H3K27me3 in flowering time loci (Figure 2A and B). In fact, TFL2 is essential for the maintenance of FT and SOC1 transcriptional repression since H3K27me3 levels were significantly lower in tfl2-1 than in WT seedlings (29). Interestingly, TFL2 and the EMF2-PRC2 complex interact in vivo through MSI1 and EMF2, and this physical link between TFL2 and PRC2 might also facilitate the recruitment of PRC2 at its target loci to maintain the repressive transcriptional state (29).
Nevertheless, how PRCs might be recruited to the replication fork for preserving the epigenetic repression is largely unknown in plants. In mammalian cells, several chromatin remodelers including PcG proteins are found in close proximity to the replication fork (76), and a physical association of PcG proteins with sites of active DNA replication has been demonstrated (77,78). Both PRC2 (an EMF2 homolog) and PRC1 (a Ring1b homolog) components have been detected at replication forks on newly synthesized DNA (37,76,79–83), revealing a possible mechanism for the mitotic maintenance of H3K27me3 mark. On the other hand, the SET domain of Drosophila CLF ortholog binds single stranded DNA (ssDNA) in vitro, which may also enable the binding of PRC2 at the replication fork (84). At the same time, PRC1 interacts with PRC2 (29). The PRC1 ortholog RING1a also binds ssDNA, and a key role during replication has been ascribed for this protein (85), suggesting that PRC1 core complex persists on chromatin during replication in vitro (70,85). Therefore, PRC complexes could be maintained close to the replication fork at two different levels, by interacting with the DNA replication machinery and also by binding to ssDNA. However, the mechanism for PRC2 complexes to preserve epigenetic chromatin states during DNA replication remains elusive. Interestingly, we have demonstrated the existence of physical interactions between Pol ϵ and PcG components, providing a plausible mechanism for maintaining the repressive transcriptional state of genomic regions during replication. How DNA polymerases may recruit PRC2 to particular loci and not generally to all replication origins during cell division is unknown and will need to be explored further.
Arabidopsis DNA Pol ϵ catalytic subunit interacts with nucleosome assemblers and chromatin remodelers to preserve epigenetic states
The phenotypic and molecular alterations displayed by the POLA5 overexpressor plants (Figure 4 A and B) resemble some of those observed in esd7 hypomorphic alleles (11) and in various PcG mutant alleles (38), suggesting a possible interference of the truncated DNA Pol ϵ catalytic subunit in the recruitment of PRCs components to the target loci, thereby preventing endogenous ESD7 protein from functioning in this process. These observations indicate a relevant role for the MIR domain-containing C-terminal region of the Pol ϵ catalytic subunit in PcG-mediated epigenetic silencing. Besides, the C-terminal domain of ICU2 also contains a putative MIR domain that is key for the epigenetic function of the DNA Pol α, since only plants overexpressing this domain of ICU2 resemble the PcG mutant phenotype (4). The PcG proteins are crucial for plant developmental processes since the loss of function of PRCs genes and some double mutant combinations between PcG genes lead to cell dedifferentiation (86,87). Intriguingly, the PcG-interaction domain of ESD7 is located in the POLA5 fragment, which includes two putative MIR motives also responsible for the interaction with the SHADOW-CHROMODOMAIN of TFL2 (11). The phenotype displayed by chromo-domain defective mutations of TFL2 is very similar to the phenotype of tfl2 null alleles, suggesting that this domain might be essential for TFL2 function (88). In animals, the HP1 SHADOW-CHROMODOMAIN allows its interaction with several factors involved in both regulation of gene expression and chromatin dynamics (50,89,90). Besides, the different isoforms of HP1 can dimerize through this domain facilitating numerous interactions with proteins involved in chromosome segregation, transcriptional regulation, DNA replication process, DNA repair or nucleosomes assembly (91–94). This array of partners of HP1 suggests that once anchored to chromatin, this protein creates a platform where a number of factors can be recruited in a flexible way. On the other hand, MIR defective mutants in several human origin recognition complex (ORC) proteins failed to localize together with the LHP1 homolog (81). Thus, the existence of a MIR domain in several DNA replication, chromatin assembly and remodeling components might be pivotal for the mutual attraction with TFL2.
The TFL2-interacting domain of Pol ϵ that resides in the C-terminal region of the protein (11) is also responsible for its binding to PRC2 components such as CLF, EMF2 and MSI1 (Figure 4 A-D; Figure 5 A and B and Table 1). However, we cannot rule out that POLA5 may also indirectly interact with PRC2 through TFL2. In fact, our proteomic analyses revealed that Arabidopsis MSI1, which is required for EMF2 PRC2 complex function (29), co-immunoprecipitated with ESD7 POLA5 fragment (Table 1 and Supplementary Table S1). Besides, the observed genetic interaction between ESD7 and MSI1 (Figure 5) supports this molecular interaction since the early flowering phenotype of esd7-1 is suppressed by the partial loss of function of msi1-1 pMSI1::MSI1-TAP, and MSI1 is also regulating FLC, FT and SOC1 expression (35), similarly to ESD7. Previous genetic interactions between FAS2 and ESD7 sustain the connection between CAF1 complex and DNA pol ϵ (11). Histone chaperones HIRA-like proteins that deposit histone H3.3 also associate to POLA5 (Table 1 and Supplementary Table S1), reinforcing the link between DNA pol ϵ and nucleosome assembly.
Among other POLA5 partners, we have identified an array of chromatin remodelers. POLA5 was found to bind MSI4/FVE peptides, a MSI1 homolog that acts redundantly with MSI1 in the repression of FLC and its homologs MADS AFFECTING FACTOR 4 and 5 (MAF4 and MAF5), promoting the floral transition (95,96). MSI4 interacts with a CLF-PRC2 complex as part of histone deacetylase and/or cullin-RING ubiquitin ligase-Damaged DNA-Binding (CUL4-DDB) complexes to silence FLC and FT expression (97). Interestingly, we could also immunoprecipitate with POLA5 additional proteins of the CUL4-DDB1 complex and a number of interactors containing DDB1-binding WD40 domains (DWD) such as VIP3 ((98); Table 1). In addition, several components of the PAF1 complex transcriptional cofactor, also involved in the repression of flowering, were identified with this approach, revealing a possible connection between DNA Pol ϵ and the PAF1 complex, proposed to antagonize the PRC2 activity (99,100).
The RAE1 protein that acts in interphase nuclei as an mRNA export factor and during mitosis as a mitotic checkpoint and spindle assembly (101), was also immunoprecipitated with POLA5, together with several nuclear pore proteins involved in mRNA export. POLA5 also pulls-down most of the components of MAC complex involved in pre-mRNA splicing, miRNA biogenesis and spliceosome assembly (58,102). Remarkably, in humans the catalytic subunit of the SWI/SNF TrxG complex, BRM, which is also pulled-down by POLA5, associates with components of the spliceosome (103). Interestingly, BRM protein has been demonstrated to antagonize the functions of Polycomb Group (PcG) proteins in Arabidopsis (104). In the same way, different subunits of HDA complexes that usually interact with MSI1 (63,105) were also found to be associated with POLA5 (Table 1 and Supplementary Table S1), suggesting that Pol ϵ may function as a scaffold for different chromatin remodeling complexes, some of them having contrasting roles in the regulation of gene expression. The finding that Pol ϵ may not be responsible for primary DNA synthesis could strengthen this hypothesis (7,106). Altogether, our proteomic analyses reveal that Pol ϵ is participating in the recruitment of different nucleosome assemblers and chromatin remodelers that are essential to preserve cell memory and support a functional connection of Pol ϵ with different chromatin remodeling complexes involved in the re-establishment of the epigenetic state and in fine-tuning gene expression, an essential process to maintain cell identity in all eukaryotic organisms. We cannot rule out that some of these interactions may be only specific for the truncated Pol ϵ catalytic subunit and may not reflect the behavior of the full length ESD7 protein. Further studies will be necessary for an in-depth understanding of how these complexes containing DNA Pol ϵ may proceed during chromatin assembly on newly replicated DNA for the re-establishment of the original epigenetic histone marks and to regulate gene expression.
In conclusion, the data presented in this work provide an insight into the mechanisms ensuring the transmission of the epigenetic code at pivotal loci in developmental control, and reveal the interplay between the DNA replication machinery and the PcG complexes in the control of developmental processes through a mechanism involving epigenetic transcriptional gene silencing. We provide an attractive model for targeting of PRC2 to newly synthesized DNA for reestablishment of epigenetic marks after dilution by replication having important implications in our understanding of epigenetic inheritance in eukaryotic organisms.
Supplementary Material
Acknowledgments
We appreciate very much Dr Lars Hennig (Swedish University of Agricultural Sciences, Upssala, Sweden) for sharing with us the EMF2 cDNA cloned into pMDC44, the pMSI1::MSI1-TAP msi1-1 line and the pMSI1::GFP-MSI1 msi1-1 mutant; Dr Martine Devic (UMR-CNRS-IRD-Université, France) for her kindly gift of pGADT7 constructs containing POLA3 and POLA5 fragments; Dr Justin Goodrich (Institute of Molecular Plant Science, University of Edinburgh, UK), for providing us with the pGBT9 clones, containing the full-length cDNAs of CLF and EMF2, the N-terminal CLF fragment cloned into pGEX-4T (GE Healthcare Bio-Sciences AB), the EMF2 fragments containing the VEFS domain (4AD and 5AD) cloned into pET30a (Novagen) and the 35S::GFP-CLF transgenic plants. We thank Pedro Crevillén (CBGP, Madrid, Spain) for the critical reading of the manuscript.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Spanish Ministerio de Economia y Competitividad MINECO [BIO2010-15589 to M.P., J.A.J.]; Spanish Ministerio de Ciencia e Innovación [BIO2013-43098R to M.P., J.A.J.]. Funding for open access charge: MINECO [ BIO2013-43098R].
Conflict of interest statement. None declared.
REFERENCES
- 1.Corpet A., Almouzni G. Making copies of chromatin: the challenge of nucleosomal organization and epigenetic information. Trends Cell Biol. 2009;19:29–41. doi: 10.1016/j.tcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.MacAlpine D.M., Almouzni G. Chromatin and DNA replication. Cold Spring Harb. Perspect. Biol. 2013;5:a010207. doi: 10.1101/cshperspect.a010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huff J.T., Zilberman D. Regulation of biological accuracy, precision, and memory by plant chromatin organization. Curr. Opin. Genet. Dev. 2012;22:132–138. doi: 10.1016/j.gde.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Hyun Y., Yun H., Park K., Ohr H., Lee O., Kim D.H., Sung S., Choi Y. The catalytic subunit of Arabidopsis DNA polymerase alpha ensures stable maintenance of histone modification. Development. 2013;140:156–166. doi: 10.1242/dev.084624. [DOI] [PubMed] [Google Scholar]
- 5.Johansson E., Dixon N. Replicative DNA polymerases. Cold Spring Harb. Perspect. Biol. 2013;5:a012799. doi: 10.1101/cshperspect.a012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunkel T.A., Burgers P.M. Delivering nonidentical twins. Nat. Struct. Mol. Biol. 2014;21:649–651. doi: 10.1038/nsmb.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson R.E., Klassen R., Prakash L., Prakash S. A major role of DNA polymerase delta in replication of both the leading and lagging DNA strands. Mol. Cell. 2015;59:163–175. doi: 10.1016/j.molcel.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenik P.D., Jurkuta R.E., Barton M.K. Interactions between the cell cycle and embryonic patterning in Arabidopsis uncovered by a mutation in DNA polymerase epsilon. Plant Cell. 2005;17:3362–3377. doi: 10.1105/tpc.105.036889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronceret A., Guilleminot J., Lincker F., Gadea-Vacas J., Delorme V., Bechtold N., Pelletier G., Delseny M., Chaboute M.E., Devic M. Genetic analysis of two Arabidopsis DNA polymerase epsilon subunits during early embryogenesis. Plant J. 2005;44:223–236. doi: 10.1111/j.1365-313X.2005.02521.x. [DOI] [PubMed] [Google Scholar]
- 10.Yin H., Zhang X., Liu J., Wang Y., He J., Yang T., Hong X., Yang Q., Gong Z. Epigenetic regulation, somatic homologous recombination, and abscisic acid signaling are influenced by DNA polymerase epsilon mutation in Arabidopsis. Plant Cell. 2009;21:386–402. doi: 10.1105/tpc.108.061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Olmo I., Lopez-Gonzalez L., Martin-Trillo M.M., Martinez-Zapater J.M., Pineiro M., Jarillo J.A. EARLY IN SHORT DAYS 7 (ESD7) encodes the catalytic subunit of DNA polymerase epsilon and is required for flowering repression through a mechanism involving epigenetic gene silencing. Plant J. 2010;61:623–636. doi: 10.1111/j.1365-313X.2009.04093.x. [DOI] [PubMed] [Google Scholar]
- 12.Fornara F., de Montaigu A., Coupland G. SnapShot: control of flowering in Arabidopsis. Cell. 2010;141:550. doi: 10.1016/j.cell.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Barrero J.M., Gonzalez-Bayon R., del Pozo J.C., Ponce M.R., Micol J.L. INCURVATA2 encodes the catalytic subunit of DNA Polymerase alpha and interacts with genes involved in chromatin-mediated cellular memory in Arabidopsis thaliana. Plant Cell. 2007;19:2822–2838. doi: 10.1105/tpc.107.054130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Ren X., Yin H., Wang Y., Xia R., Wang Y., Gong Z. Mutation in the catalytic subunit of DNA polymerase alpha influences transcriptional gene silencing and homologous recombination in Arabidopsis. Plant J. 2010;61:36–45. doi: 10.1111/j.1365-313X.2009.04026.x. [DOI] [PubMed] [Google Scholar]
- 15.Micol-Ponce R., Sanchez-Garcia A.B., Xu Q., Barrero J.M., Micol J.L., Ponce M.R. Arabidopsis INCURVATA2 regulates salicylic acid and abscisic acid signaling, and oxidative stress responses. Plant Cell Physiol. 2015;56:2207–2219. doi: 10.1093/pcp/pcv132. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias F.M., Bruera N.A., Dergan-Dylon S., Marino-Buslje C., Lorenzi H., Mateos J.L., Turck F., Coupland G., Cerdan P.D. The arabidopsis DNA polymerase delta has a role in the deposition of transcriptionally active epigenetic marks, development and flowering. PLoS Genet. 2015;11:e1004975. doi: 10.1371/journal.pgen.1004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Z., Liu J., Deng W.M., Jiao R. Histone chaperone CAF-1: essential roles in multi-cellular organism development. Cell. Mol. Life Sci. 2015;72:327–337. doi: 10.1007/s00018-014-1748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez-Parra E., Gutierrez C. The many faces of chromatin assembly factor 1. Trends Plant Sci. 2007;12:570–576. doi: 10.1016/j.tplants.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Schonrock N., Exner V., Probst A., Gruissem W., Hennig L. Functional genomic analysis of CAF-1 mutants in Arabidopsis thaliana. J. Biol. Chem. 2006;281:9560–9568. doi: 10.1074/jbc.M513426200. [DOI] [PubMed] [Google Scholar]
- 20.Murzina N., Verreault A., Laue E., Stillman B. Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell. 1999;4:529–540. doi: 10.1016/s1097-2765(00)80204-x. [DOI] [PubMed] [Google Scholar]
- 21.Quivy J.P., Roche D., Kirschner D., Tagami H., Nakatani Y., Almouzni G. A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J. 2004;23:3516–3526. doi: 10.1038/sj.emboj.7600362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudin V., Libault M., Pouteau S., Juul T., Zhao G., Lefebvre D., Grandjean O. Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development. 2001;128:4847–4858. doi: 10.1242/dev.128.23.4847. [DOI] [PubMed] [Google Scholar]
- 23.Turck F., Roudier F., Farrona S., Martin-Magniette M.L., Guillaume E., Buisine N., Gagnot S., Martienssen R.A., Coupland G., Colot V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Germann S., Blus B.J., Khorasanizadeh S., Gaudin V., Jacobsen S.E. The Arabidopsis LHP1 protein colocalizes with histone H3 Lys27 trimethylation. Nat. Struct. Mol. Biol. 2007;14:869–871. doi: 10.1038/nsmb1283. [DOI] [PubMed] [Google Scholar]
- 25.Kotake T., Takada S., Nakahigashi K., Ohto M., Goto K. Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 2003;44:555–564. doi: 10.1093/pcp/pcg091. [DOI] [PubMed] [Google Scholar]
- 26.Takada S., Goto K. Terminal flower2, an Arabidopsis homolog of heterochromatin protein1, counteracts the activation of flowering locus T by constans in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mylne J.S., Barrett L., Tessadori F., Mesnage S., Johnson L., Bernatavichute Y.V., Jacobsen S.E., Fransz P., Dean C. LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5012–5017. doi: 10.1073/pnas.0507427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sung S., He Y., Eshoo T.W., Tamada Y., Johnson L., Nakahigashi K., Goto K., Jacobsen S.E., Amasino R.M. Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat. Genet. 2006;38:706–710. doi: 10.1038/ng1795. [DOI] [PubMed] [Google Scholar]
- 29.Derkacheva M., Steinbach Y., Wildhaber T., Mozgova I., Mahrez W., Nanni P., Bischof S., Gruissem W., Hennig L. Arabidopsis MSI1 connects LHP1 to PRC2 complexes. EMBO J. 2013;32:2073–2085. doi: 10.1038/emboj.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H., Wang L., Erdjument-Bromage H., Vidal M., Tempst P., Jones R.S., Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 31.Mozgova I., Hennig L. The polycomb group protein regulatory network. Annu. Rev. Plant Biol. 2015;66:269–296. doi: 10.1146/annurev-arplant-043014-115627. [DOI] [PubMed] [Google Scholar]
- 32.Lafos M., Kroll P., Hohenstatt M.L., Thorpe F.L., Clarenz O., Schubert D. Dynamic regulation of H3K27 trimethylation during Arabidopsis differentiation. PLoS Genet. 2011;7:e1002040. doi: 10.1371/journal.pgen.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schonrock N., Bouveret R., Leroy O., Borghi L., Kohler C., Gruissem W., Hennig L. Polycomb-group proteins repress the floral activator AGL19 in the FLC-independent vernalization pathway. Genes Dev. 2006;20:1667–1678. doi: 10.1101/gad.377206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang D., Wang Y., Wang Y., He Y. Repression of FLOWERING LOCUS C and FLOWERING LOCUS T by the Arabidopsis Polycomb repressive complex 2 components. PLoS One. 2008;3:e3404. doi: 10.1371/journal.pone.0003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinbach Y., Hennig L. Arabidopsis MSI1 functions in photoperiodic flowering time control. Front. Plant Sci. 2014;5:77. doi: 10.3389/fpls.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Lucia F., Crevillen P., Jones A.M., Greb T., Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Margueron R., Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat. Rev. Genet. 2010;11:285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chanvivattana Y., Bishopp A., Schubert D., Stock C., Moon Y.H., Sung Z.R., Goodrich J. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development. 2004;131:5263–5276. doi: 10.1242/dev.01400. [DOI] [PubMed] [Google Scholar]
- 39.Chen L., Cheng J.C., Castle L., Sung Z.R. EMF genes regulate Arabidopsis inflorescence development. Plant Cell. 1997;9:2011–2024. doi: 10.1105/tpc.9.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serrano-Cartagena J., Candela H., Robles P., Ponce M.R., Perez-Perez J.M., Piqueras P., Micol J.L. Genetic analysis of incurvata mutants reveals three independent genetic operations at work in Arabidopsis leaf morphogenesis. Genetics. 2000;156:1363–1377. doi: 10.1093/genetics/156.3.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexandre C., Moller-Steinbach Y., Schonrock N., Gruissem W., Hennig L. Arabidopsis MSI1 is required for negative regulation of the response to drought stress. Mol. Plant. 2009;2:675–687. doi: 10.1093/mp/ssp012. [DOI] [PubMed] [Google Scholar]
- 42.Bouveret R., Schonrock N., Gruissem W., Hennig L. Regulation of flowering time by Arabidopsis MSI1. Development. 2006;133:1693–1702. doi: 10.1242/dev.02340. [DOI] [PubMed] [Google Scholar]
- 43.Schubert D., Primavesi L., Bishopp A., Roberts G., Doonan J., Jenuwein T., Goodrich J. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 2006;25:4638–4649. doi: 10.1038/sj.emboj.7601311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Bartolome S., Navarro P., Martin-Maroto F., Lopez-Ferrer D., Ramos-Fernandez A., Villar M., Garcia-Ruiz J.P., Vazquez J. Properties of average score distributions of SEQUEST: the probability ratio method. Mol. Cell. Proteomics. 2008;7:1135–1145. doi: 10.1074/mcp.M700239-MCP200. [DOI] [PubMed] [Google Scholar]
- 46.Navarro P., Trevisan-Herraz M., Bonzon-Kulichenko E., Nunez E., Martinez-Acedo P., Perez-Hernandez D., Jorge I., Mesa R., Calvo E., Carrascal M., et al. General statistical framework for quantitative proteomics by stable isotope labeling. J. Proteome Res. 2014;13:1234–1247. doi: 10.1021/pr4006958. [DOI] [PubMed] [Google Scholar]
- 47.Isern J., Martin-Antonio B., Ghazanfari R., Martin A.M., Lopez J.A., del Toro R., Sanchez-Aguilera A., Arranz L., Martin-Perez D., Suarez-Lledo M., et al. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep. 2013;3:1714–1724. doi: 10.1016/j.celrep.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 48.Jeong J.H., Song H.R., Ko J.H., Jeong Y.M., Kwon Y.E., Seol J.H., Amasino R.M., Noh B., Noh Y.S. Repression of FLOWERING LOCUS T chromatin by functionally redundant histone H3 lysine 4 demethylases in Arabidopsis. PLoS One. 2009;4:e8033. doi: 10.1371/journal.pone.0008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou X., Zhou J., Liu C., Liu L., Shen L., Yu H. Nuclear factor Y-mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis. Nat. Commun. 2014;5:4601. doi: 10.1038/ncomms5601. [DOI] [PubMed] [Google Scholar]
- 50.Thiru A., Nietlispach D., Mott H.R., Okuwaki M., Lyon D., Nielsen P.R., Hirshberg M., Verreault A., Murzina N.V., Laue E.D. Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 2004;23:489–499. doi: 10.1038/sj.emboj.7600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao J., Wagner D. Polycomb repression in the regulation of growth and development in Arabidopsis. Curr. Opin. Plant Biol. 2015;23:15–24. doi: 10.1016/j.pbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Mozgova I., Kohler C., Hennig L. Keeping the gate closed: functions of the polycomb repressive complex PRC2 in development. Plant J. 2015;83:121–132. doi: 10.1111/tpj.12828. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Vernaza M., Yang S., Muller R., Thorpe F., de Leau E., Goodrich J. Antagonistic roles of SEPALLATA3, FT and FLC genes as targets of the polycomb group gene CURLY LEAF. PLoS One. 2012;7:e30715. doi: 10.1371/journal.pone.0030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S.Y., Lee J., Eshed-Williams L., Zilberman D., Sung Z.R. EMF1 and PRC2 cooperate to repress key regulators of Arabidopsis development. PLoS Genet. 2012;8:e1002512. doi: 10.1371/journal.pgen.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong C.W., Roh H., Dang T.V., Choi Y.D., Fischer R.L., Lee J.S., Choi Y. An E3 ligase complex regulates SET-domain polycomb group protein activity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 2011;108:8036–8041. doi: 10.1073/pnas.1104232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohler C., Hennig L., Bouveret R., Gheyselinck J., Grossniklaus U., Gruissem W. Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J. 2003;22:4804–4814. doi: 10.1093/emboj/cdg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh S., Zhang H., Ludwig P., van Nocker S. A mechanism related to the yeast transcriptional regulator Paf1c is required for expression of the Arabidopsis FLC/MAF MADS box gene family. Plant Cell. 2004;16:2940–2953. doi: 10.1105/tpc.104.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koncz C., Dejong F., Villacorta N., Szakonyi D., Koncz Z. The spliceosome-activating complex: molecular mechanisms underlying the function of a pleiotropic regulator. Front. Plant Sci. 2012;3:9. doi: 10.3389/fpls.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrona S., Hurtado L., Bowman J.L., Reyes J.C. The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development. 2004;131:4965–4975. doi: 10.1242/dev.01363. [DOI] [PubMed] [Google Scholar]
- 60.Tian L., Chen Z.J. Blocking histone deacetylation in Arabidopsis induces pleiotropic effects on plant gene regulation and development. Proc. Natl. Acad. Sci. U.S.A. 2001;98:200–205. doi: 10.1073/pnas.011347998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waidmann S., Kusenda B., Mayerhofer J., Mechtler K., Jonak C. A DEK domain-containing protein modulates chromatin structure and function in Arabidopsis. Plant Cell. 2014;26:4328–4344. doi: 10.1105/tpc.114.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song C.P., Galbraith D.W. AtSAP18, an orthologue of human SAP18, is involved in the regulation of salt stress and mediates transcriptional repression in Arabidopsis. Plant Mol. Biol. 2006;60:241–257. doi: 10.1007/s11103-005-3880-9. [DOI] [PubMed] [Google Scholar]
- 63.Mehdi S., Derkacheva M., Ramstrom M., Kralemann L., Bergquist J., Hennig L. The WD40 domain protein MSI1 functions in a histone deacetylase complex to fine-tune abscisic acid signaling. Plant Cell. 2016;28:42–54. doi: 10.1105/tpc.15.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo M., Wang Y.Y., Liu X., Yang S., Lu Q., Cui Y., Wu K. HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J. Exp. Bot. 2012;63:3297–3306. doi: 10.1093/jxb/ers059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson L., Cao X., Jacobsen S. Interplay between two epigenetic marks. DNA methylation and histone H3 lysine 9 methylation. Curr. Biol. 2002;12:1360–1367. doi: 10.1016/s0960-9822(02)00976-4. [DOI] [PubMed] [Google Scholar]
- 66.Schatlowski N., Creasey K., Goodrich J., Schubert D. Keeping plants in shape: polycomb-group genes and histone methylation. Semin. Cell Dev. Biol. 2008;19:547–553. doi: 10.1016/j.semcdb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Annunziato A.T. Split decision: what happens to nucleosomes during DNA replication. J. Biol. Chem. 2005;280:12065–12068. doi: 10.1074/jbc.R400039200. [DOI] [PubMed] [Google Scholar]
- 68.Bonasio R., Tu S., Reinberg D. Molecular signals of epigenetic states. Science. 2010;330:612–616. doi: 10.1126/science.1191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campos E.I., Stafford J.M., Reinberg D. Epigenetic inheritance: histone bookmarks across generations. Trends Cell Biol. 2014;24:664–674. doi: 10.1016/j.tcb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Francis N.J. Does maintenance of polycomb group proteins through DNA replication contribute to epigenetic inheritance? Epigenetics. 2009;4:370–373. doi: 10.4161/epi.4.6.9374. [DOI] [PubMed] [Google Scholar]
- 71.Lanzuolo C., Orlando V. Memories from the polycomb group proteins. Annu. Rev. Genet. 2012;46:561–589. doi: 10.1146/annurev-genet-110711-155603. [DOI] [PubMed] [Google Scholar]
- 72.Lopez-Gonzalez L., Mouriz A., Narro-Diego L., Bustos R., Martinez-Zapater J.M., Jarillo J.A., Pineiro M. Chromatin-dependent repression of the Arabidopsis floral integrator genes involves plant specific PHD-containing proteins. Plant Cell. 2014;26:3922–3938. doi: 10.1105/tpc.114.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon J.A., Kingston R.E. Occupying chromatin: polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol. Cell. 2013;49:808–824. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berry S., Hartley M., Olsson T.S., Dean C., Howard M. Local chromatin environment of a Polycomb target gene instructs its own epigenetic inheritance. Elife. 2015;4:e07205. doi: 10.7554/eLife.07205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Q., Gong Z. The coupling of epigenome replication with DNA replication. Curr. Opin. Plant Biol. 2011;14:187–194. doi: 10.1016/j.pbi.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Petruk S., Sedkov Y., Johnston D.M., Hodgson J.W., Black K.L., Kovermann S.K., Beck S., Canaani E., Brock H.W., Mazo A. TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell. 2012;150:922–933. doi: 10.1016/j.cell.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petruk S., Black K.L., Kovermann S.K., Brock H.W., Mazo A. Stepwise histone modifications are mediated by multiple enzymes that rapidly associate with nascent DNA during replication. Nat. Commun. 2013;4:2841. doi: 10.1038/ncomms3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piunti A., Rossi A., Cerutti A., Albert M., Jammula S., Scelfo A., Cedrone L., Fragola G., Olsson L., Koseki H., et al. Polycomb proteins control proliferation and transformation independently of cell cycle checkpoints by regulating DNA replication. Nat. Commun. 2014;5:3649. doi: 10.1038/ncomms4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hansen K.H., Bracken A.P., Pasini D., Dietrich N., Gehani S.S., Monrad A., Rappsilber J., Lerdrup M., Helin K. A model for transmission of the H3K27me3 epigenetic mark. Nat. Cell Biol. 2008;10:1291–1300. doi: 10.1038/ncb1787. [DOI] [PubMed] [Google Scholar]
- 80.Margueron R., Justin N., Ohno K., Sharpe M.L., Son J., Drury W.J., 3rd, Voigt P., Martin S.R., Taylor W.R., De Marco V. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–767. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prasanth S.G., Shen Z., Prasanth K.V., Stillman B. Human origin recognition complex is essential for HP1 binding to chromatin and heterochromatin organization. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15093–15098. doi: 10.1073/pnas.1009945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steffen P.A., Ringrose L. What are memories made of? How Polycomb and Trithorax proteins mediate epigenetic memory. Nat. Rev. Mol. Cell Biol. 2014;15:340–356. doi: 10.1038/nrm3789. [DOI] [PubMed] [Google Scholar]
- 83.Leung K.H., Abou El Hassan M., Bremner R. A rapid and efficient method to purify proteins at replication forks under native conditions. Biotechniques. 2013;55:204–206. doi: 10.2144/000114089. [DOI] [PubMed] [Google Scholar]
- 84.Krajewski W.A., Nakamura T., Mazo A., Canaani E. A motif within SET-domain proteins binds single-stranded nucleic acids and transcribed and supercoiled DNAs and can interfere with assembly of nucleosomes. Mol. Cell. Biol. 2005;25:1891–1899. doi: 10.1128/MCB.25.5.1891-1899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lo S.M., Follmer N.E., Lengsfeld B.M., Madamba E.V., Seong S., Grau D.J., Francis N.J. A bridging model for persistence of a polycomb group protein complex through DNA replication in vitro. Mol. Cell. 2012;46:784–796. doi: 10.1016/j.molcel.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He C., Huang H., Xu L. Mechanisms guiding Polycomb activities during gene silencing in Arabidopsis thaliana. Front. Plant Sci. 2013;4:454. doi: 10.3389/fpls.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim D.H., Sung S. Polycomb-mediated gene silencing in Arabidopsis thaliana. Mol. Cells. 2014;37:841–850. doi: 10.14348/molcells.2014.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Exner V., Aichinger E., Shu H., Wildhaber T., Alfarano P., Caflisch A., Gruissem W., Kohler C., Hennig L. The chromodomain of LIKE HETEROCHROMATIN PROTEIN 1 is essential for H3K27me3 binding and function during Arabidopsis development. PLoS One. 2009;4:e5335. doi: 10.1371/journal.pone.0005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brasher S.V., Smith B.O., Fogh R.H., Nietlispach D., Thiru A., Nielsen P.R., Broadhurst R.W., Ball L.J., Murzina N.V., Laue E.D. The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 2000;19:1587–1597. doi: 10.1093/emboj/19.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smothers J.F., Henikoff S. The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr. Biol. 2000;10:27–30. doi: 10.1016/s0960-9822(99)00260-2. [DOI] [PubMed] [Google Scholar]
- 91.Fanti L., Pimpinelli S. HP1: a functionally multifaceted protein. Curr. Opin. Genet. Dev. 2008;18:169–174. doi: 10.1016/j.gde.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 92.Zeng W., Ball A.R., Jr, Yokomori K. HP1: heterochromatin binding proteins working the genome. Epigenetics. 2010;5:287–292. doi: 10.4161/epi.5.4.11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon S.H., Workman J.L. The changing faces of HP1: From heterochromatin formation and gene silencing to euchromatic gene expression: HP1 acts as a positive regulator of transcription. Bioeassays. 2011;33:280–289. doi: 10.1002/bies.201000138. [DOI] [PubMed] [Google Scholar]
- 94.Mendez D.L., Mandt R.E., Elgin S.C. Heterochromatin Protein 1a (HP1a) partner specificity is determined by critical amino acids in the chromo shadow domain and C-terminal extension. J. Biol. Chem. 2013;288:22315–22323. doi: 10.1074/jbc.M113.468413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ausin I., Alonso-Blanco C., Jarillo J.A., Ruiz-Garcia L., Martinez-Zapater J.M. Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat. Genet. 2004;36:162–166. doi: 10.1038/ng1295. [DOI] [PubMed] [Google Scholar]
- 96.Gu X., Jiang D., Yang W., Jacob Y., Michaels S.D., He Y. Arabidopsis homologs of retinoblastoma-associated protein 46/48 associate with a histone deacetylase to act redundantly in chromatin silencing. PLoS Genet. 2011;7:e1002366. doi: 10.1371/journal.pgen.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pazhouhandeh M., Molinier J., Berr A., Genschik P. MSI4/FVE interacts with CUL4-DDB1 and a PRC2-like complex to control epigenetic regulation of flowering time in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3430–3435. doi: 10.1073/pnas.1018242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H., Ransom C., Ludwig P., van Nocker S. Genetic analysis of early flowering mutants in Arabidopsis defines a class of pleiotropic developmental regulator required for expression of the flowering-time switch flowering locus C. Genetics. 2003;164:347–358. doi: 10.1093/genetics/164.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park S., Oh S., Ek-Ramos J., van Nocker S. PLANT HOMOLOGOUS TO PARAFIBROMIN is a component of the PAF1 complex and assists in regulating expression of genes within H3K27ME3-enriched chromatin. Plant Physiol. 2010;153:821–831. doi: 10.1104/pp.110.155838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu X., Michaels S.D. The Arabidopsis Paf1c complex component CDC73 participates in the modification of FLOWERING LOCUS C chromatin. Plant Physiol. 2010;153:1074–1084. doi: 10.1104/pp.110.158386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee J.Y., Lee H.S., Wi S.J., Park K.Y., Schmit A.C., Pai H.S. Dual functions of Nicotiana benthamiana Rae1 in interphase and mitosis. Plant J. 2009;59:278–291. doi: 10.1111/j.1365-313X.2009.03869.x. [DOI] [PubMed] [Google Scholar]
- 102.Zhang S., Xie M., Ren G., Yu B. CDC5, a DNA binding protein, positively regulates posttranscriptional processing and/or transcription of primary microRNA transcripts. Proc. Natl. Acad. Sci. U.S.A. 2013;110:17588–17593. doi: 10.1073/pnas.1310644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Batsche E., Yaniv M., Muchardt C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat. Struct. Mol. Biol. 2006;13:22–29. doi: 10.1038/nsmb1030. [DOI] [PubMed] [Google Scholar]
- 104.Li C., Chen C., Gao L., Yang S., Nguyen V., Shi X., Siminovitch K., Kohalmi S.E., Huang S., Wu K., et al. The Arabidopsis SWI2/SNF2 chromatin Remodeler BRAHMA regulates polycomb function during vegetative development and directly activates the flowering repressor gene SVP. PLoS Genet. 2015;11:e1004944. doi: 10.1371/journal.pgen.1004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rossi V., Locatelli S., Lanzanova C., Boniotti M.B., Varotto S., Pipal A., Goralik-Schramel M., Lusser A., Gatz C., Gutierrez C., et al. A maize histone deacetylase and retinoblastoma-related protein physically interact and cooperate in repressing gene transcription. Plant Mol. Biol. 2003;51:401–413. doi: 10.1023/a:1022090916446. [DOI] [PubMed] [Google Scholar]
- 106.Stillman B. Reconsidering DNA Polymerases at the Replication Fork in Eukaryotes. Mol. Cell. 2015;59:139–141. doi: 10.1016/j.molcel.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.