Abstract
Caries risk assessment (CRA) is widely recommended for dental caries management. Little is known regarding how practitioners use individual CRA items to determine risk and which individual items independently predict clinical outcomes in children younger than 6 y. The objective of this study was to assess the relative importance of pediatric CRA items in dental providers’ decision making regarding patient risk and in association with clinically evident caries, cross-sectionally and longitudinally. CRA information was abstracted retrospectively from electronic patient records of children initially aged 6 to 72 mo at a university pediatric dentistry clinic (n = 3,810 baseline; n = 1,315 with follow-up). The 17-item CRA form included caries risk indicators, caries protective items, and clinical indicators. Conditional random forests classification trees were implemented to identify and assign variable importance to CRA items independently associated with baseline high-risk designation, baseline evident tooth decay, and follow-up evident decay. Thirteen individual CRA items, including all clinical indicators and all but 1 risk indicator, were independently and statistically significantly associated with student/resident providers’ caries risk designation. Provider-assigned baseline risk category was strongly associated with follow-up decay, which increased from low (20.4%) to moderate (30.6%) to high/extreme risk patients (68.7%). Of baseline CRA items, before adjustment, 12 were associated with baseline decay and 7 with decay at follow-up; however, in the conditional random forests models, only the clinical indicators (evident decay, dental plaque, and recent restoration placement) and 1 risk indicator (frequent snacking) were independently and statistically significantly associated with future disease, for which baseline evident decay was the strongest predictor. In this predominantly high-risk population under caries-preventive care, more individual CRA items were independently associated with providers’ risk determination than with future caries status. These university dental providers considered many items in decision making regarding patient risk, suggesting that, in turn, these comprehensive CRA forms could also aid individualized care, linking risk assessment to disease management.
Knowledge Transfer Statement: Caries risk assessment (CRA) is widely recommended for patient-tailored, prevention-focused caries management. Studies show mixed predictive performance of pediatric CRA instruments, but little is known regarding how information captured in CRA forms guides clinical decision making. This study, in high-caries prevalence 6- to 72-mo-olds, demonstrates the following: 1) most items in a CRA instrument were independently associated with practitioners’ risk designations, 2) practitioners’ risk designations were significantly associated with future disease, and 3) of baseline measures associated with future caries, evident decay was the strongest independent indicator of future caries status. Although current disease (resulting from existing pathological and protective factor imbalance) may sufficiently predict future caries status in populations, other CRA items incorporated during risk categorization could aid practitioners to develop individualized intervention strategies against identified risk factors.
Keywords: preschool child, dental caries, epidemiology, child dentistry, decision making, risk factors
Introduction
Recent decades have seen calls for risk-based medical management of dental caries, favoring prevention-focused, minimally invasive treatments, implemented as individualized, continuous patient care (Krasse 1985; Powell 1998; Fontana et al. 2009; Brocklehurst et al. 2011). Dental education leaders have long promoted risk-based caries management in predoctoral and pediatric specialty training programs (Brown 1995; Adair 2003). Risk-based management demands that practitioners identify patient-specific caries risk factors and direct more intensive interventions toward patients at greater risk for disease. Thus, the goal of caries risk assessment (CRA) is to enable practitioners to bring patients into a favorable balance between caries predisposing and preventive factors (Featherstone 2003).
Multiple CRA forms (Ramos-Gomez et al. 2007; American Dental Association 2009–2011; Ramos-Gomez et al. 2012; American Academy of Pediatric Dentistry [AAPD] 2013) and algorithm-based programs (Bratthall and Hansel Petersson 2005; Gao et al. 2010) are available to aid practitioners in determining caries risk for children younger than 6 y. These CRA systems are based on a combination of scientific evidence and expert opinion and serve to guide practitioners in assigning risk status based on a variety of clinical and social factors. Caries Management by Risk Assessment (CAMBRA) is one approach that couples risk assessment with tailored preventive care and risk monitoring (Ramos-Gomez et al. 2007; Ramos-Gomez et al. 2012). Prospective studies have reported a range of sensitivity and specificity values across CRA instruments for predicting future decay among young children in Europe and Asia (Gao et al. 2013; Tellez et al. 2013). However, limited information is available regarding how practitioners use CRA instruments to determine patient risk status and how individual CRA items relate to patient outcomes, particularly for children younger than 6 y.
In the present study, we evaluated the relative importance of CAMBRA CRA items in dental providers’ risk determination and in disease prediction for children aged 6 to 72 mo visiting a university pediatric dentistry specialty-training program. We applied a conditional random forests tree-based regression method to describe individual variable importance. Random forests classification models can account for confounding between multiple risk factors and handle nonlinear associations and interactions (Breiman 2001; Strobl et al. 2007).
The objectives of this study were to quantify the relative importance of items included in the CAMBRA pediatric CRA form as they related to 3 outcomes: 1) dental providers’ decision to designate a patient as high caries risk, 2) clinically evident dental caries at the time of the CRA, and 3) clinically evident dental caries at the following recall visit. We also calculated unadjusted associations between baseline CRA items and clinically evident dental caries at baseline and at the following recall visit, along with the association between provider risk designation and caries status at recall.
Methods
This was an observational retrospective cohort study based on patient electronic dental records at the University of California San Francisco (UCSF) Pediatric Dentistry clinic. This clinic serves a primarily low-income, urban population and provides pediatric dentistry training to predoctoral dental students and postgraduate dental residents. Most patients are from the local geographic area; however, some patients travel significant distances, particularly for care under general anesthesia or management of complex dental or medical conditions. The UCSF Committee on Human Research (institutional review board) reviewed and approved the use of retrospective patient data. Study reporting follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for cohort studies (von Elm et al. 2007).
Eligibility criteria were: 1) a completed oral examination with CRA between January 1, 2009 (introduction of electronic CRA forms), and April 30, 2015, and 2) age ≥6 mo and <72 mo at the time of the initial (baseline) exam (Fig. 1). The longitudinal analysis was restricted to patients with a recall examination that included a second CRA. For each patient, considered were the first eligible initial exam and first eligible recall exam within the window of 4 to 36 mo after baseline.
Figure 1.

Flow diagram for participant eligibility and follow-up.
Data Source and Risk Assessment
The UCSF Program in Pediatric Dentistry stresses a risk-based, minimally invasive caries management model following CAMBRA guidelines (Ramos-Gomez et al. 2007; Ramos-Gomez et al. 2012). Briefly, CAMBRA involves first assessing each individual patient’s caries risk, followed by tailored disease management, stressing preventive, nonoperative, and minimally invasive treatments, based on expert consensus (Featherstone 2003). CRA documentation used at UCSF for children younger than 6 y consistently included 17 items with yes/no responses: risk (biological) indicators: bottle use in bed, bottle use continuously during the day, bottle use with contents other than milk or water, caregiver or sibling tooth decay, frequent snacking (≥3 times daily), inadequate salivary flow, low socioeconomic status, saliva-reducing medications, and special care needs (e.g., developmental impairment); protective items: brushes daily with fluoride dentifrice, caregiver uses xylitol, drinks fluoridated water, fluoride varnish application (past 6 mo), and lives in fluoridated community; and clinical findings (disease indicators): evident (visually obvious) tooth decay or white spot lesions, heavy dental plaque, and recently placed restorations (within 2 y).
The CAMBRA risk assessment process places patients into 1 of 4 caries risk categories (low, moderate, high, extreme) but without a rigid algorithm; rather, clinicians are guided to take into account the overall balance of risk factors, protective factors, and clinical indicators in making an informed judgment (Featherstone 2003; Ramos-Gomez et al. 2007; Ramos-Gomez et al. 2012). Caries risk assessment was stressed in the predoctoral and residency didactic and clinical curricula. Examination forms were standardized, and attending faculty members were provided CRA training.
Study Population Size
The analytic sample was fixed at the number of patients meeting eligibility criteria. Given 3,810 available patient records at baseline and 1,315 patients in the longitudinal sample, the power to estimate a 10% risk difference (e.g., outcome occurrence 70% and 60% in 2 equally sized comparison groups) would be 99% in the baseline sample and 96% in the longitudinal sample.
Statistical Analyses
First, we used the risk difference (RD) to express unadjusted bivariate associations between the baseline responses to each of the 17 CRA items and baseline high caries risk designation and between baseline CRA items and baseline caries status. For this analysis, extreme-risk patients were included in the high-risk category, due to the small number of extreme-risk patients (2.1%). Caries status was categorized using the clinically evident decay or white spot lesion item in the CRA form. Next, we calculated the RD for each baseline CRA item in relation to evident decay at the follow-up visit. For children who “aged out” from the pediatric CRA form (aged 0–6 y) at the time of follow-up, decay presence was drawn from the adult CRA form (aged ≥6 y). We obtained 95% confidence intervals (CIs) by the percentile bootstrap method (5,000 resamples).
To obtain adjusted measures of relative variable importance, we implemented a conditional random forests classification tree procedure using the cforest function in the R statistical computing environment (R Core Team 2015) in 4 separate analyses corresponding to different sets of indicators and outcomes: 1) baseline CRA indicators of high-risk designation, 2) baseline CRA indicators of baseline evident decay, 3) baseline CRA indicators of evident decay at follow-up, and 4) change in CRA items from baseline to follow-up and evident decay at follow-up, allowing 4 possible values for each CRA predictor (i.e., no-no, no-yes, yes-no, yes-yes). The fourth approach was taken, because changes in behaviors or exposures could be more strongly related to future caries than baseline values alone. In this approach, evident decay remained a baseline predictor, because change in decay status would predict the outcome by definition.
The random forests procedure is a nonparametric multivariable modeling algorithm based on conditional binary classification trees (Breiman 2001). The procedure first chooses a random subset of k = 5 (default) potential predictors and a random subset of n patients (default: 63.2% of sample). Then the procedure chooses the variable that best divides the data according to presence of the outcome, splitting into 2 nodes. The tree is expanded through recursive partitioning until no new nodes can be created with a statistically significant difference in the outcome. The procedure was repeated and averaged over m = 100 trees. We used multiple imputation (model based) for missing CRA predictors (2.5% of baseline data).
We used functions in the R package party to assign a variable importance measure to each classifier variable (Strobl et al. 2007). For each tree, classification accuracy was calculated from the subset of patients not used in that specific tree (the “hold-out” or “out-of-bag” sample). Individually, each classifier variable was randomly reassigned in the data set and accuracy recalculated. The mean decrease in accuracy (MDA) represents the increase in the proportion of misclassified observations under random classifier assignment. Larger MDA indicates greater variable importance.
To compute P values for each CRA indicator, we simulated 1,000 data sets in which outcomes were randomly assigned to each observation under the null hypothesis (i.e., no association between outcome and CRA indicators). The empirical P value is the proportion of simulated data sets (out of 1,000) that yielded an MDA equal to or exceeding the MDA from the original data set (Rehkopf et al. 2011). P values ≤0.05 were considered statistically significant.
Subgroup and Sensitivity Analysis
We repeated the random forests analysis for the outcome of evident decay at follow-up in subgroups defined by baseline age (<36 mo; ≥36 mo) and baseline caries status (with evident caries; no evident caries or recent restorations). As sensitivity checks, we assessed the robustness to 4 different modifications to the analysis. In the first, we pooled any variable categories with fewer than 25 observations in the overall sample. For example, for the special care needs CRA item, few children changed status from baseline to follow-up; thus, we collapsed categories into ever or never having special care needs. In the second, we restricted the analysis to observations with no missing values (complete cases). In the third, we applied inverse probability of censoring weights in the random forests model to account for losses to follow-up. Greater weights were assigned to observations from patients in the follow-up sample (n = 1,315) whose characteristics were more similar to patients in the baseline data set (n = 3,810), allowing inference to the initial sample. Finally, we restricted the analysis to follow-up visits that occurred 4 to 24 mo from baseline. In an exploratory analysis, we excluded baseline decay from the set of CRA indicator variables, because an exceptionally strong indicator may obscure weaker but otherwise important relationships between other variables (Aleksejuˉniene. et al. 2009).
Results
Study Population Characteristics
The study population was predominantly urban, eligible for public dental benefits, and largely identified as Asian, black, or Latino/Hispanic (Table 1). Of the 3,810 baseline caries risk assessments, 89.1% were completed by pediatric dentistry residents, 2.3% by predoctoral dental students, 0.6% by internationally trained dental students, and 7.9% by faculty dentists in university group practice. Mean patient baseline age was 42.6 mo, with 36.2% of the sample younger than 36 mo and 16.6% younger than 24 mo. About two-thirds of patients presented with clinically evident decay or white spot lesions at baseline (66.7%), and 70.9% had either evident decay or recent restorations. Three-fourths were classified as high or extreme caries risk (75.2%). Individuals with follow-up CRA data (median follow-up time, 11.7 mo) were less likely to have baseline evident decay or high caries risk status than those without a second CRA (Table 1). Nearly all children (99.4%) received a fluoride varnish application at or soon after the baseline visit.
Table 1.
Characteristics of the Study Population.
| Characteristic | Baseline Sample (n = 3,810)a | Follow-up Sample (n = 1,315)a | P Valueb |
|---|---|---|---|
| Baseline age, mean (SD) [range], mo | 42.6 (16.5) [6.3-72.0] | 41.8 (17.4) [9.5-71.7] | 0.03 |
| Sex, n (%) | |||
| Female | 1,772 (46.6) | 624 (47.6) | 0.40 |
| Male | 2,029 (53.4) | 687 (52.4) | |
| Payer type, n (%) | |||
| Private insurance | 224 (5.9) | 64 (4.9) | <0.0001 |
| Public benefits | 3,426 (90.5) | 1,213 (93.0) | |
| Cash | 129 (3.4) | 27 (2.1) | |
| Race/ethnicity, n (%) | |||
| Asian | 592 (15.5) | 247 (18.8) | <0.0001 |
| Black | 524 (13.8) | 175 (13.3) | |
| Latino/Hispanic | 1,191 (31.3) | 514 (39.1) | |
| White | 302 (7.9) | 108 (8.2) | |
| Other/no answer | 1,201 (31.5) | 271 (20.6) | |
| Residence, n (%) | |||
| City of San Francisco | 2,079 (54.6) | 848 (64.4) | <0.0001 |
| San Francisco Bay Area | 1,387 (36.4) | 383 (29.1) | |
| Outside areas | 344 (9.0) | 84 (6.4) | |
| Clinically evident dental caries at baseline, n (%) | |||
| No evident decay or recent restorations | 1,101 (29.1) | 484 (37.1) | <0.0001 |
| Recent restoration (no evident decay) | 157 (4.2) | 77 (5.9) | |
| With evident decay or white spot | 2,522 (66.7) | 743 (57.0) | |
| Baseline caries risk designation, n (%) | |||
| Low | 419 (11.0) | 196 (14.9) | <0.0001 |
| Moderate | 526 (13.8) | 252 (19.2) | |
| High | 2,786 (73.1) | 841 (64.0) | |
| Extreme | 79 (2.1) | 26 (2.0) | |
| Follow-up time, mean (SD) [range], mo | 13.8 (7.7) [4.0-35.9] | ||
| Clinically evident dental caries at follow-up, n (%) | |||
| No evident decay or recent restorations | 400 (30.8) | ||
| Recent restoration (no evident decay) | 199 (15.3) | ||
| With evident decay | 698 (53.8) | ||
Number of observations may be less for some variables due to missing information.
Chi-square test (binary variables) or t test (baseline age) for difference between follow-up sample and those without follow-up.
Cross-Sectional, Unadjusted Associations
At baseline, the most commonly recorded risk indicators were low socioeconomic status (67.5%), frequent snacking (59.5%), and tooth decay in family members (34.1%), while risk indicators related to salivary flow or special care needs were noted in <10% of the study population (Table 2). For protective items, living in a fluoridated area (91.6%) and brushing daily with fluoride toothpaste (81.3%) were widespread, but at baseline (i.e., before treatment initiation), fluoride varnish exposure (24.7%) and caregiver xylitol use (1.4%) were uncommon.
Table 2.
Bivariate Associations of Baseline Pediatric Caries Risk Assessment Items with Practitioner Risk Designation and Clinically Evident Dental Caries at Baseline and Follow-up.
| Cross-Sectional Outcomes | Longitudinal Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| High-Risk Designation | Evident Decay or White Spot (Baseline) | Evident Decay or White Spot (Follow-up) | ||||||
| Baseline CRA Items | na | % High Riskb | Risk Difference (95% CI) | % Evident Decay | Risk Difference (95% CI) | nc | % Evident Decay | Risk Difference (95% CI) |
| Risk indicators | ||||||||
| Frequent snacking | ||||||||
| Y | 2,236 | 87.2 | 29.4 (26.6, 32.2) | 78.9 | 29.8 (26.7, 32.8) | 683 | 61.6 | 15.8 (10.6, 21.1) |
| N | 1,523 | 57.5 | 49.0 | 611 | 45.7 | |||
| Low socioeconomic status | ||||||||
| Y | 2,523 | 79.6 | 13.2 (10.1, 16.2) | 72.1 | 15.5 (12.3, 18.7) | 859 | 57.6 | 10.4 (4.8, 16.2) |
| N | 1,213 | 66.4 | 56.6 | 430 | 47.2 | |||
| Caregiver or sibling tooth decay | ||||||||
| Y | 1,074 | 84.1 | 13.2 (10.4, 15.8) | 75.1 | 13.3 (10.2, 16.2) | 345 | 60.6 | 10.1 (4.4, 15.6) |
| N | 2,071 | 69.4 | 60.8 | 701 | 48.9 | |||
| Bottle for nonmilk or nonwater | ||||||||
| Y | 722 | 84.6 | 11.7 (8.7, 14.8) | 76.3 | 11.8 (8.2, 15.2) | 238 | 59.7 | 6.8 (–0.1, 13.7) |
| N | 3,017 | 72.8 | 64.5 | 1,041 | 52.9 | |||
| Salivary-reducing medications | ||||||||
| Y | 177 | 86.4 | 11.8 (6.5, 16.7) | 77.4 | 10.9 (4.3, 16.7) | 51 | 70.6 | 16.6 (3.9, 29.4) |
| N | 3,548 | 76.1 | 66.5 | 1,236 | 53.6 | |||
| Inadequate salivary flow | ||||||||
| Y | 79 | 83.5 | 8.7 (0.1, 16.4) | 75.9 | 9.4 (–0.3, 18.4) | 33 | 57.6 | 4.0 (–13.1, 20.8) |
| N | 3,594 | 75.2 | 66.7 | 1,252 | 54.0 | |||
| Bottle use in bed | ||||||||
| Y | 590 | 85.8 | 12.6 (9.3, 15.6) | 73.6 | 8.2 (4.1, 11.9) | 187 | 58.8 | 5.7 (–2.0, 13.0) |
| N | 3,143 | 73.4 | 65.6 | 1,093 | 53.7 | |||
| Bottle use continuously | ||||||||
| Y | 532 | 84.0 | 9.9 (6.4, 13.3) | 73.7 | 7.5 (3.5, 11.6) | 167 | 60.5 | 7.1 (–0.8, 14.8) |
| N | 3,149 | 74.0 | 66.0 | 1,094 | 53.7 | |||
| Special care needs | ||||||||
| Y | 340 | 84.1 | 9.8 (5.7, 13.9) | 73.2 | 7.1 (2.2, 12.0) | 110 | 54.6 | 0.6 (–9.1, 10.5) |
| N | 3,415 | 74.2 | 66.1 | 1,187 | 53.9 | |||
| Protective items | ||||||||
| Community water fluoridation | ||||||||
| Y | 3,448 | 75.6 | 6.6 (1.4, 12.0) | 67.4 | 8.0 (2.3, 13.5) | 1,213 | 54.2 | 0.6 (–10.1, 11.3) |
| N | 315 | 68.9 | 59.4 | 88 | 53.4 | |||
| Brushes daily with fluoride paste | ||||||||
| Y | 3,075 | 75.6 | 2.6 (–0.9, 6.1) | 67.9 | 5.3 (1.5, 9.2) | 1,082 | 53.5 | –4.0 (–11.3, 2.9) |
| N | 706 | 73.1 | 62.6 | 226 | 57.5 | |||
| Drinks fluoridated water | ||||||||
| Y | 3,387 | 75.2 | 0.9 (–3.5, 5.6) | 67.2 | 4.4 (–0.6, 9.6) | 1,189 | 53.7 | –4.0 (–13.5, 5.4) |
| N | 381 | 74.3 | 62.7 | 902 | 57.9 | |||
| Fluoride varnish in past 6 mo | ||||||||
| Y | 932 | 76.1 | 1.4 (–1.8, 4.6) | 68.3 | 2.1 (–1.2, 5.6) | 394 | 53.3 | –1.3 (–7.3, 4.4) |
| N | 2,834 | 74.7 | 66.2 | 902 | 54.6 | |||
| Caregiver uses xylitol | ||||||||
| Y | 52 | 65.4 | –9.5 (–22.4, 3.5) | 67.3 | 0.4 (–12.7, 13.2) | 19 | 31.6 | –22.4 (–42.3, 0.3) |
| N | 3,668 | 75.1 | 66.7 | 1,264 | 54.4 | |||
| Clinical indicators | ||||||||
| Heavy dental plaque | ||||||||
| Y | 1,678 | 92.3 | 30.8 (28.3, 33.2) | 84.9 | 32.5 (29.7, 35.2) | 493 | 64.9 | 17.6 (12.2, 22.9) |
| N | 2,108 | 61.4 | 52.4 | 812 | 47.4 | |||
| Recently placed restorations | ||||||||
| Y | 753 | 95.1 | 24.9 (22.7, 27.1) | 79.2 | 15.5 (12.1, 18.7) | 294 | 63.6 | 12.1 (5.8, 18.3) |
| N | 3,027 | 70.1 | 63.6 | 1,010 | 51.3 | |||
| Evident tooth decay or white spots | ||||||||
| Y | 2,549 | 96.1 | 63.1 (60.4, 65.8) | 752 | 77.8 | 55.1 (50.4, 59.4) | ||
| N | 1,261 | 33.0 | 563 | 22.7 | ||||
Bold text indicates statistically significant (P < 0.05).
CI, confidence interval; CRA, caries risk assessment.
Number with (Y) or without (N) each baseline CRA factor in the baseline sample.
Includes extreme risk category.
Number with (Y) or without (N) each baseline CRA factor in the follow-up sample.
All CRA risk indicators and clinical indicators were positively and statistically significantly associated both with being classified as high caries risk and with the presence of evident decay at baseline, except inadequate salivary flow, which narrowly missed statistical significance for baseline decay (Table 2). As a group, CRA protective items were not consistently associated with either cross-sectional outcome. The mean number of baseline risk indicators increased by CRA category (low: 1.0, moderate: 1.9, high: 2.5, extreme: 3.8), as did the mean number of clinical indicators (low: 0.1, moderate: 0.4, high: 1.6, extreme: 2.0), but the mean number of protective items was fairly similar by risk level (low: 2.8, moderate: 2.8, high: 2.9, extreme: 2.8).
Longitudinal, Unadjusted Associations
Practitioner baseline caries risk designation was strongly associated with evident decay at follow-up, with decay increasing in each rising CRA category: low (20.4%), moderate (30.6%), and high or extreme (68.7%). Being designated as high or extreme risk had 83.6% sensitivity for decay at follow-up (55.0% specificity), with a positive predictive value of 68.7% (negative predictive value 73.9%). All baseline CRA clinical indicators and 4 risk indicators (frequent snacking, low socioeconomic status, caregiver or sibling tooth decay, and salivary-reducing medications) were positively and statistically significantly associated with evident decay at follow-up (Table 2). None of the individual protective items were statistically significantly associated with evident decay at follow-up (Table 2).
Random Forests Analysis
Thirteen of the 17 CRA items were statistically significant independent indicators of practitioners’ high-risk designation, with the 3 clinical indicators and frequent snacking of greatest relative importance, based on MDA (Fig. 2A). Frequent snacking and dental plaque were the 2 most important CRA items associated with baseline decay (Fig. 2B). However, of the CRA items recorded at baseline, only baseline evident decay was independently and statistically significantly associated with evident decay at follow-up (Fig. 2C). In contrast, when changes in CRA items from baseline to follow-up were considered as possible classifiers, frequent snacking, plaque, and recent restorations were additional statistically significant CRA items (Fig. 2D).
Figure 2.
Relative variable importance plots for caries risk assessment (CRA) items. Horizontal axis represents mean decrease in accuracy (variable importance) from random forests models for (A) baseline CRA items and practitioners’ caries risk designation, (B) baseline CRA items and baseline caries presence, (C) baseline CRA items and follow-up caries presence, and (D) change in CRA items from baseline to follow-up and follow-up caries presence. Filled plotting symbols indicate P < 0.05. Note: interrupted scale for panels A, C, and D.
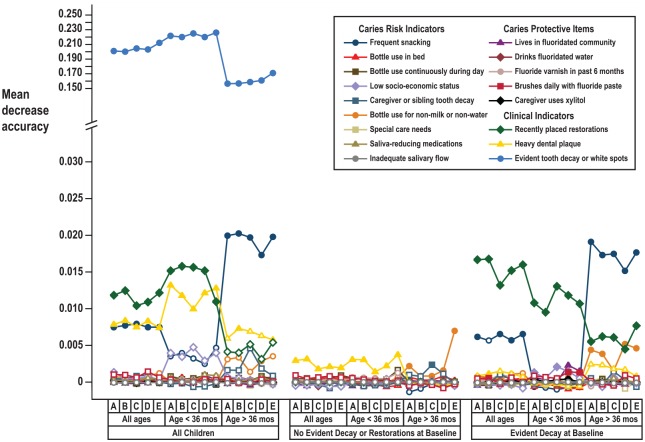
In subgroup analysis, baseline evident decay remained the most critical correlate of future decay in both older and younger children (Fig. 3). Frequent snacking was associated with greater relative importance (larger MDA) among children who were aged ≥36 mo at baseline but was not a statistically significant correlate of future decay among younger children or among children without evidence decay at baseline. Among caries-affected children, the MDA associated with recent restoration placement was greater among children initially aged <36 mo than among older children. Plaque was a statistically significant correlate among younger caries-free children. When baseline decay was excluded as a possible classifier in the total sample, low socioeconomic status joined frequent snacking, plaque, and recent restorations as statistically significant indicators. In all subgroups, results were largely unchanged under methodological variations used as sensitivity checks (Fig. 3).
Figure 3.
Relative variable importance plots for caries risk assessment (CRA) items: subgroup and sensitivity analyses. Vertical axis represents mean decrease in accuracy (variable importance) from random forests models for change in CRA items from baseline to follow-up and presence of caries at follow-up, under subgroups for age and caries status and under sensitivity checks: (A) primary analysis, (B) pooled predictor categories with <25 observations, (C) restricted to observations with no missing values, (D) inverse probability of censoring weighting for losses to follow-up, and (E) restricted to follow-up visits 4 to 24 mo from baseline. Filled plotting symbols indicate P < 0.05. Note: interrupted scale.
Discussion
In the current study, most individual items included in the CAMBRA CRA form for young children were independently associated with providers’ caries risk determination. In turn, practitioners’ assigned risk designations were strongly associated with future disease. This indicates that practitioners used information obtained during CRA to place patients into risk categories that were prognostic at the population level. Consideration of a wide spectrum of items in the CRA form has the potential to serve as the antecedent step to formulation of patient-specific preventive treatment plans tailored to observed risk factors, which is the presumed mechanism through which risk assessment may benefit patients.
In this setting, many individual CRA items were associated with baseline caries status, but fewer were independently related to caries status longitudinally. Clinical indicators, including evident decay and recent disease history, were the most important CRA items in the longitudinal analysis, and information from other items did not meaningfully improve the accuracy of the random forests models in this study. This finding is consistent with previous evaluations of existing pediatric CRA systems, in which baseline disease status was the variable most strongly related to longitudinal caries outcomes (Gao et al. 2013; Tellez et al. 2013).
The fact that only existing disease consistently and independently predicted future disease in these statistical models does not imply that other measured CRA items are not fundamental to the caries process. On the contrary, existing evident decay implies an imbalance of pathological and protective factors that, without intervention, will continue to contribute to further tooth decay (Featherstone 2003). Therefore, clinically evident decay may be the best measurable indication of a constellation of causal factors acting synergistically in the disease process (Aleksejuˉnienė et al. 2009). Independent statistical associations of individual CRA items may miss such combined effects, as inclusion of baseline disease in statistical models may block identification of critical contributions from other existing risk factors, and it is just those risk factors that the clinician aims to modify or mitigate.
Although heterogeneous by statistical approach and study population, numerous multivariable caries prediction models have been developed with moderate prognostic ability (Zero et al. 2001; Mejàre et al. 2014). Few achieved a high level of predictive accuracy (Gao et al. 2010; Gao et al. 2013); in most cases, existing caries at baseline was the single factor most related to future disease (Stewart and Stamm 1991; Disney et al. 1992; Hansel Petersson et al. 2002; Tagliaferro et al. 2006; Peres et al. 2009; Masood et al. 2012; Gao et al. 2014). However, traditional measures of diagnostic accuracy, such as sensitivity or specificity, are not necessarily ideal criteria on which to evaluate the clinical utility of a CRA instrument. Rather, success of risk-based management depends on clinical action to restore the balance in risk and protective factors in individual patients (Ramos-Gomez et al. 2012). More research is needed to evaluate how risk assessment guides this process.
In a recent review, Divaris (2015) raises the concept of “privatization of risk” (Rose 1985) in relation to risk assessment and early childhood caries (ECC), drawing a distinction between ECC risk at the population level and the likelihood that an individual patient will experience ECC at a specific time. Measureable factors associated with caries prevalence in epidemiologic studies are not equivalent to the causal determinants for specific ECC cases (Divaris 2015). For practitioners, this necessitates consideration of each patient’s disease-preventing and predisposing factors in context—a process that a formal caries risk assessment can enhance, even if precise individual-level risk quantification is elusive.
To this end, a major goal of CRA instruments is to identify potentially modifiable disease drivers that practitioners can use to develop individualized caries management strategies (Featherstone 2003; Fontana et al. 2009; Brown 2010). To improve patient outcomes, actionable caries management plans cannot emphasize nonmodifiable factors, such as past disease experience. Rather, the balance of different risk and protective factors acting in combination should be considered collectively and may be more relevant for disease prevention than single factors in isolation (Featherstone 2003). Our results show that resident providers consider items beyond current disease status when assessing patient risk, suggesting that comprehensive CRA instruments have the potential to guide clinical decision making. The present study demonstrates that dental providers in this setting incorporate multiple patient variables in assigning risk status; future investigation is necessary to show that thoughtful risk assessment leads to appropriate preventive care and improved patient outcomes.
In a recent study of predoctoral students’ use of the CAMBRA CRA form for patients aged 6 y and older, 9 CRA items were significantly associated with assigned risk status, with clinical indicators found to weigh most heavily in risk assignment (Doméjean et al. 2015). In the same setting, students’ risk designations were associated with delivery of preventive therapies (Chaffee and Featherstone 2015), consistent with a link between risk assessment and student-providers’ clinical actions. Further research is needed to assess how established dentists outside an educational environment use CRA information to evaluate patients and the extent to which risk-based preventive strategies effectively reduce caries occurrence in children. Without coupling of risk assessment to effective clinical action, CRA itself offers uncertain benefit to patients.
We speculate that additional considerations may contribute to the relatively low predictive value of some CRA items in this study. For instance, a measure of disease severity (e.g., the number of affected teeth), rather than the dichotomous outcome variable used here, might have been more sensitive to modifiable factors. Second, some risk factors, such as inadequate salivary flow, special care needs, and salivary-reducing medications, were uncommon in this population. Thus, the performance of any prediction algorithm for the population would not be greatly swayed by these items, even if such factors were highly influential for a small number of affected children. Similarly, items with a geographic component, such as community water fluoridation and consumption of fluoridated water, were widespread with little within-population variation and therefore would not be good predictors in this study, even if associated with caries outcomes across populations. In addition, protective factors measured at baseline, such as fluoride varnish application and caregiver xylitol use, were also uncommon at baseline, because CRA measures typically reflect patient status prior to engagement in continuous prevention-focused care. In fact, actions from parents and practitioners to reduce identified risk factors or add preventive factors may not be represented in baseline CRA measurements. Finally, some CRA items, such as socioeconomic status and dietary behaviors, represent complex constructs that were reduced to binary items for ease of CRA implementation. For example, it is not known what criteria individual providers used to designate patient socioeconomic status, which was correlated, but not fully concordant, with public dental benefits eligibility. It is plausible that measurement error dampened variable importance for such items.
Comprehensive caries risk assessment is an opportunity for providers to initiate caregiver counseling related to potential caries risk factors. The American Academy of Pediatric Dentistry recommends 2 pediatric CRA instruments (AAPD 2013): a 14-item form for dental providers to children aged 0 to 5 y and a 13-item form for nondental health care providers to children aged 0 to 3 y. Nondentist practitioners are key partners in caries prevention at child medical visits (Keels et al. 2008), for instance, through motivational interviewing, topical fluoride application, or underscoring the importance of oral health for parents, all of which aims to address risk factors before caries manifests (Hausen 1997). Additional research in both dental and nondental settings should examine the extent to which risk assessment with adequate decision support yields long-term caries prevention.
Some study limitations merit consideration. The CAMBRA risk assessment tool for young children does not include bacterial measures, which have been shown to be strongly associated with caries status (Gao et al. 2014). Likewise, CRA instruments have not yet been developed that incorporate genetic information or the timing of disease manifestation (Divaris 2015). Despite consistency with prior investigations (Stewart and Stamm 1991; Disney et al. 1992; Tagliaferro et al. 2006; Peres et al. 2009; Doméjean et al. 2015), not all findings are necessarily generalizable beyond this predominantly urban, low-income population from a fluoridated, high-resource area. Importantly, the student and resident dental providers at this university may not reflect the behaviors of more established dentists in practice or trainees at other universities. As is typical for clinic-based retrospective analyses, loss to follow-up was substantial, but results were largely unchanged under a sensitivity analysis that incorporated censoring weights.
Balancing these limitations, this study advantageously featured a large sample and more than 6 y of observation. The study adds evaluation of individual CRA items in relation to decision making in a pediatric setting side-by-side with analysis of items associated with providers’ risk classification and with disease status. We build on previous applications in dentistry of classification trees (Stewart and Stamm 1991; Gansky 2003) and random forests techniques (Bair et al. 2013), extending the latter approach to caries-related outcomes in children.
To conclude, in this university pediatric population, student and resident dental providers used many CRA items in classifying patients as high caries risk; however, fewer of these variables were independently associated with future disease at the population level. While clinical disease indicators alone might have obtained adequate population-level disease prediction, exclusive focus on evident disease, rather than an individual patient’s mix of pathological and protective factors that caused those disease signs, is unlikely to improve patient outcomes. Our results show that multiple elements of a comprehensive CRA tool are associated with providers’ decision making in classifying patient risk, which suggests that such an instrument may also have the potential to enable consideration of multiple caries predisposing and preventive characteristics when making decisions for personalized caries management.
Author Contributions
B.W. Chaffee, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; J.D.B. Featherstone, L. Zhan, contributed to conception, design, and data interpretation, critically revised the manuscript; S.A. Gansky, contributed to data analysis and interpretation, critically revised the manuscript; J. Cheng, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Bing Espiritu and Tom Ferris of the University of California San Francisco for technical assistance in abstracting clinical data.
Footnotes
Support to Dr. Chaffee was provided from the National Institutes of Health National Center for Advancing Translational Sciences (KL2TR000143). The supporting organization had no role in the conduct of this research. The information presented is solely the responsibility of the authors and does not necessarily represent the official views of the supporting organization.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Adair SM. 2003. The role of caries prevention protocol in pediatric dentistry specialty programs. J Calif Dent Assoc. 31(2):145–147. [PubMed] [Google Scholar]
- Aleksejuˉniene. J, Holst D, Brukiene V. 2009. Dental caries risk studies revisited: causal approaches needed for future inquiries. Int J Environ Res Public Health. 6(12):2992–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatric Dentistry (AAPD). 2013. Guideline on caries-risk assessment and management for infants, children, and adolescents. Pediatr Dent. 35(5):E157–E164. [PubMed] [Google Scholar]
- American Dental Association. 2009–2011. Caries Risk Assessment Form (Age 0–6). Chicago (IL): American Dental Association; [accessed 2016 Apr 19]. Available from: http://www.ada.org/~/media/ADA/Member%20Center/FIles/topics_caries_under6.ashx. [Google Scholar]
- Bair E, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Diatchenko L, Helgeson E, Knott C, Maixner W, Slade GD. 2013. Multivariable modeling of phenotypic risk factors for first-onset TMD: the OPPERA prospective cohort study. J Pain. 14(12, Suppl):T102–T115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D, Hansel Petersson G. 2005. Cariogram—a multifactorial risk assessment model for a multifactorial disease. Community Dent Oral Epidemiol. 33(4):256–264. [DOI] [PubMed] [Google Scholar]
- Breiman L. 2001. Random forests. Machine Learning. 45(1):5–32. [Google Scholar]
- Brocklehurst PR, Ashley JR, Tickle M. 2011. Patient assessment in general dental practice—risk assessment or clinical monitoring? Br Dent J. 210(8):351–354. [DOI] [PubMed] [Google Scholar]
- Brown JP. 1995. Developing clinical teaching methods for caries risk assessment: introduction to the topic and its history. J Dent Educ. 59(10):928–931. [PubMed] [Google Scholar]
- Brown JP. 2010. Dental caries prediction to target high-risk individuals in community-based preventive programs is problematic. J Evid Based Dent Pract. 10(4):241–243. [DOI] [PubMed] [Google Scholar]
- Chaffee BW, Featherstone JD. 2015. Long-term adoption of caries management by risk assessment among dental students in a university clinic. J Dent Educ. 79(5):539–547. [PMC free article] [PubMed] [Google Scholar]
- Disney JA, Graves RC, Stamm JW, Bohannan HM, Abernathy JR, Zack DD. 1992. The University of North Carolina Caries Risk Assessment study: further developments in caries risk prediction. Community Dent Oral Epidemiol. 20(2):64–75. [DOI] [PubMed] [Google Scholar]
- Divaris K. 2015. Predicting dental caries outcomes in children: a “risky” concept. J Dent Res. 95(3):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doméjean S, Leger S, Rechmann P, White JM, Featherstone JD. 2015. How do dental students determine patients’ caries risk level using the Caries Management By Risk Assessment (CAMBRA) system? J Dent Educ. 79(3):278–285. [PubMed] [Google Scholar]
- Featherstone JD. 2003. The caries balance: contributing factors and early detection. J Calif Dent Assoc. 31(2):129–133. [PubMed] [Google Scholar]
- Fontana M, Young DA, Wolff MS. 2009. Evidence-based caries, risk assessment, and treatment. Dent Clin North Am. 53(1):149–161. [DOI] [PubMed] [Google Scholar]
- Gansky SA. 2003. Dental data mining: potential pitfalls and practical issues. Adv Dent Res. 17:109–114. [DOI] [PubMed] [Google Scholar]
- Gao X, Di Wu I, Lo EC, Chu CH, Hsu CY, Wong MC. 2013. Validity of caries risk assessment programmes in preschool children. J Dent. 41(9):787–795. [DOI] [PubMed] [Google Scholar]
- Gao X, Hsu CY, Loh T, Hwarng B, Koh D. 2014. Role of microbiological factors in predicting early childhood caries. Pediatr Dent. 36(4):348–354. [PubMed] [Google Scholar]
- Gao XL, Hsu CY, Xu Y, Hwarng HB, Loh T, Koh D. 2010. Building caries risk assessment models for children. J Dent Res. 89(6):637–643. [DOI] [PubMed] [Google Scholar]
- Hansel Petersson G, Twetman S, Bratthall D. 2002. Evaluation of a computer program for caries risk assessment in schoolchildren. Caries Res. 36(5):327–340. [DOI] [PubMed] [Google Scholar]
- Hausen H. 1997. Caries prediction—state of the art. Community Dent Oral Epidemiol. 25(1):87–96. [DOI] [PubMed] [Google Scholar]
- Keels MA, Hale KJ, Thomas HF, Davis MJ, Czerepak CS, Weiss PA; Section on Pediatric Dentistry and Oral Health. 2008. Preventive oral health intervention for pediatricians. Pediatrics. 122(6):1387–1394. [DOI] [PubMed] [Google Scholar]
- Krasse B. 1985. Caries risk: a practical guide for assessment and control. Chicago (IL): Quintessence. [Google Scholar]
- Masood M, Yusof N, Hassan MI, Jaafar N. 2012. Assessment of dental caries predictors in 6-year-old school children—results from 5-year retrospective cohort study. BMC Public Health. 12:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejàre I, Axelsson S, Dahlén G, Espelid I, Norlund A, Tranæus S, Twetman S. 2014. Caries risk assessment: a systematic review. Acta Odontol Scand. 72(2):81–91. [DOI] [PubMed] [Google Scholar]
- Peres MA, Barros AJ, Peres KG, Araujo CL, Menezes AM. 2009. Life course dental caries determinants and predictors in children aged 12 years: a population-based birth cohort. Community Dent Oral Epidemiol. 37(2):123–133. [DOI] [PubMed] [Google Scholar]
- Powell LV. 1998. Caries risk assessment: relevance to the practitioner. J Am Dent Assoc. 129(3):349–353. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2015. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; [accessed 2016 Apr 19]. Available from: https://www.r-project.org/. [Google Scholar]
- Ramos-Gomez FJ, Crall J, Gansky SA, Slayton RL, Featherstone JD. 2007. Caries risk assessment appropriate for the age 1 visit (infants and toddlers). J Calif Dent Assoc. 35(10):687–702. [PubMed] [Google Scholar]
- Ramos-Gomez FJ, Crystal YO, Doméjean S, Featherstone JD. 2012. Minimal intervention dentistry: part 3. Paediatric dental care—prevention and management protocols using caries risk assessment for infants and young children. Br Dent J. 213(10):501–508. [DOI] [PubMed] [Google Scholar]
- Rehkopf DH, Laraia BA, Segal M, Braithwaite D, Epel E. 2011. The relative importance of predictors of body mass index change, overweight and obesity in adolescent girls. Int J Pediatr Obes. 6(2–2):e233–e242. [DOI] [PubMed] [Google Scholar]
- Rose G. 1985. Sick individuals and sick populations. Int J Epidemiol. 14(1):32–38. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Stamm JW. 1991. Classification tree prediction models for dental caries from clinical, microbiological, and interview data. J Dent Res. 70(9):1239–1251. [DOI] [PubMed] [Google Scholar]
- Strobl C, Boulesteix AL, Zeileis A, Hothorn T. 2007. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro EP, Pereira AC, Meneghim Mde C, Ambrosano GM. 2006. Assessment of dental caries predictors in a seven-year longitudinal study. J Public Health Dent. 66(3):169–173. [DOI] [PubMed] [Google Scholar]
- Tellez M, Gomez J, Pretty I, Ellwood R, Ismail AI. 2013. Evidence on existing caries risk assessment systems: are they predictive of future caries? Community Dent Oral Epidemiol. 41(1):67–78. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zero D, Fontana M, Lennon AM. 2001. Clinical applications and outcomes of using indicators of risk in caries management. J Dent Educ. 65(10):1126–1132. [PubMed] [Google Scholar]