Abstract
In the past two decades, several advancements have improved the care of HIV-infected individuals. Most importantly, the development and deployment of combination antiretroviral therapy (CART) has resulted in a dramatic decline in the rate of deaths from AIDS, so that people living with HIV today have nearly normal life expectancies if treated with CART. The term HIV-associated neurocognitive disorder (HAND) has been used to describe the spectrum of neurocognitive dysfunction associated with HIV infection. HIV can enter the CNS during early stages of infection, and persistent CNS HIV infection and inflammation probably contribute to the development of HAND. The brain can subsequently serve as a sanctuary for ongoing HIV replication, even when systemic viral suppression has been achieved. HAND can remain in patients treated with CART, and its effects on survival, quality of life and everyday functioning make it an important unresolved issue. In this Review, we describe the epidemiology of HAND, the evolving concepts of its neuropathogenesis, novel insights from animal models, and new approaches to treatment. We also discuss how inflammation is sustained in chronic HIV infection. Moreover, we suggest that adjunctive therapies — treatments targeting CNS inflammation and other metabolic processes, including glutamate homeostasis, lipid and energy metabolism — are needed to reverse or improve HAND-related neurological dysfunction.
Several advances have dramatically improved the care and prognosis of HIV-positive (HIV+) individuals in the past 20 years, changing the course of HIV from a life-limiting infection that was frequently complicated by fatal opportunistic infections and malignancies to a chronic infection that can be managed, is associated with a near-normal lifespan and in which opportunistic infections are rare1.
The first major advancement was an understanding of the direct relationship between HIV replication and subsequent immunological and clinical progression. This finding emphasized the need to completely suppress HIV replication in order to control disease progression.
The second major advancement was the development and deployment of combination antiretroviral therapy (CART), which can provide effective systemic suppression of HIV replication. The introduction of CART in the mid-1990s resulted in a 50% decline in the rate of death from AIDS, substantial decreases in rates of maternal–infant transmission, reduced incidence of opportunistic infections, and a 40–50% decrease in the incidence of HIV-associated dementia (HAD), which was previously common and is the most severe form of cognitive impairment associated with the infection2.
The third major change in the care of HIV+ patients was the ability to monitor the efficacy of CART through the reliable and widespread measurement of CD4+ helper T cells, plasma HIV RNA levels and antiretroviral resistance profiles, all of which are now fully integrated into routine clinical care in the developed world and used to optimize treatment for individual patients. Plasma viral load is now routinely monitored in HIV+ patients to ensure complete systemic viral suppression, and resistance profiles are used to monitor for the development of resistance to antiretroviral therapy and to choose CART regimens with optimal effectiveness in a given patient3.
The latest major advancement in the care of HIV+ individuals is the recommendation to begin CART as soon as a patient is willing to commit to this lifelong therapy, regardless of CD4+ T cell count3. This recommendation is bolstered by results from the recent Strategic Timing of Antiretroviral Therapy (START) trial, which proved the benefits and safety of earlier CART initiation on overall outcomes in HIV infection4. In this international study of >4,600 CART-naive HIV+ patients with CD4+ T cell counts >500 cells/μL, immediate initiation of CART resulted in fewer AIDS-related and non-AIDS related events than did deferring initiation of CART until CD4+ T cell counts fell below 350 cells/μL.
Key points.
Despite entering the era of combination antiretroviral therapy (CART), HIV-associated neurocognitive disorder (HAND) remains prevalent; however, less severe forms of HAND now predominate, and the most severe form, HIV-associated dementia, is rare
In individuals treated with CART, the risk of HAND increases with age and in the presence of cardiovascular disease risk factors
Latent HIV can persist in the brain even when systemic virological control is achieved with CART, thereby hampering efforts to eradicate HIV
Animal models of CNS HIV infection — such as macaques infected with simian immunodeficiency virus — develop severe HAND, viral encephalitis and neuronal apoptosis, and are central to understanding the immunopathogenesis of HIV-induced CNS damage
A growing body of work indicates that mild HAND can be modelled in immunocompetent mice infected with chimeric HIV (a model known as EcoHIV), and in chronically HIV-infected immunodeficient mice reconstituted with human immune systems
To date, clinical trials of HAND therapies have been unsuccessful, but further trials for the treatment of HAND are forthcoming, including a trial of intranasal insuli
For almost a decade, the term HIV-associated neurocognitive disorder (HAND) has been used to describe the range of neurocognitive dysfunction associated with HIV infection5. Just as the course of HIV or AIDS has changed significantly over the past two decades, so has the course of HAND (FIG. 1). Nevertheless, despite our increasing knowledge and understanding of HAND, there is still no definitive marker or specific treatment: CART is the only option to prevent or delay the progression of HAND, but it is effective only in a subset of patients. The development of HAND remains an important issue for HIV+ patients, as it affects not only survival and quality of life, but also everyday functioning6. Worldwide, HAND remains a common cause of cognitive impairment and has persisted even in individuals who have received CART7,8. As CART becomes more widely distributed in resource-limited settings and improves survival, the long-term global impact of HAND will become even more significant. In addition, early HIV infection of the CNS is believed to contribute to the development of HAND, and evidence suggests that the CNS can subsequently serve as a reservoir for ongoing HIV replication, thereby limiting the opportunity for a sterilizing cure or eradication9.
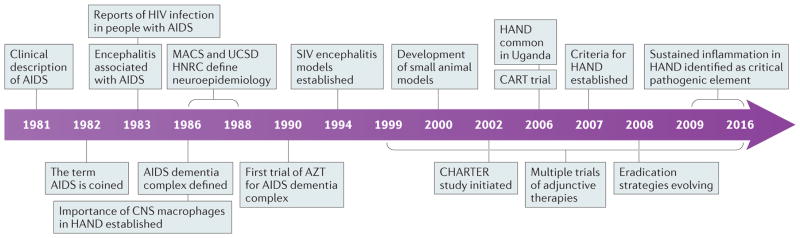
Figure 1. Timeline of advances in neuro-AIDS research.
Since the discovery of AIDS in 1981 and HIV in 1983, important advances have been made in research into and the prevention and treatment of HIV-associated neurocognitive disorder (HAND). AZT, azidothymidine; CART, combination antiretroviral therapy; HNRC, HIV Neurobehavioral Research Center; MACS, Multicenter AIDS Cohort Study; UCSD, University of California San Diego.
This Review will focus on HAND, describing its changing epidemiology and its neuropathogenesis, including recent insights from animal models. We will review known risk factors for HAND and consider projections of the epidemiology in resource-limited countries and among the ageing population with HIV infection. We will also detail the evidence for early brain infection and the brain as a sanctuary for HIV, as well as considering how and why inflammation is sustained in chronic HIV infection, even when systemic virological control is achieved. Finally, we will discuss new approaches to the treatment of HAND and their implications in an era when HIV eradication might be feasible. In particular, adjunctive therapies targeting CNS inflammation and other metabolic processes, including glutamate homeostasis, lipid and energy metabolism, could be necessary to prevent or improve HAND-related neurological dysfunction10.
Clinical features of HAND
Epidemiology and risk factors
HAND refers to a spectrum of neurocognitive impairment that includes asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD), and is diagnosed using neuropsychological testing and functional status assessments5 (TABLE 1). In the pre-CART era, HAD was the most common form of HAND and was almost invariably fatal11,12. However, the prevalence of HAD has substantially declined with the widespread implementation of CART. Before 1991, 20% of participants enrolled in the Multicenter AIDS Cohort Study (MACS) met the criteria for HAD, but only 5% met the criteria in 2001–2003 (REFS 13,14). As a result, HAD — the most severe form of HAND — is rare in the developed world today.
Table 1.
Classification of HIV-associated neurocognitive disorders
| HIV-associated neurocognitive dysfunction (HAND) type* | Prevalence in CART-treated HIV+ individuals | Diagnostic criteria5 |
|---|---|---|
| Asymptomatic neurocognitive impairment (ANI) | 30% |
|
| Mild neurocognitive disorder (MND) | 20–30% |
|
| HIV-associated dementia (HAD) | 2–8% |
|
SD, standard deviation.
With no evidence of other cause. Adapted from Antinori, A. et al. Neurology 69, 1789–1799 (2007).
Changes in HAND severity in the CART era
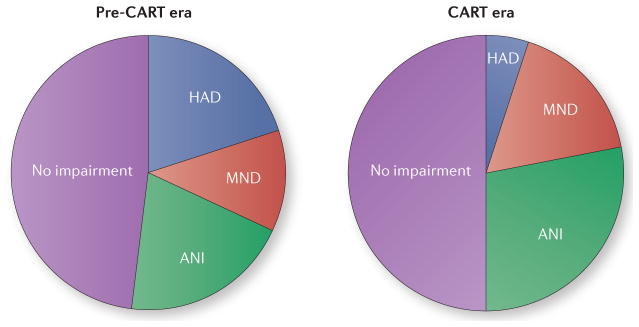
Despite improved life expectancies and a dramatic decline in the rates of CNS opportunistic infections in HIV+ people as a result of CART, HAND remains a major cause of morbidity: 15–55% of HIV+ individuals are estimated to have HAND — a proportion that remains similar to that in the pre-CART era7,14 (FIG. 2). It should be noted, though, that these HAND cases primarily represent the more mild forms of the condition7,14. In both the USA and Sub-Saharan Africa, patients receiving CART have much better neuropsychological function than CART-naive patients or individuals treated with zidovudine monotherapy13,15. As a result, the prevalence of milder forms of HAND has increased so that ANI now accounts for approximately 70% of all forms of HAND7.
Figure 2. More-effective therapies have reduced the severity of HIV-associated the severity of HIV-associated neurocognitive disorders.
Since the introduction of combination antiretroviral therapies (CARTs) in 1996, the proportion of HIV+ individuals with neurocognitive symptoms has remained unchanged, but the proportion of people with severe symptoms has declined so that HIV-associated dementia (HAD) is much less common and asymptomatic neurocognitive impairment (ANI) now accounts for the majority of cases. MND, mild neurocognitive disorder. Adapted from McArthur, J. C. et al. Ann. Neurol. 67, 699–714 (2010).
Conversion from asymptomatic to symptomatic HAND
Despite being asymptomatic, ANI is clinically relevant because individuals with ANI can transition to one of the more severe forms of HAND: for example, participants of the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study who had ANI at baseline were two to six times more likely to develop symptomatic HAND during several years of follow-up than those who were neurocognitively normal at baseline16. The increased risk of conversion to symptomatic dementia for individuals with ANI could reflect the finding that some individuals have very early involvement of the brain after HIV infection. For example, structural brain changes can sometimes be identified by neuroimaging within 100 days of primary infection, even in the absence of symptomatic involvement17,18. However, the term ANI should be reserved for research studies, as its use in clinical settings remains controversial.
HAND and immunosuppression
Besides the reduced severity of HAND, other epidemiological features of the condition have also changed in the CART era. For example, in the pre-CART era, HAD was primarily seen in advanced HIV disease19. Although HAD is much less prevalent in patients receiving CART, when it does occur, it now often does so in patients with less severe immunosuppression20. Moreover, in the pre-CART era, low CD4+ T cell counts21 and high plasma and cerebrospinal fluid (CSF) viral loads20,22 were associated with HAD, but these biomarkers of viral infection are not consistently associated with cognitive impairment in CART-treated patients20, and new predictive biomarkers are being sought. On the other hand, CD4+ T cell count nadir remains strongly associated with HAND, even in virologically suppressed patients on CART, and a history of clinically-defined AIDS is associated with onset of cognitive impairment at a younger age (<50 years)7,20,23,24.
HAND progression
Perhaps surprisingly, the true impact of CART on HAND remains ill-defined. HAND generally remains stable during CART, but rarely resolves completely. A 4-year study of 197 CART-treated individuals demonstrated that 77% remained neurocognitively stable, with only 13% deteriorating to a more severe form of HAND, and 10% improving25. Thus, HAND is typically not progressive in the majority of aviraemic HIV+ individuals on CART. The fact that lower CD4+ T cell nadir is a risk factor for HAND7 suggests that earlier HIV treatment to prevent severe immunosuppression could reduce the severity of HAND (that is, shift the phenotype from HAD to MND or ANI). However, the recent START trial failed to confirm a major effect of early CART on HAND4,26. Although immediate initiation of CART resulted in fewer AIDS-related events than did deferred initiation of CART, performance on neuropsychological testing did not differ between these groups after a mean of 3.3 years of follow-up, indicating that earlier treatment might not markedly affect the development of HAND27.
Clinical characteristics of and risk factors for HAND have also changed with CART. Typically, HAND presents with executive dysfunction and memory impairment with prominent disruption of attention, multitasking, impulse control, judgement and memory encoding and retrieval. HAND can also be associated with motor dysfunction, including bradykinesia, loss of coordination and gait imbalance. Whereas deficits in motor skills and psychomotor speed were the most common manifestations of HAND before CART, deficits in learning and/or memory and executive function are more common symptoms today20.
Risk factors for HAND
Cardiovascular risk factors
Cardiovascular risk factors were linked to poorer cognitive performance in the MACS and other cohort studies, and central obesity and diabetes were important risk factors for HAND in the CHARTER cohort28,29. In a study of 245 HIV+ individuals in Italy, diabetes, carotid intima-media thickness and cardiovascular risk factors — including hyperlipidaemia and tobacco use — were strongly associated with lower cognitive performance30.
Age
Older age is associated with an increased risk of HAND. Older HIV+ adults (>50 years) in the Hawaii Ageing Cohort were twice as likely as their younger HIV+ counterparts to have HAD after adjusting for other known dementia risk factors31,32. Older age has also been associated with increased risk of HAND in several South African cohorts33,34; however, the effect of age on HAND risk is likely confounded by the increased prevalence of cerebrovascular risk factors at older ages26,35. In the CHARTER cohort, older age, elevated systolic blood pressure, high BMI, high serum cholesterol, and a diagnosis of AIDS were associated with worse global neuropsychological performance, suggesting that small or large vessel atherosclerotic disease could be contributing to cognitive impairment in older HIV+ individuals36.
Hepatitis C virus infection
In one cohort, hepatitis C co-infection was found to nearly double the risk of cognitive impairment in HIV+ individuals compared with those without hepatitis C37; however, a later study found hepatitis C infection to have no effect on the cognitive performance of HIV+ individuals38. With the prospect of a true cure for hepatitis C virus with new and potent antiviral regimens, this controversial point needs further research.
Additional risk factors
Substances of abuse, particularly methamphetamine, have deleterious effects on cognition, which are more pronounced when combined with HIV infection39. Cognitive reserve could also be important: in the MACS, cognitive impairment was observed in 38% of HIV+ participants with less than a high-school education, but only 17% of HIV+ participants with at least a high-school education13. Clinically, several comorbidities can contribute to cognitive impairment in HIV+ individuals (BOX 1). If these comorbidities are present, it is difficult to ascertain with certainty whether cognitive impairment is caused by direct effects of HIV, direct effects of the comorbidities, or a combination of both, thereby making a definitive diagnosis of HAND more difficult. Additional risk factors for HAND in the CART era are summarized in BOX 2.
Box 1. Comorbidities to be considered in HAND.
Age-related cognitive deficits
Alcohol and substance abuse
Viral co-infection: HCV, HIV-2 and HTLV-I
Nutritional and vitamin deficiencies
Accelerated atherosclerosis
Traumatic brain injury
Obstructive sleep apnoea, disturbed sleep
Psychiatric illnesses: anxiety disorders, major depression, bipolar disorder
HAND, HIV-associated neurocognitive syndrome; HCV, hepatitis C virus; HIV-2, human immunodeficiency virus type 2; HTLV, human T-lymphotropic virus I.
Box 2. Risk factors for HAND in the CART era.
Advanced age
Low CD4+ T cells nadir
Use of illicit drugs
Hepatitis C co-infection
Cerebrovascular disease risk factors: diabetes, hypertension, hypercholesterolaemia, obesity
Sleep disorders: insomnia, obstructive sleep apnoea, sleep fragmentation
Psychiatric comorbidity: major depression, anxiety disorders, bipolar disease
CART, combination antiretroviral therapy; HAND, HIV-associated neurocognitive disorder.
HAND in the global setting
More than 70% of the world’s HIV+ population lives in Sub-Saharan Africa, and HIV+ individuals throughout this region tend to have worse neuropsychological function than HIV-uninfected (HIV−) counterparts33,40–45. In a study of HIV+ outpatients in Uganda, nearly one-third met the criteria for HAD, with advanced age and low CD4+ T cell count conferring increased risk46. In a South African study, 25% of CART-naive HIV+ individuals met the criteria for HAD, and an additional 42% met the criteria for MND33. Large longitudinal studies from resource-limited settings are lacking, but if these proportions are accurate, HAND would be the most common form of young-age neurocognitive impairment worldwide. In addition, owing to an increased risk of HAND with advancing age, the burden of HAND in the developing world might continue to increase as life expectancy rises with increased availability of CART. However, this change could be offset by the wider deployment of CART: the AIDS Clinical Trials Group (ACTG) A5199 trial tested the effects of different CART regimens in resource-limited settings and showed improved neuropsychological performance, regardless of the specific regimen47. CART was also shown to improve cognitive performance and everyday function in a clinical setting in Uganda15.
Biomarkers in HAND
Although a variety of potential biomarkers for HAND have been identified (TABLE 2), the majority of these are actually markers associated with HAD rather than ANI and MND, which currently represent much more common forms of cognitive impairment. Validated biomarkers for these more common forms are desperately needed to more accurately diagnose and delineate the early stages of HAND (given that it is often very challenging to distinguish ANI and MND from other comorbidities) or, more importantly, to predict the trajectory of cognitive function in HIV+ patients. Biomarkers that could identify a preclinical stage of HAND or predict cognitive worsening (expected in 23% of patients with ANI48) would open the possibility of treatment at the earliest stage of neurological decline, when interventions are likely to have the greatest impact. Equally important is the identification of biomarkers that are associated with cognitive improvement, which would enable more accurate assessment of the interventions in phase I/II clinical trials that are too short for reliable assessment of effectiveness with neuropsychological tests. Finally, understanding the underlying molecular mechanisms and how biomarkers differ across the spectrum of HAND will ultimately facilitate the identification and development of precision therapeutics.
Table 2.
Surrogate biomarkers for cognitive status in HIV
| Pathophysiological mechanism | Primary infection | ANI/MND | HIV-associated dementia |
|---|---|---|---|
| Cell stress | Not determined | ||
| Neuronal injury/protection | Not determined | ||
| Oxidative stress | Not determined | ||
| Energy metabolism | Brain choline57,224 | Brain choline79 | |
| Immune activation | |||
| Glutamate regulation | Brain glutamate224 |
ANI, asymptomatic neurocognitive impairment; sCD, soluble cluster of differentiation; CSF, cerebrospinal fluid; Glx, glutamate/glutamine complex; HLA DR, human leukocyte antigen–antigen D related; IFN, interferon; iNOS, inducible nitrous oxide synthase; IP, inducible protein; MCP, monocyte specific chemokine; MMP, matrix metalloprotease; MND, mild neurocognitive disorder; NAA, N-acetyl aspartate; NFL, neurofilament light chain; p-tau, phosphorylated tau; sAPPβ, soluble amyloid precursor protein beta; STAT, signal transducers and activators of transcription; SOD 1, superoxide dismutase 1; TNF, tumour necrosis factor; t-tau, total tau.
Changes in these markers indicate cognitive decline in HIV+ patients.
Changes in these markers indicate cognitive improvement in HIV+ patients.
Some of the tested molecules were associated with cognitive decline, some with cognitive improvement.
Biomarkers for HAND can be broadly classified into four groups: soluble markers of immune activation, markers of metabolic or cellular stress, neuronal injury markers, and neuroimaging markers. As several recent reviews have summarized the current knowledge of neuroimaging markers at various stages of HAND49–52, we will only briefly discuss selected imaging markers that are related to soluble indicators. Our focus here is to briefly discuss biomarkers in the context of HAND staging, and the progress made in informatics approaches that attempt to incorporate biological markers with clinical and demographic features so as to identify groups of prognostic indicators for change in cognitive status.
Innovative approaches to foster community networking between populations that are at risk of HIV infection have provided unique opportunities to study biological and neuropsychological changes that occur at very early time points following HIV infection. These studies have revealed that neurological involvement occurs very rapidly following infection. Early changes in brain structure, including increased permeability of the blood–brain barrier, reductions in brain volume17,53 and decreases in diffusion measures of white matter54, can appear within the first few months following infection. These structural modifications are accompanied by increases in circulating levels of inflammatory cytokines, immune activation17,55,56, evidence of acute metabolic disturbances57, and measurable deficits in cognitive and psychomotor functions58. Many of these structural, cognitive and inflammatory modifications do not improve to a clinically relevant degree following the initiation of CART57–59 and can persist or even worsen over time in some individuals, despite systemic viral suppression60–64. These pathological modifications have considerable individual variability, and it is currently not known how these very early events affect the development and/or trajectory of cognitive impairments later in life. Continuing to follow these patients from very early infection through long-term CART will provide valuable information on how individual responses to infection affect long-term outcomes.
Biomarkers implicated in HAND
In individuals who develop ANI and MND, markers of immune65–67 and cytokine activation68,69 are more pronounced than in HIV+ individuals without cognitive impairment. Likewise, changes in bioenergetics, as measured with neuroimaging70–72 and metabolomic appraches73,74, are readily apparent in individuals with ANI or MND compared with cognitively normal HIV+ individuals, as are accumulations of bioactive lipids, such as ceramide, and sterol markers of cell stress75,76. Brain structural changes84 and progressive impairments in energy and lipid metabolism75,77–80, immune regulation67,81,82 and metabolism74,83 worsen with age and duration of infection50,85–89. Whether markers of neuronal and axonal injury are elevated during acute infection is not entirely clear. Initial studies reported that markers of neuronal injury, such as neurofilament light chain protein, tau and amyloid precursor proteins, were not elevated during early stages of ANI, or at early time points following the initiation of CART90,91. Rather, these markers typically appear in the mid to late stages of the disease process91–93 and correlate with low CD4+ T cell counts94 and MRI markers of neuronal damage95. However, ultrasensitive measures of neurofilament light chain levels identified that 44% of people with primary HIV infection did show elevated markers of neuronal damage, suggesting that a subset of newly infected individuals show signs of neurological injury. These findings demonstrate that neurological involvement occurs within months of initial HIV infection95, but the precise relationship between this early involvement and the onset of cognitive impairment is unknown. The interval between early involvement and clinical HAND symptoms suggests the existence of a lengthy therapeutic window, during which targeted interventions could preserve cognitive function.
The need for composite biomarkers
Our current lack of clinically validated biomarkers for ANI and MND suggests that any single biomarker might be insufficient to identify early stages of HAND, and that alternative methods that can identify combinations of markers might facilitate efforts to reliably identify the earliest stages of HAND in clinical practice. Advanced statistical approaches, such as machine learning and multivariate statistical modelling, have been increasingly used to interrogate complex biological and clinical data. For neurodegenerative conditions, these approaches are based on the notion that complex diseases such as HAND can be better understood by incorporating multiple biological and clinical variables using nonparametric approaches. Studies using these approaches have begun to identify clinical and demographic factors, including time-dependent treatment effects, historical or current comorbid conditions and metabolic pathways associated with lipid metabolism, bioenergetics and inflammation, that are progressively perturbed during the onset and worsening of ANI and MND73,75,96. Validation of these models and of the underlying mechanistic pathways identified will be critical in assessing their utility for clinical practice and the identification of molecular targets for precision therapeutics.
Pathogenesis in HAND
The neuropathology of HAND has changed considerably since the introduction of CART: the frequency with which HIV encephalitis is observed at autopsy has reduced from 54% before CART to 15% in the CART era97. Encephalitis and outright neuronal loss were historically thought to have central roles in HAND, but these factors are no longer sufficient to explain neurological dysfunction in the CART era, as these pathologies are no longer typical98. The paucity of overt neuropathology specific to HIV infection in CART-treated patients suggests that the underlying pathophysiology of HAND is more likely to be associated with functional alterations in neurons (FIG. 3). This paradigm shift necessitates new therapeutic strategies tailored to preserve brain function in CART-treated patients.
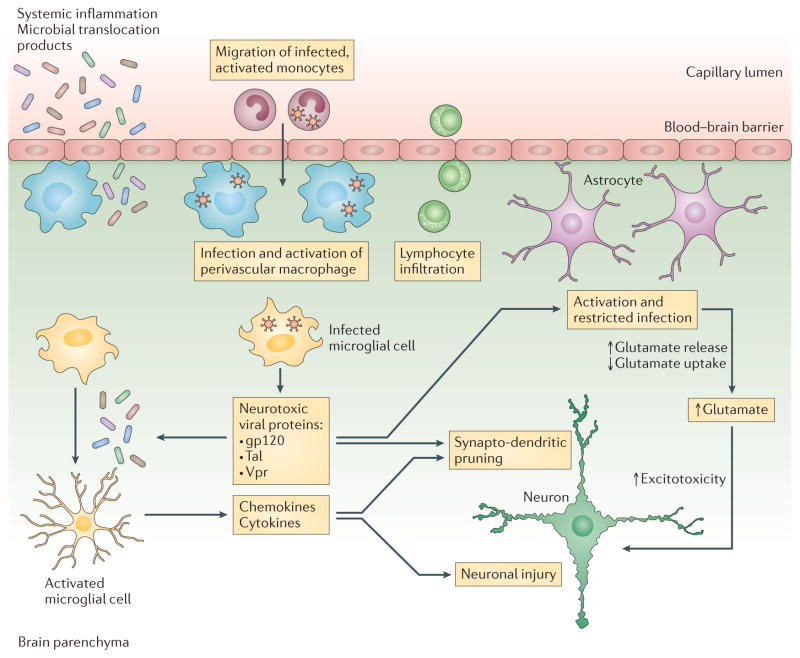
Figure 3. Neuropathogenic mechanisms that contribute to HIV-associated neurocognitive disorders.
HIV-infected macrophages and microglial cells release neurotoxic viral proteins that trigger astrocyte activation, which results in increased glutamate release and reduced glutamate uptake. Elevated extracellular glutamate levels cause neuronal bioenergetic disturbances that lead to aberrant synaptodendritic pruning and neuronal injury. Moreover, systemic inflammation and microbial translocation products lead to microglial activation and increased production of chemokines and cytokines that contribute to neuronal injury. Adapted from Williams, D. W. et al. Curr. HIV Res. 12, 85–96 (2014).
Inflammation and HIV neurotoxicity in HAND
Compelling evidence suggests that inflammation has an important role in triggering events that lead to neurodegeneration in HIV infection. However, robust inflammation is not always seen in HAND, especially early in the disease process.
Navia et al.99 provided one of the first comprehensive neuropathological studies of HAD in 1986, describing their findings as follows: “Most commonly noted was diffuse pallor in the white matter, which in the pathologically milder cases was accompanied by scanty perivascular infiltrates of lymphocytes and brown-pigmented macrophages, and in the most advanced cases by clusters of foamy macrophages and multinucleated cells associated with multifocal rarefaction of the white matter. However, in nearly one third of the demented cases the histopathological findings were remarkably bland in relation to the severity of clinical dysfunction. In addition, similar mild changes were noted in over one half of the non-demented patients, consistent with subclinical involvement”. Their observations remain relevant today. Contemporary pathological studies in CART-treated individuals do not usually report white matter pallor, but more-subtle changes in white matter integrity are apparent on diffusion tensor imaging; these changes seem to worsen with increasing age and duration of infection85,100–105. Activated circulating monocytes continue to have a critical role, both for the introduction of HIV into the brain via transmigration across the blood–brain barrier in response to chemotactic signals expressed within the parenchyma, and for the subsequent establishment of infection within CNS perivascular macrophages106–108, microglia109 and astrocytes110. Although astrocytes do not seem capable of producing intact virions under normal conditions, they can produce and export non-structural proteins such as tat (an HIV transcription factor), Rev, and Nef, all of which promote inflammation and neuronal damage111–113.
One question that remains unanswered is why CNS inflammation is sustained even when the initial stimulus — viral replication — is suppressed by CART95,114. According to one hypothesis, the inflammatory responses initiated by HIV direct the proteasome to become an ‘immunoproteasome’ that impedes turnover of folded proteins in brain cells and affects cellular homeostasis and response to stress115, resulting in perturbed neuronal and synaptic protein dynamics, possibly contributing to HAND. Another postulated mechanism for the sustained CNS inflammation is microglial priming from circulating microbial translocation products derived from gut bacteria and a disturbed microbiome116. It has also been suggested that the CNS inflammation in CART-treated individuals could be an attenuated form of immune reconstitution inflammatory syndrome1. The role of genetic control of inflammatory responses, specifically, polymorphisms in genes encoding CCL3L1 and CCR5, has also been invoked to explain the individual variability in the course of HAND as well as the occurrence of HAND in only a subset of HIV+ individuals117.
The brain as a reservoir for HIV persistence
The importance of the brain as a potential reservoir for persistent HIV infection has gained renewed importance, with the intense focus of efforts being on eradication strategies. Clearly, a true sterilizing cure cannot be achieved if the brain harbours latent HIV that can be reactivated and can then reseed systemic infection. Simian immunodeficiency virus (SIV) models (see below) have produced robust evidence for the persistence of SIV DNA even after prolonged suppression of viral replication with CART118. Numerous studies of HIV RNA levels in the CSF have also suggested the presence of latent infection in the brain. The phenomenon of CSF viral escape supports the concept of a CNS reservoir9. This phenomenon can occur in as many as 5–10% of CART recipients and is associated with immune activation119 and major depressive disorder120.
Clearance of both latent and productive HIV from the brain must underpin successful eradication. Macrophages and microglia, the cells within the brain that harbour HIV and produce infectious virions, are very long-lived, with turn-over rates of months or years121. The importance of the relative penetrance of different antiretrovirals into the brain remains debated122, but it is certainly plausible that lower concentrations of antiretrovirals within the CNS might lead to sub-optimal virological suppression. The cellular pharmacology of CART is relatively understudied with regard to tissue macrophages. A much higher maximal effective concentration (EC50) in macrophages than in lymphocytes might reduce CART efficacy in this cell type123. Furthermore, up to 20% of astrocytes isolated directly from autopsy brain tissues of HIV+ individuals contain integrated HIV124. The ability of HIV-1 to integrate into terminally differentiated astrocytes suggests a permanent reservoir of provirus in the brain that influences the development and likely success of strategies aimed at eradicating HIV-1.
Early and progressive disturbances of bioenergetics
The sparing of neurons in CART-treated individuals with HAND suggests that functional changes underlie cognitive impairment in HIV+ individuals. Several lines of evidence support the notion that a loss of bioenergetic homeostasis could be an early event that primes the CNS for functional deficits. Brain gene expression profiling studies have identified that HIV+ patients exhibit widespread alterations in the expression of genes that regulate brain energy metabolism, and dysregulation progresses over time125. PET studies have revealed varying degrees of reduced glucose uptake in the mesial frontal gyrus126, as well as evidence for small but consistent age-related reductions of glucose uptake in the anterior cingulate cortex71 in individuals with undetectable plasma viral loads. Moreover, magnetic resonance spectroscopy imaging studies have identified progressive abnormalities in levels of choline, N-acetylaspartate, glutamate and glutamine-containing compounds in multiple brain regions of HIV-infected individuals on CART77,78; these abnormalities correlated with deficits in motor and psychomotor speed, attention and working memory70,77,78. High field-strength nuclear magnetic resonance (NMR) spectroscopy analysis of energy metabolites in the CSF has revealed accumulation of specific tricarboxylic acid cycle and glycolytic intermediates that were associated with changes in cognitive status in CART-treated HIV+ patients73. The pattern of change in these metabolic features suggested that worsening cognitive function is associated with increased aerobic glycolysis, and improvements in cognitive function are associated with a shift in metabolism to promote anaerobic glycolysis. These disruptions in cellular energetics could explain abnormal accumulation of sphingolipids and proteins, such as amyloid-β, in dysfunctional endolysosomal compartments72,75,76,80,127–129: reduced or modified cellular energy production would impair the function of the proton pumps that are dependent on adenosine triphosphate (ATP) — these pumps are necessary to maintain an acidic luminal pH, which is required for efficient functioning of >50 hydrolytic enzymes in lysosomes that degrade cellular products128,130,131. The discussed studies have identified several possible targets for therapeutic intervention that include modulators of glucose metabolism (intranasal insulin), ceramide metabolism72,132,133 and endolysosomal function131. Small-molecule therapeutics designed to affect these targets are in early to mid-stage development131,134, and a clinical trial is planned to determine the effectiveness of intranasal insulin to treat ANI and MND in HIV+ patients.
Evidence for abnormal glutamate homeostasis
Both viral and host factors are thought to perturb brain glutamate metabolism and neurotransmission, and thereby have an important role in the development of HAND135,136. In support of this notion, CSF levels of glutamate are fivefold greater in HIV+ individuals than in healthy controls137, and recent studies of HIV+ patients who received CART revealed selective increases in CSF levels of glutamate in patients with HAND compared with patients without neurocognitive impairment74. Tat and envelope glycoprotein gp120 have been shown to decrease glial and synaptic glutamate uptake138,139, stimulate glutamate release from nerve endings138,140, and phosphorylate glutamate receptors, thus potentiating the toxicity of the neurotransmitter141. Although glutamate levels in the CSF are increased in HIV+ patients with cognitive impairment, glutamate levels are selectively lower in the parietal grey matter, basal ganglia and cortex77,78. These findings are consistent with incomplete recycling of glutamate by the glutamate–glutamine shuttle, leading to increased output of glutamate into the extracellular space and a reduction in the total amount of intraneuronal glutamate. HIV-infected macrophages have been shown to release ATP, which triggers production of neurotoxic levels of glutamate142 and decreases expression of the cytoprotective enzyme haem-oxygenase-1.
Several strategies for modulating glutamate-mediated neuronal toxicity have been evaluated. Early work focused on N-methyl-D-aspartate (NMDA) receptor antagonism, in particular the use of 1-amino-3,5-dimethyladamantane (memantine), a noncompetitive, low-affinity antagonist approved for treatment of Alzheimer disease. Unfortunately, despite preclinical studies suggesting a promising efficacy143–145, initial clinical trials in HAND showed no effect146. One alternative to direct receptor blockade is the modulation of enzymes that are responsible for production of glutamate, such as glutaminase and glutamate carboxypeptidase II. Glutaminase mRNA and protein are strongly upregulated following HIV infection, and inhibition of this upregulation blocks glutamate release and provides neuroprotection in pre-clinical models of HAND147,148. Similarly, small-molecule inhibitors of glutamate carboxypeptidase II, which blocks conversion of the abundant neuropeptide N-acetyl-aspartylglutamate into glutamate, have been shown to protect against gp120-induced toxicity149. Another suggested approach is to regulate the transporters responsible for modulation of extracellular glutamate, such as the cystine–glutamate transporter, which is profoundly upregulated in microglia that are activated by tat150. Unfortunately, no clinically available brain-penetrating inhibitors exist to test this hypothesis in patients, but preclinical development is underway151,152.
Animal models of neuro-HIV
Primate models
SIV-infected macaque models are of great value for studying the pathogenesis of HAND, including attempts to discover neuroprotective host genes and predictive plasma and CSF biomarkers153. SIV-induced neuropathology closely resembles HIV-induced alterations, including multifocal perivascular aggregates in the brain that are composed of macrophages and multi-nucleate giant cells that contain replicating virus154. Macaque models are particularly useful for studying the neuropathogenesis of HIV because plasma, CSF, and CNS samples can be obtained at multiple time points throughout infection, from acute through asymptomatic to terminal stages. In addition, SIV-infected macaques can be treated with suppressive antiretroviral therapy to study HAND in the context of treatment155.
Various SIV models have been established to study HIV-induced CNS damage. One such model uses intravenous inoculation of pigtailed macaques with both a neurovirulent clone, SIVmac/17E-Fr, and an immunosuppressive swarm, SIV/DeltaB670. With this inoculation combination, approximately two-thirds of macaques develop SIV encephalitis within 84 days156,157. Of interest is the fact that, though most studies of SIV pathogenesis use rhesus macaques, pigtailed macaques develop CNS disease more often than do rhesus macaques that receive the same SIV inoculum158. Key features of the pigtailed macaque SIV model include development of CNS inflammation that correlates with high viral load in the brain, cognitive and motor deficits typical of HIV, and the classic lesions of HIV encephalitis156,159–163. Furthermore, the pigtailed macaque SIV model enables the impact of antiretroviral therapy on the CNS to be studied, as neuroinflammation persists despite suppression of plasma and CSF viral load155. In another model, SIV encephalitis is induced in most SIVmac251-infected rhesus macaques through depletion of CD8+ cells164–166. Although this model illustrates key role of CD8+ cells in the neuropathogenesis and has been informative with respect to CNS macrophage biology, the elimination of both CD8+ T cells and natural killer cells constrains the study of cell-mediated immunity in the CNS.
The physiological relevance of primate models to mild HAND in CART-treated individuals has not yet been established. Starting CART for infected animals as early as 4 days after infection had significant benefits for acute brain disease, including suppression of SIV expression in the brain and improvements in inflammation and immunological aspects of the disease155,167. Initiation of CART treatment after acute infection reduced SIV burden in the brain and prevented neurophysiological and locomotional alterations168. Longer-term CART regimens and neurocognitive assessments of SIV-infected macaques treated with CART might, therefore, be necessary to demonstrate mild HAND in this model.
Rodent models
Rodents lack the receptors and some cellular factors to support high-level productive HIV infection169. The first mouse model made to study the impact of HIV on the brain included selective expression of envelope (env)170, a pathogenic HIV protein, in astrocytes under the control of the glial fibrillary acidic promoter (GFAP)171. These transgenic animals showed evidence of neurotoxicity and behavioural deficits171–174, as well as defective neurogenesis173,175. A different mouse model, based on inducible doxycycline-dependent expression of tat under the control of GFAP175–177,175, resulted in synaptic pathology, learning and memory deficits, and anxiety178–181.
Limitations of rodent models
Although a great deal about the pathophysiological effects of HIV env and tat proteins has been learned from rodent models182,183 and these models continue to be relevant for research into some pathologies associated with HAND, they also have limitations. For example, such transgenic models cannot mimic complex aspects of HIV infection in the human host, such as invasion of the brain by activated HIV-infected and uninfected monocytes and macrophages and by free virus, which are key events in the development of HAND184.
Towards better rodent models of neuro-HIV
Alternative approaches to address the limitations of rodent models include the expression of human receptors and co-receptors, tat-cofactors, and/or the viral genome in rodents185–187. Mice and rats that carry a gag-pol deleted HIV transgene show a broad range of pathologies, including nephropathy, pulmonary disease and brain abnormalities188–190. The transgenic HIV rat model, in particular, has been used extensively by several research groups as a model for HIV-associated brain disease; the phenotypic alterations of these rats include HAND-like gene expression profiles, changes in energy metabolism in the brain, synaptodendritic damage and behavioural deficits191–193. These pathologies are probably caused by HIV long terminal repeat (LTR)-driven expression of viral RNA and multiple viral proteins in host cells194, so might at least partially reflect the physiological complexity of HIV–host interactions in human disease. However, there are also important limitations of the model, including the presence of an incomplete HIV genome, absence of natural infectious processes, host immune tolerance to HIV transgene products, and the fact that the HIV genome is present in all cells and expressed in a variety of cell types and tissues. In a better representation of the natural HIV infection process, mice and rats carrying human CD4 and CCR5 transgenes exhibited low-level HIV infection and expression in vivo185,195. As in humans, HIV infection in these animals can be blocked by antiretroviral drugs185,195. There are indications, however, that HIV infection of CD4 or CCR5 transgenic rodents is limited and does not propagate196, and there have been no reports of HIV brain entry and neuropathogenesis in this model to date.
In another approach, a mouse model of HIV infection was created by causing HIV tropism in mice by introducing HIV with gp120 replaced by the ecotropic murine leukaemia virus gp80 envelope gene197. This chimeric virus, called EcoHIV, gains entry into murine cells through the cationic amino acid transporter-1 (mCAT)198. Conventional immunocompetent mice that are exposed to EcoHIV acquire efficient HIV infection that can be prevented by treatment with antiretroviral drugs that are in clinical use199. Despite widespread expression of mCAT in mouse tissues198, persistent HIV infection is preferentially detected in lymphoid tissues and the brain, specifically in CD4+ T cells, macrophages and microglia, but not in the liver or lung197,200,201. Infected mice seroconvert and develop CD8+ T cell-mediated responses, which limit systemic virus expression. Similar to processes observed in patients receiving CART, these animals do not show progression to immunodeficiency and AIDS197,199,201,202. Despite immune control, HIV spreads to lymphoid tissues and the brain, and residual virus remains infective197,199. Virus burden in the brain was low, and no gross brain pathology was observed, but gene expression tests in brain tissue revealed low-level inflammatory and type I interferon responses199,201. Efficient HIV expression and microglia and astrocyte activation were observed after stereotactic EcoHIV inoculation into mouse basal ganglia, but these changes were limited by type I interferon responses201.
Thus, the EcoHIV model might capture many of the features observed in individuals with HAND who are receiving CART, including maintenance of functional immunity, viral persistence at low levels in the periphery and brain, and minimal brain pathology despite the presence of molecular changes that are associated with neuroinflammation and cognitive dysfunction199,201,202. Some distinctions from human disease are clear. Brain abnormalities in EcoHIV-infected mice occur in the presence of a functional host immune system155,160,202, so some proposed determinants of mild HAND in humans (for example, CD4+ T cell nadir (REF. 24)), might not be required in this model. Absence of gp120 from EcoHIV prevents analysis of the contributions that env makes to neuropathogenesis during viral infection of mice.
Research in rodent models has been stimulated by improvements in the efficiency and stability of human haematopoietic stem cell grafts into immunodeficient mice203,204. NOD/Scid IL-2R-gamma null (NSG) mice that are engrafted with human CD34+ stem cells (NSG-hCD34+), which differentiate into mature human T lymphocytes, monocytes and macrophages, can be efficiently infected with HIV in a sustained manner203–206. Chronic HIV infection in this model is characterized by high HIV plasma burdens, CD4+ T cell depletion, and low-level HIV neuroinvasion205,206. The latter is probably linked to transmigration of HIV-infected monocytes and macrophages into the mouse CNS. These human cells localize predominantly in the meninges and perivascular spaces and, to a lesser extent, in brain parenchyma205,207. Despite low viral burdens, chronically HIV-infected NSG-hCD34+ mice show several important characteristics of HAND, including activation of resident microglia and astrocytes in some brain regions, limited brain pathology in some mice, elevated markers of neuroinflammation, and evidence of neuronal injury and neurodegeneration, assessed with brain metabolite analysis and immunofluorescence staining205–207. Some of these changes were reversed by nanoparticle-based CART208. This model has also reproduced some aspects of the cognitive deficits of HAND; for example, animals exhibit increased anxiety in an open field exploratory behaviour test207. Physical fragility of NSG-hCD34+ mice precludes testing of cognitive impairment in infected mice with more conventional tests of learning and memory, such as the Morris water maze205. Despite the potential limitations of this model, which also include variability in the efficiency of human cell engraftment and the low rates of graft-versus-host disease, it holds promise for studies of prognostic and diagnostic translatable neuroimaging and biomarkers, and for providing a model in which to test novel therapeutic approaches to on-going cognitive impairment or its prevention175,209.
Each of the mouse models discussed above should be aided by the recent development of sophisticated and reproducible behavioural tests for executive dysfunction and attention deficits in mice. Impaired performance in these tests can serve as markers of cognitive impairment in these models210–212.
Therapeutic advances for HAND
The widespread implementation of CART means that it is more important than ever to consider HAND therapy in the context of ageing HIV+ patients who have received CART for years or even decades, but have persistent systemic and CNS inflammation. The development of validated biomarkers or clinical neurocognitive tests that can accurately stratify the risk of developing HAND will be important steps in improving therapy.
Eradication strategies
As discussed above, advancements in HIV-eradication strategies have drawn attention to the CNS as a potentially important reservoir for HIV. While CD4+ memory T cells are clearly the major viral reservoir, other sites — including gut-associated lymphoid tissue, peripheral blood, bone marrow and the brain — could also be important reservoirs. The SIV encephalitis model provides convincing evidence that viral DNA persists even after complete suppression of SIV in the blood and CSF118. It is critical not to overlook the CNS as a potential reservoir site when eradication strategies are deployed9. A central premise of HIV eradication is that latent viral reservoirs might need to be activated so as to be targeted for elimination. Novel latency-reversing agents (LRAs) might be used to activate latent viruses and purge persistent reservoirs in resting memory CD4+ T cells and throughout the body, but the degree of reservoir reduction that is necessary for a true ‘cure’ is unknown213. In addition, LRAs might pose a challenge in the CNS, as activating latent viral reservoirs in the brain in immunocompetent patients might result in an overabundant inflammatory response that leads to brain oedema and profound neurological complications.
CNS escape and CNS CART penetration
In some people with chronic HIV infection, HIV-1 RNA can be found at higher concentrations in the CSF than in the blood, possibly as a result of poor delivery of antiretroviral drugs into the CNS. Published reports have identified that low-level HIV is present in the CSF in up to 28% of adults receiving CART214,215. Nevertheless, the clinical relevance of this CSF viral escape is not well understood, because the phenomenon has no consistent correlation with CNS penetration of CART or with the development of HAND.
The extent to which antiretroviral drug distribution and toxicity in the CNS affect clinical outcomes is also debated. CART regimens with high CNS penetration–effectiveness (CPE) have been associated with a reduced proportion of patients with detectable CSF viral loads216. By contrast, one large cohort of 51,938 HIV+ individuals who were CART-naive at enrolment found a 74% increased risk of HAD in those receiving CART with high CPE. A trial that focused on optimizing the CNS penetration of CART regimens failed to show an effect of this strategy on neurocognitive performance217. Given the potent CART agents available today and this conflicting evidence, in clinical practice we use the simplest, most potent and least toxic regimens in HIV+ patients with or without HAND, and do not consider the theoretical CPE of a given CART regimen.
Neurotoxicity of CART
The suggestion that neurocognitive function is worse with high CPE CART regimens has prompted concerns that antiretrovirals themselves might be neurotoxic, thus contributing to the persistence of HAND in the CART era218. Some in vitro investigations have supported these concerns. For example, in one study, MAP-2 staining, dendritic arborization complexity, and neural responses to exogenous calcium were used as markers for neuronal damage and revealed neuronal toxicity of 15 different antiretroviral drugs from different drug classes219. Our research group has also shown that metabolites of efavirenz, a commonly used antiretroviral, may induce neuronal injury in vitro220, and clinical observations have suggested negative neurocognitive effects of this drug221. Whether these observations are clinically relevant for other CART regimens is uncertain. The use of CART is undoubtedly life-saving, so CART should not be interrupted or deferred on the theoretical grounds of neurotoxicity.
Drug discovery in HAND
One of the challenges that the HAND research community has faced is the lack of interest from the pharmaceutical industry in the development of therapeutics for HAND, principally because the condition has not been perceived as a viable target. This reluctance of the commercial pharmaceutical sector has imposed an increasing burden on the academic sector to develop new therapeutics for HAND. One example of the efforts led by academic researchers is the development of intranasal insulin as a possible therapeutic agent for HAND, which we are studying in preclinical and human studies. A number of studies have successfully used intranasal insulin to improve cognitive function in healthy individuals, and in individuals with impaired cognitive performance as a result of ageing or Alzheimer disease222. The mechanistic explanation for these protective effects is not well understood, but insulin has a variety of metabolic and trophic effects and might directly protect neurons and dampen inflammatory cytokine expression223. These multi-target effects of insulin, coupled with intranasal delivery to selectively target the CNS, make intranasal insulin an attractive candidate for a neuroprotective therapy in HAND.
Conclusions
Although significant progress has been made in understanding the clinical features, epidemiology, and neuropathogenesis of HAND, a number of critical and unanswered questions remain (BOX 3). We hope that in the next decade, substantial progress will be made in the development of validated biomarkers and an effective adjunctive therapy that can be added to CART regimens to prevent and/or ameliorate the neurocognitive deficits of HAND.
Box 3. Critical unanswered questions regarding HAND.
In the setting of complete, durable systemic virological suppression with CART, do individuals with HIV infection continue to develop HAND?
Despite improved understanding of the pathogenic mechanisms that underlie HAND, why are there no definitive adjunctive treatments?
Can in vitro or in vivo models be used to more effectively develop and translate novel therapeutics agents for clinical trials?
Can validated surrogate markers be used to improve the efficiency of clinical trials for HAND?
Can screening tests and methods to assess for HAND be optimized to identify those at risk of developing HAND and those at risk of progression of HAND?
How can knowledge of optimal screening tests and methods of assessment be more widely and effectively disseminated to HIV care providers around the world?
CART, combination antiretroviral therapy; HAND, HIV-associated neurocognitive syndrome.
Acknowledgments
Victoria Maranto and Heather Thomas assisted with the preparation of this manuscript by collating references and assisting with preparation of figures. The authors of this manuscript have been supported by NIH grants: 2P30MH075673 (to J.C.M, B.S., N.H., A.B., N.S.); 1P01MH105280 (J.C.M, N.H., N.S.); 271201000036C-5-0-1, MH22005 and R21MH083465 (J.C.M., A.B.); 5R01MH099733, U01AI035042, NS081196 and MH107345 (N.S.); 5R01DA040390, 2R01MH077542, 1R01MH096636 and R03MH103985 (N.H.); DA037611, DA017618 and MH105145 (D.V.); AG034852, R21NS07062 and R03DA032470 (B. S.); R01MH083728 (M. P.); P50MH-094268 (M.H.), P01 MH070306 (J.M.); R01NS077869 and R01NS089482 (J.M.). J.C.M. and A.B. are supported by Johns Hopkins University Center for AIDS Research (P30AI094189). M.P. is supported by the Stanley Medical Research Institute, Chevy Chase, Maryland, USA.
Glossary
- HIV-associated dementia (HAD)
Marked cognitive impairment involving at least two cognitive domains that substantially interferes with daily functioning
- Sterilizing cure
Elimination of all HIV-infected cells from the individual
- Asymptomatic neurocognitive impairment (ANI)
Cognitive impairment involving at least two cognitive domains that does not interfere with everyday functioning
- Mild neurocognitive disorder (MND)
Cognitive impairment involving at least two cognitive domains that produces at least mild interference in daily functioning
- CSF viral escape
Presence of detectable HIV in the cerebrospinal fluid (CSF) despite undetectable HIV RNA levels in the plasma
Footnotes
Author contributions
D.S. and A.M.D. contributed equally to this manuscript. All authors researched the literature for the article and contributed to discussion of the content. D.S., A.M.D., N.H., B.S., M.P., A.B., D.V. and J.C.M. wrote the manuscript. D.S., A.M.D., N.S., N.H., D.V., J.C.M. reviewed and/or edited the manuscript.
Competing interests statement
The authors declare no competing interests.
References
- 1.Fauci AS, Marston HD. Ending the HIV-AIDS pandemic — follow the science. N Engl J Med. 2015;373:2197–2199. doi: 10.1056/NEJMp1502020. [DOI] [PubMed] [Google Scholar]
- 2.Maschke M, et al. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. AIDSinfo. 2015 [online], https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 4.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. This article updated the research nosology for HIV-associated neurocognitive disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton RK, et al. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group HIV Neurobehavioral Research Center. Psychosom Med. 1994;56:8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Heaton R, et al. HIV-associated neurocognitive disorders (HAND) persist in the era of potent antiretroviral therapy: The CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. This study reported that HIV-associated neurocognitive disorder remains prevalent even in individuals treated with combination antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tozzi V, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 9.Fois AF, Brew BJ. The potential of the CNS as a reservoir for HIV-1 infection: implications for HIV eradication. Curr HIV/AIDS Rep. 2015;12:299–303. doi: 10.1007/s11904-015-0257-9. [DOI] [PubMed] [Google Scholar]
- 10.McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 2010;24:1367–1370. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- 11.Grant I, et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections: studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1987;107:828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- 12.Ellis RJ, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. Arch Neurol. 1997;54:416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- 13.Becker JT, et al. Cohort profile: recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol. 2015;44:1506–1516. doi: 10.1093/ije/dyu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McArthur JC, et al. Dementia in AIDS patients: incidence and risk factors. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 15.Sacktor N, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in Sub-Saharan Africa. Neurology. 2006;67:311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- 16.Grant I, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82:2055–2062. doi: 10.1212/WNL.0000000000000492. This study revealed that asymptomatic neurocognitive impairment increases the risk of symptomatic HIV-associated neurocognitive disorder in the future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragin AB, et al. Brain alterations within the first 100 days of HIV infection. Ann Clin Transl Neurol. 2015;2:12–21. doi: 10.1002/acn3.136. This manuscript highlights neuroimaging and cerebrospinal fluid cytokine findings in primary HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vo QT, et al. Neuropsychological test performance before and after HIV-1 seroconversion: the Multicenter AIDS Cohort Study. J Neurovirol. 2013;19:24–31. doi: 10.1007/s13365-012-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42:1472–1476. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- 20.Heaton R, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Childs E, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 22.McArthur JC, et al. Relationship between human immunodeficiency virus — associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 23.Molsberry SA, et al. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. AIDS. 2015;29:713–721. doi: 10.1097/QAD.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis RJ, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacktor N, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86:334–340. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright EJ, et al. Factors associated with neurocognitive test performance at baseline: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):97–108. doi: 10.1111/hiv.12238. [DOI] [PubMed] [Google Scholar]
- 27.Wright EJ, et al. No difference between the effects of immediate versus delayed ART on neuropsychological test performance in HIV+ adults with CD4 counts above 500 cells/microliter: the Strategic Timing of Anti-Retroviral (START) Neurology Substudy. Presented at the 15th European AIDS Conference; Barcelona. 2015. [Google Scholar]
- 28.McCutchan JA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker JT, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabbiani M, et al. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med. 2013;14:136–144. doi: 10.1111/j.1468-1293.2012.01044.x. [DOI] [PubMed] [Google Scholar]
- 31.Fazeli PL, et al. Cognitive functioning in adults aging with HIV: a cross-sectional analysis of cognitive subtypes and influential factors. J Clin Res HIV AIDS Prev. 2015;1:155–169. doi: 10.14302/issn.2324-7339.jcrhap-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valcour V, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. This study showed that older age more than doubles the risk of HIV-associated neurocognitive disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joska JA, et al. Characterization of HIV-associated neurocognitive disorders among individuals starting antiretroviral therapy in South Africa. AIDS Behav. 2011;15:1197–1203. doi: 10.1007/s10461-010-9744-6. [DOI] [PubMed] [Google Scholar]
- 34.Joska JA, et al. Neuropsychological outcomes in adults commencing highly active anti-retroviral treatment in South Africa: a prospective study. BMC Infect Dis. 2012;12:39. doi: 10.1186/1471-2334-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marquine MJ, et al. The veterans aging cohort study index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr. 2014;65:190–197. doi: 10.1097/QAI.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaton R, et al. Aging amplifies HIV neurocognitive impairment: the effects may be related to vascular and metabolic factors. J Neurovirol. 2012;18:S46. [Google Scholar]
- 37.Vivithanaporn P, et al. Hepatitis C virus co-infection increases neurocognitive impairment severity and risk of death in treated HIV/AIDS. J Neurol Sci. 2012;312:45–51. doi: 10.1016/j.jns.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Clifford DB, et al. Absence of neurocognitive effect of hepatitis C infection in HIV-coinfected people. Neurology. 2015;84:241–250. doi: 10.1212/WNL.0000000000001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber E, et al. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol. 2013;19:65–74. doi: 10.1007/s13365-012-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawler K, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. 2010;13:15. doi: 10.1186/1758-2652-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwasa J, et al. Lessons learned developing a diagnostic tool for HIV-associated dementia feasible to implement in resource-limited settings: pilot testing in Kenya. PLoS ONE. 2012;7:e32898. doi: 10.1371/journal.pone.0032898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Royal W, 3rd, et al. Clinical features and preliminary studies of virological correlates of neurocognitive impairment among HIV-infected individuals in Nigeria. J Neurovirol. 2012;18:191–199. doi: 10.1007/s13365-012-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akolo C, et al. Neurocognitive impairment associated with predominantly early stage HIV infection in Abuja, Nigeria. J Neurovirol. 2014;20:380–387. doi: 10.1007/s13365-014-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanmogne GD, et al. HIV-associated neurocognitive disorders in Sub-Saharan Africa: a pilot study in Cameroon. BMC Neurol. 2010;10:60. doi: 10.1186/1471-2377-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly CM, et al. HIV associated neurocognitive disorders (HAND) in Malawian adults and effect on adherence to combination anti-retroviral therapy: a cross sectional study. PLoS ONE. 2014;9:e98962. doi: 10.1371/journal.pone.0098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong MH, et al. Frequency of and risk factors for HIV dementia in an HIV clinic in Sub-Saharan Africa. Neurology. 2007;68:350–355. doi: 10.1212/01.wnl.0000252811.48891.6d. This study found that HIV-associated neurocognitive disorder is common in sub-Saharan Africa. [DOI] [PubMed] [Google Scholar]
- 47.Robertson K, et al. Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis. 2012;55:868–876. doi: 10.1093/cid/cis507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heaton RK, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60:473–480. doi: 10.1093/cid/ciu862. This longitudinal study highlights the changing epidemiology of HIV-associated neurocognitive disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol. 2014;34:89–102. doi: 10.1055/s-0034-1372346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol. 2012;18:291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ances BM, Hammoud DA. Neuroimaging of HIV-associated neurocognitive disorders (HAND) Curr Opin HIV AIDS. 2014;9:545–551. doi: 10.1097/COH.0000000000000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McIntosh RC, Rosselli M, Uddin LQ, Antoni M. Neuropathological sequelae of Human Immunodeficiency Virus and apathy: a review of neuropsychological and neuroimaging studies. Neurosci Biobehav Rev. 2015;55:147–164. doi: 10.1016/j.neubiorev.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Ragin AB, et al. Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79:2328–2334. doi: 10.1212/WNL.0b013e318278b5b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly SG, et al. Early suppressive antiretroviral therapy in HIV infection is associated with measurable changes in the corpus callosum. J Neurovirol. 2014;20:514–520. doi: 10.1007/s13365-014-0261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S, et al. Matrix metalloproteinase levels in early HIV infection and relation to in vivo brain status. J Neurovirol. 2013;19:452–460. doi: 10.1007/s13365-013-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burdo TH, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sailasuta N, et al. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. 2012;7:e49272. doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kore I, et al. Neuropsychological impairment in acute HIV and the effect of immediate antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70:393–399. doi: 10.1097/QAI.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang L, et al. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antiviral Ther. 2003;8:17–26. [PubMed] [Google Scholar]
- 60.Hestad K, et al. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta Neurol Scand. 1993;88:112–118. doi: 10.1111/j.1600-0404.1993.tb04201.x. [DOI] [PubMed] [Google Scholar]
- 61.Jernigan TL, et al. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen RA, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paul RH, et al. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc. 2008;14:725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 65.Lyons JL, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burdo TH, et al. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamat A, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sacktor N, et al. Impact of minocycline on cerebrospinal fluid markers of oxidative stress, neuronal injury, and inflammation in HIV-seropositive individuals with cognitive impairment. J Neurovirol. 2014;20:620–626. doi: 10.1007/s13365-014-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan L, et al. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol. 2013;19:144–149. doi: 10.1007/s13365-013-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohamed MA, et al. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging. 2010;28:1251–1257. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Towgood KJ, et al. Regional cerebral blood flow and FDG uptake in asymptomatic HIV-1 men. Hum Brain Mapp. 2013;34:2484–2493. doi: 10.1002/hbm.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haughey NJ, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 73.Dickens AM, et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS. 2015;29:559–569. doi: 10.1097/QAD.0000000000000580. This study showed that changes in bioenergetics are associated with cognitive performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cassol E, Misra V, Dutta A, Morgello S, Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. 2014;28:1579–1591. doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bandaru VVR, et al. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology. 2013;81:1492–1499. doi: 10.1212/WNL.0b013e3182a9565e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bandaru VV, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–1487. doi: 10.1212/01.wnl.0000260610.79853.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gongvatana A, et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol. 2013;19:209–218. doi: 10.1007/s13365-013-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang L, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. NeuroImage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 80.Mielke MM, Bandaru VV, McArthur JC, Chu M, Haughey NJ. Disturbance in cerebral spinal fluid sphingolipid content is associated with memory impairment in subjects infected with the human immunodeficiency virus. J Neurovirol. 2010;16:445–456. doi: 10.3109/13550284.2010.525599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh J, et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J Neuroinflammation. 2014;11:199. doi: 10.1186/s12974-014-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McGuire JL, Gill AJ, Douglas SD, Kolson DL. Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol. 2015;21:439–448. doi: 10.1007/s13365-015-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cassol E, et al. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis. 2013;13:203. doi: 10.1186/1471-2334-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Archibald S, et al. Brain morphometric correlates of metabolic variables in HIV: the CHARTER study. J Neurovirol. 2014;20:603–611. doi: 10.1007/s13365-014-0284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seider TR, et al. Age exacerbates HIV-associated white matter abnormalities. J Neurovirol. 2015 doi: 10.1007/s13365-015-0386-3. http://dx.doi.org/10.1007/s13365-015-0386-3. [DOI] [PMC free article] [PubMed]
- 86.Chang L, et al. Effects of APOE ε4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014;82:2213–2222. doi: 10.1212/WNL.0000000000000526. This article links glial dysfunction and changes seen on magnetic resonance spectroscopy to HIV-associated cognitive impairment and age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Canizares S, Cherner M, Ellis RJ. HIV and aging: effects on the central nervous system. Semin Neurol. 2014;34:27–34. doi: 10.1055/s-0034-1372340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfefferbaum A, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2014;35:1755–1768. doi: 10.1016/j.neurobiolaging.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80:1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peluso MJ, et al. Absence of cerebrospinal fluid signs of neuronal injury before and after immediate antiretroviral therapy in acute HIV infection. J Infect Dis. 2015;212:1759–1767. doi: 10.1093/infdis/jiv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peterson J, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS ONE. 2014;9:e116081. doi: 10.1371/journal.pone.0116081. This study demonstrated change in markers of neuronal injury as HIV-associated neurocognitive disorderprogresses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abdulle S, et al. CSF neurofilament protein (NFL) — a marker of active HIV-related neurodegeneration. J Neurol. 2007;254:1026–1032. doi: 10.1007/s00415-006-0481-8. [DOI] [PubMed] [Google Scholar]
- 93.Angel TE, et al. The cerebrospinal fluid proteome in HIV infection: change associated with disease severity. Clin Proteomics. 2012;9:3. doi: 10.1186/1559-0275-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jessen Krut J, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS ONE. 2014;9:e88591. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peluso MJ, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207:1703–1712. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marcotte TD, et al. A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J Neuroimmune Pharmacol. 2013;8:1123–1135. doi: 10.1007/s11481-013-9504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vago L, et al. Pathological findings in the central nervous system of AIDS patients on assumed antiretroviral therapeutic regimens: retrospective study of 1597 autopsies. AIDS. 2002;16:1925–1928. doi: 10.1097/00002030-200209270-00009. This pathological patient series study highlights the impact of combination antiretroviral therapy on reducing the frequency of the pathological effects of HIV infection. [DOI] [PubMed] [Google Scholar]
- 98.Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: encephalitis and neurodegeneration reconsidered. Curr HIV/AIDS Rep. 2015;12:272–279. doi: 10.1007/s11904-015-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathol Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. This classic clinicopathological patient series provided the first detailed descriptions of HIV-associated dementia, termed AIDS dementia complex. [DOI] [PubMed] [Google Scholar]
- 100.Chang L, et al. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stubbe-Drger B, et al. Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol. 2012;12:23. doi: 10.1186/1471-2377-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM. The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS. 2012;26:1501–1508. doi: 10.1097/QAD.0b013e3283550bec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoare J, et al. Relationship between apolipoprotein E4 genotype and white matter integrity in HIV-positive young adults in South Africa. Eur Arch Psychiatry Clin Neurosci. 2013;263:189–195. doi: 10.1007/s00406-012-0341-8. [DOI] [PubMed] [Google Scholar]
- 104.Kamat R, et al. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J Clin Exp Neuropsychol. 2014;36:854–866. doi: 10.1080/13803395.2014.950636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lentz MR, et al. Diffusion tensor and volumetric magnetic resonance measures as biomarkers of brain damage in a small animal model of HIV. PLoS ONE. 2014;9:e105752. doi: 10.1371/journal.pone.0105752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown A. Understanding the MIND phenotype: macrophage/microglia inflammation in neurocognitive disorders related to human immunodeficiency virus infection. Clin Transl Med. 2015;4:7. doi: 10.1186/s40169-015-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gelman IH, Zhang J, Hailman E, Hanafusa H, Morse SS. Identification and evaluation of new primer sets for the detection of lentivirus proviral DNA. AIDS Res Hum Retroviruses. 1992;8:1981–1989. doi: 10.1089/aid.1992.8.1981. [DOI] [PubMed] [Google Scholar]
- 108.Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14:318–326. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schuenke K, Gelman BB. Human microglial cell isolation from adult autopsy brain: brain pH, regional variation, and infection with human immunodeficiency virus type 1. J Neurovirol. 2003;9:346–357. doi: 10.1080/13550280390201056. [DOI] [PubMed] [Google Scholar]
- 110.Churchill MJ, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- 111.Pu H, et al. HIV-1 tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 112.Chompre G, et al. Astrocytic expression of HIV-1 Nef impairs spatial and recognition memory. Neurobiol Dis. 2013;49:128–136. doi: 10.1016/j.nbd.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Marle G, et al. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen TP, Soukup VM, Gelman BB. Persistent hijacking of brain proteasomes in HIV-associated dementia. Am J Pathol. 2010;176:893–902. doi: 10.2353/ajpath.2010.090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ferrington DA, Gregerson DS. Immunoproteasomes: structure, function, and antigen presentation. Prog Mol Biol Transl Sci. 2012;109:75–112. doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ancuta P, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gonzalez E, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 118.Clements JE, Gama L, Graham DR, Mankowski JL, Zink MC. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Curr Opin HIV AIDS. 2011;6:37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dahl V, et al. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28:2251–2258. doi: 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hammond ER, et al. Persistent CSF but not plasma HIV RNA is associated with increased risk of new-onset moderate-to-severe depressive symptoms; a prospective cohort study. J Neurovirol. 2016 doi: 10.1007/s13365-015-0416-1. http://dx.doi.org/10.1007/s13365-015-0416-1. [DOI] [PMC free article] [PubMed]
- 121.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 122.Caniglia EC, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83:134–141. doi: 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gavegnano C, Fromentin E, Schinazi EA. Lower levels of nucleoside analog triphosphates in primary human macrophages compared to human leukocytes could impair potency of antiretroviral drugs in human viral reservoirs. Glob Antiviral J. 2008;4(Suppl 1):70. [Google Scholar]
- 124.Churchill MJ, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- 125.Borjabad A, et al. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 2011;7:e1002213. doi: 10.1371/journal.ppat.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andersen AB, et al. Cerebral FDG-PET scanning abnormalities in optimally treated HIV patients. J Neuroinflammation. 2010;7:13. doi: 10.1186/1742-2094-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gelman BB, et al. Potential role for white matter lysosome expansion in HIV-associated dementia. J Acquir Immune Def Syndr. 2005;39:422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- 128.Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging. 2013;34:2370–2378. doi: 10.1016/j.neurobiolaging.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Achim CL, et al. Increased accumulation of intraneuronal amyloid β in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hui L, Chen X, Haughey NJ, Geiger JD. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro. 2012;4:243–252. doi: 10.1042/AN20120017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bae M, et al. Activation of TRPML1 clears intraneuronal Aβ in preclinical models of HIV infection. J Neurosci. 2014;34:11485–11503. doi: 10.1523/JNEUROSCI.0210-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–9540. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Figuera-Losada M, et al. Cambinol, a novel inhibitor of neutral sphingomyelinase 2 shows neuroprotective properties. PLoS ONE. 2015;10:e0124481. doi: 10.1371/journal.pone.0124481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen D, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vazquez-Santiago FJ, Noel RJ, Jr, Porter JT, Rivera-Amill V. Glutamate metabolism and HIV-associated neurocognitive disorders. J Neurovirol. 2014;20:315–331. doi: 10.1007/s13365-014-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferrarese C, et al. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- 138.Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997;411:107–109. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- 139.Melendez RI, Roman C, Capo-Velez CM, Lasalde-Dominicci JA. Decreased glial and synaptic glutamate uptake in the striatum of HIV-1 gp120 transgenic mice. J Neurovirol. 2015 doi: 10.1007/s13365-015-0403-6. http://dx.doi.org/10.1007/s13365-015-0403-6. [DOI] [PMC free article] [PubMed]
- 140.Musante V, et al. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb Cortex. 2010;20:1974–1984. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- 141.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 142.Gill AJ, Kovacsics CE, Vance PJ, Collman RG, Kolson DL. Induction of heme oxygenase-1 deficiency and associated glutamate-mediated neurotoxicity is a highly conserved HIV phenotype of chronic macrophage infection that is resistant to antiretroviral therapy. J Virol. 2015;89:10656–10667. doi: 10.1128/JVI.01495-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lipton SA. Memantine prevents HIV coat protein-induced neuronal injury in vitro. Neurology. 1992;42:1403–1405. doi: 10.1212/wnl.42.7.1403. [DOI] [PubMed] [Google Scholar]
- 144.Muller WE, Schroder HC, Ushijima H, Dapper J, Bormann J. gp120 of HIV-1 induces apoptosis in rat cortical cell cultures: prevention by memantine. Eur J Pharmacol. 1992;226:209–214. doi: 10.1016/0922-4106(92)90063-2. [DOI] [PubMed] [Google Scholar]
- 145.Anderson ER, Gendelman HE, Xiong H. Memantine protects hippocampal neuronal function in murine human immunodeficiency virus type 1 encephalitis. J Neurosci. 2004;24:7194–7198. doi: 10.1523/JNEUROSCI.1933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schifitto G, et al. A placebo-controlled study of memantine for the treatment of human immunodeficiency virus-associated sensory neuropathy. J Neurovirol. 2006;12:328–331. doi: 10.1080/13550280600873835. [DOI] [PubMed] [Google Scholar]
- 147.Erdmann N, et al. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J Neurochem. 2007;102:539–549. doi: 10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Erdmann JB, et al. Replicative intermediates of Maize streak virus found during leaf development. J Gen Virol. 2010;91(Pt 4):1077–1081. doi: 10.1099/vir.0.017574-0. [DOI] [PubMed] [Google Scholar]
- 149.Thomas AG, Bodner A, Ghadge G, Roos RP, Slusher BS. GCP II inhibition rescues neurons from gp120IIIB-induced neurotoxicity. J Neurovirol. 2009;15:449–457. doi: 10.3109/13550280903350598. [DOI] [PubMed] [Google Scholar]
- 150.Gupta S, et al. HIV-Tat elicits microglial glutamate release: role of NAPDH oxidase and the cystine-glutamate antiporter. Neurosci Lett. 2010;485:233–236. doi: 10.1016/j.neulet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shukla SM, Sharma SK. Sinomenine inhibits microglial activation by Aβ and confers neuroprotection. J Neuroinflammation. 2011;8:117. doi: 10.1186/1742-2094-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thomas AG, et al. High-throughput assay development for cystine-glutamate antiporter (xc−) highlights faster cystine uptake than glutamate release in glioma cells. PLoS ONE. 2015;10:e0127785. doi: 10.1371/journal.pone.0127785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beck SE, et al. Paving the path to HIV neurotherapy: predicting SIV CNS disease. Eur J Pharmacol. 2015;759:303–312. doi: 10.1016/j.ejphar.2015.03.018. This article reviews factors predicting simian immunodeficiency virus CNS disease, including neuroprotective host genes and biomarkers in blood and cerebrospinal fluid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sharer LR, et al. Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol. 1988;23:S108–S112. doi: 10.1002/ana.410230727. [DOI] [PubMed] [Google Scholar]
- 155.Zink MC, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202:161–170. doi: 10.1086/653213. This simian immunodeficiency virus macaque model study demonstrated that the CNS was a reservoir for latent virus in macaques treated with combination antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zink MC, et al. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mankowski JL, Clements JE, Zink MC. Searching for clues: tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J Infect Dis. 2002;186:S199–S208. doi: 10.1086/344938. [DOI] [PubMed] [Google Scholar]
- 158.Beck SE, et al. Macaque species susceptibility to simian immunodeficiency virus: increased incidence of SIV central nervous system disease in pigtailed macaques versus rhesus macaques. J Neurovirol. 2015;21:148–158. doi: 10.1007/s13365-015-0313-7. [DOI] [PubMed] [Google Scholar]
- 159.Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175:963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 160.Ellis RJ, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 161.McArthur JC, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 162.Mankowski JL, Queen SE, Tarwater PM, Fox KJ, Perry VH. Accumulation of β-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol. 2002;61:85–90. doi: 10.1093/jnen/61.1.85. [DOI] [PubMed] [Google Scholar]
- 163.Weed MR, et al. Central nervous system correlates of behavioral deficits following simian immunodeficiency virus infection. J Neurovirol. 2003;9:452–464. doi: 10.1080/13550280390218751. [DOI] [PubMed] [Google Scholar]
- 164.Schmitz JE, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 165.Ratai EM, et al. CD8+ lymphocyte depletion without SIV infection does not produce metabolic changes or pathological abnormalities in the rhesus macaque brain. J Med Primatol. 2011;40:300–309. doi: 10.1111/j.1600-0684.2011.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol. 2012;7:363–371. doi: 10.1007/s11481-011-9330-3. This article provides an overview of the role of monocytes and macrophages in the CNS in an simian immunodeficiency virus model. [DOI] [PubMed] [Google Scholar]
- 167.Graham DR, et al. Initiation of HAART during acute simian immunodeficiency virus infection rapidly controls virus replication in the CNS by enhancing immune activity and preserving protective immune responses. J Neurovirol. 2011;17:120–130. doi: 10.1007/s13365-010-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marcondes MC, et al. Early antiretroviral treatment prevents the development of central nervous system abnormalities in simian immunodeficiency virus-infected rhesus monkeys. AIDS. 2009;23:1187–1195. doi: 10.1097/QAD.0b013e32832c4af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 171.Toggas SM, et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 172.Lee MH, et al. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol Dis. 2011;41:678–687. doi: 10.1016/j.nbd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Okamoto S, et al. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 174.D’hooge R, Franck F, Mucke L, De Deyn P. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- 175.Jaeger LB, Nath A. Modeling HIV-associated neurocognitive disorders in mice: new approaches in the changing face of HIV neuropathogenesis. Dis Model Mech. 2012;5:313–322. doi: 10.1242/dmm.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim BO, et al. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fitting S, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fitting S, et al. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. This article highlights the prospects of the use of Tat-transgenic mice in the study of HIV-associated neurocognitive disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Paris JJ, Singh HD, Carey AN, McLaughlin JP. Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res. 2015;291:209–218. doi: 10.1016/j.bbr.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 2014;231:2349–2360. doi: 10.1007/s00213-013-3385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mucke L, Masliah E, Campbell IL. Transgenic models to assess the neuropathogenic potential of HIV-1 proteins and cytokines. Curr Top Microbiol Immunol. 1995;202:187–205. doi: 10.1007/978-3-642-79657-9_13. [DOI] [PubMed] [Google Scholar]
- 183.Killian MS, Levy JA. HIV/AIDS: 30 years of progress and future challenges. Eur J Immunol. 2011;41:3401–3411. doi: 10.1002/eji.201142082. [DOI] [PubMed] [Google Scholar]
- 184.Lipton SA. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. NeuroReport. 1992;3:913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 185.Seay K, et al. Mice transgenic for CD4-specific human CD4, CCR5 and cyclin T1 expression: a new model for investigating HIV-1 transmission and treatment efficacy. PLoS ONE. 2013;8:e63537. doi: 10.1371/journal.pone.0063537. This study highlights the prospects of the use of transgenic mice with human HIV co-receptors to study HIV-associated neurocognitive disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hanna Z, et al. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 187.Goffinet C, et al. Primary T-cells from human CD4/CCR5-transgenic rats support all early steps of HIV-1 replication including integration, but display impaired viral gene expression. Retrovirology. 2007;4:53. doi: 10.1186/1742-4690-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kopp JB, et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci USA. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lund AK, Lucero J, Herbert L, Liu Y, Naik JS. Human immunodeficiency virus transgenic rats exhibit pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2011;301:L315–L326. doi: 10.1152/ajplung.00045.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 2015;48:336–349. doi: 10.1016/j.bbi.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li MD, et al. Transcriptome sequencing of gene expression in the brain of the HIV-1 transgenic rat. PLoS ONE. 2013;8:e59582. doi: 10.1371/journal.pone.0059582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fields JA, et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol Dis. 2015;86:154–169. doi: 10.1016/j.nbd.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8:119–134. doi: 10.1007/BF03033824. [DOI] [PubMed] [Google Scholar]
- 195.Goffinet C, Allespach I, Keppler OT. HIV-susceptible transgenic rats allow rapid preclinical testing of antiviral compounds targeting virus entry or reverse transcription. Proc Natl Acad Sci USA. 2007;104:1015–1020. doi: 10.1073/pnas.0607414104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goffinet C, et al. Antagonism of CD317 restriction of human immunodeficiency virus type 1 (HIV-1) particle release and depletion of CD317 are separable activities of HIV-1 Vpu. J Virol. 2010;84:4089–4094. doi: 10.1128/JVI.01549-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Potash M, et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci USA. 2005;102:3760–3765. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Devés R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 199.Hadas E, et al. Transmission of chimeric HIV by mating in conventional mice: prevention by pre-exposure antiretroviral therapy and reduced susceptibility during estrus. Dis Model Mech. 2013;6:1292–1298. doi: 10.1242/dmm.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Potash MJ, et al. Transmission of chimeric HIV by mating in conventional mice: prevention by pre-exposure antiretroviral therapy and reduced susceptibility during estrus. Dis Model Mech. 2014;7:178–179. doi: 10.1242/dmm.014167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.He H, et al. Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses. J Neuropathol Exp Neurol. 2014;73:59–71. doi: 10.1097/NEN.0000000000000026. This article highlights the prospects of the use of the EcoHIV mouse model to study HIV-associated neurocognitive disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kelschenbach JL, et al. Mice chronically infected with chimeric HIV resist peripheral and brain superinfection: a model of protective immunity to HIV. J Neuroimmune Pharmacol. 2012;7:380–387. doi: 10.1007/s11481-011-9316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marsden MD, Zack JA. Studies of retroviral infection in humanized mice. Virology. 2015;479–480:297–309. doi: 10.1016/j.virol.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Akkina RK, et al. Improvements and limitations of humanized mouse models for HIV research: NIH/NIAID ‘Meet the Experts’ 2015 Workshop Summary. AIDS Res Hum Retroviruses. 2015;32:109–119. doi: 10.1089/aid.2015.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gorantla S, et al. Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis. Am J Pathol. 2010;177:2938–2949. doi: 10.2353/ajpath.2010.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dash P, et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J Neurosci. 2011;31:3148–3157. doi: 10.1523/JNEUROSCI.5473-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Boska MD, et al. Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV-1 infection of humanized mice. Mol Neurodegener. 2014;9:58. doi: 10.1186/1750-1326-9-58. This study highlights the prospects of the use of the NSG-hsCD34+ mouse model to study HIV-associated neurocognitive disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dash PK, et al. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS. 2012;26:2135–2144. doi: 10.1097/QAD.0b013e328357f5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rongvaux A, et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Arguello PA, Gogos JA. Cognition in mouse models of schizophrenia susceptibility genes. Schizophr Bull. 2010;36:289–300. doi: 10.1093/schbul/sbp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Snigdha S, et al. A preclinical cognitive test battery to parallel the National Institute of Health Toolbox in humans: bridging the translational gap. Neurobiol Aging. 2013;34:1891–1901. doi: 10.1016/j.neurobiolaging.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Young JW, Amitai N, Geyer MA. Behavioral animal models to assess pro-cognitive treatments for schizophrenia. Handb Exp Pharmacol. 2012;213:39–79. doi: 10.1007/978-3-642-25758-2_3. [DOI] [PubMed] [Google Scholar]
- 213.Hill AL, Rosenbloom DI, Fu F, Nowak MA, Siliciano RF. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci USA. 2014;111:13475–13480. doi: 10.1073/pnas.1406663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 215.Yilmaz A, Svennerholm B, Hagberg L, Gisslen M. Cerebrospinal fluid viral loads reach less than 2 copies/ml in HIV-1-infected patients with effective antiretroviral therapy. Antivir Ther. 2006;11:833–837. [PubMed] [Google Scholar]
- 216.Letendre SL, et al. ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis. 2014;59:1032–1037. doi: 10.1093/cid/ciu477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ellis RJ, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis. 2014;58:1015–1022. doi: 10.1093/cid/cit921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Etherton MR, Lyons JL, Ard KL. HIV-associated neurocognitive disorders and antiretroviral therapy: current concepts and controversies. Curr Infect Dis Rep. 2015;17:485. doi: 10.1007/s11908-015-0485-6. [DOI] [PubMed] [Google Scholar]
- 219.Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18:388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tovar-y-Romo LB, et al. Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther. 2012;343:696–703. doi: 10.1124/jpet.112.195701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ma Q, et al. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol. 2015 doi: 10.1007/s13365-015-0382-7. http://dx.doi.org/10.1007/s13365-015-0382-7. [DOI] [PMC free article] [PubMed]
- 222.Craft S, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Craft S, Cholerton B, Baker LD. Insulin and Alzheimer’s disease: untangling the web. J Alzheimers Dis. 2013;33:S263–S275. doi: 10.3233/JAD-2012-129042. [DOI] [PubMed] [Google Scholar]
- 224.Young AC, et al. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology. 2014;83:1592–1600. doi: 10.1212/WNL.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gisslen M, et al. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 226.Heyes MP, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 227.Sacktor N, et al. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 228.Turchan J, et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- 229.Boven LA, et al. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- 230.Li W, et al. Nitrosative stress with HIV dementia causes decreased L-prostaglandin D synthase activity. Neurology. 2008;70:1753–1762. doi: 10.1212/01.wnl.0000282761.19578.35. [DOI] [PubMed] [Google Scholar]
- 231.Sevigny JJ, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 232.Cinque P, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 233.Ryan LA, et al. Plasma levels of soluble CD14 and tumor necrosis factor-α type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001;184:699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 234.Hagberg L, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Burdo TH, Ellis RJ, Fox HS. Osteopontin is increased in HIV-associated dementia. J Infect Dis. 2008;198:715–722. doi: 10.1086/590504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Erichsen D, et al. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138:144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 237.Huang Y, et al. Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci. 2011;31:15195–15204. doi: 10.1523/JNEUROSCI.2051-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pemberton LA, Brew BJ. Cerebrospinal fluid S-100β and its relationship with AIDS dementia complex. J Clin Virol. 2001;22:249–253. doi: 10.1016/s1386-6532(01)00196-2. [DOI] [PubMed] [Google Scholar]