Great articles often begin with an intriguing paradox, describe an elegant experimental approach, provide lasting and important data, and change the course of their discipline. Sturtevant and Beadle (1936) meets all of those criteria and stands as a paradigm for the genetic analysis of chromosome behavior in Drosophila. It began with two paradoxes, both of which were vexing but not obviously connected. First, females heterozygous for paracentric inversions, which do not include the centromere, failed to produce progeny bearing single crossovers within the inversion but did produce progeny bearing double crossovers. There was no change in the number of eggs hatched, ruling out inviability of eggs containing single crossover chromosomes as an explanation. Second, although such females only rarely produced progeny bearing two maternal X chromosomes, they frequently produced progeny with no maternal X chromosomes (patroclinous males). How was inversion heterozygosity producing such an odd set of meiotic anomalies?
Sturtevant and Beadle demonstrated that single crossovers did indeed occur within the inverted segments by characterizing crossing over in attached-X chromosomes, where both the normal X and its inversion-bearing homolog were attached to a single centromere. The arms of attached-X chromosomes (being homologs) undergo pairing and crossing over. These authors found that the occurrence of single crossovers within the inverted region in such chromosomes generated ring-X chromosomes at expected frequencies for the larger inversions and substantial frequencies for the smaller ones.
These observations led Sturtevant and Beadle to conclude that in the inversion heterozygotes, “…single crossover chromatids are selectively eliminated during the meiotic process.” But how? And is that “selective elimination” tied to the production of those patroclinous exceptions? Sturtevant and Beadle proposed that the mechanism for selective elimination lay in the fact that meiosis in Drosophila involves only nuclear division within the oocyte—no cell division occurs (Huettner 1924). The four meiotic nuclei are arranged in a row perpendicular to the egg cortex. Only the innermost nucleus participates in fertilization; the other three are eliminated.
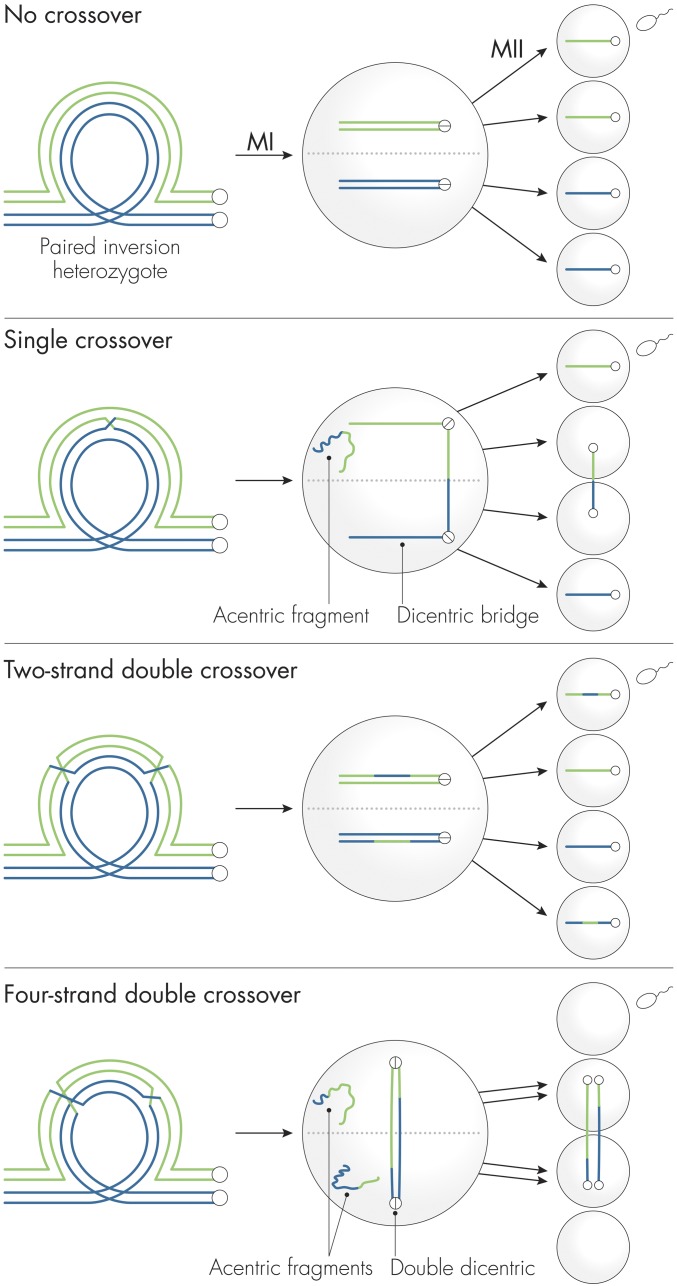
A single crossover within a paracentric inversion generates two (noncrossover) parental types and two recombinant products: one acentric fragment lacking a centromere and a complementary dicentric chromosome with a chromatin bridge connecting two homologous centromeres. The acentric fragment cannot attach to the meiotic spindle and is lost, but what becomes of the dicentric chromosome and the two nonrecombinant chromatids that compose the meiotic tetrad? Sturtevant and Beadle proposed that
A single chromatid tie at the first meiotic division results in orientation of the spindle attachments in such a manner that only chromatids with a single spindle attachment get into the terminal nuclei, one of which will become the egg nucleus.
This hypothesis explains both the selective elimination of single crossover chromosomes and the failure of that loss to cause egg mortality because the dicentric chromatids are relegated to the inner two nuclei that never participate in fertilization anyway (Figure 1).
Figure 1.
Outcomes of crossing over in Drosophila females heterozygous for a paracentric inversion. In each panel, only the innermost nucleus following meiosis II will become an oocyte nucleus and participate in fertilization. Without crossing over, homologs and sister chromatids segregate normally at the first and second meiotic divisions, respectively. Depending on initial orientation, either homolog has an equal probability of segregating to the oocyte. A single crossover within the inversion produces two noncrossover chromatids, an acentric fragment that is lost, and a dicentric bridge that is relegated to the two central nuclei at meiosis II; only a noncrossover chromatid can segregate to the oocyte. Thus, single-crossover progeny are not recovered from inversion heterozygotes and there is no increase in egg mortality. A double crossover involving the same two nonsister chromatids (two-strand double) results in two double recombinant and two noncrossover chromatids. The double recombinant chromatids can segregate normally to the oocyte nucleus. However, a double crossover involving all four strands (four-strand double) produces two acentric fragments and a double dicentric bridge. As shown, both chromatids composing the dicentric remain stuck in the central nuclei, resulting in an oocyte nucleus that is nullo-X and will produce a patroclinous male when fertilized by an X-bearing sperm. The known ratios of two-, three-, and four-strand double crossover bivalents allowed Sturtevant and Beadle to make their famous prediction that the ratio of double recombinant progeny to patroclinous males should be 3:2.
Four-strand double crossovers, which involve all four chromatids, generate a double dicentric chromosome in which both pairs of sister centromeres are connected to their homolog by chromatid bridges. These dicentric chromatids are unable to segregate at meiosis II and both remain stuck in the central nuclei. Sturtevant and Beadle thus proposed that
A double chromatid tie results in the formation of end nuclei with no X chromosome, and a no-X egg will result.
Such a no-X egg will, if fertilized by an X-bearing sperm, produce a patroclinous male. But not all double crossovers within the inversion involve four-strand doubles: two-strand and three-strand doubles also occur at predicted frequencies. By considering the outcome of all possible double crossover events, Sturtevant and Beadle predicted that the ratio of viable double crossover progeny to patroclinous males should be 3:2. The fit of this hypothesis to their experimental data was astounding. Not only did Sturtevant and Beadle beautifully explain both paradoxes, but their analysis also served as a paradigm for subsequent examination of other complex meiotic chromosome mechanisms by many investigators.
Sturtevant and Beadle (1936) stands as a classic in the exacting analytical process known as “doing genetics.” Few papers exemplify the beauty of genetic analysis as well as this gem.
Acknowledgments
We thank Angela Miller for illustration and editorial assistance.
Footnotes
Communicating editor: C. Gelling
ORIGINAL CITATION
Alfred H. Sturtevant and George W. Beadle
GENETICS September 1, 1936 21: 554–604
Image of George Beadle (left) and Alfred Sturtevant (right) in 1951. Courtesy of the Archives, California Institute of Technology.
Literature Cited
- Huettner A. F., 1924. Maturation and fertilization in Drosophila melanogaster. J. Morphol. 39: 249–265. [Google Scholar]
- Sturtevant A. H., Beadle G. W., 1936. The Relations of Inversions in the X Chromosome of Drosophila Melanogaster to Crossing over and Disjunction. Genetics 21: 554–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading in GENETICS
- Doebley J., 2001. George Beadle’s Other Hypothesis: One-Gene, One-Trait. Genetics 158: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz N. H., Berg P., Singer M., Lederberg J., Susman M., et al. , 2004. A centennial: George W. Beadle, 1903–1989. Genetics 166: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi J. C., 1994. Sturtevant’s mantle and the (lost?) art of chromosome mechanics. Genetics 136: 707–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine W. B., 1991. Alfred Henry Sturtevant and crosses between Drosophila melanogaster and Drosophila simulans. Genetics 129: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B. S., 2016. Biochemical Genetics and Molecular Biology: The Contributions of George Beadle and Edward Tatum. Genetics 203: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Other Articles in GENETICS by A. H. Sturtevant and G. W. Beadle
- Beadle G. W., 1932a The Relation of Crossing over to Chromosome Association in Zea-Euchlaena Hybrids. Genetics 17: 481–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., 1932b Genes in Maize for Pollen Sterility. Genetics 17: 413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., 1935. Crossing over near the Spindle Attachment of the X Chromosomes in Attached-X Triploids of Drosophila Melanogaster. Genetics 20: 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., 1937. Development of Eye Colors in Drosophila: Fat Bodies and Malpighian Tubes in Relation to Diffusible Substances. Genetics 22: 587–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., Coonradt V. L., 1944. Heterocaryosis in Neurospora Crassa. Genetics 29: 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., Emerson S., 1935. Further Studies of Crossing over in Attached-X Chromosomes of Drosophila Melanogaster. Genetics 20: 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., Ephrussi B., 1936. The Differentiation of Eye Pigments in Drosophila as Studied by Transplantation. Genetics 21: 225–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G. W., Ephrussi B., 1937. Development of Eye Colors in Drosophila: Diffusible Substances and Their Interrelations. Genetics 22: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T., Beadle G. W., 1936. Studies on Hybrid Sterility IV. Transplanted Testes in Drosophila Pseudoobscura. Genetics 21: 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T., Sturtevant A. H., 1938. Inversions in the Chromosomes of Drosophila Pseudoobscura. Genetics 23: 28–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S., Sturtevant A. H., 1932. The Linkage Relations of Certain Genes in Oenothera. Genetics 17: 393–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., Beadle G. W., 1937a Development of Eye Colors in Drosophila: Transplantation Experiments on the Interaction of Vermilion with Other Eye Colors. Genetics 22: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., Beadle G. W., 1937b Development of Eye Colors in Drosophila: Production and Release of cn+ Substance by the Eyes of Different Eye Color Mutants. Genetics 22: 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlahan M. B., Beadle G. W., Calhoun H. G., 1949. Linkage Studies with Biochemical Mutants of Neurospora Crassa. Genetics 34: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1917. Crossing over without Chiasmatype? Genetics 2: 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1920. Genetic Studies on DROSOPHILA SIMULANS. I. Introduction. Hybrids with DROSOPHILA MELANOGASTER. Genetics 5: 488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1921a Genetic Studies on DROSOPHILA SIMULANS. II. Sex-Linked Group of Genes. Genetics 6: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1921b Genetic Studies on DROSOPHILA SIMULANS. III. Autosomal Genes. General Discussion. Genetics 6: 179–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1925. The Effects of Unequal Crossing over at the Bar Locus in Drosophila. Genetics 10: 117–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1928. A Further Study of the so-Called Mutation at the Bar Locus of Drosophila. Genetics 13: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1936. Preferential Segregation in Triplo-IV Females of Drosophila Melanogaster. Genetics 21: 444–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1940. Genetic Data on Drosophila Affinis, with a Discussion of the Relationships in the Subgenus Sophophora. Genetics 25: 337–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1945. A Gene in Drosophila Melanogaster That Transforms Females into Males. Genetics 30: 297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant, A. H., 1946. On the dot chromosomes of Drosophila repleta and D. hydei. Genetics 31: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1956. A Highly Specific Complementary Lethal System in Drosophila Melanogaster. Genetics 41: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant, A. H., 1963. “Genetics” from 1916 to 1962. Genetics 48: 7–8. [PubMed] [Google Scholar]
- Sturtevant A. H., 2001. Reminiscences of T. H. Morgan. Genetics 159: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., Dobzhansky T., 1936. Geographical Distribution and Cytology of “Sex Ratio” in Drosophila Pseudoobscura and Related Species. Genetics 21: 473–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., Novitski E., 1941. The Homologies of the Chromosome Elements in the Genus Drosophila. Genetics 26: 517–541. [DOI] [PMC free article] [PubMed] [Google Scholar]