Abstract
The eukaryotic cytoplasm contains a variety of ribonucleoprotein (RNP) granules in addition to the better-understood membrane-bound organelles. These granules form in response to specific stress conditions and contain a number of signaling molecules important for the control of cell growth and survival. However, relatively little is known about the mechanisms responsible for, and the ultimate consequences of, this protein localization. Here, we show that the Hrr25/CK1δ protein kinase is recruited to cytoplasmic processing bodies (P-bodies) in an evolutionarily conserved manner. This recruitment requires Hrr25 kinase activity and the Dcp2 decapping enzyme, a core constituent of these RNP granules. Interestingly, the data indicate that this localization sequesters active Hrr25 away from the remainder of the cytoplasm and thereby shields this enzyme from the degradation machinery during these periods of stress. Altogether, this work illustrates how the presence within an RNP granule can alter the ultimate fate of the localized protein.
Keywords: ribonucleoprotein granules, processing bodies, protein kinase, protein stability, Dcp2 decapping enzyme, casein kinase 1
THE eukaryotic cell is subdivided into distinct functional areas by the presence of a variety of organelles. The best understood of these are the membrane-bound structures, like the nucleus, endoplasmic reticulum, and mitochondria. These traditional compartments are relatively stable and essential for the proper compartmentalization of the different reactions occurring in the cytoplasm. However, the cell also contains a collection of nonmembraneous organelles that are more dynamic in nature and form in response to particular cellular and environmental stimuli. Perhaps the two best characterized of these are the centrosome and nucleolus, which act as a microtubule-organizing center and a subnuclear site of ribosome assembly, respectively (Greenan et al. 2010; Brangwynne 2011; Brangwynne et al. 2011). This latter class also includes a number of recently identified cytoplasmic ribonucleoprotein (RNP) granules like the processing body (P-body) and stress granule (Anderson and Kedersha 2009; Balagopal and Parker 2009; Thomas et al. 2011). These cytoplasmic structures have been conserved through evolution and have been linked to a variety of human diseases, including certain neurodegenerative disorders, cancers, and autoimmune conditions (Li et al. 2013; Anderson et al. 2015). Despite this importance to human health, relatively little is known about the manner in which these RNP granules influence biological processes in the cell.
These cytoplasmic RNP granules typically assemble in response to environmental stress or particular developmental cues (Thomas et al. 2011). Granule formation occurs as a consequence of the regulated coalescence of specific sets of proteins and translationally repressed mRNAs at discrete sites in the cytoplasm (Anderson and Kedersha 2009; Balagopal and Parker 2009). The presence of these core proteins often depends upon specific RNA-binding motifs and/or prion-like self-assembly elements that may also have roles in the final aggregation necessary for granule formation (Gilks et al. 2004; Decker et al. 2007; Pilkington and Parker 2008; Reijns et al. 2008; Jonas and Izaurralde 2013). This coalescence is thought to lead to a phase transition that produces granules in a gel-like or condensed liquid phase, with contents present at concentrations higher than that in the surrounding environment (Brangwynne et al. 2009; Kato et al. 2012; Weber and Brangwynne 2012; Hyman et al. 2014). P-bodies, in particular, contain proteins involved in messenger RNA (mRNA) decay including the exonuclease Xrn1, the major decapping enzyme Dcp1/Dcp2, and decapping enhancers like Edc3 and Pat1 (Bashkirov et al. 1997; Ingelfinger et al. 2002; Eystathioy et al. 2003; Sheth and Parker 2003; Cougot et al. 2004; Eulalio et al. 2007a; Parker and Sheth 2007). As a result, P-bodies were originally proposed to be cytoplasmic sites of mRNA decay (Sheth and Parker 2003; Franks and Lykke-Andersen 2008). However, subsequent work has demonstrated that mRNA turnover occurs normally in cells lacking P-body foci and thus the biological functions of these granules remain unclear (Stoecklin et al. 2006; Decker et al. 2007; Eulalio et al. 2007b; Ramachandran et al. 2011). More recent studies have suggested that the activities of P-bodies, and other RNP granules, may be determined by the presence of the more peripheral proteins associated with these structures. For example, these cytoplasmic granules have been found to influence cell physiology by recruiting signaling molecules important for the normal regulation of growth and survival (Arimoto et al. 2008; Takahara and Maeda 2012; Kedersha et al. 2013; Thedieck et al. 2013; Wippich et al. 2013; Shah et al. 2014). The reasons for this recruitment are likely manifold and similar to those that apply to the membrane-bound compartments in the cytoplasm. These include the recruited protein being responsible for a specific activity within the granule, being sequestered away from its normal site of action (and thus down-regulated), and being stored or protected within the RNP structure. As a result, it is essential that we identify the proteins localized to these RNP structures, the underlying machinery governing this recruitment, and the physiological consequences of this protein localization.
To begin to answer these questions, we undertook an examination of a representative constituent of P-body foci, the Hrr25/CK1δ protein kinase. Hrr25 belongs to the casein kinase 1 (CK1) family of protein kinases and is the yeast ortholog of the mammalian CK1δ enzyme (DeMaggio et al. 1992). The Hrr25/CK1δ proteins have conserved roles in ribosome maturation, vesicle trafficking, DNA repair, and chromosome segregation during meiosis (Hoekstra et al. 1991; Petronczki et al. 2006; Schafer et al. 2006; Ray et al. 2008; Grozav et al. 2009; Biswas et al. 2011; Isoda et al. 2011; Lord et al. 2011). We report here that this critical regulator of cell proliferation is efficiently recruited to P-bodies in both yeast and mammalian cells under all conditions examined. This localization depends upon a specific interaction with Dcp2, the catalytic subunit of the primary decapping enzyme in eukaryotes and a conserved core component of these RNP granules. This recruitment also requires Hrr25 kinase activity, suggesting that only the active enzyme can be localized to P-body foci. Finally, experiments with targeting-deficient variants of Hrr25 indicate that a failure to associate with P-bodies results in the rapid degradation of this kinase in stressed cells. Therefore, the P-body appears to provide a type of safe haven that can offer protection from the cytoplasmic degradation machinery during periods of duress.
Materials and Methods
Yeast growth conditions and strain construction
Standard Escherichia coli and yeast media and growth conditions were used throughout this study. Synthetic complete medium with 2% glucose, referred to as SC-dextrose (SCD), was used for yeast culture unless otherwise noted. The glucose starvation medium was SCD without the added glucose and was referred to as SC. Expression from the CUP1 promoter was induced by the addition of 100 μM CuSO4 to the growth medium. For the indicated stress treatments, yeast strains were grown to midlog phase in SCD medium and then transferred for 20 min to SC medium lacking glucose (−Glc), H2O, SC containing 2% galactose (SCGal), or SCD medium containing 3 mM H2O2, 0.5% NaN3, or 0.4% HCl.
To reduce Hrr25 protein levels in cells, we used a previously described strain, KKY387, that expresses an unstable, degron-tagged version of Hrr25 (Hrr25degron) under the control of a galactose-inducible promoter (Kafadar et al. 2003; Ray et al. 2008). Briefly, this strain was grown to midlog phase in SCGal medium and then transferred to SCD for 8 hr to repress Hrr25degron expression and arrest growth. These growth-arrested cells were then transferred to SC medium lacking glucose and P-body formation was assessed by fluorescence microscopy. Cells carrying the wild-type HRR25 locus were subjected to the same experimental regimen and served as the +Hrr25 control for these experiments.
The yeast strains used are listed in Supplemental Material, Table S1, Table S2, and File S1. Tandem affinity purification (TAP) or mCherry-tagged yeast strains were generated with a previously described PCR-based strategy using a TAP (pPHY4174) or mCh (pPHY3932) plasmid, respectively (Longtine et al. 1998). To construct strains with chromosomally encoded mutant alleles of HRR25, we first introduced a single-copy URA3 plasmid carrying a HRR25-mCh construct (pPHY3741) into the wild-type BY4741 strain. Second, the same PCR-based deletion method was used to replace the chromosomal HRR25 locus with the LEU2 gene (Longtine et al. 1998). Third, mEGFP-tagged versions of different hrr25 alleles were introduced on the integrating plasmid, pRS403, and targeted to the HRR25 locus. This plasmid contains the HIS3 gene and successful integration was indicated by the selection for growth on media lacking histidine. These strains were subsequently plated onto SCD plates containing 0.8 mg/ml 5-fluoroorotic acid (5-FOA; US Biological) to counterselect against the URA3 plasmid carrying the wild-type HRR25 locus. Finally, cells were examined by fluorescence microscopy and those with an mEGFP signal, but no mCh fluorescence, were saved. The presence of the hrr25 mutations in each strain were further confirmed by a PCR analysis using the appropriate mutation-specific primers.
Mammalian cell culture and transfection
Cell culture and transfection were performed as described (Majumder and Fisk 2013; Varia et al. 2013). Briefly, HeLa S3 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin antibiotic. The RFP-RCK1 construct (a gift from Daniel Schoenberg) was transfected into the cells using FuGENE (Promega, Madison, WI) as per the manufacturer’s instructions. The cells were incubated at 37° with 5% CO2.
Plasmid construction
The plasmids used in this study are listed in Table S3. The PAT1-mCh (pPHY3785, CEN, URA3) and PAT1-GFP (pPHY3648, CEN, URA3) plasmids were described previously (Ramachandran et al. 2011; Shah et al. 2014). The DCP2-RFP (pPHY3714, CEN, LEU2), DHH1-GFP (pPHY2659, CEN, URA3), LSM1-mCh (pPHY3698, CEN, LEU2) and EDC3-mCh (pPHY3660 CEN, URA3) plasmids were provided by Claudio de Virgilio, Tien-Hsien Chang, Anita Hopper, and Roy Parker, respectively (Beckham et al. 2007; Buchan et al. 2011). Plasmids used for PCR-based deletion or tagging of specific loci were derived from the pFA6a series; these constructs had LEU2 replacing KANMX6 and either mCh or the TAP tag replacing GFP (Longtine et al. 1998). To construct the HRR25 plasmids used in this study, HRR25 fragments were generated with a PCR reaction using genomic DNA as template and then inserted into vectors containing the HRR25 promoter, an appropriate in-frame tag, such as GFP, mCherry, or 3× HA, and finally the ADH1 terminator. The HRR25 promoter was replaced by the promoter from the CUP1 or GPD genes, where needed. Mutagenesis was performed with the GeneArt site-directed mutagenesis system (Life Technologies) or by a gap-repair strategy where the mutations were incorporated into a particular PCR primer.
Microscopy and data analysis
Cells expressing the indicated fusion constructs were grown to early log phase (0.6–0.8 OD600 units/ml) before exposure to the indicated stress or drug treatment. The cells were then collected by centrifugation and spotted onto a microscope slide. The time between the start of each treatment and image capture was 20–30 min unless otherwise noted. Two microscope systems were used to collect the images presented here. Most images were taken with a spinning disk confocal system (UltraVIEW Vox CSUX1 system; Perkin-Elmer, Norwalk, CT) with 405-, 488-, and 561-nm solid state lasers and dual back-thinned EM CCD cameras (C9100-13; Hamamatsu Photonics) using a Nikon Ti-E inverted microscope without binning, under single camera mode with ×100/1.4 N.A. Plan-Apo objective lenses (Nikon, Garden City, NY). For the initial screening of deletion strains and the disassembly analysis, an inverted microscope (Eclipse Ti; Nikon) equipped with an Andor Zyla digital camera, Nikon HC filters and a ×100/1.45 N.A. Plan-Apo objective lens (Nikon) was used.
Images were taken with the Volocity software package (Perkin-Elmer) and analyzed with ImageJ software (National Institutes of Health) as described (Wang et al. 2014). Images in figures were maximum-intensity projections of z-sections spaced at 0.25 μm except where otherwise noted. For the quantitation of the fraction of cells that contain foci, the data represent the average of two or three experiments that examined at least 100 cells in each case. To quantify P-body foci intensity, images were sum-slice projections of z-sections spaced at 0.45 μm. The area of each P-body focus was selected and measured with ImageJ to determine the total intensity. The background within two radii of each focus was measured and subtracted from the total to generate the final intensity of the focus. Ten representative cells (based on the distribution of foci number) were selected for analysis and the average fluorescence intensity was calculated for each time point. The data shown represent the average of three such independent measurements.
Immunofluorescence
Indirect immunofluorescence was performed as described (Kedersha and Anderson 2007; Varia et al. 2013). Briefly, the cells were grown on glass coverslips in a six-well dish and harvested at 50–60% confluency. Cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. Methanol was immediately added and the cells were incubated for 10 min at −20°. The cells were rinsed three times with PBS containing 0.5% normal goat serum (blocking reagent) and then incubated with the primary antibody, rabbit anti-CK1δ (Pierce Chemical, Rockford, IL), for 1 hr at room temperature. After three washes with PBS containing normal goat serum (PBS/NGS), the cells were incubated with the secondary antibody, donkey anti-rabbit conjugated with FITC (Alexa Fluor 488; Invitrogen, Carlsbad, CA). DAPI was added to the final PBS wash in order to label DNA.
Western immunoblotting and co-immunoprecipitation
Protein samples for Western blotting were prepared with a glass bead lysis method, separated on 7.5% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes (GE Healthcare) as described (Budovskaya et al. 2002, 2004). The membranes were then probed with the appropriate primary and secondary antibodies. The Supersignal chemiluminescent substrate (Pierce Chemical) was subsequently used to detect the reactive bands. Extracts for co-immunoprecipitation were prepared by resuspending cells in lysis buffer [25 mM Tris-HCl (pH 7.4), 140 mM NaCl, 1% Tween-20, 1 mM PMSF] and agitating with glass beads. TAP- or HA-tagged proteins were immunoprecipitated with sheep anti-mouse IgG magnetic Dynabeads (Invitrogen) or anti-HA Sepharose (Roche). Protease and phosphatase inhibitors were present at all steps. The extent of interaction was then assessed by Western blotting with the anti-GFP antibody (Roche).
Examining the rate of Hrr25 protein degradation
lmmunoprecipitations from yeast cells labeled with Tran35S-label (MP Biomedicals) were performed as described previously (Herman and Emr 1990; Simon and Kornitzer 2014). Briefly, expression from a CUP1pro-HRR25-HA construct was induced by the addition of 100 μM CuSO4 for 15 min at 30°. At this time, 50 OD600 units of cells were collected by centrifugation and placed in a medium containing the Tran35S-label mix at a final concentration of 20 μCi/OD600 for 15 min at 30°. This labeling reaction was terminated by the addition of a chase solution that resulted in final methionine and cysteine concentrations of 5 mM and 1 mM, respectively. The cells were then divided into five aliquots, washed, and moved to SC media containing chase solution but without glucose for the starvation. Samples were collected at the indicated time points and immunoprecipitation was performed as described above. The precipitated radiolabeled proteins were separated by electrophoresis on 7.5% SDS-polyacrylamide gels. Following electrophoresis, the gels were fixed and dried before autoradiography. The relative radioactivity present was quantified with the ImageJ software package.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
The localization of Hrr25/CK1δ to P-bodies is an evolutionarily conserved phenomenon
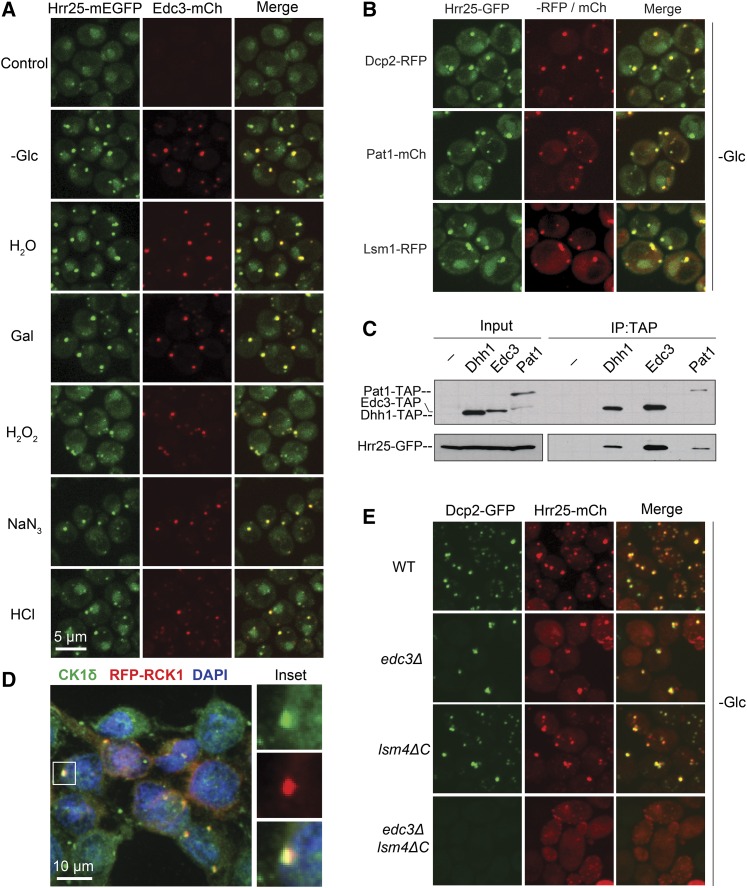
We have previously shown that Hrr25 is present specifically in P-body foci in stationary phase cells (Shah et al. 2014). However, these cytoplasmic granules are induced by a variety of different stress conditions and we tested here whether this protein kinase was associated with P-body foci at these times. For this analysis, we used a yeast strain expressing an mEGFP-tagged Hrr25 protein and an mCherry-tagged Edc3 (Edc3-mCh) reporter that served as the marker for P-body foci. Both fusion constructs were integrated into the genome and expressed from their respective endogenous promoters. For each condition, we found that Hrr25 was associated with P-body foci and that the colocalization between Hrr25 and Edc3 was >90% (Figure 1A; Figure S1A). This level of colocalization was equivalent or higher than that observed between any pair of P-body reporters (Mitchell et al. 2013; Shah et al. 2014). This observation was not restricted to Edc3, as Hrr25 exhibited a similar degree of colocalization with other P-body constituents, including Dcp2, Pat1, and Lsm1 (Figure 1B). Consistent with this localization, Hrr25 was detected in immunoprecipitates with several different P-body proteins (Figure 1C; Figure S1B). Previous larger-scale studies had identified potential interactions between Hrr25 and particular P-body constituents (Ito et al. 2001; Ho et al. 2002; Cary et al. 2015). Moreover, we found that the mammalian ortholog of Hrr25, CK1δ, was also found in P-body foci (Figure 1D). For this experiment, P-bodies were induced by the overexpression of RCK1, the mammalian ortholog of the yeast Dhh1 RNA helicase (Cougot et al. 2004). Both Dhh1 and RCK1 have been shown to be associated with P-body foci (Sheth and Parker 2003; Cougot et al. 2004). Finally, the presence of Hrr25 in foci was found to be dependent upon P-body formation as very few Hrr25 puncta were detected in cells defective for the assembly of these cytoplasmic granules (Figure 1E; Figure S1, C–F). Altogether, these data indicated that Hrr25 was a constant constituent of P-bodies in yeast cells and that this recruitment to P-body foci has been evolutionarily conserved from yeast to humans.
Figure 1.
The localization of the Hrr25/CK1δ protein kinase to P-bodies is an evolutionarily conserved phenomenon. (A) Hrr25 was efficiently recruited to P-bodies in all stress conditions examined. Cells expressing Hrr25-mEGFP and Edc3-mCh were grown to midlog phase and then subjected to the indicated stress conditions. Focus formation was then assessed by confocal microscopy. (B) Hrr25 exhibited a significant level of colocalization with multiple P-body reporters. Strains expressing Hrr25-mEGFP and the indicated P-body reporters were grown to midlog phase and then transferred to a medium lacking glucose to induce P-body formation. (C) Hrr25 was detected in immunoprecipitates with multiple P-body proteins. The indicated TAP-tagged P-body proteins were precipitated from yeast cell extracts and the relative level of the associated Hrr25-GFP protein was assessed by Western blotting. (D) Human CK1δ was associated with P-bodies. P-body formation was induced in HeLa cells by the overexpression of an RCK1-RFP construct and an antibody to the CK1δ enzyme was used for immunofluorescence. RCK1 is the mammalian ortholog of the yeast Dhh1 RNA helicase (Sheth and Parker 2003; Cougot et al. 2004). The panels at the right show the CK1δ, RFP-RCK1, and merged images for the indicated focus in the main panel. (E) Hrr25 foci were absent from cells defective for P-body assembly. The localization of Dcp2-GFP and Hrr25-mCh was determined by confocal microscopy after the indicated strains had been transferred to a medium lacking glucose for 20 min. Quantitation of these data and the +Glc control images are shown in Figure S1, C and D, respectively. P-body assembly has been shown to be compromised in both the edc3Δ lsm4ΔC and pat1Δ mutants (Sheth and Parker 2003; Decker et al. 2007; Ramachandran et al. 2011).
Hrr25 is not required for P-body formation and disassembly
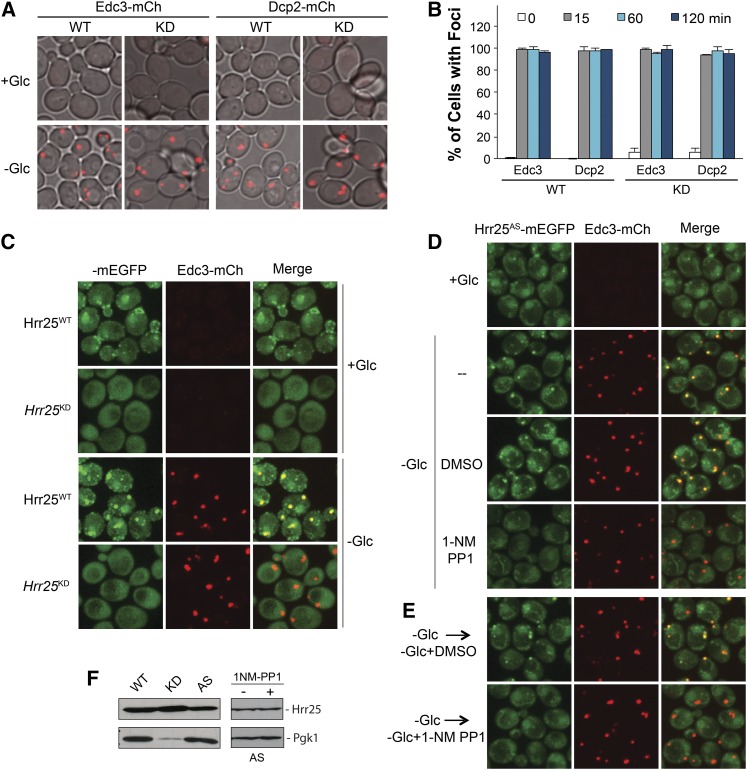
To test whether Hrr25 has a role in P-body assembly, we examined the consequences of altering either Hrr25 protein or activity levels in cells. Since this protein kinase is required for viability (Figure S2A), we took advantage of a yeast strain that expresses an unstable, degron-tagged version of this enzyme (Giaever et al. 2002; Kafadar et al. 2003). This variant, Hrr25degron, was expressed from an inducible GAL promoter that is strongly repressed by the presence of glucose. A shift to a glucose-containing medium therefore results in a growth arrest due to the shutdown of Hrr25 production and the rapid turnover of the preexisting pool of this protein kinase (Kafadar et al. 2003). Following this arrest, the cells were transferred to a medium lacking glucose to induce P-body formation. Using this regimen, we found that P-bodies were produced at a similar level in the arrested cells, suggesting that Hrr25 was not needed for efficient P-body assembly (Figure S2B). To confirm this result, we also examined P-body formation in a strain that expressed a kinase-defective variant of Hrr25 (Hrr25KD) where lysine-38 in the catalytic site is replaced with an alanine residue (Murakami et al. 1999). Cells that express only this version of Hrr25 are viable but exhibit a significant growth defect (Figure S2A). Consistent with the above results, we found that P-body assembly (and disassembly) was not affected by the diminished levels of Hrr25 activity in this strain (Figure 2, A and B; Figure S2C). Finally, we found that the overexpression of Hrr25 did not have a significant effect on P-body numbers in either stressed or nonstressed cells (Figure S2D). In all, these results indicated that neither Hrr25 nor its kinase activity was needed for efficient P-body assembly.
Figure 2.
Hrr25 kinase activity was required for its recruitment to P-body foci. (A and B) P-body assembly did not require Hrr25 kinase activity. Strains expressing either the wild-type (WT) or a kinase-defective (KD) Hrr25 enzyme were grown to midlog phase and then transferred to a medium lacking glucose for the indicated times. P-body formation was then assessed by confocal microscopy at the indicated times (B); images at the 60-min time point are shown in A. The Hrr25KD protein has an alanine replacing the critical lysine-38 residue in the catalytic site (Murakami et al. 1999). (C) A kinase-defective variant of Hrr25 did not localize to P-body foci. Strains expressing either the WT or a KD Hrr25 enzyme were grown to midlog phase and then transferred to a medium lacking glucose to induce P-body formation. (D) Hrr25 kinase activity was required for P-body localization. Cells expressing an analog-sensitive version of Hrr25, Hrr25AS, were transferred to a medium lacking glucose with or without the drug, 1-NM PP1, for 45 min. P-body foci formation was then assessed by confocal microscopy. (E) The inhibition of Hrr25 kinase activity resulted in the rapid loss of Hrr25 from P-body foci. Cells expressing the Hrr25AS protein were starved for glucose to induce P-body formation and then treated with the drug, 1-NM PP1, for 15 min to inhibit Hrr25 activity. The localization of Hrr25AS-mEGFP and Edc3-mCh were then assessed by confocal microscopy. (F) Western blot analysis of the levels of WT, KD, and AS versions of Hrr25 in midlog phase cultures of each strain. The low level of Pgk1 in the hrr25-kd mutant is most likely a consequence of the significant growth defect associated with this strain. The right hand blots show that the levels of the Hrr25AS protein were similar after 45 min of glucose starvation in the presence or absence of the 1-NM PP1 inhibitor.
The kinase activity of Hrr25 is required for its efficient localization to P-bodies
In contrast to the above results, we found that Hrr25 kinase activity was required for its own recruitment to P-bodies. For example, the kinase-defective variant, Hrr25KD, was not recruited to P-body foci upon glucose deprivation (Figure 2C). These results were confirmed by studies with a strain expressing a conditional allele of HRR25 that renders the encoded protein sensitive to the ATP analog, 1-NM PP1 (Bishop et al. 2001; Petronczki et al. 2006). This protein, Hrr25AS, has a glycine residue replacing the isoleucine at position 82 (I82G) and is referred to as an analog-sensitive version of this protein kinase (Bishop et al. 2001; Petronczki et al. 2006). Here, we found that an exposure to this drug resulted in a significant defect in Hrr25AS localization to P-bodies (Figure 2D; Figure S2, E and F). This result suggested that the above observations with the constitutively inactive variant of Hrr25 were not due to secondary consequences associated with the long-term loss of Hrr25 activity. We also found that Hrr25 kinase activity was needed for the continued presence of this protein in P-body foci. Specifically, the addition of 1-NM PP1 to cells that already contained P-bodies resulted in the rapid return of the foci-associated Hrr25AS back to the cytosol (Figure 2E). Collectively, these results indicated that Hrr25 kinase activity was necessary for its localization to P-bodies.
Hrr25 recruitment to P-bodies requires the presence of Dcp2, the catalytic subunit of the decapping enzyme
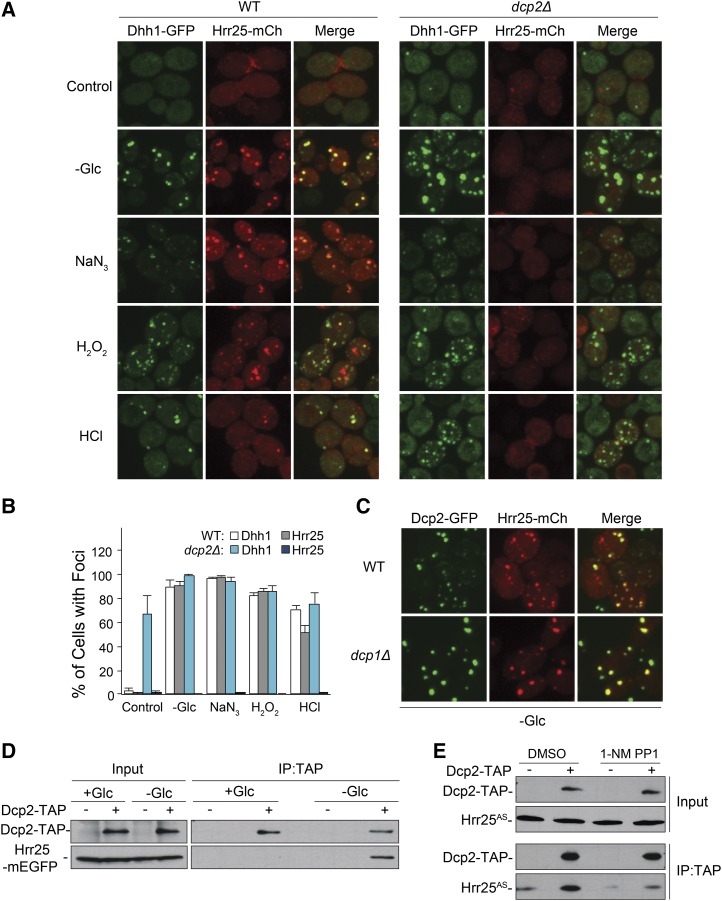
Hrr25 could be recruited to P-bodies as a result of an interaction with either a protein or mRNA component of these structures. However, Hrr25 does not possess any known RNA-binding motif and no RNA-binding activity has been demonstrated for this protein. Therefore, we tested whether Hrr25 localization to P-bodies was dependent upon any of the known protein constituents of these granules. For this analysis, we examined 43 yeast deletion strains, each lacking a particular P-body protein (Table S1) (Mitchell et al. 2013). These studies identified only one candidate, the dcp2Δ mutant, that lacks the catalytic subunit of the decapping enzyme. Dcp2 was one of the first proteins identified in P-body foci and is generally thought of as a core constituent of these granules (van Dijk et al. 2002; Sheth and Parker 2003; Cougot et al. 2004). The dcp2Δ strain exhibited a severe defect in Hrr25 localization, as there were almost no visible Hrr25 foci following glucose deprivation (Figure 3, A and B; Figure S3A). A similar defect was observed under all other stress conditions examined, indicating that the presence of Dcp2 was generally required for Hrr25 localization to P-bodies (Figure 3, A and B).
Figure 3.
The recruitment of Hrr25 to P-bodies required Dcp2, the catalytic subunit of the decapping enzyme. (A and B) The presence of Dcp2 was generally required for Hrr25 localization to P-bodies. P-bodies were induced by the indicated stress conditions in either wild-type or dcp2Δ cells. P-body foci were visualized by confocal microscopy (A) and the fraction of cells with foci were quantified (in B). (C) Hrr25 localization to P-bodies did not require Dcp1. Hrr25-mCh localization was assessed by confocal microscopy after glucose deprivation of wild-type or dcp1Δ cells. (D) Hrr25 was associated with Dcp2 specifically in glucose-deprived cells. TAP-tagged Dcp2 was precipitated from extracts prepared from either midlog phase cells (+Glc) or cells that had been starved for glucose for 30 min (−Glc). The amount of coprecipitating Hrr25-mEGFP was assessed by Western blotting with an antibody to GFP. (E) The inhibition of Hrr25 kinase activity disrupted the Hrr25-Dcp2 interaction. TAP-tagged Dcp2 was precipitated from glucose-starved cells expressing the Hrr25AS protein in the presence or absence of the drug, 1-NM PP1. The relative levels of the associated Hrr25AS protein were subsequently assessed by Western blotting.
Since Dcp2 is part of the decapping enzyme, the failure of Hrr25 to associate with P-body foci in dcp2Δ mutants could be due to the decreased levels of uncapped mRNA in this strain. However, two results suggested that the defect was more likely due to the absence of the Dcp2 protein proper. First, we found that Hrr25 was efficiently localized to P-bodies in a dcp1Δ strain (Figure 3C). This mutant lacks Dcp1, the regulatory subunit of the Dcp1/Dcp2 decapping complex, and therefore possesses diminished levels of decapping activity (Beelman et al. 1996; van Dijk et al. 2002; She et al. 2006). The second observation was that Hrr25 was detected in Dcp2 immunoprecipitates specifically in cells that had been deprived of glucose and thus contained P-body foci (Figure 3D). Very little, if any, Hrr25 was associated with Dcp2 in log-phase cells where P-body numbers are very low. In addition, this interaction required Hrr25 kinase activity, as less Hrr25AS protein was associated with Dcp2 after an exposure to the drug, 1-NM PP1 (Figure 3E). These data are therefore consistent with a model where Dcp2 interacts, either directly or indirectly, with Hrr25 and thereby recruits this protein kinase to P-body foci. Additional support for this model was provided by an analysis of two mutants, xrn1Δ and sbp1Δ, that form P-body-related structures in log-phase cells (Sheth and Parker 2003; Segal et al. 2006). The key point here is that these log-phase foci or aggregates differ with respect to the amount of associated Dcp2. In particular, whereas Dcp2 was readily apparent in the xrn1Δ foci, it was either absent or present at very low levels in the log-phase foci in sbp1Δ cells (Figure S3B). Correspondingly, Hrr25-GFP fluorescence was detectable only in the log-phase xrn1Δ foci (albeit at a low level). Finally, we also detected Hrr25 in the P-body foci that are found in log-phase cells of the dcp1Δ mutant (Figure S3C) (Teixeira and Parker 2007). These latter foci also contain the Dcp2 protein. Altogether, the data here suggest that Hrr25 was recruited to P-body foci as a result of an interaction with the Dcp2 protein.
Hrr25 recruitment to foci occurs after that of the core constituents
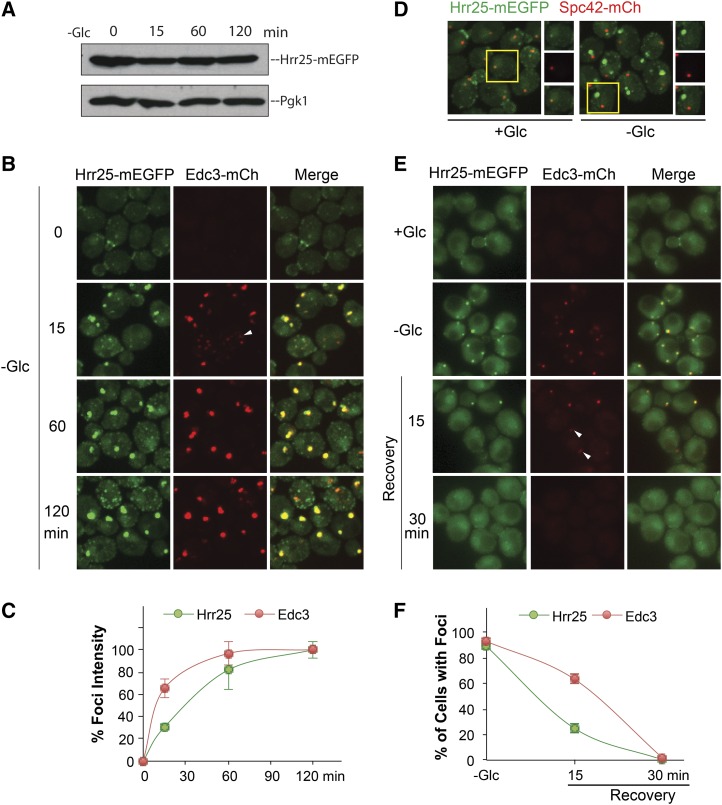
The above results suggested that Hrr25 might be a more peripheral component of the P-body granule. To examine this possibility further, we assessed the timing of Hrr25 recruitment relative to that of the core constituents, Edc3 and Dcp2. For this analysis, the relative intensity of the foci-associated fluorescence was assessed at different times up to 120 min after transfer to a medium lacking glucose. A Western blot analysis indicated that the levels of Hrr25 did not change appreciably during this time period (Figure 4A). In general, we found that cells contained multiple smaller foci after 10–15 min of glucose deprivation and that these foci appeared to coalesce into a smaller number of larger structures at later times (Figure 4B; Figure S4A). The maximum level of fluorescence was generally achieved within 120 min and the value at this time was set as the 100% level for our analysis. Using this approach, we found that Hrr25 reached its maximum fluorescence at a rate that was slower than that for both Edc3 and Dcp2 (Figure 4, B and C; Figure S4, A and B). This recruitment to P-bodies was accompanied by a loss of Hrr25 at the spindle pole body and bud neck, two structures that Hrr25 is known to associate with in log-phase cells (Figure 4D) (Kafadar et al. 2003; Lusk et al. 2007). An analogous result was observed with a time-course analysis of P-body disassembly. In this case, the decrease in foci-associated fluorescence was assessed after the readdition of a glucose-containing medium. The glucose concentration used was 0.2% instead of the standard 2%, in order to slow down the disassembly process and facilitate the detection of any potential differences (Figure S4C). We found that Hrr25 foci number decreased at a faster rate than that observed for either Edc3 or Dcp2, suggesting that Hrr25 left the granule before these two core constituents (Figure 4, E and F; Figure S4, D and E). These timing experiments are therefore consistent with the above data, indicating that Dcp2 was required for Hrr25 localization to these RNP granules.
Figure 4.
The kinetics of Hrr25 recruitment to P-body foci. (A) Hrr25 protein levels were constant for the entire 120-min incubation period. Midlog phase cells were transferred to a medium lacking glucose for the indicated times and the levels of Hrr25-mEGFP were assessed by Western blotting. (B and C) Hrr25 was recruited to P-body foci with slower kinetics than Edc3. Cells expressing Hrr25-mEGFP and Edc3-mCh were transferred to a medium lacking glucose and visualized by confocal microscopy at the indicated times. The graph in C indicates the average fluorescence intensities of foci at the indicated times. (D) Hrr25 was not associated with the spindle pole body (SPB) following glucose deprivation. Cells expressing Hrr25-mEGFP and Spc42-mCh were analyzed by confocal microscopy in midlog phase (+Glc) or after transfer to a medium lacking glucose (−Glc). Hrr25 colocalized with the SPB marker, Spc42-mCh, in dividing cells but not in the glucose-starved cells where P-bodies were forming. (E and F) The loss of Hrr25 foci was more rapid than that observed for Edc3 following glucose readdition. Cells expressing Hrr25-mEGFP and Edc3-mCh were transferred from a medium lacking glucose to SC minimal containing 0.2% glucose to induce P-body disassembly (Recovery). Cells were visualized by fluorescence microscopy at the indicated times (E) and the percentage of cells with foci were quantified (F). The white arrowheads indicate Edc3-containing foci with little, if any, Hrr25-associated fluorescence.
Two sequence elements in the central domain of Hrr25 are required for P-body localization
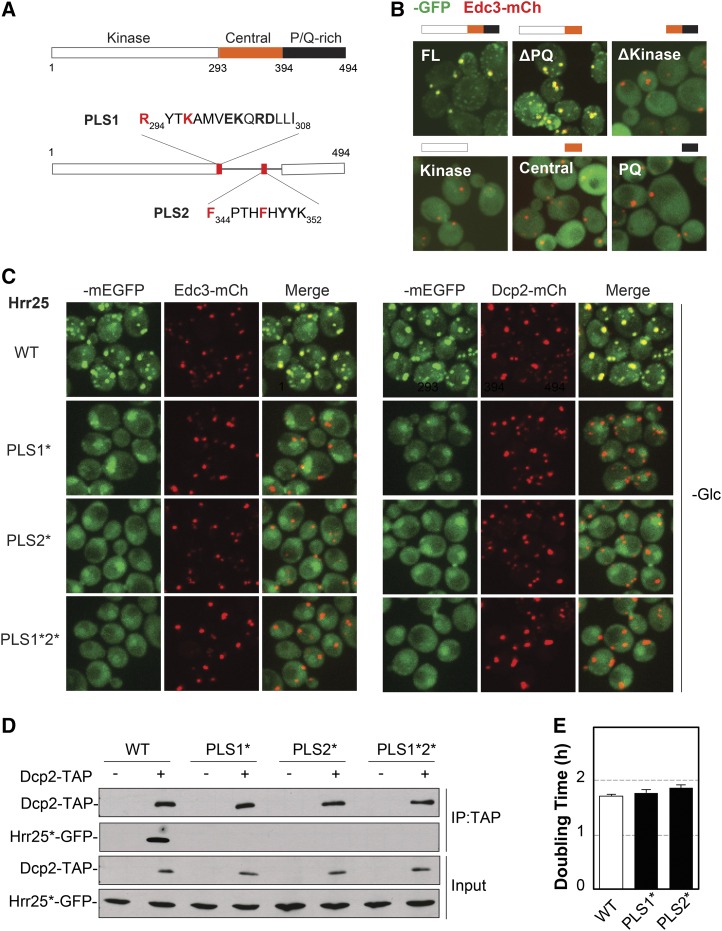
A structure–function analysis of Hrr25 was carried out to determine the domains required for the localization to P-bodies. This protein contains three recognizable domains: the protein kinase domain (residues 1–293); a central region of unknown function (294–394); and a C-terminal domain that is rich in proline and glutamine residues (P/Q rich, 395–494) (Figure 5A) (Petronczki et al. 2006). To determine which of these was important for P-body localization, we generated GFP-tagged versions of the Hrr25 truncations shown in Figure 5B. The analysis of these constructs suggested that both the kinase and central domains were required for P-body association (Figure 5B). However, neither of these domains when expressed alone was sufficient for this targeting. The fact that the C-terminal P/Q-rich domain was dispensable for P-body localization was somewhat surprising, as this region is predicted to be intrinsically disordered and to have the potential to form prion-like structures (Michelitsch and Weissman 2000). These types of domains are present in a number of RNP granule constituents and have been shown to be important in several cases for the localization to, and even assembly of, these granules (Jonas and Izaurralde 2013).
Figure 5.
Two distinct sequence elements in the central domain of Hrr25 were necessary for P-body localization. (A) The domain structure of Hrr25 (top) and the location and sequence of the PLS1 and PLS2 localization signals (bottom). The two residues altered in the PLS1* and PLS* variants are indicated in red. (B) The kinase and central domains of Hrr25 were required for P-body localization. Wild-type cells were transformed with plasmids expressing the indicated GFP-tagged Hrr25 truncation constructs. The localization of these fusion proteins was assessed by fluorescence microscopy after glucose starvation. FL, full-length Hrr25. (C) Alterations in either PLS1 or PLS2 resulted in a loss of Hrr25 localization to P-bodies. Cells expressing the indicated mEGFP-tagged Hrr25 proteins were grown to midlog phase, transferred to a medium lacking glucose, and then visualized by confocal microscopy. (D) Both the PLS1 and PLS2 elements were required for the Hrr25 interaction with Dcp2. TAP-tagged Dcp2 was precipitated from cell extracts and the relative levels of the associated Hrr25 proteins were assessed by Western blotting. (E) Alterations in PLS1 or PLS2 that disrupted P-body localization (PLS1* and PLS2*) did not have a significant effect on the mitotic growth rate. The doubling times shown in the graph are the averages obtained from three independent growth curve determinations.
A more-detailed truncation analysis identified two sequence elements in the central domain that were critical for Hrr25 localization to P-bodies (Figure 5A; Figure S5A). These motifs were designated P-body localization signals, PLS1 and PLS2. To examine the importance of particular amino acids in these elements, a series of alterations were constructed that changed two or more biochemically similar residues to alanines (Figure S5B). These experiments identified two positively charged residues in PLS1 (R294 and K297) and two phenylalanines in PLS2 (F344 and F348) as critical for P-body localization. Changing these residues in either localization element alone resulted in a significant defect in Hrr25 recruitment to P-bodies; these variants were designated PLS1* and PLS2*, respectively (Figure 5, A and C; Figure S5C). These alterations also disrupted the Hrr25 interaction with Dcp2 that was detected in co-immunoprecipitation assays (Figure 5D). Therefore, both PLS1 and PLS2 appear to be necessary for the proper localization of Hrr25 to P-body foci. It is important to note that these alterations in PLS1 and PLS2 did not have a significant effect on cell growth or the number of P-body foci (Figure 5E; Figure S5E). These results therefore suggested that the localization defects associated with PLS1* or PLS2* were not due to effects on Hrr25 kinase activity. Instead, these studies identified Hrr25 alterations that disrupt P-body localization without having a significant effect on the essential mitotic activities of this protein.
The recruitment to P-bodies protected the active Hrr25 enzyme from degradation
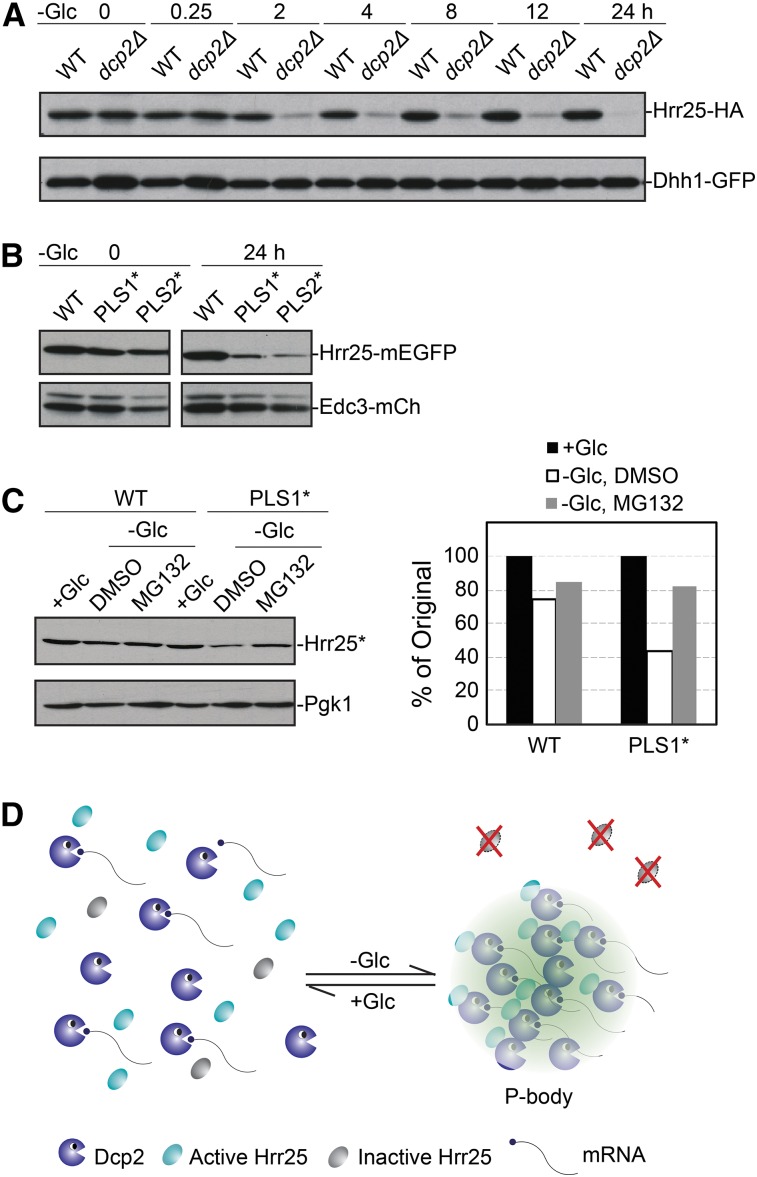
To assess the biological significance of Hrr25 recruitment to P-bodies, we examined the fate of this protein in the dcp2Δ mutant that lacks the likely targeting subunit for this protein kinase. For this experiment, wild-type and dcp2Δ cells were transferred to a medium lacking glucose for up to 24 hr and Hrr25 levels were assessed by Western blotting at the indicated times. We found that the Hrr25 protein was unstable in the dcp2Δ mutant and that most of this protein was degraded within 2 hr of glucose starvation (Figure 6A). In contrast, the levels of Hrr25 remained relatively constant over the entire 24-hr starvation period in the wild-type control. It is unlikely that the higher Hrr25 levels in the wild-type strain are due to new synthesis as protein translation rates decrease by >90% following glucose deprivation (Ashe et al. 2000). To ensure that this instability was due to the loss of P-body localization and not some other consequence associated with the absence of Dcp2, we also examined strains expressing the PLS1* and PLS2* variants that are defective for P-body localization (see Figure 5A). We found that these localization-defective variants were also unstable relative to the wild-type protein in glucose-starved cells (Figure 6B). These studies therefore suggested that a failure to localize to P-bodies resulted in a more rapid turnover of the Hrr25 protein. To directly test this possibility, we assessed the rate of Hrr25 decay with a pulse-chase experiment performed with wild-type and dcp2Δ cells under glucose starvation conditions. These studies indicated that the Hrr25 protein was indeed more rapidly turned over in the dcp2Δ mutant (Figure S6). Moreover, we found that the turnover of the PLS1* variant was significantly slowed by the addition of an inhibitor of the proteasome complex (Figure 6C). Altogether, these data are consistent with Hrr25 being degraded in a proteasome-dependent manner when it is not sequestered within the P-body. This sequestration may therefore allow the cell to maintain a functional reserve of this enzyme. Since our previous data showed that P-bodies are required for the long-term survival of stationary phase cells, we tested whether this survival was compromised in cells expressing only the PLS1* variant of Hrr25 (Ramachandran et al. 2011). However, we found no significant defect in these cells, suggesting that this sequestered pool of Hrr25 must be required for some other yet to be determined activity of the P-body. Nonetheless, the data here demonstrate how the localization within an RNP granule can influence the ultimate fate of a cytoplasmic protein.
Figure 6.
A failure to localize to P-bodies resulted in the rapid turnover of Hrr25. (A) Hrr25 protein levels significantly decreased in dcp2Δ cells upon glucose starvation. WT and dcp2Δ cells were starved for glucose for the indicated times and the levels of Hrr25 were then assessed by Western blotting. (B) Alterations of the PLS1 or PLS2 motifs resulted in the destabilization of Hrr25 upon glucose starvation. Strains expressing the indicated Hrr25 proteins were starved for glucose for 24 hr and the relative level of each protein was then assessed by Western blotting. (C) Inhibition of the proteasome stabilized the PLS1* variant upon glucose starvation. Strains expressing the indicated Hrr25 proteins were starved for glucose for 24 hr with or without the proteasome inhibitor, MG-132 (100 μM). The relative level of each protein was assessed by Western blotting (left) and quantified with the ImageJ software package (right). (D) A model showing how the localization to P-bodies sequesters Hrr25 away from the degradation machinery in the cytoplasm.
Discussion
RNP granules, like P-bodies, are nonmembranous compartments that result from the concentration of particular mRNAs and proteins at discrete sites in the cytoplasm. Although an ever-increasing number of proteins are being found associated with these structures (Kedersha et al. 2013; Mitchell et al. 2013; Shah et al. 2014), relatively little is known about the mechanisms responsible for, and the ultimate consequences of, this recruitment. In this study, we found that the Hrr25/CK1δ protein kinase, an essential regulator of cell proliferation, is recruited to cytoplasmic P-bodies through a specific interaction with Dcp2, the major decapping enzyme in eukaryotes and a core constituent of these granules. This is the first detailed characterization of the mechanisms underlying the recruitment of a signaling molecule to this RNP structure. Interestingly, the data here indicate that this association serves to sequester active Hrr25 away from the remainder of the cytoplasm and thereby protects Hrr25 from the degradation machinery during these periods of stress (Figure 6D).
This localization to P-bodies was found to require Hrr25 kinase activity and two short sequence motifs in the central domain of this protein. Alterations affecting any one of these three regions resulted in a significant defect in P-body association. Although these alterations could affect granule localization indirectly, the simplest interpretation of the data is that PLS1 and PLS2 are P-body targeting determinants. Based on a recent crystal structure, both PLS1 and PLS2 are located within a well-ordered alpha-helical domain that is packed against the C-terminal lobe of the Hrr25 protein kinase domain (K. Corbett, personal communication). The residues that make up PLS1 (294–308) are solvent exposed and could directly contact Dcp2 and thereby recruit Hrr25 to P-body foci. The requirement for kinase activity is also significant, as it suggests that the Hrr25 protein in P-bodies could be active. Moreover, this observation argues against the possibility that it is only misfolded, nonfunctional forms of Hrr25 that are being recruited to these foci. Instead, these data are consistent with a Hrr25-catalyzed phosphorylation being critical for the P-body localization of this protein kinase. Identifying the target of this activity will be important for a full understanding of the mechanisms governing Hrr25 recruitment to these RNP granules.
The key question that remains is what are the physiological consequences of this protein localization? The data here indicate that the presence of Hrr25 in P-bodies may serve to maintain the levels of this enzyme by limiting its turnover in the cytoplasm. Targeting-defective variants of Hrr25 that fail to localize to P-bodies were degraded when cells were exposed to a granule-inducing stress. Thus, the decision to associate with an RNP granule can influence the ultimate fate of the recruited protein. The sequestration in the granule would provide the cell with a pool of active Hrr25 that could be deployed when the initiating stress is removed. This work therefore advances our understanding of the mechanisms controlling protein recruitment to RNP granules and the potential consequences for the localized protein. Determining the broader physiological relevance of this pool of protein is the next step in the analysis and is a primary focus of our current research efforts. Since we know rather little currently about the biological roles of the P-body, the continuation of this work should further our understanding of these and related RNP granules in the eukaryotic cell.
Acknowledgments
We thank Tien-Hsien Chang, Martha Cyert, Harold Fisk, Anita Hopper, Roy Parker, Daniel Schoenberg, Jeremy Thorner, and Claudio de Virgilio for reagents used in this study; Harold Fisk, Anita Hopper, and Jian-Qiu Wu for access to their equipment; Kevin Corbett for sharing Hrr25 structural data prior to publication; Megan Emerson and Anna Butler for technical support; and members of the Herman lab, especially Regina Nostramo, for helpful discussions and comments on the manuscript. This work was supported by grants GM-101191 and GM-065227 from the National Institutes of Health (to P.K.H.) and a graduate student fellowship from the Pelotonia Fellowship Program (to B.Z.).
Footnotes
Communicating editor: D. J. Lew
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.187419/-/DC1.
Literature Cited
- Anderson P., Kedersha N., 2009. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 10: 430–436. [DOI] [PubMed] [Google Scholar]
- Anderson P., Kedersha N., Ivanov P., 2015. Stress granules, P-bodies and cancer. Biochim. Biophys. Acta 1849: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimoto K., Fukuda H., Imajoh-Ohmi S., Saito H., Takekawa M., 2008. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 10: 1324–1332. [DOI] [PubMed] [Google Scholar]
- Ashe M. P., De Long S. K., Sachs A. B., 2000. Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell 11: 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal V., Parker R., 2009. Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr. Opin. Cell Biol. 21: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashkirov V. I., Scherthan H., Solinger J. A., Buerstedde J. M., Heyer W. D., 1997. A mouse cytoplasmic exoribonuclease (mXRN1p) with preference for G4 tetraplex substrates. J. Cell Biol. 136: 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham C. J., Light H. R., Nissan T. A., Ahlquist P., Parker R., et al. , 2007. Interactions between brome mosaic virus RNAs and cytoplasmic processing bodies. J. Virol. 81: 9759–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelman C. A., Stevens A., Caponigro G., LaGrandeur T. E., Hatfield L., et al. , 1996. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature 382: 642–646. [DOI] [PubMed] [Google Scholar]
- Bishop A. C., Buzko O., Shokat K. M., 2001. Magic bullets for protein kinases. Trends Cell Biol. 11: 167–172. [DOI] [PubMed] [Google Scholar]
- Biswas A., Mukherjee S., Das S., Shields D., Chow C. W., et al. , 2011. Opposing action of casein kinase 1 and calcineurin in nucleo-cytoplasmic shuttling of mammalian translation initiation factor eIF6. J. Biol. Chem. 286: 3129–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P., 2011. Soft active aggregates: mechanics, dynamics and self-assembly of liquid-like intracellular protein bodies. Soft Matter 7: 3052–3059. [Google Scholar]
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., et al. , 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324: 1729–1732. [DOI] [PubMed] [Google Scholar]
- Brangwynne C. P., Mitchison T. J., Hyman A. A., 2011. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 108: 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan J. R., Yoon J. H., Parker R., 2011. Stress-specific composition, assembly and kinetics of stress granules in Saccharomyces cerevisiae. J. Cell Sci. 124: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya Y. V., Hama H., DeWald D. B., Herman P. K., 2002. The C terminus of the Vps34p phosphoinositide 3-kinase is necessary and sufficient for the interaction with the Vps15p protein kinase. J. Biol. Chem. 277: 287–294. [DOI] [PubMed] [Google Scholar]
- Budovskaya Y. V., Stephan J. S., Reggiori F., Klionsky D. J., Herman P. K., 2004. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J. Biol. Chem. 279: 20663–20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary G. A., Vinh D. B., May P., Kuestner R., Dudley A. M., 2015. Proteomic Analysis of Dhh1 Complexes Reveals a Role for Hsp40 Chaperone Ydj1 in Yeast P-Body Assembly. G3 (Bethesda) 5: 2497–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot N., Babajko S., Seraphin B., 2004. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 165: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C. J., Teixeira D., Parker R., 2007. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J. Cell Biol. 179: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaggio A. J., Lindberg R. A., Hunter T., Hoekstra M. F., 1992. The budding yeast HRR25 gene product is a casein kinase I isoform. Proc. Natl. Acad. Sci. USA 89: 7008–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E., 2007a P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8: 9–22. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E., 2007b P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 27: 3970–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T., Jakymiw A., Chan E. K., Seraphin B., Cougot N., et al. , 2003. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA 9: 1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks T. M., Lykke-Andersen J., 2008. The control of mRNA decapping and P-body formation. Mol. Cell 32: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., Chu A. M., Ni L., Connelly C., Riles L., et al. , 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. [DOI] [PubMed] [Google Scholar]
- Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., et al. , 2004. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15: 5383–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenan G., Brangwynne C. P., Jaensch S., Gharakhani J., Julicher F., et al. , 2010. Centrosome size sets mitotic spindle length in Caenorhabditis elegans embryos. Curr. Biol. 20: 353–358. [DOI] [PubMed] [Google Scholar]
- Grozav A. G., Chikamori K., Kozuki T., Grabowski D. R., Bukowski R. M., et al. , 2009. Casein kinase I delta/epsilon phosphorylates topoisomerase IIalpha at serine-1106 and modulates DNA cleavage activity. Nucleic Acids Res. 37: 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D., 1990. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol. Cell. Biol. 10: 6742–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., et al. , 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415: 180–183. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., et al. , 1991. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science 253: 1031–1034. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Weber C. A., Julicher F., 2014. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30: 39–58. [DOI] [PubMed] [Google Scholar]
- Ingelfinger D., Arndt-Jovin D. J., Luhrmann R., Achsel T., 2002. The human LSm1–7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 8: 1489–1501. [PMC free article] [PubMed] [Google Scholar]
- Isoda M., Sako K., Suzuki K., Nishino K., Nakajo N., et al. , 2011. Dynamic regulation of Emi2 by Emi2-bound Cdk1/Plk1/CK1 and PP2A–B56 in meiotic arrest of Xenopus eggs. Dev. Cell 21: 506–519. [DOI] [PubMed] [Google Scholar]
- Ito T., Chiba T., Ozawa R., Yoshida M., Hattori M., et al. , 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S., Izaurralde E., 2013. The role of disordered protein regions in the assembly of decapping complexes and RNP granules. Genes Dev. 27: 2628–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafadar K. A., Zhu H., Snyder M., Cyert M. S., 2003. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 17: 2698–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Han T. W., Xie S., Shi K., Du X., et al. , 2012. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149: 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N., Anderson P., 2007. Mammalian stress granules and processing bodies. Methods Enzymol. 431: 61–81. [DOI] [PubMed] [Google Scholar]
- Kedersha N., Ivanov P., Anderson P., 2013. Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci. 38: 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. R., King O. D., Shorter J., Gitler A. D., 2013. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 201: 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., et al. , 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Lord C., Bhandari D., Menon S., Ghassemian M., Nycz D., et al. , 2011. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 473: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk C. P., Waller D. D., Makhnevych T., Dienemann A., Whiteway M., et al. , 2007. Nup53p is a target of two mitotic kinases, Cdk1p and Hrr25p. Traffic 8: 647–660. [DOI] [PubMed] [Google Scholar]
- Majumder S., Fisk H. A., 2013. VDAC3 and Mps1 negatively regulate ciliogenesis. Cell Cycle 12: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelitsch M. D., Weissman J. S., 2000. A census of glutamine/asparagine-rich regions: implications for their conserved function and the prediction of novel prions. Proc. Natl. Acad. Sci. USA 97: 11910–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S. F., Jain S., She M., Parker R., 2013. Global analysis of yeast mRNPs. Nat. Struct. Mol. Biol. 20: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A., Kimura K., Nakano A., 1999. The inactive form of a yeast casein kinase I suppresses the secretory defect of the sec12 mutant. Implication of negative regulation by the Hrr25 kinase in the vesicle budding from the endoplasmic reticulum. J. Biol. Chem. 274: 3804–3810. [DOI] [PubMed] [Google Scholar]
- Parker R., Sheth U., 2007. P bodies and the control of mRNA translation and degradation. Mol. Cell 25: 635–646. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Matos J., Mori S., Gregan J., Bogdanova A., et al. , 2006. Monopolar attachment of sister kinetochores at meiosis I requires casein kinase 1. Cell 126: 1049–1064. [DOI] [PubMed] [Google Scholar]
- Pilkington G. R., Parker R., 2008. Pat1 contains distinct functional domains that promote P-body assembly and activation of decapping. Mol. Cell. Biol. 28: 1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V., Shah K. H., Herman P. K., 2011. The cAMP-dependent protein kinase signaling pathway is a key regulator of P body foci formation. Mol. Cell 43: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P., Basu U., Ray A., Majumdar R., Deng H., et al. , 2008. The Saccharomyces cerevisiae 60 S ribosome biogenesis factor Tif6p is regulated by Hrr25p-mediated phosphorylation. J. Biol. Chem. 283: 9681–9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijns M. A., Alexander R. D., Spiller M. P., Beggs J. D., 2008. A role for Q/N-rich aggregation-prone regions in P-body localization. J. Cell Sci. 121: 2463–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer T., Maco B., Petfalski E., Tollervey D., Bottcher B., et al. , 2006. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature 441: 651–655. [DOI] [PubMed] [Google Scholar]
- Segal S. P., Dunckley T., Parker R., 2006. Sbp1p affects translational repression and decapping in Saccharomyces cerevisiae. Mol. Cell. Biol. 26: 5120–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. H., Nostramo R., Zhang B., Varia S. N., Klett B. M., et al. , 2014. Protein kinases are associated with multiple, distinct cytoplasmic granules in quiescent yeast cells. Genetics 198: 1495–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She M., Decker C. J., Chen N., Tumati S., Parker R., et al. , 2006. Crystal structure and functional analysis of Dcp2p from Schizosaccharomyces pombe. Nat. Struct. Mol. Biol. 13: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U., Parker R., 2003. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300: 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E., Kornitzer D., 2014. Pulse-chase analysis to measure protein degradation. Methods Enzymol. 536: 65–75. [DOI] [PubMed] [Google Scholar]
- Stoecklin G., Mayo T., Anderson P., 2006. ARE-mRNA degradation requires the 5′-3′ decay pathway. EMBO Rep. 7: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara T., Maeda T., 2012. Transient sequestration of TORC1 into stress granules during heat stress. Mol. Cell 47: 242–252. [DOI] [PubMed] [Google Scholar]
- Teixeira D., Parker R., 2007. Analysis of P-body assembly in Saccharomyces cerevisiae. Mol. Biol. Cell 18: 2274–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thedieck K., Holzwarth B., Prentzell M. T., Boehlke C., Klasener K., et al. , 2013. Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells. Cell 154: 859–874. [DOI] [PubMed] [Google Scholar]
- Thomas M. G., Loschi M., Desbats M. A., Boccaccio G. L., 2011. RNA granules: the good, the bad and the ugly. Cell. Signal. 23: 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E., Cougot N., Meyer S., Babajko S., Wahle E., et al. , 2002. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 21: 6915–6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varia S., Potabathula D., Deng Z., Bubulya A., Bubulya P. A., 2013. Btf and TRAP150 have distinct roles in regulating subcellular mRNA distribution. Nucleus 4: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Lo Presti L., Zhu Y. H., Kang M., Wu Z., et al. , 2014. The novel proteins Rng8 and Rng9 regulate the myosin-V Myo51 during fission yeast cytokinesis. J. Cell Biol. 205: 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S. C., Brangwynne C. P., 2012. Getting RNA and protein in phase. Cell 149: 1188–1191. [DOI] [PubMed] [Google Scholar]
- Wippich F., Bodenmiller B., Trajkovska M. G., Wanka S., Aebersold R., et al. , 2013. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152: 791–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.