Abstract
Species differentiation and the underlying genetics of reproductive isolation are central topics in evolutionary biology. Hybrid sterility is one kind of reproductive barrier that can lead to differentiation between species. Here, we analyze the complex genetic basis of the intraspecific hybrid male sterility that occurs in the offspring of two distant natural strains of Arabidopsis thaliana, Shahdara and Mr-0, with Shahdara as the female parent. Using both classical and quantitative genetic approaches as well as cytological observation of pollen viability, we demonstrate that this particular hybrid sterility results from two causes of pollen mortality. First, the Shahdara cytoplasm induces gametophytic cytoplasmic male sterility (CMS) controlled by several nuclear loci. Second, several segregation distorters leading to allele-specific pollen abortion (pollen killers) operate in hybrids with either cytoplasm. The complete sterility of the hybrid with the Shahdara cytoplasm results from the genetic linkage of the two causes of pollen mortality, i.e., CMS nuclear determinants and pollen killers. Furthermore, natural variation at these loci in A. thaliana is associated with different male-sterility phenotypes in intraspecific hybrids. Our results suggest that the genomic conflicts that underlie segregation distorters and CMS can concurrently lead to reproductive barriers between distant strains within a species. This study provides a new framework for identifying molecular mechanisms and the evolutionary history of loci that contribute to reproductive isolation, and possibly to speciation. It also suggests that two types of genomic conflicts, CMS and segregation distorters, may coevolve in natural populations.
Keywords: cytoplasmic male sterility, hybrid sterility, pollen killer, reproductive barrier, segregation distorsion
REPRODUCTIVE isolation is an important component of species differentiation, and mechanisms that create reproductive barriers between once-conspecific organisms have attracted interest since evolutionary biology emerged as a field of study (Coyne 1992; Orr 1996). Hybrid incompatibility, due to genetic divergence between the hybridizing parents, as theorized by Bateson, Dobzhansky, and Muller (Orr 1996), is commonly observed between subspecies or distinct populations of the same species (Cutter 2012). Hybrid incompatibility is therefore thought to contribute to the differentiation between incipient species. Reproductive barriers translate into interspecific hybrid unviability, weakness, or sterility, and recent studies on several types of hybrid incompatibility have shed light on their mechanisms in various taxa (Maheshwari and Barbash 2011). The genetic basis of hybrid sterility has been studied in yeast (Chou et al. 2010), fruit flies (Dobzhansky 1936; Larracuente and Presgraves 2012), mice (Mihola et al. 2009), and several plants, including Solanum (Moyle and Nakazato 2008), rice (Oryza) (Ouyang et al. 2010), Mimulus (Barr and Fishman 2010; Lowry and Willis 2010), Arabidopsis lyrata (Leppala and Savolainen 2011), and A. thaliana (Torjek et al. 2006; Durand et al. 2012). In this last species, male sterility observed in the progeny of crosses between C24 or Shahdara (Sha) and Col-0 accessions results from an incompatible interaction between two duplicated genes located on different chromosomes (Durand et al. 2012), as in a classical two-locus Bateson–Dobzhansky–Muller (BDM) interaction. In rice, many reproductive barriers have been observed between species and subspecies, including several types of hybrid sterility (Ouyang and Zhang 2013). Classical two-locus BDM interactions between differentially inactivated duplicated genes appear to cause pollen sterility in hybrids between Oryza sativa and O. glumaepatula (Yamagata et al. 2010) and between the two O. sativa subspecies japonica and indica (Mizuta et al. 2010). In both of these BDM interactions, a particular combination of alleles present at the two loci is deleterious at the haploid stage, leading to the production of deficient pollen grains in the hybrid plant. In addition, at least two examples of one-locus hybrid sterility have been reported in rice (Long et al. 2008; Yang et al. 2012). In these cases, hybrid sterility results from the abortion of the gametophytes (pollen grains in one case, embryo sacs in the other) that carry one of the parental alleles, when in the presence of the other allele in the hybrid. Such loci, leading to segregation distortions, were named gamete killers (Cameron and Moav 1957). Segregation distorters are considered selfish genetic elements because they enhance their own transmission to progeny at the expense of the fitness of the organism, creating an intragenomic conflict (Frank 2000).
Another type of hybrid sterility involves cytoplasmic male sterility (CMS), which generates an intragenomic conflict between the cytoplasmic and nuclear genomes. In this case, pollen abortion is induced by the presence of maternally inherited mitochondrial genes, which enhance their fitness by impairing resource allocation to the male function (Cosmides and Tooby 1981; Saur Jacobs and Wade 2003). Nuclear restorers of fertility (Rf) that inhibit the action of sterilizing mitochondrial genes are selected under the pressure to restore male function. In some cases, Rf genes have a deleterious effect on fitness compared with their nonrestorer alleles, resulting in a cost of restoration (McCauley and Bailey 2009). When Rfs are not fixed in populations, gynodioecy (the co-occurrence of females and hermaphrodites) is observed. However, fixation of Rfs leads to undetectable, cryptic CMS, revealed only by crosses between individuals from allopatric hermaphrodite populations. Cryptic CMS has been reported in Mimulus (Fishman and Willis 2006) and A. lyrata (Leppala and Savolainen 2011; Aalto et al. 2013). In A. thaliana, we previously discovered a cryptic CMS by crossing two distant A. thaliana accessions, Sha and Mr-0, originating respectively from Tajikistan and Sicily. The reciprocal F1 hybrids give different reproductive phenotypes: F1 plants with the Sha cytoplasm are unable to produce pollen and, consequently, seeds (because A. thaliana is a selfing species). In contrast, the reciprocal F1 plants with Mr-0 cytoplasm have full seed set. A gene present in the mitochondrial genome of Sha, called orf117Sha, has been identified as the cytoplasmic factor that induces male sterility; in the nuclear genome, two large regions on chromosomes 1 and 3 are associated with the sterility phenotype (Gobron et al. 2013).
Here, we dissect the complex genetic bases of the Sha × Mr-0 hybrid male sterility. We confirm that the Sha cytoplasm induces male sterility in the presence of Mr-0 nuclear alleles and narrow down the two main nuclear regions involved in the hybrid sterility. We also uncover nuclear segregation distorters that lead to the death of pollen grains carrying Sha alleles (pollen killers; PKs) at the same loci previously identified as involved in the CMS. By conducting a QTL analysis, we identify several additional genomic regions involved in Sha × Mr-0 hybrid sterility. Finally, by studying other crosses in A. thaliana, we explore the links between the sterility phenotypes of the hybrids and natural variation at the loci involved in the sterility. Our results indicate that the hybrid sterility observed in the Sha × Mr-0 F1 results from the combination of gametophytic CMS and PK effects whose nuclear determinants are genetically linked.
Materials and Methods
Nomenclature
Hereafter, crosses are always written in the following, conventional order: female parent × male parent. When needed, the origin of the cytoplasmic (mitochondrial and chloroplastic) genomes of the crossed plants is indicated in brackets before the name that designates the nuclear genotype: a plant carrying the cytoplasm from parent A and the nuclear genome from parent B is designated [A]B. The genotype at a specified locus Lx is designated LxM, LxS, or LxH for homozygous Mr-0, homozygous Sha, or heterozygous, respectively. BCp denotes paternal backcross, and BCm denotes maternal backcross.
Plant materials
The [Sha]Mr-0 and [Mr-0]Sha cytolines were obtained after recurrent paternal backcrosses of Sha × Mr-0 and Mr-0 × Sha F1s followed by genotyping with 384 SNP markers distributed throughout the genome (see Supplemental Material, File S1). The SNPs used are available at https://www.versailles.inra.fr/ijpb/crb/anatool/ (Simon et al. 2012).
Due to the poor fertility of plants heterozygous on chromosome 1 and chromosome 3 in the Sha cytoplasm, segregating populations needed in this work were obtained through complex cross plans described below.
Two populations, PopL1 and PopL3, were constructed with the aim of narrowing down the intervals containing the two main loci involved in hybrid sterility, hereafter named L1 and L3 (Figure S1). They had the Sha cytoplasm and a nuclear background mainly homozygous for the Sha alleles, except on chromosome 1 and chromosome 3. In PopL1, chromosome 1 segregated heterozygous and homozygous Sha genotypes between 10.7 Mb and the south telomere, whereas chromosome 3 was homozygous Mr-0 from 15.2 Mb to the south telomere. Conversely, in PopL3, chromosome 1 was homozygous Mr-0 from 10.7 Mb to the south telomere, and chromosome 3 segregated heterozygous and homozygous Sha genotypes between 15.2 Mb and the south telomere.
Near-isogenic lines with a Sha nuclear background but heterozygous at L1, L3, or both L1 and L3 were created in the two cytoplasmic backgrounds, in order to investigate segregation distortion at these two loci (see File S1, Figure S1, and Figure S2).
The Sha × Mr-0 F2* mapping population was composed of plants carrying the Sha cytoplasm, homozygous Mr-0 at L1 and L3, and segregating elsewhere. To obtain this population, a plant [Sha]L1ML3M, selected from the progeny of the [Sha]L1HL3H × [Mr-0]L1HL3H cross, was first crossed with Mr-0 in both ways. The Sha × Mr-0 F2* population was then obtained by crossing the two resulting F1 plants, using the F1 carrying the Sha cytoplasm as the mother, because it produces very few seeds via selfing.
Growth conditions
Before sowing, seeds were stratified in the dark at 4°C for 3 days in a water solution containing 0.1% agar and 7 mM KNO3 to overcome the dormancy that is particularly strong in seeds with Mr-0 alleles. Plants were grown in soil in a greenhouse under long-day conditions (16-h day, 8-h night) with additional artificial light (105 µE/m2/sec) when necessary.
Genotyping
SNP genotyping was performed at the genomics facility at INRA Toulouse, France (http://www.genotoul.fr); genomic DNA preparation, genotyping, and data analysis were carried out as described by Simon et al. (2012). For microsatellite genotyping, DNA extractions were conducted on leaves from 15-day-old seedlings as described by Loudet et al. (2002); the microsatellite markers used are described in Table S1. The presence of orf117Sha was detected by PCR as described by Gobron et al. (2013). Allele-specific PCRs at L1 and L3 were performed with the primer pairs described in Table S1 (L1MrF/L1MrR and L1ShaF/L1ShaR, specific to Mr-0 and Sha alleles, respectively, at L1, and L3MrF/L3MrR and L3ShaF/L3ShaR, specific to Mr-0 and Sha alleles, respectively, at L3) on equal amounts of DNA; pollen was isolated as described by Honys and Twell (2004).
Phenotyping
Plant overall fertility was scored using a visual estimate of the number and size of the fruits (siliques) that developed. Scores were based on the following scale (with fractional scores if necessary): 0, plant completely sterile (no developed siliques); 1, a few developed siliques; 2, roughly half of the siliques are developed; 3, a few aborted siliques; 4, plant fully fertile (Figure S3). Sha and Mr-0 plants were included in every experiment as fully fertile controls. Scoring was carried out independently by two experimenters. For each plant, scoring was carried out after three stems had flowered. To take into account the possible variation in the fertility phenotype during development, plants were scored one or two times per week for at least 2 or 3 weeks to obtain a minimum of five scores per plant, averaged to obtain the final score.
Pollen viability was estimated from flower buds harvested just before anthesis. Sha and Mr-0 plants were used as fertile controls. For each plant, anthers of two buds (8–12 anthers/plant) were dissected and observed under a light microscope after Alexander staining (Alexander 1969). The cytoplasm of viable pollen grains was colored in red, and the pollen wall was colored in green, thus dead pollen grains appeared greenish. For each anther, pollen viability was scored using the following scale: 0, all pollen grains aborted; 1, a few viable pollen grains; 2, about half of the pollen grains are viable; 3, a few aborted pollen grains; 4, all pollen grains are viable. The final score of the plant was the average of all anther scores.
According to their cytoplasms and nuclear genotypes, mainly at L1 and L3, the plants from crosses between Sha and Mr-0 showed different degrees of overall and/or pollen fertility. The fertility of the genotypes, scored at the overall plant level and at the pollen viability level as described above, is summarized in Table 1. Some plants with a maximum overall fertility score (i.e., 4) showed low pollen viability.
Table 1. Fertility phenotype of genotypes from crosses between Sha and Mr-0.
| Plant fertility score | Pollen viability score | Figure | |
|---|---|---|---|
| [Mr-0]Sha | 4 | 4 | Figure 1, A and C |
| [Sha]Mr-0 | 0 | 0.1 | Figure 1, A and C |
| Sha × Mr-0 F1 | 0 | 0.1 | Figure 3 |
| Mr-0 × Sha F1 | 4 | 1.6 | Figure 3 |
| [Sha]L1HL3H | 0 to >2a | 0 to >1.5a | Figure 6 |
| [Mr-0]L1HL3H | 4 | 2.1 | Figure 6 |
| [Sha]L1ML3M | 4 | 3.6 | — |
During their development, these plants are first sterile and then later produce some siliques with seeds.
L1 and L3 mapping
At both L1 and L3, the Sha homozygous state was associated with an overall fertility score of 3 or higher, whereas heterozygotes were mainly sterile (overall fertility score of 1 or lower). Genotyping of the 276 plants from PopL1 led to the identification of 85 recombinants between markers CIW1 (18.4 Mb) and F5I14 (24.4 Mb). Similarly, genotyping of the 294 plants from PopL3 identified 43 recombinants between markers MSAT3.58 (18.7 Mb) and MSAT3.70 (23.4 Mb). Because we suspected that the sterility phenotype of homozygotes could necessitate several determinants, we used only sterile recombinants to reduce the intervals. Therefore, for L1 and L3 mapping, markers homozygous for Sha alleles in mainly sterile recombinants (an overall fertility score of 1 or lower) were excluded from the candidate interval.
QTL analysis
Phenotyping and genotyping data of the Sha × Mr-0 F2* population were analyzed with the R/qtl package (Broman et al. 2003), using the Haley–Knott regression method. The QTL model was implemented and refined using the addqtl, addint, and refineqtl functions, by adding significant new QTL and/or interactions to the QTL first detected with the scanone function.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Cytolines confirm the Sha/Mr-0 CMS
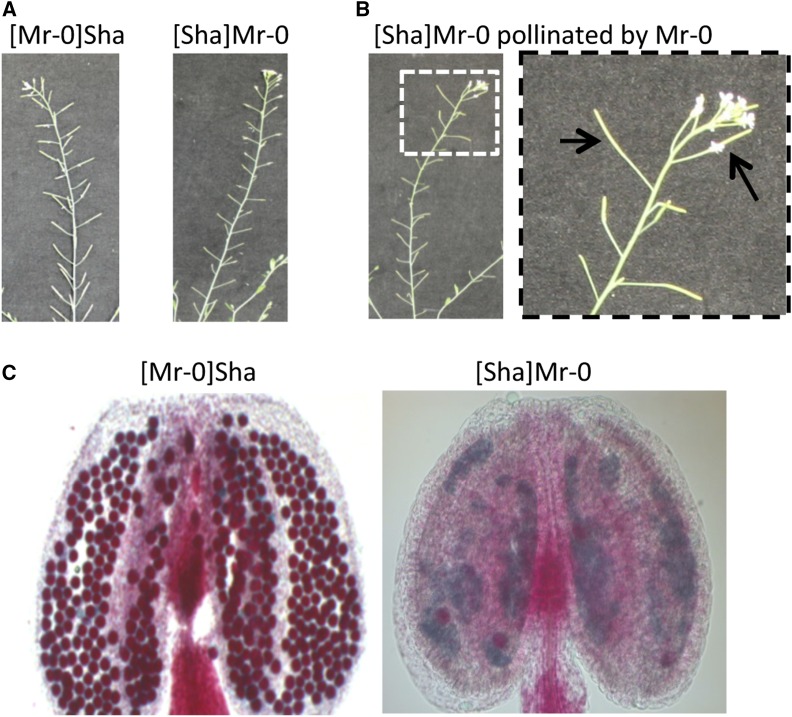
Our previous work (Gobron et al. 2013) showed that the Sha × Mr-0 hybrid is completely sterile, in contrast to the Mr-0 × Sha hybrid, which has full seed set. From each of these two F1 hybrids, we created a line combining the nuclear genome of one accession with the cytoplasmic genomes of the other via a series of recurrent backcrosses with the male parent. Such genotypes are hereafter referred to as cytolines (Grace et al. 1994). In the greenhouse, the cytolines [Sha]Mr-0 and [Mr-0]Sha did not show any growth or developmental defects, but [Sha]Mr-0 was sterile, producing no seeds, whereas [Mr-0]Sha was fertile (Figure 1A). Seed production was restored in [Sha]Mr-0 after manual pollination with Mr-0 pollen, indicating a male dysfunction in [Sha]Mr-0 (Figure 1B). Cytological observations of anthers showed that the [Sha]Mr-0 cytoline produced almost no viable pollen, while all pollen grains were viable in [Mr-0]Sha (Figure 1C). This pattern confirmed that the Sha cytoplasm is responsible for a male sterility, which can be suppressed by factors that are present in the Sha nuclear genome (so-called Rfs) but are absent or nonfunctional in the Mr-0 nuclear genome.
Figure 1.
Reproductive phenotypes of reciprocal cytolines. (A) The [Mr-0]Sha cytoline presents normal fruit (silique) development whereas the [Sha]Mr-0 cytoline is totally sterile. (B) Manual pollination of the sterile [Sha]Mr-0 cytoline with Mr-0 pollen (dashed box) leads to a fertile, restored phenotype (arrows). (C) Alexander staining (observation under a light microscope, ×10) of anthers. The cytoline with the Mr-0 cytoplasm has only viable (red) pollen grains, whereas its reciprocal shows no viable pollen grains.
The two nuclear loci L1 and L3 are required for Sha × Mr-0 hybrid male sterility
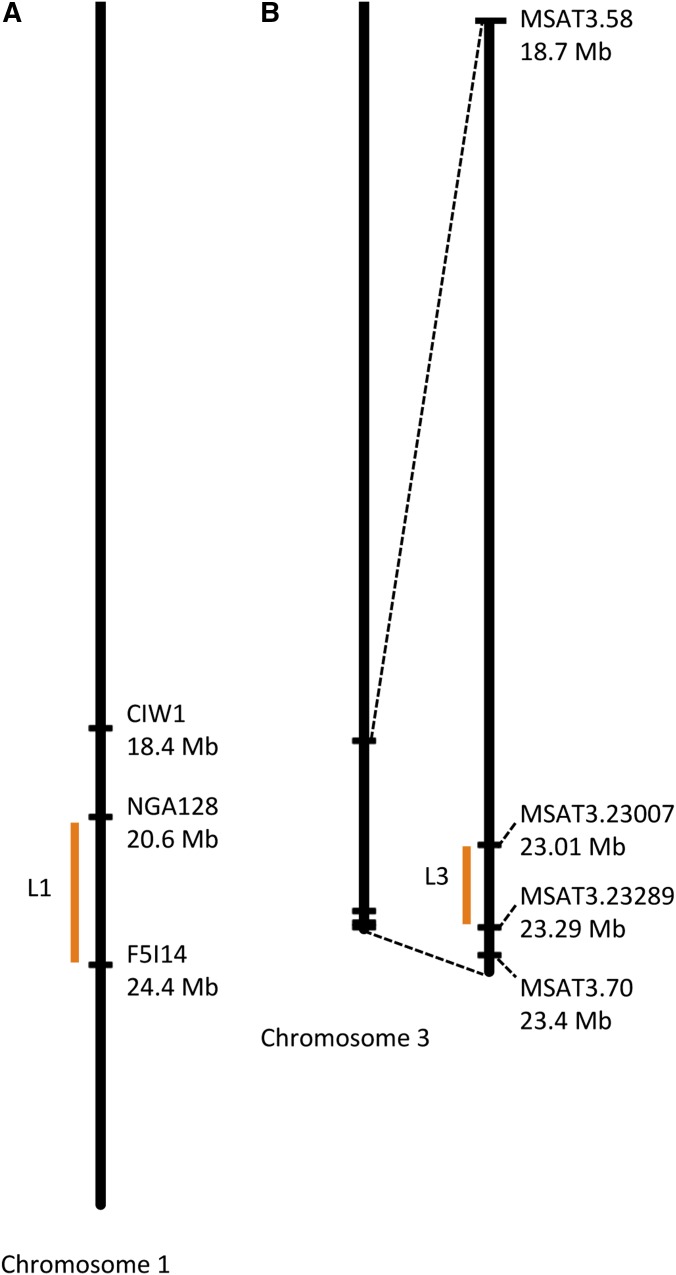
A preliminary study suggested that two regions on the southern arms of chromosome 1 and chromosome 3 carried major genetic factors for hybrid sterility (Gobron et al. 2013). To map these two loci independently, we created two mapping populations in the Sha cytoplasm, PopL1 and PopL3. In PopL1, the L1 region segregated heterozygous and homozygous Sha genotypes, whereas the L3 region was fixed for Mr-0. Similarly, in PopL3 the L3 region segregated heterozygous and homozygous Sha genotypes whereas L1 was fixed for Mr-0 (Figure S1). In each mapping population, plants that were homozygous Sha at the segregating region had normal seed sets. Thus, we were able to reduce the size of the region of interest by excluding regions that were homozygous Sha in sterile recombinant plants. This mapping strategy confirmed that the two regions L1 and L3 carry major factors required for Sha × Mr-0 hybrid sterility, and narrowed down the causal loci to 3.8 Mb on chromosome 1 (Figure 2A) and to 0.28 Mb on chromosome 3 (Figure 2B). The L1 locus remained rather large due to the lack of sterile recombinants in this interval, suggesting that it carries more than one determinant needed for hybrid sterility.
Figure 2.
Mapping of the L1 and L3 loci involved in male sterility. (A) The L1 locus was narrowed down to the interval between the NGA128 and the F5I14 markers. (B) The L3 locus was narrowed down to the interval between the MSAT3.23007 and the MSAT3.23289 markers.
L1 and L3 carry PKs active in both cytoplasms
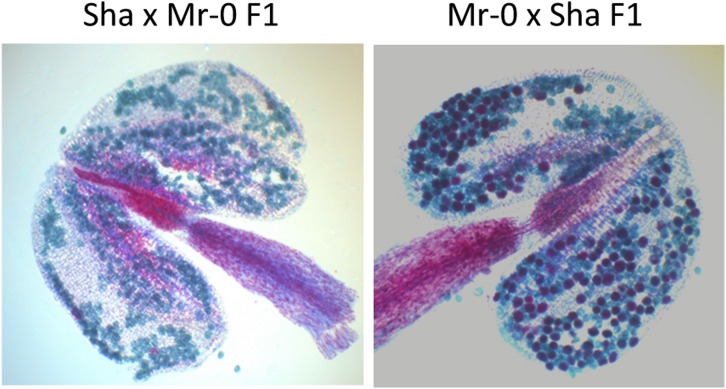
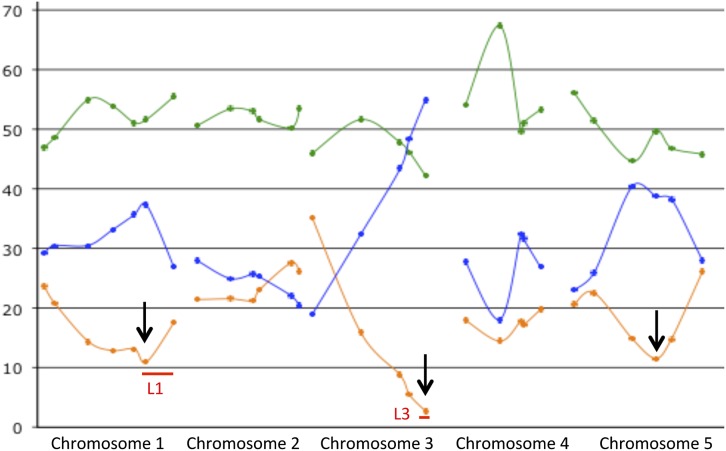
In the Mr-0 × Sha F1 plants, although they set seeds normally, more than half of pollen grains were aborted (Table 1 and Figure 3). When genotyping a Mr-0 × Sha F2 population with genome-wide markers, significant deficits in the Sha homozygous genotype were found at three loci (Figure 4 and Table S2). Two of these loci colocalized with L1 and L3, also involved in the sterility of the Sha × Mr-0 F1. Biases against the Sha alleles were also observed at L1 and L3 in the progeny of a cross using the Mr-0 × Sha F1 as the male parent and the sterile [Sha]Mr-0 cytoline as the female parent (Table 2), suggesting a relationship between segregation distortion and pollen mortality in Mr-0 × Sha F1 plants. Pollen grains produced by a heterozygous plant and that carried the Mr-0 allele at L1 and/or L3 contributed more than expected to the next generation, indicating that the nonviable pollen grains observed in the anthers of Mr-0 × Sha F1 plants carried Sha alleles. This was validated by genotyping mature pollen of Mr-0 × Sha F1 plants at both loci (Figure 5).
Figure 3.
Pollen viability of reciprocal Sha/Mr-0 F1 plants. In the Sha × Mr-0 F1, all pollen grains are aborted (green), whereas the reciprocal presents a mixture of aborted (green) and viable (red) pollen grains.
Figure 4.
Genome-wide analysis of allele segregation in the Mr-0 × Sha F2 family. The plots show the percentage of plants heterozygous (green), homozygous Mr-0 (blue), and homozygous Sha (orange) at each marker. Arrows indicate the markers where the bias against the Sha allele is maximal (see Table S2 for genotyping data). Red bars indicate the locations of the L1 and L3 loci.
Table 2. Genotype segregation at L1 and L3 in the [Sha]Mr-0 × (Mr-0 × Sha) family.
| Locus | Chromosome | Marker | Position (Mb) | Number of Hz | Number of Mr-0 | Number of plants | P (χ2) | Observed Hz frequency (%) | Expected Hz frequency (%) |
|---|---|---|---|---|---|---|---|---|---|
| L1 | 1 | NGA128 | 20.6 | 52 | 125 | 177 | 4 × 10–8 | 29 | 50 |
| L3 | 3 | MSAT3.23007 | 23.1 | 2 | 179 | 181 | 2 × 10–39 | 1 | 50 |
The segregation biases at L1 and L3 were measured in the male descent of a heterozygous plant to verify the male origin of the biases observed in its selfing progeny. Hz, heterozygote.
Figure 5.
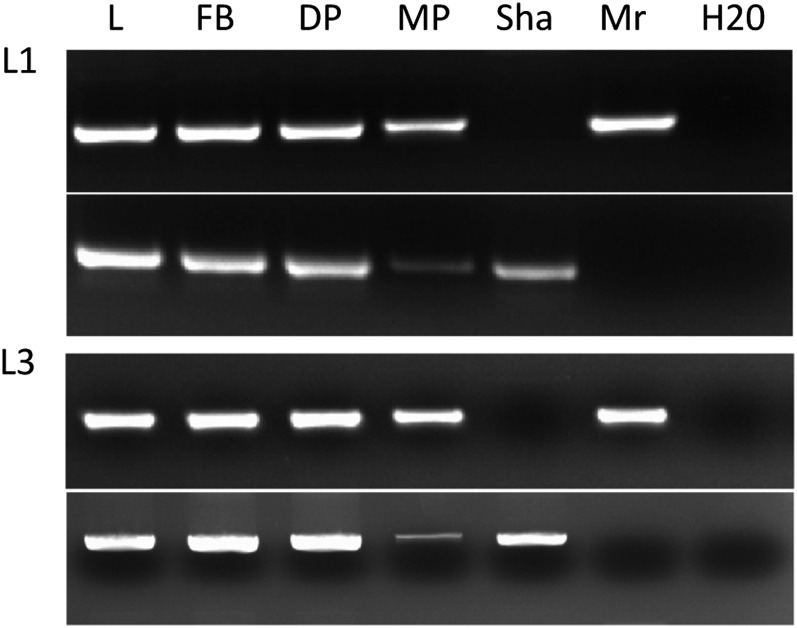
Genotyping at L1 and L3 of pollen from Mr-0 × Sha hybrid plants. At each locus, two different pairs of primers, respectively specific to the Mr-0 (top) or the Sha (bottom) allele, were used to amplify genomic DNA isolated from leaves (L), floral buds (FB), developing pollen (DP), and mature pollen (MP) of heterozygous plants. Sha and Mr, DNA from Sha and Mr-0 plantlets, respectively; H2O, negative control without DNA. The Sha alleles are barely detectable in mature pollen but amplified together with the Mr-0 alleles in immature pollen and vegetative tissues. Most of the pollen grains carrying the Sha alleles were thus eliminated at the mature pollen stage.
The segregation distortions against Sha alleles at L1 and L3 in the Mr-0 cytoplasmic background were confirmed in the selfing progenies of plants that were homozygous Sha apart from L1 and L3 ([Mr-0]L1HL3H) and of plants segregating only at either L1 or L3 ([Mr-0]L1HL3S or [Mr-0]L1SL3H) (Table 3). In all of these genotypes, unviable pollen was observed after Alexander staining (Figure 6 and Figure S4), further linking segregation biases to pollen mortality.
Table 3. Genotype segregation at L1 and L3 in the progeny of plants heterozygous at L1 and/or L3.
| Family | Locus | Chromosome | Marker | Position (Mb) | Number of Sha | Number of Hz | Number of Mr-0 | Number of plants | P (χ2) | Observed Sha frequency (%) | Expected Sha frequency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [Mr-0]L1HL3H | L1 | 1 | NGA128 | 20.6 | 15 | 85 | 70 | 170 | 2 × 10–8 | 9 | 25 |
| [Mr-0]L1HL3H | L3 | 3 | MSAT3.23007 | 23.0 | 31 | 79 | 61 | 171 | 3 × 10–3 | 18 | 25 |
| [Mr-0]L1HL3S | L1 | 1 | NGA128 | 20.6 | 5 | 100 | 78 | 183 | 1 × 10–13 | 3 | 25 |
| [Mr-0]L1SL3H | L3 | 3 | MSAT3.23007 | 23.0 | 18 | 87 | 73 | 178 | 4 × 10–8 | 10 | 25 |
| [Sha]L1HL3H | L1 | 1 | NGA128 | 20.6 | 4 | 29 | 21 | 54 | 4 × 10–3 | 7 | 25 |
| [Sha]L1HL3H | L3 | 3 | MSAT3.23007 | 23.0 | 2 | 25 | 29 | 56 | 2 × 10–6 | 4 | 25 |
| [Sha]L1HL3S | L1 | 1 | NGA128 | 20.6 | 10 | 91 | 83 | 184 | 2.6 × 10–13 | 5 | 25 |
| [Sha]L1SL3H | L3 | 3 | MSAT3.23007 | 23.0 | 0 | 91 | 87 | 178 | 3.3 × 10–19 | 0 | 25 |
The biases at L1 and L3 were observed in both cytoplasmic backgrounds and independently of each other. The observed biases are compatible with a pollen lethal effect (closer to 1:1 Hz:Mr-0 segregation). Alexander staining of the pollen from these plants is presented in Figure 6 and Figure S4. Hz, heterozygote.
Figure 6.
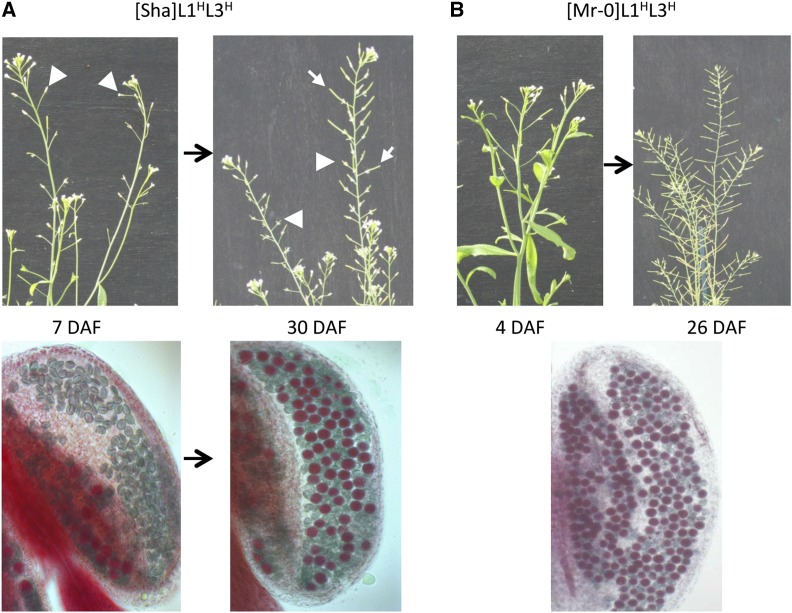
Reproductive phenotypes in [Sha]L1HL3H and [Mr-0]L1HL3H plants. Overall fertility phenotypes at two developmental stages (top panels) and pollen viability in close-up views of typical anthers after Alexander staining (bottom panels). DAF, day after flowering. (A) The [Sha]L1HL3H plants are initially completely sterile (left panel, top), with anthers harboring only aborted (green) pollen grains (left panel, bottom). Later on, the anthers also contain viable (red) pollen grains (right panel, bottom) and plants produce fertile flowers (right panel, top). White arrows indicate developed siliques that will produce seeds; white arrowheads indicate aborted fruits. (B) [Mr-0]L1HL3H plants are fully fertile throughout their development (top), and their anthers present a mixture of aborted and viable pollen grains throughout the reproductive phase (bottom).
In the Sha cytoplasmic background, we could not examine segregation biases in the male lineage because the Sha × Mr-0 F1 produced no viable pollen. Plants heterozygous only at L1 and L3 and homozygous Sha in the rest of their nuclear genome ([Sha]L1HL3H), although mainly sterile, produced some selfing seeds in late developmental stages (Table 1), and we detected biases against plants homozygous Sha at L1 and L3 (Table 3). These biases were confirmed in the selfing progenies of plants heterozygous only at either locus ([Sha]L1HL3S or [Sha]L1SL3H, Table 3).
Among the pollen grains produced by a plant heterozygous at L1 or L3, those carrying Sha alleles were outcompeted by those carrying Mr-0 alleles in either cytoplasm. We consistently observed pollen lethality in plants producing biased progenies (Figure 6 and Figure S4), and Sha alleles were under-represented in mature pollen from plants heterozygous at L1 and L3 (Figure 5), indicating that dead pollen grains carried Sha alleles at L1 and/or L3. Furthermore, both the Sha accession and the [Mr-0]Sha cytoline produced only viable pollen (Figure 1C); therefore, pollen grains carrying Sha alleles at these specific loci are killed when, and only when, they are produced by a plant heterozygous at these loci, leading to a deficit in Sha alleles in the progeny. This kind of segregation distorter has previously been defined as a PK, which acts at the gametophytic stage to eliminate one parental allele from the pollen production of a hybrid (Cameron and Moav 1957). A PK relies on the interaction of two types of actors: a “killer,” carried by one genotype, has a lethal effect on a “target,” carried by the other genotype that does not contain the killer; in the present case, the Mr-0 alleles at L1 and L3 are killers, whereas Sha alleles are targets. We analyzed the F2 progenies of crosses between Mr-0 and Cvi-0, known to be devoid of Rfs for the CMS (Gobron et al. 2013). These progenies showed significant biases against Cvi-0 alleles both at L1 and L3 (Table 4), indicating that the presence of Mr-0 alleles induces elimination of alleles from other natural accessions independently of their restorer function.
Table 4. Genotype segregation at L1 and L3 in the Cvi-0 × Mr-0 and Mr-0 × Cvi-0 F2 families.
| Family | Locus | Chromosome | Marker | Position (Mb) | Number of Cvi-0 | Number of Hz | Number of Mr-0 | Number of plants | P (χ2) | Observed Cvi-0 frequency (%) | Expected Cvi-0 frequency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cvi-0 × Mr-0 F2 | L1 | 1 | NGA128 | 20.6 | 18 | 98 | 62 | 178 | 8 × 10–6 | 10 | 25 |
| Mr-0 × Cvi-0 F2 | L1 | 1 | NGA128 | 20.6 | 20 | 101 | 60 | 181 | 4 × 10–5 | 11 | 25 |
| Cvi-0 × Mr-0 F2 | L3 | 3 | MSAT3.23007 | 23.0 | 13 | 81 | 85 | 179 | 1 × 10–13 | 7 | 25 |
| Mr-0 × Cvi-0 F2 | L3 | 3 | MSAT3.23007 | 23.0 | 11 | 98 | 69 | 178 | 2 × 10–9 | 6 | 25 |
PKs and CMS jointly participate in the Sha × Mr-0 F1 male sterility
The loss of pollen due to the PKs in Mr-0 × Sha F1 plants was not sufficient to alter their overall fertility (Table 1). However, Sha × Mr-0 F1 plants produced no viable pollen and no seeds. This contrast indicates that, in addition to PKs, other sterilizing factors act in the hybrid with the Sha cytoplasm and corroborates the involvement of the [Sha]Mr-0 CMS in hybrid sterility, leading to the death of pollen grains carrying Mr-0 alleles in the Sha cytoplasm. Further, we observed a difference in fertility between [Mr-0]L1HL3H plants, which set seeds normally, despite their loss of pollen due to PKs, and [Sha]L1HL3H plants, which were much less fertile (Table 1). PKs were active in all of these plants; therefore, their fertility difference, due to the CMS in the Sha cytoplasm, indicated that Sha had specific restorer genes at L1 and L3. We concluded that the two main loci involved in hybrid sterility, L1 and L3, carry both cytoplasm-independent PKs that lead to the death of Sha pollen grains and restorers of CMS that cause Mr-0 pollen to abort in the Sha cytoplasm. These two kinds of factors contribute together to the hybrid sterility of the Sha × Mr-0 F1.
L1 and L3 are not sufficient to produce full F1 male sterility
The sterility of the plants [Sha]L1HL3H changed during their development: they produced only sterile flowers during the first 2–3 weeks after flowering, but subsequently developed some siliques with seeds (Figure 6). Heterozygosity at L1 and L3 was thus not sufficient to maintain complete sterility when the rest of the genome is homozygous Sha. In addition, surprisingly, [Sha]L1ML3M plants, homozygous Mr-0 at both L1 and L3, were fertile (Table 1). These results strongly suggested the existence of additional Rf and potentially PKs in the genome, outside L1 and L3.
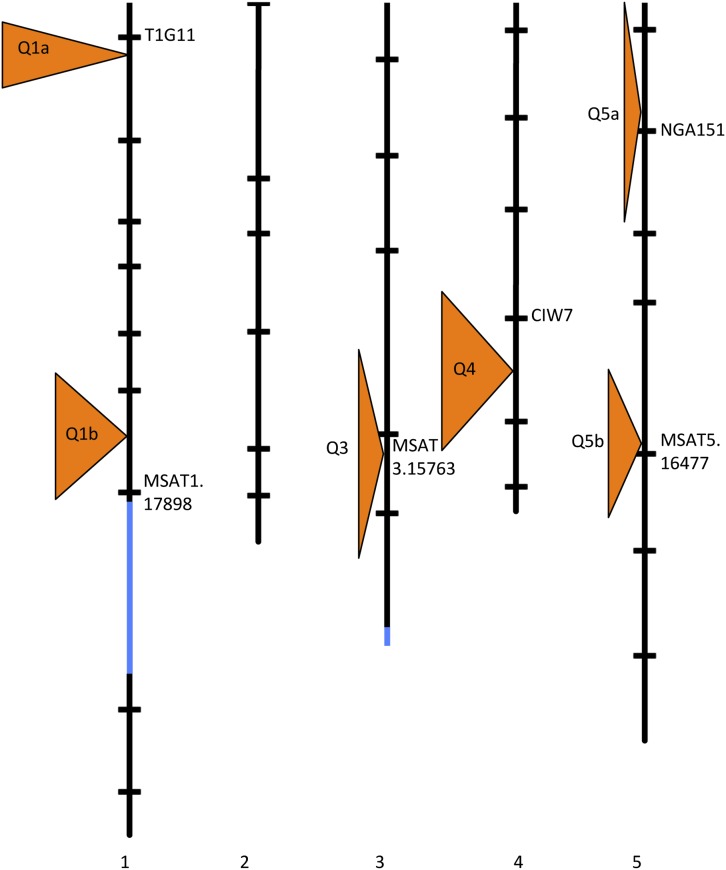
We used a quantitative genetic approach to look for additional loci involved in the fertility phenotype. To do so, we generated a mapping population (named Sha × Mr-0 F2*, see Materials and Methods) in the Sha cytoplasm, where plants were homozygous Mr-0 at L1 and L3, to eliminate previously identified PK effects and fix the nonrestorer alleles at these loci, and segregating elsewhere. The Sha × Mr-0 F2* family showed low overall fertility. We scored the overall fertility of 184 plants throughout their reproductive development (see Materials and Methods), and genotyped them with 33 markers scattered across the genome (Table S3). The QTL analysis identified six QTL, and one QTL–QTL interaction, together explaining 65.5% of the phenotypic variation (Figure 7 and Table S4). For every QTL, the Sha allele was associated with higher fertility. Interestingly, Q5b is located in a region at around 17 Mb on chromosome 5, hereafter named L5, where a strong bias against the Sha allele was observed in the Mr-0 cytoplasmic background (Figure 4, Table S2, and Table S5). We also observed a strong bias against the Sha allele at this locus in the progeny of a plant [Sha]L1ML3ML5H (Table S6). We suspect that this locus is also associated with an Rf (in Sha) and a PK (whose killer element is in the Mr-0 genome).
Figure 7.
QTL affecting the fertility phenotype in the Sha × Mr-0 F2* family. The regions that do not segregate in this family (homozygous for Mr-0 alleles) are indicated in blue. The orange triangles indicate the most likely positions of the QTL; their height corresponds to the 1.8 LOD support interval and their width is proportional to the LOD score of the corresponding QTL. The positions of markers are indicated as horizontal bars. The name of the marker closest to each QTL is given.
We conclude that the pollen lethality observed in the progeny of crosses between Sha and Mr-0 results from a complex genetic determinism involving different genomic regions. Moreover, pollen lethality is caused by a plurality of factors that act concurrently: gametophytic CMS relying on several nuclear loci in interaction with the Sha cytoplasm, plus PKs acting in either cytoplasm. Remarkably, our analyses indicated that both kinds of factors colocalized at several loci.
The separation of the PK and the CMS factors suppresses hybrid sterility
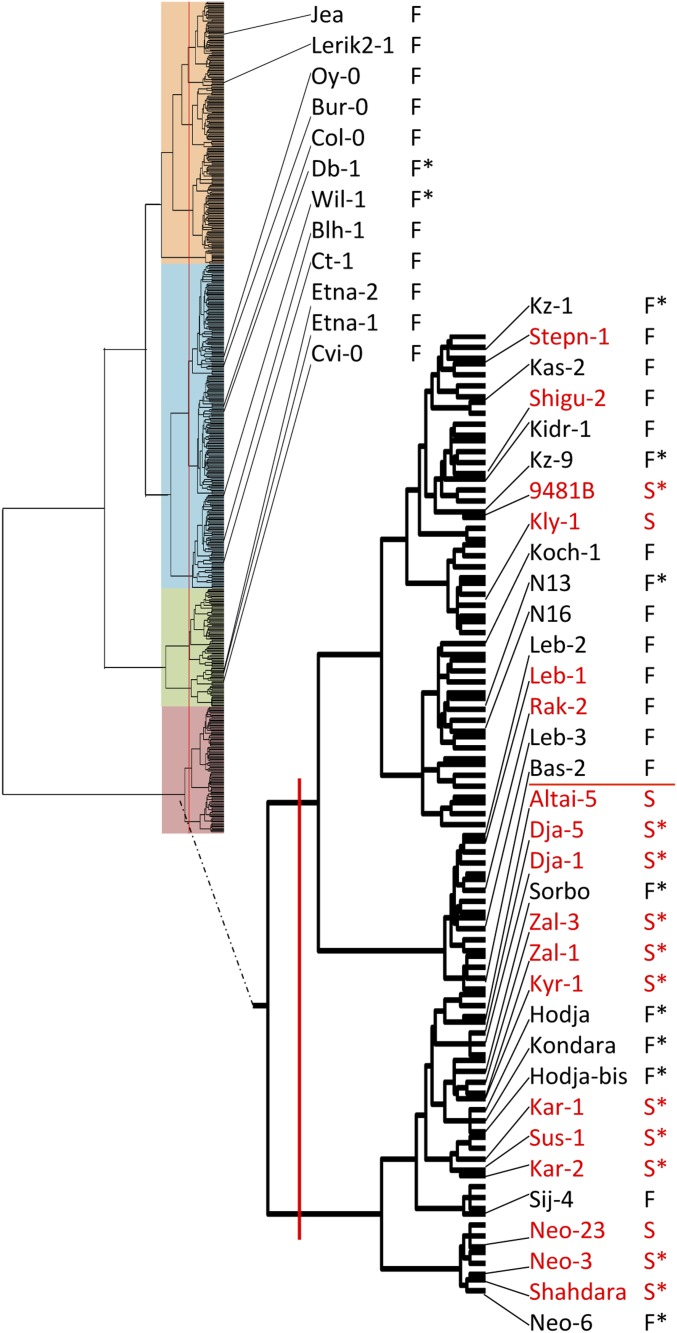
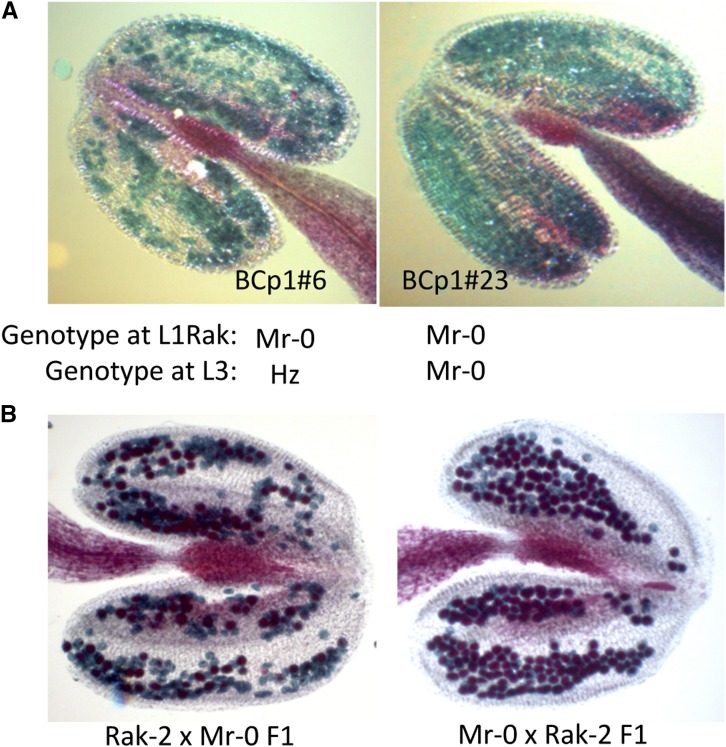
Studying the fertility of reciprocal crosses between Mr-0 and a panel of 21 accessions, Gobron et al. (2013) observed that the 11 accessions that do not carry the mitochondrial gene orf117Sha give rise to fertile F1s, whereas the 10 accessions carrying the orf117Sha produce male sterile F1s when crossed with Mr-0 as the male parent. In the present work, we performed 25 additional two-way crosses between accessions and Mr-0. Among the 46 accessions studied in total, the 28 accessions that do not carry the mitochondrial orf117Sha produced fertile F1s. In contrast, 14 out of the 18 crosses between Mr-0 as a male parent and accessions carrying the orf117Sha as females behaved like the Sha × Mr-0 cross: they produced completely male-sterile F1 hybrids, whereas reciprocal crosses were fertile (Figure 8). Four accessions, although carrying orf117Sha, gave fertile F1s when crossed to Mr-0 (Figure 8). They all belonged to the same subgroup of nuclear diversity, and we chose for further study the representative Rak-2 × Mr-0 cross, which was fertile although Rak-2 carries the same cytoplasm as Sha at all markers analyzed. We performed a paternal backcross (BCp1) of Rak-2 × Mr-0 F1 with Mr-0, and observed that 9 out of 27 BCp1 plants were completely male sterile (Table S7). The Rak-2 cytoplasm thus induces male sterility, as expected from its similarity to the Sha cytoplasm, and the difference in fertility between the hybrids Sha × Mr-0 and Rak-2 × Mr-0 is likely due to different nuclear factors controlling pollen viability in these two crosses.
Figure 8.
Fertility of 46 crosses with Mr-0 as the male parent. The accessions are organized in four main clusters (color shaded) corresponding to groups of genetic similarity obtained by clustering 598 natural accessions using 341 SNP markers (Simon et al. 2012). The accessions in red carry the mitochondrial sterilizing factor orf117Sha; they all belong to the same nuclear diversity group. The F1 phenotype is indicated beside the name of the maternal parent by “F” for fertile F1 or “S” for sterile F1. A red horizontal line separates the two differentiated subgroups of nuclear diversity in this group. *F1 phenotype from Gobron et al. (2013).
We genotyped 182 plants of each reciprocal Rak-2/Mr-0 F2 at the loci L1 and L3 (Table 5). L3 showed a strong bias against the Rak-2 allele in both cytoplasms, as observed for the Sha allele in Sha/Mr-0 crosses, and thus most likely resulting from a similar PK effect. L1 showed a strong segregation distortion against the Mr-0 allele in the Rak-2 × Mr-0 family only, which implied an interaction with the Rak-2 cytoplasm. Among 182 F2 plants of this family, all producing normal seed sets, there were no plant Mr-0 at markers ind1.22788 (22.8 Mb) and F5I14, an interval of 158 Kb included in L1, hereafter called L1Rak. This genetic behavior is expected for a unique Rf locus in gametophytic CMS. In addition, all sterile BCp1 plants were homozygous Mr-0 between ind1.22788 and F5I14 (Table S6), indicating that the pollen grains Mr-0 at L1Rak had died. Accordingly, we observed no viable pollen in the anthers of these BCp1 plants (Figure 9A). The anthers of the Rak-2 × Mr-0 F1 plants carried more aborted pollen grains than those of the reciprocal F1 (Figure 9B), which is consistent with a combination of two deleterious effects in the former (i.e., PK at L3 plus CMS at L1Rak) compared to the sole action of a PK at L3 in the latter. Furthermore, among BCp1 plants heterozygous at L1Rak, we observed more aborted pollen grains in the plants that were also heterozygous at L3 than in those that were Mr-0 at L3 (Table S7), although in every case the amount of dead pollen was not sufficient to alter the overall fertility of the plants.
Table 5. Genotype segregation at L1 and L3 in the Rak-2 × Mr-0 and Mr-0 × Rak-2 F2 families.
| Family | Locus | Chromosome | Marker | Position (Mb) | Number of Rak-2 | Number of Hz | Number of Mr-0 | Number of plants | P (χ2) | Observed Mr-0 frequency (%) | Expected Mr-0 frequency (%) | Observed Rak-2 frequency (%) | Expected Rak-2 frequency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rak-2 × Mr-0 F2 | L1 | 1 | NGA128 | 20.6 | 69 | 103 | 10 | 182 | 1 × 10–9 | 6 | 25 | – | – |
| L3 | 3 | MSAT3.23289 | 23.3 | 3 | 91 | 88 | 182 | 6 × 10–18 | – | – | 2 | 25 | |
| Mr-0 × Rak-2 F2 | L1 | 1 | NGA128 | 20.6 | 50 | 87 | 45 | 182 | 0.73 | 25 | 25 | – | – |
| L3 | 3 | MSAT3.23289 | 23.3 | 3 | 89 | 90 | 182 | 8 × 10–19 | – | – | 2 | 25 |
Figure 9.
Pollen viability of plants from crosses between Rak-2 and Mr-0. Alexander staining (observation under a light microscope, ×10) of anthers. Aborted pollen grains are stained green, while viable pollen grains are stained red. Hz, heterozygote. (A) Sterile Rak-2 × Mr-0 BCp1 plants (see also Table S6). (B) Rak-2/Mr-0 reciprocal F1 hybrids.
We conclude that in Rak-2 × Mr-0, as in Sha × Mr-0, pollen lethality results from a combination of both CMS and cytoplasm-independent PKs. L1 and L3, the two main loci involved in pollen mortality in the crosses between Sha and Mr-0, also participate in the sterility in the crosses between Rak-2 and Mr-0. However, remarkably, the plants heterozygous Rak-2/Mr-0 at L1 did not present any PK effect at this locus. Inversely, the progeny of plants heterozygous at L3 presented a strong bias against the Rak-2 allele (Table 5), typical of the PK observed at L3 in Sha/Mr-0 hybrids, whereas the Rak-2 allele at L3 had no restorer effect (Table S7). The dissociation of PK and CMS effects very likely contributes to the residual production of pollen in Rak2 × Mr-0 F1 plants, allowing normal seed set, unlike in Sha × Mr-0 F1 plants.
Discussion
The Sha × Mr-0 F1 accumulates different causes of pollen sterility
We explored the genetic determinants of male sterility observed in the offspring of two distant A. thaliana accessions. We showed that the absence of seed set in the Sha × Mr-0 hybrid results from the combination of two different kinds of causes, both acting on male gametophyte development: gametophytic CMS leading to the death of pollen carrying Mr-0 nonrestorer alleles in a Sha cytoplasmic background, and at least two PK loci leading to the specific abortion of pollen grains carrying the Sha alleles, independently of the cytoplasmic background. We confirmed the coexistence of these two mechanisms by isolating them from each other. First, the abortion of all pollen in the [Sha]Mr-0 cytoline indicates that the inability of the Mr-0 nuclear genome to restore fertility in the Sha cytoplasm is sufficient to achieve male sterility without the action of any segregation distorter. Second, heterozygotes at PK loci produce unviable pollen carrying the Sha alleles, even in a nonsterilizing cytoplasm. The normal seed set of the Mr-0 × Sha F1, despite pollen mortality due to PKs, shows that the combination of the two mechanisms is necessary for the total sterility of the reciprocal Sha × Mr-0 F1. L1 and L3, the two major loci involved in the Sha × Mr-0 hybrid sterility, effectively carry genetic factors for both mechanisms. In addition, in the Sha × Mr-0 F2* population, in which these two major loci were fixed for nonrestorer Mr-0 alleles, we detected six additional QTL for fertility, indicating a complex genetic determinism for the Sha × Mr-0 hybrid sterility.
Segregation distortions result from PKs active at several loci in the same intraspecific cross
Segregation distortions were observed in Sha/Mr-0 hybrids at three loci, L1, L3, and L5 (Figure 4, Table 2, Table 3, Table S2, Table S5, and Table S6). The under-representation of the Sha allele at L1 and L3 in mature pollen (Figure 5) showed that the distortions result from allele-dependent pollen death. Such distortions may result from the fitness costs of Rf genes, expressed at the pollen viability level (Montgomery et al. 2014) or from the action of PKs that eliminate the Sha alleles. Here, no pollen defect was observed in plants carrying fixed Sha alleles, in either cytoplasm. Furthermore, the observation of biases against Cvi-0 alleles at L1 and L3 in Cvi-0/Mr-0 hybrids (Table 4) demonstrates that the Mr-0 alleles have killer behavior at these loci, and that the elimination of target alleles is not due to an Rf, because Cvi-0 alleles are not restorers (Gobron et al. 2013). We also observed the elimination of Rak-2 nonrestorer alleles at L3 in the Rak-2/Mr-0 F2s (Table 5). Taken together, these results show that PKs are the main cause of the segregation biases at L1 and L3 in the Sha/Mr-0 heterozygotes, although we cannot exclude an additional cost effect of Rfs at these loci and/or at L5.
Single-locus segregation distorters usually rely on the tight genetic linkage of several interacting genes, leading to their transmission as self-sufficient loci. Such loci have been documented in animals, especially in Drosophila (Orr et al. 2007; Larracuente and Presgraves 2012), and in Ascomycetes (Turner and Perkins 1979; Raju 1994). In plants, after the first description of a PK in a hybrid between two Solanum species (Cameron and Moav 1957), gamete killers (most often PKs) have been reported, especially in hybrids between cultivated plants and their wild relatives, where they often constitute obstacles to the breeding of desirable traits (Maan 1975; Sano 1990). In rice in particular, a number of gamete killers have been described in crosses between cultivated rice (O. sativa) and its wild relatives (Sano 1990; Hu et al. 2006; Garavito et al. 2010), or between O. sativa subspecies (Oka 1974; Zhang et al. 2006). The genes underlying two of the latter have been identified (Chen et al. 2008; Long et al. 2008; Yang et al. 2012). Gamete killers are also found in wild species, as in the interspecific cross between Mimulus guttatus and M. nasutus (Fishman and Saunders 2008), and, at the intraspecific level, in some populations of the dioecious Silene latifolia (Taylor and Ingvarsson 2003). Recently, several single-locus gametic transmission distorters were reported in interpopulation crosses of the A. thaliana relative A. lyrata, most of which affect pollen, although the causes of segregation distortion remain to be elucidated (Leppala et al. 2013).
We are not aware of any reports of gamete killers in intraspecific hybrids of A. thaliana, despite the large number of crosses between natural accessions available. However, we suspect that gamete killers have not been identified as such in previous studies. For example, strong segregation distortions reported in a Lov-5 × Sha F2 (Salome et al. 2012) at two loci on chromosome 1 (0.5% Lov-5 and 2% Sha remaining) suggest gamete-killer effects. Strikingly, the location of the second distorted locus is consistent with it corresponding to L1, which also leads to the elimination of the Sha allele at this locus.
Sha and Rak-2 possess different Rfs
Because Rak-2 carries the same cytoplasm as Sha, in particular the mitochondrial sterility-inducing orf117Sha, we assumed that the Sha and Rak-2 cytoplasms belong to the same CMS-inducing system. However, the genetics of fertility restoration in Sha × Mr-0 is different from that in Rak-2 × Mr-0. Whereas restoration is complex in the Sha × Mr-0 cross, in the Rak-2 × Mr-0 CMS it is controlled by a unique Rf locus, L1Rak, included in the L1 interval: paternal backcross plants were fully sterile when, and only when, homozygous Mr-0 at L1Rak (Table S7 and Figure 9A); and the Mr-0 allele at L1Rak was not transmitted through pollen in the Rak-2 cytoplasm. Therefore, the restorer genes carried by the nuclear genomes of Sha and Rak-2 are different. The presence of different sets of Rf genes in related genotypes has been reported in other species. For example, in maize, three restorers, Rf1, Rf8, or Rf*, are able to restore fertility, in combination with Rf2 (Wise et al. 1999). In radish, Ogura CMS was first described to be controlled by a unique gene in some Japanese radish cultivars (Ogura 1968), but then found to rely on two or three major restoration loci, along with minor ones, in populations from United States or European germplasms (Nieuwhof 1990; Bett 2004).
Here, the L1 locus, which encompasses L1Rak, colocalizes in Col-0 with a region carrying a cluster of no fewer than 15 mitochondria-targeted Rf-like pentatricopeptide repeat (PPR) genes (O’Toole et al. 2008; Fujii et al. 2011). The Rf-like PPR genes, defined on the basis of their homology to restorer genes identified in several species, obviously provide excellent candidates for the restoration of fertility. In addition, the Rf-like PPR genes are known to evolve more rapidly than other PPR genes, mostly by duplication events, and possibly under diversifying selection (O’Toole et al. 2008; Fujii et al. 2011). Evidence for diversifying selection under cytonuclear conflict has been reported for some PPR genes in A. lyrata (Foxe and Wright 2009). If PPR genes do underlie the fertility restoration at L1 and L1Rak, their rapid evolving behavior may explain the difference in the genetics of restoration between the two accessions. In rice, the Rf-1 restoration locus of the BT-CMS line is a large cluster of highly similar PPR genes containing two related functional restorers, Rf-1A and Rf-1B (Wang et al. 2006), and different rice cultivars, subspecies, and species exhibit different structural organizations and copy numbers of the Rf-1 complex locus (Kato et al. 2007). The identification of Rf genes in Rak-2 and Sha will undoubtedly shed light on their evolutionary relationship.
Genetically linked PK and Rf loci concurrently contribute to Sha × Mr-0 hybrid sterility
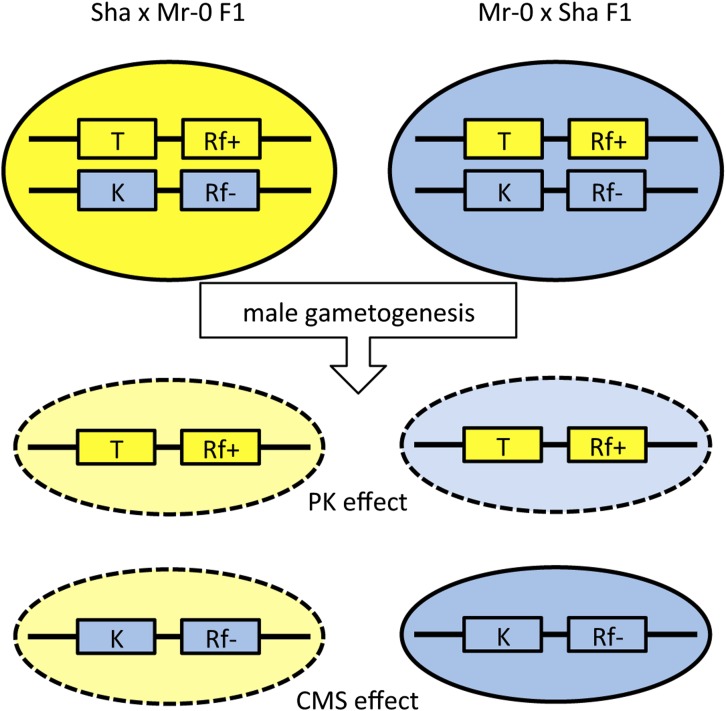
Our results show that the complete male sterility of the Sha × Mr-0 F1 hybrid results from the following features: PKs eliminate pollen carrying Sha alleles, CMS eliminates pollen carrying Mr-0 alleles, and PK and Rf loci are genetically linked, so that all pollen grains eventually abort (Figure 10). Because PKs act in both cytoplasms, whereas the CMS is active only in the Sha cytoplasm, all pollen grains produced by the Sha × Mr-0 hybrid die, but the Mr-0 pollen produced by the Mr-0 × Sha hybrid remains viable. Based on these observations, the fertility of the Rak-2 × Mr-0 F1 plants is the result of the lack of PKs at L1 and the lack of a major restorer at L3: the absence of genetic linkage between the major Rf locus (L1Rak) and the PK at L3 permits enough residual pollen production to allow normal seed set in the hybrid. Furthermore, this model also provides an explanation for the absence of sterile recombinants at L1 in the PopL1 mapping population: recombinants in this interval likely separated PK and Rf factors, by either loss of the Mr-0 killer factor of the PK or its linkage with a restorer allele, allowing the rescue of pollen grains.
Figure 10.
Genetic model of hybrid male sterility resulting from the combination of CMS and PKs. For clarity, we assumed only one nuclear locus in the genome with PK and gametophytic Rf, represented by boxes, tightly linked. The color of the boxes, and that of the ovals for cytoplasmic backgrounds, represents the parent of origin: yellow, Sha; blue, Mr-0. At the PK locus, the Sha allele is the target (T), and Mr-0 is the killer (K). For the CMS locus, the Sha allele restores fertility (Rf+), Mr-0 does not (Rf–). Heterozygotes produce only two pollen types, due to the tight genetic linkage between the two factors. The abortion of pollen is represented by a light background color and a dashed line. PK effect: the presence of the K (Mr-0) factor in the mother plant induces the abortion of pollen with the T (Sha) allele in either cytoplasm. The CMS kills pollen carrying the Rf– (Mr-0) allele only in the Sha (sterilizing) cytoplasm. Therefore, all pollen grains produced by the Sha × Mr-0 F1 die, but from different causes, resulting in the sterility of the hybrid. Conversely, the Mr-0 × Sha F1 produces enough viable pollen (carrying the Mr-0 allele) to ensure normal seed set.
The degree of genetic linkage between PKs and Rfs probably varies among the different loci. We suspect that PK and Rf at L1 have become dissociated by recombination between NGA128 and F5I14, which are 3.8 Mb apart in Col-0, whereas both PK and Rf factors at L3 reside in a 280 kb interval (Figure 2). In crosses between A. lyrata ssp. lyrata and petraea, in which transmission ratio distorters are observed at male fertility QTL (Leppala and Savolainen 2011), a major Rf locus and a segregation distorter have been mapped 2 cM apart. It is noteworthy that this distorter eliminates the allele from the sterilizing cytoplasm donor, i.e., the allele linked to the Rf (Aalto et al. 2013).
We consider it unlikely that the genetic linkage of Rf and PK factors at the two loci L1 and L3 in a single cross is fortuitous. The corresponding genomic regions may be prone to carrying factors affecting pollen viability, for example by concentrating genes involved in pollen development. Colocalization also suggests the possibility that restoration of CMS and PKs shares a common determinant. This could be an expression signal (e.g., a pollen-specific promoter), or a protein-coding sequence. For example, the Drosophila ovd gene has been identified as the cause of both male sterility and segregation distortion in crosses between bogota and USA subspecies of D. pseudoobscura (Phadnis and Orr 2009). Male sterility is visible only when bogota females are crossed with USA males, revealing asymmetrical behavior reminiscent of the asymmetrical hybrid sterility in Sha/Mr-0 A. thaliana crosses.
One of the loci described here colocalizes with a cluster of Rf-like PPR genes on chromosome 1. As a result of the conflict between cytoplasm and nuclear genomes in CMS (Touzet and Budar 2004; Hernandez Mora et al. 2010; Fujii et al. 2011), clusters of Rf-like PPR genes are prone to major structural rearrangements between related species or subspecies (Geddy and Brown 2007; Kato et al. 2007) or within species (Hernandez Mora et al. 2010). These allelic structural variations likely hinder meiotic recombination in these regions, a feature that may favor the stabilization of segregation distorter loci (Schwander et al. 2014).
Genomic conflicts potentially underlie reproductive barriers between Sha and Mr-0 accessions
Sha and Mr-0 have accumulated genetic differences at two PK loci at least, at which intragenomic conflicts are suspected, in addition to a cryptic CMS that very likely drove the selection of eight identified restorer loci, at least two of which are genetically linked to PKs. Our results show that these loci concurrently create reproductive barriers between these two accessions. The contribution of genomic conflicts to reproductive isolation has recently been emphasized (Crespi and Nosil 2013). In that regard, a recent study of hybrid male sterility between Drosophila species proposed that intragenomic conflicts at segregation distorter loci drive genome divergence, leading to reproductive barriers (Zhang et al. 2015). These Drosophila loci controlling male fertility are tightly linked to sex-ratio distortion at six genomic regions, reminiscent of our results for A. thaliana divergent strains.
Information on the level of divergence between A. thaliana accessions is provided by diversity and genetic structure analyses conducted using natural variants from around the world. Sha and Mr-0 originate from geographically distant allopatric populations (Tajikistan and Sicily, respectively). Both areas were putative glacial refugia during the Pleistocene (Beck et al. 2008). Geographic isolation in distinct refugia is predicted to be a major factor favoring the divergence between accessions, corroborated by the highly significant genetic differentiation (FST) values between Italian and Central Asian accessions (Beck et al. 2008). Sha groups with other accessions from Central Asia in a highly differentiated cluster (Nordborg et al. 2005; Platt et al. 2010; Simon et al. 2012; Brennan et al. 2014). Mr-0 clusters with some Mediterranean and Cape Verde accessions (Simon et al. 2012), and its genetic structure is very different from all other accessions (Nordborg et al. 2005). Therefore, Sha and Mr-0 may come from lineages that experienced different genomic conflicts: CMS in the Sha lineage and PKs in the Mr-0 lineage.
It is also conceivable that PKs and Rf coevolved in the ancestral lineage of Sha and Mr-0. In this case, their genetic linkage may have influenced their evolutionary trajectories. For instance, the presence of a nearby killer allele may protect a nonrestorer allele from being swept out. In gametophytic CMS, heterozygotes do not transmit the nonrestorer allele through pollen. Hence, gametophytic restorer alleles are likely easily fixed, sweeping out nonrestorer ones. The presence of a linked PK favoring the CMS nonrestorer allele may slow down the fixation of restorer alleles, allowing longer survival of nonrestorer ones. The genetic variability that we observe at the locus would then be a remnant of the evolutionary history of intertwined intragenomic conflicts.
Geographically widespread selfing taxa, such as A. thaliana, are expected to give rise to cryptic species complexes, as has been observed in the related genus Draba in which similar genetic mechanisms appear to be contributing to the evolution of reproductive barriers (Skrede et al. 2008). Our results make it plausible that Sha and Mr-0 are currently becoming cryptic species. The identification and evolutionary analysis of the genes responsible for the Sha × Mr-0 hybrid sterility will provide clues on their functions as well as valuable information on the evolutionary dynamics of reproductive isolation in this species. This type of analysis will undoubtedly contribute to our understanding of some of significant mechanisms involved in speciation.
Acknowledgments
We gratefully acknowledge J. Jimenez-Gomez, O. Loudet, R. Mercier, G. Pelletier, and E. Téoulé for their critical reading of the manuscript. The Institut Jean-Pierre Bourgin benefits from the support of the Labex SPS (Saclay Plant Science) (ANR-10-LABX-0040-SPS).
Footnotes
Communicating editor: L. C. Moyle
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.183707/-/DC1
Literature Cited
- Aalto E. A., Koelewijn H. P., Savolainen O., 2013. Cytoplasmic male sterility contributes to hybrid incompatibility between subspecies of Arabidopsis lyrata. G3 (Bethesda) 3: 1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. P., 1969. Differential staining of aborted and nonaborted pollen. Stain Technol. 44: 117–122. [DOI] [PubMed] [Google Scholar]
- Barr C. M., Fishman L., 2010. The nuclear component of a cytonuclear hybrid incompatibility in Mimulus maps to a cluster of pentatricopeptide repeat genes. Genetics 184: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. B., Schmuths H., Schaal B. A., 2008. Native range genetic variation in Arabidopsis thaliana is strongly geographically structured and reflects Pleistocene glacial dynamics. Mol. Ecol. 17: 902–915. [DOI] [PubMed] [Google Scholar]
- Bett E. K., 2004. Mapping and genetic characterization of loci controlling the restoration of male fertility in Ogura CMS radish. Mol. Breed. 13: 125–133. [Google Scholar]
- Brennan A. C., Mendez-Vigo B., Haddioui A., Martinez-Zapater J. M., Pico F. X., et al. , 2014. The genetic structure of Arabidopsis thaliana in the south-western Mediterranean range reveals a shared history between North Africa and southern Europe. BMC Plant Biol. 14: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman K. W., Wu H., Sen S., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. [DOI] [PubMed] [Google Scholar]
- Cameron D. R., Moav R. M., 1957. Inheritance in Nicotiana Tabacum Xxvii. Pollen killer, an alien genetic locus inducing abortion of microspores not carrying it. Genetics 42: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ding J., Ouyang Y., Du H., Yang J., et al. , 2008. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. Proc. Natl. Acad. Sci. USA 105: 11436–11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Hung Y. S., Lin K. H., Lee H. Y., Leu J. Y., 2010. Multiple molecular mechanisms cause reproductive isolation between three yeast species. PLoS Biol. 8: e1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides L. M., Tooby J., 1981. Cytoplasmic inheritance and intragenomic conflict. J. Theor. Biol. 89: 83–129. [DOI] [PubMed] [Google Scholar]
- Coyne J. A., 1992. Genetics and speciation. Nature 355: 511–515. [DOI] [PubMed] [Google Scholar]
- Crespi B., Nosil P., 2013. Conflictual speciation: species formation via genomic conflict. Trends Ecol. Evol. 28: 48–57. [DOI] [PubMed] [Google Scholar]
- Cutter A. D., 2012. The polymorphic prelude to Bateson–Dobzhansky–Muller incompatibilities. Trends Ecol. Evol. 27: 209–218. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T., 1936. Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21: 113–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S., Bouche N., Perez Strand E., Loudet O., Camilleri C., 2012. Rapid establishment of genetic incompatibility through natural epigenetic variation. Curr. Biol. 22: 326–331. [DOI] [PubMed] [Google Scholar]
- Fishman L., Saunders A., 2008. Centromere-associated female meiotic drive entails male fitness costs in monkeyflowers. Science 322: 1559–1562. [DOI] [PubMed] [Google Scholar]
- Fishman L., Willis J. H., 2006. A cytonuclear incompatibility causes anther sterility in Mimulus hybrids. Evolution 60: 1372–1381. [DOI] [PubMed] [Google Scholar]
- Foxe J. P., Wright S. I., 2009. Signature of diversifying selection on members of the pentatricopeptide repeat protein family in Arabidopsis lyrata. Genetics 183: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. A., 2000. Polymorphism of attack and defense. Trends Ecol. Evol. 15: 167–171. [DOI] [PubMed] [Google Scholar]
- Fujii S., Bond C. S., Small I. D., 2011. Selection patterns on restorer-like genes reveal a conflict between nuclear and mitochondrial genomes throughout angiosperm evolution. Proc. Natl. Acad. Sci. USA 108: 1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito A., Guyot R., Lozano J., Gavory F., Samain S., et al. , 2010. A genetic model for the female sterility barrier between Asian and African cultivated rice species. Genetics 185: 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddy R., Brown G. G., 2007. Genes encoding pentatricopeptide repeat (PPR) proteins are not conserved in location in plant genomes and may be subject to diversifying selection. BMC Genomics 8: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobron N., Waszczak C., Simon M., Hiard S., Boivin S., et al. , 2013. A cryptic cytoplasmic male sterility unveils a possible gynodioecious past for Arabidopsis thaliana. PLoS One 8: e62450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace K. S., Allen J. O., Newton K. J., 1994. R-type plasmids in mitochondria from a single source of Zea luxurians teosinte. Curr. Genet. 25: 258–264. [DOI] [PubMed] [Google Scholar]
- Hernandez Mora J. R., Rivals E., Mireau H., Budar F., 2010. Sequence analysis of two alleles reveals that intra-and intergenic recombination played a role in the evolution of the radish fertility restorer (Rfo). BMC Plant Biol. 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D., Twell D., 2004. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Xu P., Deng X., Zhou J., Li J., et al. , 2006. Molecular mapping of a pollen killer gene S29(t) in Oryza glaberrima and co-linear analysis with S22 in O. glumaepatula. Euphytica 151: 273–278. [Google Scholar]
- Kato H., Tezuka K., Feng Y. Y., Kawamoto T., Takahashi H., et al. , 2007. Structural diversity and evolution of the Rf-1 locus in the genus Oryza. Heredity 99: 516–524. [DOI] [PubMed] [Google Scholar]
- Larracuente A. M., Presgraves D. C., 2012. The selfish Segregation Distorter gene complex of Drosophila melanogaster. Genetics 192: 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppala J., Savolainen O., 2011. Nuclear–cytoplasmic interactions reduce male fertility in hybrids of Arabidopsis lyrata subspecies. Evolution 65: 2959–2972. [DOI] [PubMed] [Google Scholar]
- Leppala J., Bokma F., Savolainen O., 2013. Investigating incipient speciation in Arabidopsis lyrata from patterns of transmission ratio distortion. Genetics 194: 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Zhao L., Niu B., Su J., Wu H., et al. , 2008. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl. Acad. Sci. USA 105: 18871–18876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet O., Chaillou S., Camilleri C., Bouchez D., Daniel-Vedele F., 2002. Bay-0 × Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis. Theor. Angew. Genet. 104: 1173–1184. [DOI] [PubMed] [Google Scholar]
- Lowry D. B., Willis J. H., 2010. A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biol. 8: e1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan S. S., 1975. Exclusive preferential transmission of an alien chromosome in common wheat. Crop Sci. 15: 287–292. [Google Scholar]
- Maheshwari S., Barbash D. A., 2011. The genetics of hybrid incompatibilities, pp. 331–355 in Annual Review Genetics, Vol. 45, edited by Bassler B. L., Lichten M., Schupbach G. Annual Reviews, Palo Alto, CA. [DOI] [PubMed] [Google Scholar]
- McCauley D. E., Bailey M. F., 2009. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Ann. Bot. (Lond.) 104: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihola O., Trachtulec Z., Vlcek C., Schimenti J. C., Forejt J., 2009. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 323: 373–375. [DOI] [PubMed] [Google Scholar]
- Mizuta Y., Harushima Y., Kurata N., 2010. Rice pollen hybrid incompatibility caused by reciprocal gene loss of duplicated genes. Proc. Natl. Acad. Sci. USA 107: 20417–20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery B. R., Bailey M. F., Brown G. G., Delph L. F., 2014. Evaluation of the cost of restoration of male fertility in Brassica napus. Botany 92: 847–853. [Google Scholar]
- Moyle L. C., Nakazato T., 2008. Comparative genetics of hybrid incompatibility: sterility in two Solanum species crosses. Genetics 179: 1437–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwhof M., 1990. Cytoplasmic–genetic male sterility in radish (Raphanus sativus L.). Identification of maintainers, inheritance of male sterility and effect of environmental factors. Euphytica 47: 171–177. [Google Scholar]
- Nordborg M., Hu T. T., Ishino Y., Jhaveri J., Toomajian C., et al. , 2005. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 3: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A., 1968. Studies on the new male sterility in Japanese radish, with special references to utilization of this sterility towards the practical raising of hybrid seeds. Mem. Fac. Agric. Kagoshima Univ. 6: 39–78. [Google Scholar]
- Oka H., 1974. Analysis of genes controlling f(1) sterility in rice by the use of isogenic lines. Genetics 77: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. A., 1996. Dobzhansky, Bateson, and the genetics of speciation. Genetics 144: 1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. A., Masly J. P., Phadnis N., 2007. Speciation in Drosophila: from phenotypes to molecules. J. Hered. 98: 103–110. [DOI] [PubMed] [Google Scholar]
- O’Toole N., Hattori M., Andres C., Iida K., Lurin C., et al. , 2008. On the expansion of the pentatricopeptide repeat gene family in plants. Mol. Biol. Evol. 25: 1120–1128. [DOI] [PubMed] [Google Scholar]
- Ouyang Y., Zhang Q., 2013. Understanding reproductive isolation based on the rice model. Annu. Rev. Plant Biol. 64: 111–135. [DOI] [PubMed] [Google Scholar]
- Ouyang Y., Liu Y. G., Zhang Q., 2010. Hybrid sterility in plant: stories from rice. Curr. Opin. Plant Biol. 13: 186–192. [DOI] [PubMed] [Google Scholar]
- Phadnis N., Orr H. A., 2009. A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science 323: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt A., Horton M., Huang Y. S., Li Y., Anastasio A. E., et al. , 2010. The scale of population structure in Arabidopsis thaliana. PLoS Genet. 6: e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju N. B., 1994. Ascomycete spore killers: chromosomal elements that distort genetic ratios among the products of meiosis. Mycologia 86: 461–473. [Google Scholar]
- Salome P. A., Bomblies K., Fitz J., Laitinen R. A., Warthmann N., et al. , 2012. The recombination landscape in Arabidopsis thaliana F2 populations. Heredity 108: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y., 1990. The genic nature of gamete eliminator in rice. Genetics 125: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur Jacobs M., Wade M. J., 2003. A synthetic review of the theory of gynodioecy. Am. Nat. 161: 837–851. [DOI] [PubMed] [Google Scholar]
- Schwander T., Libbrecht R., Keller L., 2014. Supergenes and complex phenotypes. Curr. Biol. 24: R288–R294. [DOI] [PubMed] [Google Scholar]
- Simon M., Simon A., Martins F., Botran L., Tisne S., et al. , 2012. DNA fingerprinting and new tools for fine-scale discrimination of Arabidopsis thaliana accessions. Plant J. 69: 1094–1101. [DOI] [PubMed] [Google Scholar]
- Skrede I., Brochmann C., Borgen L., Rieseberg L. H., 2008. Genetics of intrinsic postzygotic isolation in a circumpolar plant species, Draba nivalis (Brassicaceae). Evolution 62: 1840–1851. [DOI] [PubMed] [Google Scholar]
- Taylor D. R., Ingvarsson P. K., 2003. Common features of segregation distortion in plants and animals. Genetica 117: 27–35. [DOI] [PubMed] [Google Scholar]
- Torjek O., Witucka-Wall H., Meyer R. C., von Korff M., Kusterer B., et al. , 2006. Segregation distortion in Arabidopsis C24/Col-0 and Col-0/C24 recombinant inbred line populations is due to reduced fertility caused by epistatic interaction of two loci. Theor. Appl. Genet. 113: 1551–1561. [DOI] [PubMed] [Google Scholar]
- Touzet P., Budar F., 2004. Unveiling the molecular arms race between two conflicting genomes in cytoplasmic male sterility? Trends Plant Sci. 9: 568–570. [DOI] [PubMed] [Google Scholar]
- Turner B. C., Perkins D. D., 1979. Spore killer, a chromosomal factor in neurospora that kills meiotic products not containing it. Genetics 93: 587–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zou Y., Li X., Zhang Q., Chen L., et al. , 2006. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18: 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. P., Gobelman-Werner K., Pei D., Dill C. L., Schnable P. S., 1999. Mitochondrial transcript processing and restoration of male fertility in T-cytoplasm maize. J. Hered. 90: 380–385. [DOI] [PubMed] [Google Scholar]
- Yamagata Y., Yamamoto E., Aya K., Win K. T., Doi K., et al. , 2010. Mitochondrial gene in the nuclear genome induces reproductive barrier in rice. Proc. Natl. Acad. Sci. USA 107: 1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Y., Zhao X. B., Cheng K., Du H. Y., Ouyang Y. D., et al. , 2012. A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 337: 1336–1340. [DOI] [PubMed] [Google Scholar]
- Zhang L., Sun T., Woldesellassie F., Xiao H., Tao Y., 2015. Sex ratio meiotic drive as a plausible evolutionary mechanism for hybrid male sterility. PLoS Genet. 11: e1005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. S., Lu Y. G., Liu X. D., Feng J. H., Zhang G. Q., 2006. Cytological mechanism of pollen abortion resulting from allelic interaction of F1 pollen sterility locus in rice (Oryza sativa L.). Genetica 127: 295–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.