Abstract
Expanding efforts to develop preventive gonorrhea vaccines is critical because of the dire possibility of untreatable gonococcal infections. Reverse vaccinology, which includes genome and proteome mining, has proven very successful in the discovery of vaccine candidates against many pathogenic bacteria. However, progress with this approach for a gonorrhea vaccine remains in its infancy. Accordingly, we applied a comprehensive proteomic platform—isobaric tagging for absolute quantification coupled with two-dimensional liquid chromatography and mass spectrometry—to identify potential gonococcal vaccine antigens. Our previous analyses focused on cell envelopes and naturally released membrane vesicles derived from four different Neisseria gonorrhoeae strains. Here, we extended these studies to identify cell envelope proteins of N. gonorrhoeae that are ubiquitously expressed and specifically induced by physiologically relevant environmental stimuli: oxygen availability, iron deprivation, and the presence of human serum. Together, these studies enabled the identification of numerous potential gonorrhea vaccine targets. Initial characterization of five novel vaccine candidate antigens that were ubiquitously expressed under these different growth conditions demonstrated that homologs of BamA (NGO1801), LptD (NGO1715), and TamA (NGO1956), and two uncharacterized proteins, NGO2054 and NGO2139, were surface exposed, secreted via naturally released membrane vesicles, and elicited bactericidal antibodies that cross-reacted with a panel of temporally and geographically diverse isolates. In addition, analysis of polymorphisms at the nucleotide and amino acid levels showed that these vaccine candidates are highly conserved among N. gonorrhoeae strains. Finally, depletion of BamA caused a loss of N. gonorrhoeae viability, suggesting it may be an essential target. Together, our data strongly support the use of proteomics-driven discovery of potential vaccine targets as a sound approach for identifying promising gonococcal antigens.
Despite current prevention and management strategies, infections caused by sexually transmitted pathogens affect hundreds of millions of women and men in both resource-constrained and developed countries (1). Gonorrhea remains a leading public health burden with an estimated 78 million new cases annually worldwide (2). Major challenges in eradicating this ancient human disease include increasing multidrug-resistance among Neisseria gonorrhoeae strains and the high incidence of asymptomatic infections that contributes to the spread of gonorrhea (3, 4). Gonococcal infections often have devastating sequelae in women including pelvic inflammatory disease, ectopic pregnancy, and infertility (5, 6). In addition, gonorrhea during pregnancy causes chorioamnionitis, which can be complicated further by a septic abortion, premature membrane rupture, and preterm delivery. Infants born to mothers with cervical gonorrhea have an increased risk of neonatal gonococcal conjunctivitis, which can lead to corneal scarring and blindness. In men, untreated urethritis may evolve into penile edema, urethral stricture and epididymitis (7, 8). Furthermore, gonorrhea facilitates the transmission and acquisition of HIV (9). For these reasons, it is critical to develop effective interventions against gonorrhea. Currently, strains resistant to the last effective option for empiric monotherapy—third-generation cephalosporins—are emerging and clinical treatment failures have been documented in several countries (4, 10). History indicates that the incremental development of antibiotic resistance in N. gonorrhoeae inevitably continues to challenge antibiotic therapy (4). The World Health Organization recently recognized the escalating problem of the spread of antimicrobial resistance in N. gonorrhoeae and highlighted the importance of novel approaches to identify alternative strategies for the treatment and prevention of N. gonorrhoeae infections, including a gonorrhea vaccine (11).
Pioneered by Rappuoli and colleagues, reverse vaccinology—which initially included genome and later proteome mining—has proven to be very successful in the discovery of vaccine candidates against many pathogenic bacteria (12–15). In particular, these methodologies paved the way for the newly developed group B meningococcal vaccine, which was a formidable effort for many years. Among the 28 antigens that were discovered and that elicited bactericidal antibodies against group B meningococci in vitro were the Neisserial heparin-binding antigen (NHBA), factor H-binding protein (fHbp), and the Neisserial adhesin A (NadA). These three proteins are formulated as part of the Bexsero meningococcal group B vaccine approved by the European commission in 2013 and the United States in February of 2015 (12, 15, 16). Unfortunately, N. gonorrhoeae homologs of these proteins are not suitable vaccine targets (17). Further, in contrast to meningococcal vaccines, progress on gonococcal vaccines has been hampered primarily by the absence of a vaccine-targetable surface capsule, exceptional variability of several surface antigens, a poor understanding of protective responses, and until relatively recently, the lack of a small laboratory animal model to systematically test potential vaccine candidates (16). However, recent potential breakthroughs—such as the availability of transgenic mice to alleviate some host restrictions, new insights into immunosuppression mechanisms used by N. gonorrhoeae, and growing evidence that induction of Th1 response may be critical for vaccine efficacy—justify re-visiting gonorrhea vaccine development and initiating a significant focus in this area (16, 18–20).
Currently, ∼12 potential gonorrhea vaccine antigens are being pursued (16). However, this is a very limited repertoire considering that, during the development of the Bexsero vaccine, out of nearly 600 candidates selected by in silico analysis, 350 recombinant meningococcal proteins were successfully expressed in Escherichia coli and evaluated for their surface exposure and ability to induce bactericidal antibodies (12). Thus, a comprehensive antigen discovery program would be exceedingly valuable toward making a gonorrhea vaccine a reality. We are approaching this goal by applying proteomics-driven reverse vaccinology. In our previous study, a quantitative proteome analysis of cell envelopes and naturally released membrane vesicles derived from well-recognized N. gonorrhoeae laboratory strains FA1090, F62, MS11, and 1291 revealed a myriad of novel proteins, including 21 predicted outer membrane proteins (21). To extend these studies, herein we report high-throughput profiling of the N. gonorrhoeae cell envelope to identify ubiquitously and differentially expressed proteins in response to environmental cues relevant to infection including different oxygen tensions (aerobic, anaerobic), iron deprivation, and the presence of normal human serum. In addition, a subset of five identified proteins was evaluated for the capacity to induce antibodies that recognize a collection of diverse clinical isolates and have bactericidal activity against serum resistant and serum sensitive N. gonorrhoeae strains.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
The following N. gonorrhoeae strains were used in this study: FA1090 (22), MS11 (23), 1291 (24), F62 (25), FA19 (26), the clinical isolates LGB1, LG14, LG20, and LG26, which were collected from two public health clinics in Baltimore from 1991 to 1994 and differ in porB variable region type and pulsed gel electrophoresis patterns (21, 27), 13 isolates from patients attending the Public Health-Seattle & King County Sexually Transmitted Disease clinic from 2011 to 2013 (supplemental Table S1), and the WHO 2015 reference strains (10, 28, 29).
N. gonorrhoeae isolates were cultured from frozen stocks stored at −80 °C onto gonococcal base agar solid medium (GCB1, Difco, Sparks, MD) containing Kellogg's supplements I (1:100) and II (1:1000) (30). After incubation for 18–20 h at 37 °C in a humid, 5% CO2 atmosphere, transparent colonies with piliated or nonpiliated colony morphologies were sub-cultured onto GCB plates. Piliated N. gonorrhoeae variants were used for transformation, whereas nonpiliated bacteria were used in all other experiments. Following ∼18 h of incubation as described above, bacteria were harvested from the solid media using a polyester-tipped applicator (Puritan, Guilford, ME), and suspended to a final OD600 of 0.1 in gonococcal base liquid (GCBL) medium containing Kellogg's supplements I and II and sodium bicarbonate (at a final concentration of 0.042%) (30, 31). Subsequently, gonococci were propagated as stated in the text: in GCBL at 37 °C or aerobically on GCB; GCB with Kellogg's supplement I and with deferoxamine mesylate salt (Desferral, Sigma, St. Louis, MO) at 5 μm final concentration [iron limited conditions (32)]; GCB with the addition of 7.5% normal human serum [NHS (33)]; and anaerobically on GCB with 1.2 mm nitrite as a terminal electron acceptor (34).
E. coli NEB5α and BL21(DE3) were used for genetic manipulations and as a host for heterologous protein expression, respectively. The E. coli strains were grown in Luria-Bertani (LB) medium (Difco) or maintained on LB agar plates at 37 °C supplemented with appropriate antibiotics. Antibiotics were used at the following concentrations: N. gonorrhoeae kanamycin (40 μg/ml) and erythromycin (0.5 μg/ml); E. coli kanamycin (50 μg/ml).
Isolation of Cell Envelopes
For proteomic analysis of the N. gonorrhoeae cell envelope composition, N. gonorrhoeae FA1090 was first cultured on GCB, bacteria were collected from plates, suspended in GCBL to an OD600 of 0.1, and 100 μl aliquots were spread on solid media for simultaneous growth under aerobic (standard GCB, iron limited, and in the presence of human serum) and anaerobic (in the presence of nitrite) conditions, as described above. Bacteria were harvested when the colonies reached approximately similar size (after 18 and 36 h for aerobic and anaerobic conditions, respectively), and the cell envelope fractions were isolated as described previously (21, 35). Protein concentrations were measured using 2D Quant Kit (GE Healthcare, Piscataway, NJ).
Two-dimensional Liquid Chromatography and Mass Spectrometry (2D LC-MS/MS)
After isolation, the cell envelope-associated proteins were precipitated, trypsinized and labeled with iTRAQ reagents (AB Sciex, Waltham, MA) according to the procedures reported by Zielke et al. (21, 35). The following iTRAQ tags were used to label peptides in the cell envelope fractions derived from N. gonorrhoeae cultured on GCB under four growth conditions: 114 for standard aerobic; 115 for growth in the presence of 7.5% normal human serum; 116 for iron-limited; and 117 for anaerobic. iTRAQ-labeled peptides were separated using strong cation exchange (SCX) chromatography (21, 35). Desalted SCX fractions were analyzed by LC/ESI MS/MS with a ThermoScientific Easy-nLC II (Thermo Scientific) nano HPLC coupled to a hybrid Orbitrap Elite ETD (Thermo Scientific) mass spectrometer using an instrument configuration as described in (36). In-line desalting was accomplished using a reversed-phase trap column (100 μm × 20 mm) packed with Magic C18AQ (5-μm, 200 Å resin; Michrom Bioresources, Auburn, CA), followed by peptide separations on a reversed-phase column (75 μm × 250 mm) packed with Magic C18AQ (5-μm, 100 Å resin; Michrom Bioresources) directly mounted on the electrospray ion source. A 90-min gradient from 7% to 35% acetonitrile in 0.1% formic acid at a flow rate of 400 nL/min was used for chromatographic separations. The heated capillary temperature was set to 300 °C and a spray voltage of 2250 V was applied to the electrospray tip. The Orbitrap Elite instrument was operated in the data-dependent mode, switching automatically between MS survey scans in the Orbitrap (AGC target value 1,000,000; resolution 60,000; and injection time 250 msec) and MS/MS scans in the OrbiTrap (AGC target value of 50,000; 15,000 resolution; and injection time 250 msec). The 15 most intense ions from the survey scan were selected for fragmentation by higher energy collisionally activated dissociation (HCD) with normalized collision energy of 40%. Selected ions were dynamically excluded for 45 s with a list size of 500 and exclusion mass-by-mass width ± 0.5.
Proteomic Data Analysis
Data analysis was performed using Proteome Discoverer 1.4 (Thermo Scientific). The data were searched against a SwissProt N. gonorrhoeae FA1090 database with 1963 protein entries (downloaded on January, 17, 2012) that included common contaminants [the common Repository of Adventitious Proteins (cRAP)]. Trypsin was set as the enzyme with maximum missed cleavages set to 2. The precursor ion tolerance and the fragment ion tolerance were set to 10 ppm and 0.8 Da, respectively. Variable modifications included iTRAQ4Plex (+144.102 Da) on any N-Terminus, oxidation on methionine (+15.995 Da), methyl methanethiosulfonate on cysteine (+46.988 Da), and iTRAQ4Plex on lysine (+144.102 Da). Data were searched using Sequest HT. All search results were run through Percolator for scoring. Quantification was performed using the iTRAQ 4plex method built into Proteome Discoverer. The mass spectrometry data have been deposited in the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository ProteomeXchange with the data set identifier PXD001944. Additionally, supplemental Table S2 lists all identified proteins with their accession number, a brief description, calculated score, protein coverage, number of unique and total peptides, iTRAQ ratios, molecular mass, and calculated pI. All statistical analyses were performed as we described previously (21, 35, 37). Briefly, only proteins identified with 1% false discovery rate (FDR) based on at least one unique peptide with ≥95% confidence were recorded. The default bias-correction was used and all quantitative variables were analyzed by the Proteome Discoverer 1.4. Subsequently, average ratios and standard deviations were calculated for proteins identified in three independent experiments. Proteins were considered ubiquitously expressed if the iTRAQ ratios were between 0.5 and 2, and were designated as differentially expressed when the values were below 0.5 or above 2 with the corresponding p values < 0.05. The relative protein abundance heat maps were created using MultiExperiment Viewer (version 4.9) software (http://www.tm4.org/mev.html) (38).
Bioinformatic Analysis
Subcellular localization of identified proteins was assessed as described previously (21, 35, 37) using PSORTb 3.0.2 (39), SOSUIGramN (40), and CELLO 2.5 (41), and majority-votes strategy was used for protein localization assignment. In cases where these three software systems predicted different subcellular localizations for a specific protein, the protein was assigned to a group of “unknown subcellular localization.” The predicted amino acid sequences of identified proteins were analyzed for the presence of a signal peptide using the SignalP v.4.1 server (42). Phylogenetic classification of identified proteins to the clusters of orthologous groups (COGs) functional categories was achieved using COGnitor.
Subcellular Fractionation Procedures
Nonpiliated, transparent N. gonorrhoeae colonies of wild type strain FA1090 were harvested from solid media, suspended in 500 ml of GCBL to an OD600 of 0.1 and cultured at 37 °C with aeration (220 rpm) to OD600 of 0.6–0.8. Cells were separated from culture supernatants by centrifugation (20 min, 6000 × g), and the cell envelopes were purified from whole-cell lysates (21, 35). The naturally secreted membrane vesicles (MVs) were harvested from the supernatants as described previously (21). The isolated soluble supernatant fractions (SS; containing secreted proteins and proteins originating from cell lysis) were stored at −20 °C until used, whereas insoluble material containing MVs was reconstituted in PBS containing 0.4% SDS. The total protein amount in each isolated subproteome (cytoplasmic, cell envelopes, MVs, and SS) was measured using a DC Protein Assay Kit (BioRad, Hercules, CA).
Conservation of Candidate Vaccine Antigens in a Panel of N.gonorrhoeae Isolates
The conservation of BamA, LptD, TamA, NGO2054, and NGO2139 was assessed at both nucleotide and predicted amino acid levels between FA1090, NCCP11945 (http://www.ncbi.nlm.nih.gov/), the draft genome sequences of 14 different GC strains (downloaded from http://www.broadinstitute.org/), and the 14 WHO 2015 reference strains (10, 28, 29) using ClustalOmega (http://www.ebi.ac.uk/).
Genetic Manipulations
All genetic manipulations used to engineer different N. gonorrhoeae mutants utilized in this study are described in Supplemental Experimental Procedures.
Cloning and Purification of Recombinant Protein Variants
The genes lptD, bamA, tamA, ngo2054, ngo2139, ngo0994 (laz), and ngo0277 (bamD) were individually amplified using appropriate primers (supplemental Table S3). The subsequent PCR products were digested with NcoI and EcoRI or NcoI and HindIII, as indicated, and ligated into similarly cut pET28a(+) to create C-terminal-6×His-tagged fusions. All constructed plasmids were moved to E. coli BL21(DE3).
For the overproduction of individual proteins, overnight cultures of E. coli BL21(DE3) carrying pET-LptD, pET-BamA, pET-TamA, pET-NGO2054, pET-NGO2139, pET-Laz or pET-BamD were used to inoculate 1 L of LB supplemented with kanamycin. Protein overproduction was induced by the addition of 1 mm IPTG when the cultures reached an OD600 of about 0.5. After 3 h of growth at 37 °C, bacterial cells were pelleted by centrifugation and cell pellets were stored at −80 °C until used. Recombinant NGO2054, NGO2139, Laz and BamD proteins were purified under native conditions whereas LptD, BamA and TamA were purified under denaturing conditions. All proteins were purified using the NGC Scout Chromatography system (Bio-Rad). For native conditions, bacterial cells were resuspended in lysis buffer (20 mm Tris pH 8.0, 500 mm NaCl, 10 mm imidazole) and lysed by five passages through a French pressure cell at 12,000 p.s.i. Cell lysates were clarified by centrifugation and loaded onto Bio-Scale Mini Profinity IMAC cartridges (Bio-Rad). Loosely bound proteins were removed with 10 column volumes of wash buffer (20 mm Tris pH 8.0, 500 mm NaCl, 40 mm imidazole) and proteins were eluted with 40–250 mm imidazole gradient.
For proteins purified under denaturing conditions, cell pellets were reconstituted in denaturing lysis buffer (6 m urea, 300 mm KCl, 50 mm KH2PO4, 5 mm imidazole, pH 8) and lysed as described above. Lysates were cleared from cell debris by centrifugation at 6000 × g for 20 min at 4 °C and applied to Bio-Scale Mini Profinity IMAC cartridges (Bio-Rad) equilibrated with denaturing lysis buffer. Columns were washed with ten column volumes of denaturing wash buffer (6 m urea, 300 mm KCl, 50 mm KH2PO4, 10 mm imidazole, pH 8) and finally proteins were eluted with five column volumes of denaturing elution buffer (6 m urea, 300 mm KCl, 50 mm KH2PO4, 250 mm imidazole, pH 8).
Fractions containing the purified recombinant protein of interest were dialyzed against 20 mm Tris pH 8.0, 500 mm NaCl, and 10% glycerol for 24 h with three consecutive changes of the buffer. Proteins were stored at −80 °C. The N. gonorrhoeae Obg protein, ObgGC, used in this study as a cytoplasmic protein marker, was purified as described (43).
Antisera Preparations
Rabbit polyclonal antisera against the individual proteins were prepared by Pacific Immunology (Ramona, CA) using about 2 mg of the purified recombinant versions of BamA, LptD, TamA, NGO2054, NGO2139, Laz and BamD and a 13-week antibody production protocol was approved by IACUC Animal Protocol #1, in a certified animal facility (USDA 93-R-283), as well as the National Institute of Health Animal Welfare Assurance Program (#A4182–01). The polyclonal anti-ObgGC antiserum was obtained previously (43).
Bactericidal Assays
Nonpiliated colonies of N. gonorrhoeae FA1090 and MS11 were inoculated into 5 ml of GCBL broth to an OD600 of 0.1. Cultures were incubated with aeration at 37 °C until the mid-logarithmic phase of growth (OD600≈0.6) was reached. The immune and respective preimmune sera were heat inactivated by incubation for 30 min at 56 °C and diluted in GCBL medium. Bacterial cells [2 × 104 colony forming units (CFUs)/ml)] in 40 μl were mixed with diluted antisera or preimmune sera and incubated for 15 min at 37 °C. Following incubation, 10 μl of NHS (Cellgro, Manassas, VA) were added to each tube. As a control 10 μl of heat-inactivated NHS (HI-NHS) was used. Samples were incubated for additional 30 min at 37 °C. Finally, 50 μl of each suspension were spread onto GCB plates and the CFUs were determined after 18–20 h incubation. Controls included bacteria incubated with: test sera with HI-NHS, pre-immune sera with NHS, and pre-immune sera with HI-NHS. The average percent killing was determined from at least four independent experiments and was calculated as the number of CFUs in samples incubated with antigen specific antisera and NHS to the number of CFUs recovered from samples treated with rabbit postimmune sera and HI-NHS (44). N. gonorrhoeae viability was not affected in any of the control samples.
Dot Blotting
Non-piliated colonies of N. gonorrhoeae FA1090 were suspended to an OD600 of 0.1 in GCBL pre-warmed to 37 °C and incubated for 3 h with shaking at 220 rpm at 37 °C. Bacterial cells were harvested and suspended in GCBL to an OD600 of 2.0. To obtain whole-cell lysates, bacteria were incubated at 100 °C for 5 min followed by sonication (30 s, amplitude 50). Suspensions (5 μl) of intact and lysed cells were spotted onto a nitrocellulose membrane. The membrane was allowed to dry for 30 min at room temperature, blocked, and probed with respective polyclonal antibodies, as described below.
Protease Treatment of Intact N. gonorrhoeae Cells
The surface accessibility studies were performed using modified protocols described previously (45, 46). After 3 h of growth in GCBL, N. gonorrhoeae FA1090 cells were harvested by centrifugation for 10 min at 2000 × g and 4 °C. The pellet was suspended in PBS (pH 8.0) to an OD600 of 2.5 and 500 μl of this solution were incubated with 0, 40, or 80 μg/ml of Trypsin-UltraTM [l-(tosylamido-2-phenyl) ethyl chloromethyl ketone-treated trypsin, NEB, Ipswich, MA] for 1 h at 37 °C. The reactions were stopped by the addition of 10 μl of 50 mm phenylmethanesulfonylfluoride (PMSF) and cells were washed with PBS. Finally, the N. gonorrhoeae cells were suspended in PBS and the OD600 of the suspension was measured. The cells were pelleted again, lysed in SDS sample buffer and subjected to SDS-PAGE and immunoblotting analysis, as outlined below.
SDS-PAGE and Immunoblotting Analysis
Whole-cell lysates were obtained from N. gonorrhoeae grown in GCBL with aeration and on GCB plates cultured under conditions specified in the text. Bacteria were harvested from liquid media either at different time points of growth or at desired OD600 values, as indicated. Different N. gonorrhoeae clinical isolates were collected from GCB plates, followed by suspension in prewarmed GCBL, and measurement of the cell density at OD600.Fractions containing either cytoplasmic, cell envelope, MVs, or secreted proteins (the same amount of total protein loaded per well), or whole-cell lysates matched by equivalent OD600 units, were prepared in SDS sample buffer in the presence of 50 mm dithiothreitol and separated in either 10–20% Criterion Tris-Tricine TGX (BioRad) or 4–20% Mini-PROTEAN TGX precast gels (Bio-Rad). The proteins were transferred onto 0.2 μm nitrocellulose membrane (Bio-Rad) using a Trans-blot Turbo (Bio-Rad). A solution of 5% milk in phosphate buffered saline pH 7.0 (PBS, Li-Core, Lincoln, NE) supplemented with 0.1% Tween 20 (PBST) was used for blocking. Following 1 h of incubation, polyclonal rabbit antisera against LptD (1:5,000), BamA (1:10,000), TamA (1:5,000), NGO2054 (1:10,000), NGO2139 (1:20,000), ObgGC [1:5,000, (43)], polyclonal anti-AniA antibodies [1:10,000;(21)], monoclonal mouse anti-MtrE antisera (1:10,000), monoclonal mouse anti-Ng-MIP antibodies (1:10,000; a gift of Mariagrazia Pizza, Novartis Vaccines, Italy), and polyclonal rabbit anti-TbpB antisera (1:1,000; a gift of Cynthia Cornelissen, Virginia Commonwealth University, Richmond) diluted in PBST as indicated in parenthesis were added to the membranes. Horseradish peroxidase conjugate of goat anti-rabbit IgG antiserum (BioRad) or goat anti-mouse IgG antibody (ThermoFisher Scientific), correspondingly, was utilized as the secondary antibody at a 1:10,000 dilution. The reactions were developed using Clarity Western ECL-Substrate (BioRad) and a Chemi-DocTM MP System (BioRad) was used for Western blot imaging.
Experimental Design and Statistical Rationale
All studies to identify potential gonorrhea vaccine antigens including iTRAQ LC-MS/MS experiments were performed on three separate occasions. Statistical analysis was performed as described above. All other experiments were conducted at least in biological triplicates and mean values with corresponding S.E. were presented.
RESULTS AND DISCUSSION
Experimental Strategy
N. gonorrhoeae is an exclusive human pathogen that primarily infects the lower genitourinary tract. Ascending infections are also common in women, as are pharyngeal and rectal infections in both men and women. Conjunctival infections can also occur (5). During colonization within these microecological niches, N. gonorrhoeae encounters areas of different oxygen tension (aerobic and anaerobic sites of infection), iron deprivation and, in the event of disseminated infection, exposure to human serum (5, 47, 48). An effective vaccine with a broad spectrum of protection would preferentially consist of antigens that are not only highly conserved among different N. gonorrhoeae strains but are also utilized by the bacteria to persist at anatomically distinct sites within its sole human host. Accordingly, herein we performed proteomic studies to identify potential vaccine antigens that are ubiquitously expressed or induced by these environmental cues. The experimental strategy is outlined in Fig. 1. N. gonorrhoeae FA1090 was spread on GCB plates and maintained concurrently under standard growth conditions (SGC), GCB supplemented with 7.5% normal human serum (+NHS), iron-depleted GCB (-Iron), and anaerobically in the presence of nitrite as terminal electron acceptor (-O2). The plate-grown gonococci were collected when the colonies reached approximately similar size, lysed, and the membrane proteins were isolated using sodium carbonate extraction coupled with ultracentrifugation. This methodology enables enrichment of the cell envelope proteins and yields samples compatible with downstream proteomic applications, including iTRAQ labeling and MS/MS analysis (21, 35, 49, 50). Isolated proteins from bacteria cultured under individual growth conditions were subsequently digested with trypsin, and the acquired peptides were labeled with the following iTRAQ tags: SGC - 114; NHS - 115; -Iron - 116; and -O2 - 117 (Fig. 1). The labeled samples were pooled and the peptide mixtures were fractionated in two dimensions using off-line strong-cation exchange chromatography followed by reversed-phase chromatography performed in-line with the mass spectrometer. Finally, the MS/MS analysis was accomplished using an Orbitrap Elite mass spectrometer. These experimental procedures were repeated on three separate occasions and all raw data were deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository ProteomeXchange with the data set identifier PXD001944.
Fig. 1.
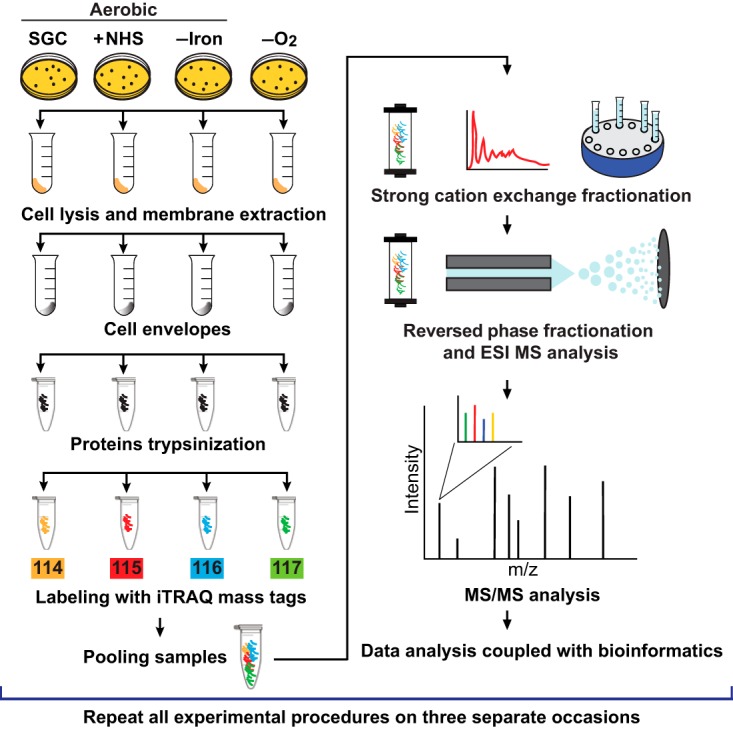
Experimental design of quantitative proteomic profiling of the Neisseria gonorrhoeae cell envelopes across different growth conditions. N. gonorrhoeae strain FA1090 was cultured on gonococcal base agar solid medium (GCB) aerobically under standard growth conditions (SGC); on GCB with the addition of 7.5% normal human serum (+NHS); iron limited [GCB without ferric nitrate in Kellogg's Supplements and with deferoxamine mesylate salt at 5 μm final concentration (-Iron)]; and anaerobically on GCB with nitrite as a terminal electron acceptor (-O2). Bacteria were harvested from solid media when the colonies reached approximately the same size. Following lysis, cell envelope proteins were enriched using a sodium carbonate extraction procedure and ultracentrifugation. The same amounts of proteins (100 μg) in each sample were subjected to trypsin digestion. Peptides in different samples were labeled with the following iTRAQ tags: 114 for SGC, 115 for NHS, 116 for -Iron, 117 for -O2. Following labeling, samples were pooled and the peptides were separated by strong cation exchange and reversed-phase chromatography. The Orbitrap Elite was used to collect mass spectra and proteins were identified and quantified using Proteome Discoverer. All experiments described above were repeated on three separate occasions.
Proteomic Data Analyses
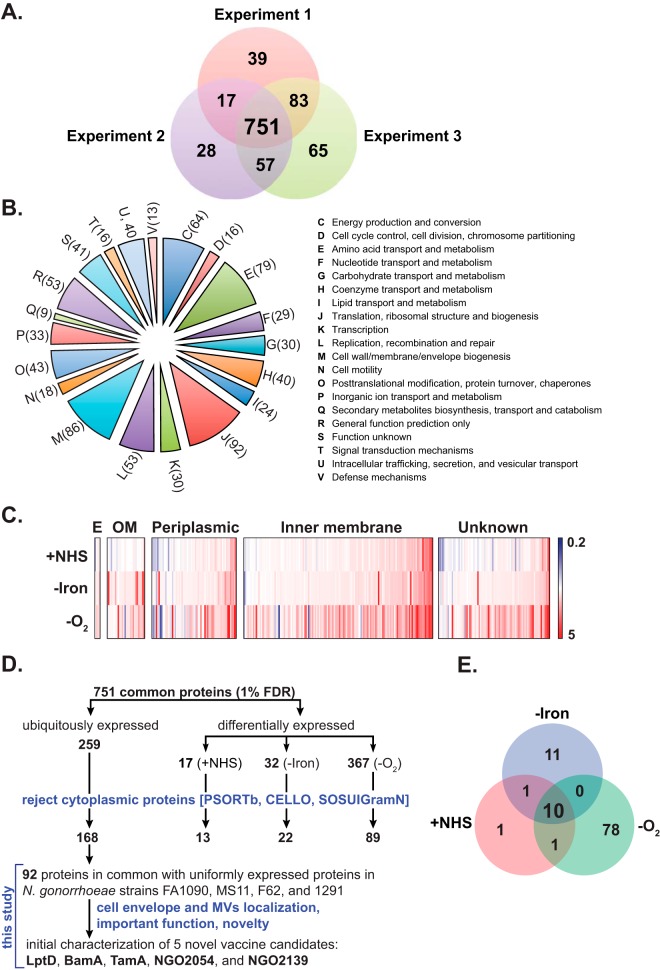
The total number of proteins identified based on at least two unique peptides filtered to a 1% FDR was 890, 853, and 956 in the first, second, and third independent biological experiment, respectively (Fig. 2A). Further analyses were applied solely to a group of 751 common proteins identified during four growth conditions in biological triplicate experiments.
Fig. 2.
Analysis of identified proteins. A, Venn diagram illustrating the distribution of proteins identified in independent triplicate proteomic experiments. A total of 890, 853, and 956 individual protein species were identified in Experiment 1, 2, and 3; respectively. Further analyses were applied to 751 common proteins identified in all experiments. B, A phylogenetic classification of common proteins was accomplished using the Clusters of Orthologous Groups of proteins (COGs). Letters and digits displayed on the pie chart represent individual COGs and numbers of proteins assigned to each phylogenetic group. C, Heat map illustrating the changes in N. gonorrhoeae proteome upon exposure to the environmental cues relevant to infection. Relative abundance of proteins identified during different growth conditions (NHS, -Iron, and -O2) was compared with the levels of corresponding protein species found in SGC. The color scale covers from fivefold down-regulation (blue), through no change (white), to fivefold up-regulation (red). D, Decision tree to select gonorrhea vaccine candidates from the group of 751 common N. gonorrhoeae cell envelope-associated proteins identified with 1% FDR in three independent experiments via high-throughput proteomic mining. This group contains 259 uniformly expressed and 416 specifically induced proteins. Cytoplasmic proteins were eliminated using bioinformatics predictions and literature searches. Vaccine candidates were ultimately selected from 92 ubiquitously expressed proteins identified by comparing the 168 uniformly expressed cell envelope proteins of this study with the 305 previously identified cell envelopes proteins isolated from FA1090, MS11, F62, and 1291 (21). Subsequently, five of these proteins that were localized to both cell envelopes and MVs fractions (21), and either had pivotal role(s) in other bacterial species (BamA, LptD, TamA) or unknown function (NGO2054 and NGO2139) were subjected to initial assessments as potential gonorrhea vaccine candidates. E, Analysis of anaerobic, iron, and NHS-responsive stimulons of N. gonorrhoeae. Differentially expressed proteins in response to anoxia, iron deprivation, and the presence of NHS were compared with identify the common anaerobic, iron-depleted, and NHS-responsive protein stimulon.
Protein Functional Categories
Functional classification using COG clustering identified twenty different phylogenetic protein groups (Fig. 2B), with the largest subsets in category J (translation, ribosomal structure and biogenesis; 92 proteins), category M (cell wall/membrane/envelope biogenesis; 86 proteins), and category E (amino acid transport and metabolism; 79 proteins). Furthermore, 52 proteins were assigned only a putative function (category R), whereas 41 proteins remained without an allocated biological role (category S; Fig. 2B).
Assessment of Protein Expression
The relative levels (iTRAQ ratios) of identified proteins were obtained by comparing N. gonorrhoeae proteome profiles under individual test conditions to the corresponding protein expression at SGC, as follows: +NHS/SGC (115/114), -Iron/SGC (116/114), and -O2/SGC (117/114). Similarly, to our previous studies (21, 37), a >2.0-fold cutoff threshold in the iTRAQ ratios was chosen as a criterion for differential protein abundance. Overall, our experiments revealed that N. gonorrhoeae responds to the environmental cues tested, particularly anaerobiosis, with dramatic changes in the cell envelope proteome (Fig. 2C). Expression of 17, 32, and 367 proteins in the presence of NHS, upon iron deprivation, and during anaerobic growth, respectively, was characterized as significantly different compared with SGC (Fig. 2D, Tables I–II and supplemental Tables S4 and S6). Further, 259 proteins were ubiquitously expressed under all conditions tested (Fig. 2D, supplemental Table S5 and S7).
Table I. Differentially expressed Neisseria gonorrhoeae cell envelope proteins in response to normal human serum.
| Accession | Name | Gene | Average ratio ± S.D.a | SPb |
|---|---|---|---|---|
| Periplasmic | ||||
| Q5F7X1c | Putative uncharacterized protein | NGO1044 | 2.2 ± 1.74 | N |
| Q5FAC5c | Cytochrome c4 | cyc4, NGO0101 | 2.5 ± 2.06 | Y |
| Inner membrane | ||||
| Q5F4Z5c | ATP synthase C chain | atpE, NGO2145 | 2.1 ± 2.25 | N |
| Q5F9V4c | Putative uncharacterized protein | NGO0284 | 2.8 ± 2.69 | N |
| Q5F9N2c | Putative HemY protein | NGO0361 | 2.9 ± 1.19 | N |
| Q5F6Y2c | Putative uncharacterized protein | NGO1411 | 3 ± 4.04 | N |
| Q5F767 | Putative paraquot-inducible protein A | pqiA, NGO1319 | 3.5 ± 1.77 | N |
| Q5F6V9c | Putative cadmium resistance protein | cadD, NGO1435 | 3.7 ± 2.29 | N |
| Q5F6K9 | Penicillin-binding protein 2 | penA, NGO1542 | 5.3 ± 4.53 | N |
| Q5FA49c | Sec-independent protein translocase protein TatA | tatA, NGO0183 | 5.8 ± 7.8 | N |
| Q5F6M1c,d | Cell division protein FtsQ | ftsQ, NGO1530 | 2.8 ± 1.77 | N |
| Unknown localization | ||||
| Q5F9C2c | Putative uncharacterized protein | NGO0477 | 3.5 ± 3.65 | N |
| Q5FAD1c | Pilin assembly protein | pilP, NGO0095 | 5.1 ± 7.36 | Y |
a Average ratios and standard deviations were calculated for proteins identified in three independent experiments.
b Predictions of signal peptide cleavage site using SignalP (42).
c iTRAQ ratios were 0.5<proteins<2 in one of the biological triplicate experiments.
d The inner membrane localization of FtsQ was verified experimentally (103).
Table II. Differentially expressed Neisseria gonorrhoeae cell envelope proteins in response to iron deprivation.
| Accession | Name | Gene | Average ratio ± S.D.a | SPb | Microarray (−/+ Fe)c | Microarray (−/+ Fe)d |
|---|---|---|---|---|---|---|
| Outer membrane | ||||||
| Q5F526e | Putative uncharacterized protein | NGO2111 | 2.3 ± 0.37 | N | N/A | N/A |
| Q5F674e | Putative outer membrane protein OmpU | NGO1688 | 2.6 ± 0.57 | Y | N/A | 3.67; 2.96; 3.27; 2.13 |
| Q5F6Q4 | Transferrin-binding protein A | tbpA, NGO1495 | 3.8 ± 2.56 | Y | 1.87 | 4.43; 4.45; 5.20; 4.88 |
| Q5F950 | Putative TonB-dependent receptor | tdfG, NGO0553 | 4.5 ± 3.9 | Y | 1.58 | 2.74; 1.88; 2.16 |
| Q5F543 | Ferric enterobactin receptor | fetA, NGO2093 | 7.6 ± 6.91 | Y | 1.73 | 50.9; 107.5; 39.9; 15.5 qRT-PCR |
| Q5F6Q3f | Transferrin-binding protein B | tbpB, NGO1496 | 11.4 ± 4.27 | N | 1.51 | 7.45; 7.06; 12.44; 11.48 |
| Periplasmic | ||||||
| Q5F5F7e | Putative mafB-like adhesin | NGO1971 | 2.1 ± 1.25 | Y | N/A | 2.3 |
| Q5F544e | Ferric enterobactin periplasmic binding protein | fetB, NGO2092 | 2.8 ± 1.4 | N | 1.59 | 2.92; 3.40; 3.86; 4.00 |
| Q5F7X1e | Putative uncharacterized protein | NGO1044 | 2.9 ± 2.88 | N | N/A | N/A |
| Q5FA17e | ABC transporter, periplasmic binding protein, iron related | fbpA, NGO0217 | 5.2 ± 4.15 | Y | 1.58 | 2.01; 8.72 |
| Inner membrane | ||||||
| Q5F6S5e | NAD(P) transhydrogenase subunit beta | pntB, NGO1472 | 2.1 ± 0.87 | N | N/A | 1.97 |
| Q5F4Z5e | Putative ATP synthase C chain | atpE, NGO2145 | 2.1 ± 2.35 | N | N/A | 1.88 |
| Q5F6K9e | Penicillin-binding protein 2 | penA, NGO1542 | 2.2 ± 1.22 | N | N/A | N/A |
| Q5F9N2e | Putative HemY protein | NGO0361 | 2.5 ± 1.88 | N | N/A | N/A |
| Q5F6Y2e | Putative uncharacterized protein | NGO1411 | 2.9 ± 4.27 | N | N/A | N/A |
| Q5F6V9e | Putative cadmium resistance protein | cadD, NGO1435 | 2.9 ± 3.98 | N | N/A | N/A |
| Q5F9V4e | Putative uncharacterized protein | NGO0284 | 3.2 ± 3.68 | N | N/A | N/A |
| Q5F767e | Putative paraquot-inducible protein A | pqiA, NGO1319 | 3.3 ± 2.34 | N | N/A | N/A |
| Q5FA49e | Sec-independent protein translocase protein TatA | tatA, NGO0183 | 6 ± 7.33 | N | N/A | N/A |
| Q5F711 | Transport protein | exbB, NGO1378 | 7.7 ± 4.57 | N | 1.53 | 3.94; 3.27; 6.50; 6.31 |
| Unknown localization | ||||||
| Q5F9C2e | Putative uncharacterized protein | NGO0477 | 2.3 ± 1.8 | N | N/A | N/A |
| Q5FAD1e | Pilin assembly protein | pilP, NGO0095 | 5 ± 6.85 | Y | N/A | 3.53 |
a Average ratios and standard deviations were calculated for proteins identified in three independent experiments.
b Predictions of signal peptide cleavage site using SignalP (42).
c Data derived from Ducey et al. (2005), (61).
d Data derived from Jackson et al. (2010), (57).
e iTRAQ ratios were 0.5<proteins<2 in one of the biological triplicate experiments.
f The outer membrane localization of TbpB was verified experimentally (104).
Elimination of Cytoplasmic Proteins
Combining bioinformatic prediction of protein localization with profiling of bacterial subproteomes (cell envelopes, naturally released MVs, surface-exposed proteins, and secreted proteins) is an important part of data analysis because enrichments are never completely free from cytosolic proteins despite thoroughly executed experimental procedures (21, 37, 51–53). In addition, even minute amounts (low femtomoles) of proteins present in a sample can be detected by current MS instruments (54). Some of the cytoplasmic proteins arise from cell lysis during culture or copurify with large protein complexes tethered to the cell envelope; others may be translocated to the cell surface by noncanonical secretory pathways (51, 52).
To eliminate cytoplasmic proteins, a step that follows proteomic mining in our decision tree of vaccine candidates evaluation (Fig. 2D), the subcellular localization of all identified proteins was analyzed using predictive web-based software including PSORTb 3.0.2 (55), SOSUIGramN (56), and CELLO 2.5 (41). The primary reason for including these three systems and the majority-votes strategy for proteins assignment was that each of the programs employs different methods for prediction of protein localization.
In our analysis, proteins were allocated to a particular subcellular compartment when at least two of the software systems predicted the same localization. In cases where PSORTb, SOSUIGramN, and CELLO 2.5 assigned different subcellular locations for a particular protein, the protein was allocated to a group of proteins with “unknown subcellular localization”. This approach yielded assignment of 4 extracellular-, 26 outer membrane-, 53 periplasmic-, 116 inner membrane-, 481 cytoplasmic-, and 71 proteins with unknown localization (Fig. 2C, Tables I–II and supplemental Tables S4–S7).
Differentially Expressed Proteins
After excluding cytosolic proteins, a total of 13, 22, and 89 proteins showed differential expression during exposure of N. gonorrhoeae to NHS, iron deprivation, and anaerobic conditions, respectively (Fig. 2E).
Analysis of the Common NHS, Iron-depleted, and Anaerobic Stimulon
Comparison of data sets revealed 10 significantly stimulated proteins in all tested conditions compared with standard aerobic growth (Fig. 2E). The NHS, iron-responsive, and anaerobic stimulon includes F0F1-type ATP synthase C subunit (AtpE, NGO2145), paraquat inducible protein A (PqiA, NGO1319), a putative cadmium resistance protein (CadD, NGO1435), penicillin-binding protein 2 (PBP2, NGO1542), pilin assembly protein PilP (NGO0095), Sec-independent protein translocase protein TatA (NGO0183), a putative HemY protein (NGO0361), and putative uncharacterized proteins NGO0284, NGO0477, and NGO1411 (Tables I–III). None of these proteins were included in the previously described common iron, hydrogen peroxide, and anaerobic stimulon, which consisted of 14 genes and was constructed based on separate global transcriptome analyses (57–59). The poor correspondence between direct comparisons of different data sets has been noted before (59) and is related to bacterial culture methods (solid versus liquid media, different growth phases), sample preparation, sensitivity, detection limit, and cut-off settings of differential expression, as well as the occurrence of post-transcriptional regulatory mechanisms. An example of this latter challenge is exemplified by studies elucidating the manganese-dependent oxidative stress resistance of N. gonorrhoeae where proteomic approaches identified a set of 96 differentially regulated proteins, whereas no changes in gene expression were detected by microarray analysis and qPCR (60).
Table III. Analysis of polymorphic sites in vaccine candidate proteins among 36 Neisseria gonorrhoeae isolates.
| Name/Gene | Length |
Polymorphic sites |
||
|---|---|---|---|---|
| bp | AA | Nucleotides (%) | AA (%) | |
| LptD | 2403 | 801 | 18 (0.75) | 8 (1) |
| BamA | 2376 | 792 | 21 (0.88) | 7 (0.88) |
| TamA | 1905 | 635 | 14 (0.73) | 5 (0.79) |
| NGO2054 | 270–273 | 90–91 | 8 (2.9) | 4 (4.4) |
| NGO2139 | 864 | 288 | 2 (0.23) | 0 (0) |
NHS Responsive Proteins
In response to NHS, expression of a total of 13 cell envelope-associated proteins was significantly elevated within a range from 2.1- to 5.8-fold (Fig. 2E, Table I). In addition to the 10 members of the proteome-based stimulon (discussed above), expression of cytochrome c4 (NGO0101), cell division protein FtsQ (NGO1530), and the uncharacterized protein NGO1044 was considerably induced. The increased levels of cytochrome c4 and NGO1044 were also observed during anaerobic growth and upon iron limitation, respectively, whereas FtsQ was solely up-regulated by the presence of NHS (Fig. 2E, Tables I–II, supplemental Table S4).
Effect of Iron Deprivation on the Cell Envelope Protein Profile
With the application of a >2.0-fold cutoff for differential protein expression, six outer membrane proteins, four periplasmic proteins, 10 inner membrane proteins, and two proteins with unknown localization were designated as up-regulated during growth of N. gonorrhoeae under iron limiting conditions (Table II). Among these proteins, 12 were identified as part of the Fur regulon by an examination of the steady-state levels of mRNA in response to iron availability and by microarray (57, 61). Some of these proteins have been well-characterized, including iron acquisition transporters such as the transferrin receptor system composed of TbpA and TbpB, ferric enterobactin receptor FetA and periplasmic iron-binding transporter FetB, ferric binding protein A (FbpA), TonB-dependent transporter TdfG, and ExbB (62–66). The TbpA and TbpB proteins are being pursued as gonorrhea vaccine candidates because of their conservation within specific regions among different isolates, importance in N. gonorrhoeae pathophysiology, and abilities to elicit cross-reactive and bactericidal antibodies (48, 67).
Importantly, however, our proteomic approach revealed an additional 10 proteins with expression profiles that were positively regulated by low-iron conditions, including predicted outer membrane proteins, NGO2111 and NGO1688, both containing DUF560 domain of unknown function (Table II). These proteins were identified using iTRAQ-2D-LC-MS/MS as being ubiquitously present in the cell envelopes of N. gonorrhoeae strains FA1090, MS11, F62, and 1291 maintained under routine aerobic cultivation in GCBL (21).
Differentially Expressed Proteins During Anaerobic Growth
In our proteomic profiling, a total of 367 proteins (about 19% of the N. gonorrhoeae FA1090 genome) were differentially regulated during anaerobic growth (supplemental Table S4 and supplemental Table S6). Deep sequencing analysis performed on similarly cultured gonococci revealed differential regulation of 198 genes (59). This discrepancy suggests that expression of many of these proteins is regulated at the post-transcriptional level. After excluding cytoplasmic proteins, 89 proteins (four outer membrane, 11 periplasmic, 47 inner membrane, and 27 with unknown localization) showed significantly altered quantities and eight of them were also identified using a RNA-seq approach (supplemental Table S4, (59)). Within this group only NGO0372 and NGO2012 were downregulated, with average ratios (± S.D.) of 0.4 ± 0.06 and 0.2 ± 0.11, respectively. Not surprisingly, proteins that participate in the anaerobic denitrification pathway, including outer membrane proteins AniA (68) and lipid-modified azurin, Laz (69); inner membrane protein NorB (70); and different types of cytochromes, as well as proteins involved in cytochrome biogenesis (71–73), were the most highly up-regulated, with induction reaching 15-fold in comparison to SGC (supplemental Table S4). It is worth noting that AniA is currently being investigated as a gonorrhea vaccine candidate because of its pivotal function in bacterial survival under oxygen-limiting conditions in the presence of nitrite, expression in vivo, surface localization, conservation, and ability to induce functional antibodies that inactivate the AniA nitrite reductase activity (45, 74, 75). In addition to AniA, our proteomic profiling revealed induced expression of three outer membrane proteins including vaccine antigens NspA (NGO0203) and porin PI.B [NGO1812, (16)], as well as TdfJ [NGO1205, (76)], which is a homolog of the N. meningitidis zinc uptake protein ZnuD (77). The presence of TdfJ was not required for survival of N. gonorrhoeae within cervical epithelial cells (76). Its expression, however, was significantly elevated in biofilms, where gonococci utilize the anaerobic respiration pathway (78), thus the function of TdfJ in N. gonorrhoeae pathophysiology remains to be further elucidated.
Ubiquitously Expressed Proteins
In comparison to SGC, the expression of 168 proteins (four extracellular, 18 outer membrane, 37 periplasmic, 67 inner membrane, and 42 with unknown localization) remained unaltered (iTRAQ ratios of 0.5<proteins<2) upon exposure of N. gonorrhoeae to NHS, iron depletion and oxygen deprivation (supplemental Table S5). In our previous proteomic analysis, 305 proteins were designated as ubiquitously present in the cell envelopes isolated from FA1090, MS11, F62, and 1291 (21). A comparison of these two data sets revealed 92 ubiquitously expressed proteins in common (Fig. 2D). This group contains known outer membrane residents such as Phospholipase A (PldA, NGO1492); two potential gonorrhea vaccine antigens: pilus-associated adhesin PilC and an outer membrane channel for pilus extrusion PilQ (16); Omp3; and MafA (NGO1067). Ubiquitous proteins also included homologs of LPS assembly protein LptD (OstA, ImpA, NGO1715); components of the β-barrel assembly machinery (BAM) including BamA and BamD (ComL, NGO0277); the Translocation and Assembly Module [TAM, (79)] consisting of TamA (NGO1956) and TamB (NGO1955); hypothetical proteins NGO1344 and NGO2121; and predicted lipoproteins NGO0834, NGO1985, NGO2054, and NGO2139 [supplemental Table S5 and (21)].
Among the newly identified cell envelope-associated proteins was a predicted outer membrane protein, NGO0952 [TdfH, (76)], comprised of conserved domains TIGR01785 and cI21487, which are characteristic of TonB-dependent heme/hemoglobin receptors/transporters and outer membrane channels, respectively. The function of TdfH remains unknown (76), but global transcriptome analysis revealed that its expression might be subjected to repression by the AraC transcriptional regulator MpeR (76, 80). A homolog of TdfH from N. meningitidis, designated as CbpA, has been identified as a receptor for calprotectin and thus plays a role in zinc acquisition (81). Moreover, the levels of efflux pumps MtrCDE and FarAB remained unaltered despite different growth conditions (supplemental Table S5). The MtrCDE efflux pump transports structurally diverse hydrophobic compounds outside the bacterial cell, whereas FarAB is required for resistance against antimicrobial long chained fatty acids (82–84). The surface exposed MtrE serves as an outer membrane channel for both efflux systems (84), and is being developed as a gonorrhea vaccine candidate (16).
Selected Vaccine Candidates
Our constructed antigen decision tree contains both uniformly expressed and specifically induced gonorrhea vaccine candidates (Fig. 2D). In this report, we subjected to initial assessments as potential vaccine targets five proteins: BamA, LptD, TamA, NGO2054, and NGO2139 (Fig. 2D). These candidates were uniformly expressed during different growth conditions (supplemental Table S5), displayed similar abundance in four N. gonorrhoeae strains, were localized to both cell envelopes and MVs fractions (21), and had either pivotal role(s) in other bacterial species (BamA, LptD, TamA) or unknown functions (NGO2054 and NGO2139).
The Bam complex, BamA-E in E. coli, folds and inserts beta-barrel proteins into the outer membrane [reviewed in (85)]. The Omp85 protein family member BamA is found in all Gram-negative bacteria (85, 86). Despite the overall functional conservation, BamA homologs recognize their protein substrates in a species-specific manner and display highly divergent sequences located within the surface-exposed loops (87–89). The finding that BamA of Burkholderia pseudomallei induced protective immunity in mice (90) highlights the potential of this protein as a vaccine target. We reasoned that BamA of N. gonorrhoeae, similar to other bacterial species, is an essential protein. To test this hypothesis, we engineered a strain of FA1090 in which a copy of bamA was placed under the control of the IPTG-inducible promoter and inserted within an intergenic chromosomal region between the lctP and aspC genes (91). Subsequently, the native bamA was replaced in-frame with the nonpolar kanamycin resistance cassette using homologous recombination. As expected, the resultant strain FA1090 ΔbamA/Plac::bamA grew robustly in the presence of IPTG, whereas without the inducer, the bacteria failed to grow on solid media and halted proliferation in liquid media within about 6 h from back dilution (data not shown and supplemental Fig. S1, respectively), concomitant to depletion of BamA levels (Fig. 3).
Fig. 3.
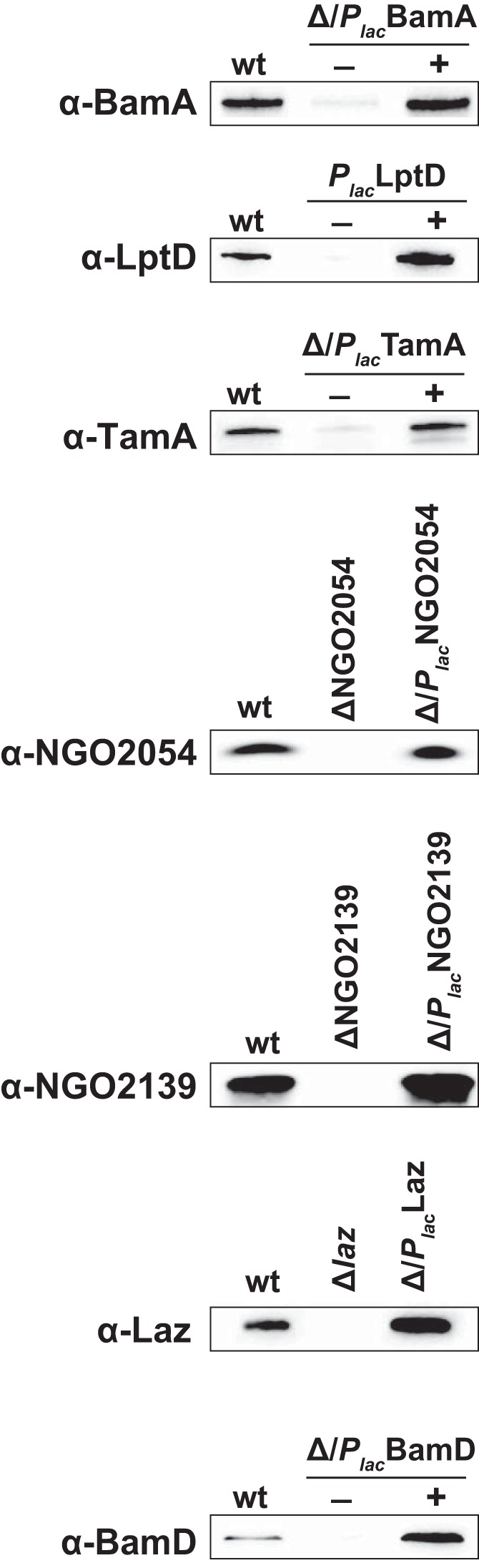
Generation of polyclonal antibodies and immunoblotting analysis. Recombinant versions of vaccine candidates BamA, LptD, TamA, NGO2054, and NGO2139, as well as proteins employed as controls in the experiments (Laz and BamD) were purified by Ni2+ affinity chromatography and subsequently used to obtain polyclonal rabbit antisera. Wild type, isogenic conditional mutants, or knockouts, as well as complemented variants of FA1090 (as indicated above each immunoblot) were harvested from GCB. Whole-cell lysates matched by equivalent OD600 units were separated in 4–20% Mini-PROTEAN TGX precast gels, transferred onto nitrocellulose membranes and probed with different polyclonal rabbit antibodies.
Our second vaccine candidate, LptD, is a low-abundance outer membrane β-barrel protein involved in transport of lipopolysaccharide/lipooligosaccharide into the outer leaflet of the outer membrane (92–94). We have demonstrated that, in contrast to N. meningitidis (93) and similar to E. coli, LptD is an essential protein for N. gonorrhoeae viability (21).
TAM is a newly discovered archetypal protein transport system comprised of TamA (an Omp85 family protein) and TamB. TAM promotes efficient secretion of autotransporters and is required for virulence in proteobacteria (79, 95). Functional characterization of the complex in E. coli, Citrobacter rodentium, and Salmonella enterica demonstrated that TamA is an integral outer membrane protein whereas TamB is a large protein that is encoded in an operon along with the gene for TamA and is located in the inner membrane. A similar genetic organization of a putative TAM complex exists in N. gonorrhoeae with TamA and TamB in an operon encoded by NGO1956 and NGO1955, respectively. We discovered both these proteins as being uniformly present in the cell envelopes of different gonococcal strains (21). In agreement with other organisms studied so far, the TAM complex is likely not essential for N. gonorrhoeae viability as conditional or in-frame deletion mutants of tamA or tamB, respectively, did not display noticeable growth defects [data not shown and (21)].
Finally, although bioinformatics predictions allocated NGO2054 and NGO2139 to a group of proteins with unknown subcellular location [supplemental Table S5 and (21)], both proteins contain predicted signal peptides with a lipoprotein box containing the indispensable cysteine residue, which suggest their outer membrane localization (96). The NGO2054 protein encompasses a LambdaBor motif (amino acid residues 3 to 36), a bacterial virulence determinant encoded by lysogenic coliphage lambda (97), whereas NGO2139 is annotated in KEGG as a d-methionine transport system substrate binding protein. The crystal structure of its homolog in N. meningitidis (GNA1946) was recently solved and surprisingly revealed an l-methionine bound into the protein cleft (98). Both proteins remain functionally uncharacterized but were found dispensable for N. gonorrhoeae viability during SGC in liquid and on solid media (21).
Characterization of Antigen-specific Polyclonal Antisera
Recombinant variants of each of the five vaccine candidates that contained a C-terminal 6×His extension tag to enable purification from E. coli cell lysates using Ni-NTA affinity chromatography were generated. To provide additional controls for our experiments, recombinant BamD and Laz were also prepared.
The chimeric BamA, LptD, and TamA formed insoluble aggregates and were purified under denaturing conditions, whereas NGO2054, NGO2139, BamD and Laz were obtained under native conditions. Subsequently, all proteins were used to immunize rabbits and obtain polyclonal antisera. The reactivity of these newly generated antibodies was examined using wild type N. gonorrhoeae FA1090 and isogenic conditional or in-frame deletion mutants, as well as corresponding complemented strains. As expected, the individual antisera cross-reacted with whole-cell lysates originating from the wild type and complemented gonococci but not from the mutant strains (Fig. 3).
Secondary Validation of iTRAQ Results
The reliability of protein quantitation using iTRAQ on MALDI- and ESI-based MS instrumentation has been demonstrated for alterations of up to two orders of magnitude in a wide range of biological samples assessed in technical and biological replicates. However, it has been considered that low-signal species may have higher relative variability. In addition, underestimation of large changes when using an iTRAQ-based protein quantitation approach has also been reported [reviewed in (99)]. Therefore, to provide additional validation of protein expression profiles, the cell envelopes extracted from FA1090 cultured concurrently under SGC, in the presence of NHS, during iron deprivation, and anaerobically were subjected to SDS-PAGE and immunoblotting analyses (supplemental Fig. S2). SDS-PAGE coupled with colloidal Coomassie G-250 staining showed that, as expected (Fig. 2C, supplemental Table S4), the most altered was the overall cell envelope protein profile of N. gonorrhoeae maintained under anaerobic conditions (supplemental Fig. S2A). In agreement with the iTRAQ analyses (supplemental Table S5), similar levels of MtrE, BamA, LptD, TamA, NGO2054, NGO2139, BamD, and Ng-MIP were detected by immunoblotting during all tested conditions (supplemental Fig. S2B). As controls for differentially expressed proteins, we utilized antisera against well-recognized protein markers for anaerobic [AniA (21) and Laz (68)] and iron-limited [TbpB (48)] conditions. The iTRAQ quantitation indicated that in comparison to SGC, the relative levels of AniA and Laz increased on average 9.5 ± 2.81 and 2.9 ± 0.59 fold, respectively (supplemental Table S4), whereas there was 11.4 ± 4.27 fold increase in TbpB abundance during iron limitation (Table II). Corroborating these findings, as shown in supplemental Fig. S2B, increased amounts of AniA and Laz, as well as TbpB were detected in the cell envelope fractions of gonococci grown anaerobically and during iron deprivation, correspondingly.
Additionally, to examine the expression of our vaccine candidates throughout N. gonorrhoeae growth, wild type FA1090 was maintained under routine aerobic cultivation in GCBL. Bacterial proliferation was monitored by measurements of cell density at OD600 within 6 h of the experiment (supplemental Fig. S3A). Every hour samples were withdrawn and the whole cell lysates were probed with anti-BamA, anti-LptD, anti-TamA, anti-NGO2054, and anti-NGO2139 antisera. The representative immunoblots (supplemental Fig. S3B) showed that the vaccine candidates were constitutively expressed throughout the duration of the experiment.
Together, these analyses further supported iTRAQ-based proteome relative quantitation and demonstrated that the selected candidates are ubiquitously expressed during exposure of N. gonorrhoeae to different environmental cues as well as throughout different growth phases.
Assessment of Vaccine Candidates' Localization and Surface Exposure
Bioinformatic predictions of proteins' subcellular locations are invaluable tools for creating a “decision tree” to evaluate vaccine candidates. Experimental verification, however, is essential for final subcellular protein assignment. The subcellular localization of AniA and Ng-MIP are two great examples of discrepancies between in silico predictions and experimental evidence. According to the predictive bioinformatic tools (SOSUIGramN, PSORTb, and CELLO) and the majority-votes strategy, both proteins were assigned to the periplasmic compartment (supplemental Tables S3 and S4, respectively). In contrast, Shewell, L.K. et al. (45) demonstrated the outer membrane localization and surface-exposure of AniA by immune-S.E.M. and trypsin-accessibility studies. Likewise, subcellular fractionation of N. gonorrhoeae cells coupled with immunoblotting and flow-cytometry experiments revealed the surface-localization of Ng-MIP (100).
Therefore, the subcellular localization and surface exposure of BamA, LptD, TamA, NGO2054, and NGO2139 were investigated. In these experiments, controls included protein markers for surface-exposed (Ng-MIP, MtrE, and AniA), periplasmic side of the outer membrane [BamD, (85)] and cytoplasmic [ObgGC (43)] localization, respectively. The N. gonorrhoeae FA1090 cells were harvested at mid-logarithmic phase of growth in GCBL, lysed and the whole cell lysate was subjected to a carbonate extraction method to enrich for outer membrane proteins in the cell envelope fraction (21). Separation of MVs from soluble proteins was achieved by high-speed ultracentrifugation of culture supernatant (21). The same total amounts of purified subproteomes were loaded onto SDS-PAGE followed by immmunoblotting analyses with antisera against individual proteins. As expected, the ObgGC was found exclusively in the cytosolic protein fraction (Fig. 4). None of the proteins were detected in the soluble fraction of the supernatant. Ng-MIP, MtrE, AniA, BamA, LptD, TamA, NGO2054, NGO2139, and BamD were found predominantly in the cell envelopes. In addition, these proteins were also identified in MVs and some of them were detected in the cytosol, which suggests that during the exponential phase they were still tightly associated with the ribosomes.
Fig. 4.
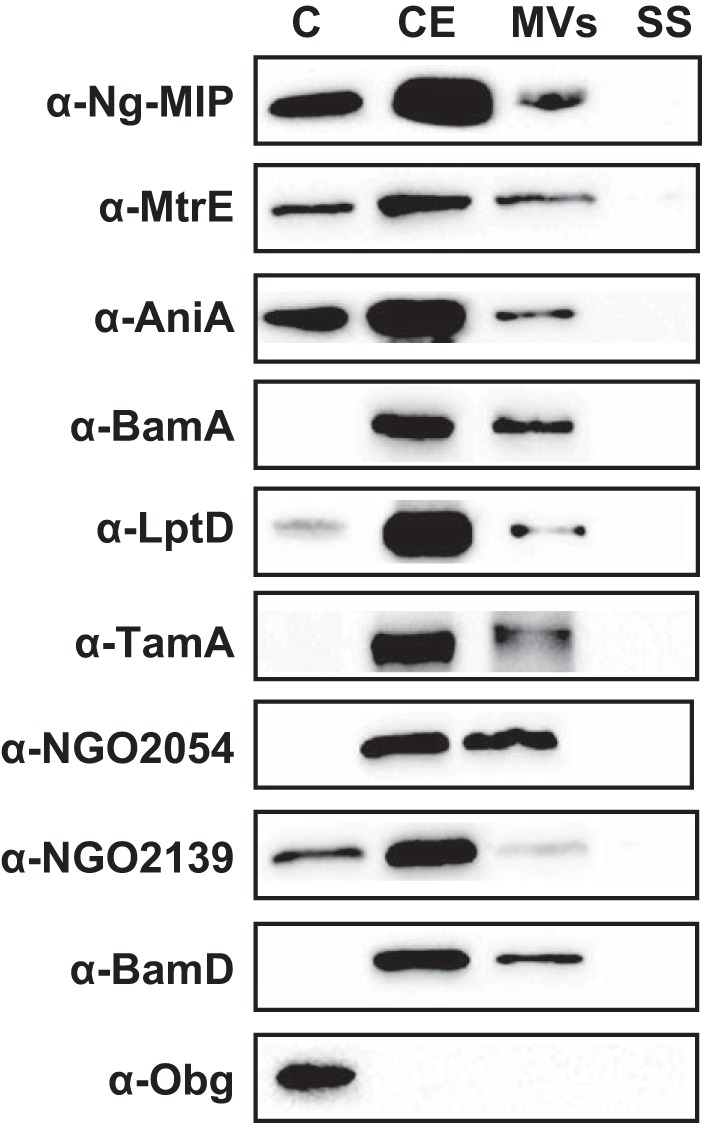
Subcellular localization of selected vaccine candidates. Equal amounts of subproteome fractions (C-cytoplasmic, CE-cell envelopes, MVs-naturally released membrane vesicles, SS-soluble fractions of supernatants) derived from FA1090 cultured under standard growth conditions in GCBL were separated in 4–20% gradient gels. The proteins were transferred to nitrocellulose and probed with individual polyclonal rabbit antibodies against tested proteins, as indicated on the left. Controls included protein markers for the periplasmic face of the outer membrane (BamD) and cytoplasm (ObgGC) protein.
Subsequently, to investigate the surface accessibility of selected vaccine candidates, dot blotting experiments and protease accessibility studies were conducted (Fig. 5A). In the first approach, intact or lysed N. gonorrhoeae FA1090 cells, cultured as described above, were spotted on the nitrocellulose membrane and probed with primary antisera against individual vaccine candidates as well as proteins utilized as controls. The antibodies against BamA, LptD, TamA, NGO2054, and NGO2139 cross-reacted with intact gonococci similarly to anti-Ng-MIP, anti-MtrE, and anti-AniA antisera (Fig. 5B). In contrast, BamD and ObgGC were not detected unless cell lysates were applied to the nitrocellulose and probed with the respective antibodies (Fig. 5C).
Fig. 5.
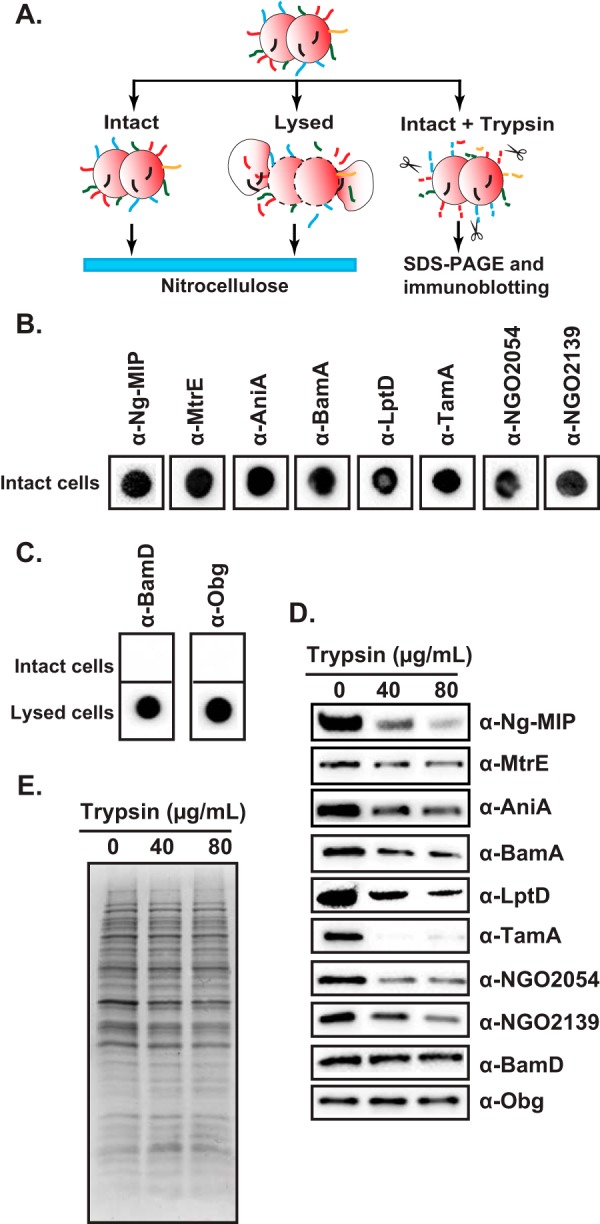
Assessment of surface exposure of candidate antigens BamA, LptD, TamA, NGO2054, and NGO2139. A, Experimental outline of dot blotting of either intact or lysed N. gonorrhoeae FA1090 cells and protease accessibility studies using intact cells. B, Intact cells of FA1090 were spotted on nitrocellulose and probed with different polyclonal antibodies, as shown above individual dot blots. C, Intact and lysed cells were used to detect BamD and Obg, which were utilized as periplasmic and cytoplasmic protein markers, respectively. D, Intact FA1090 cells were incubated with increasing concentrations of trypsin (as indicated), lysed, separated in 4–20% Tris-glycine precast gels and probed with individual antisera. E, Intactness of the cells during trypsin treatment was verified by separation of total-cell lysates by SDS-PAGE and visualization of protein profiles with colloidal Coomassie G-250 staining.
In the second approach, intact N. gonorrhoeae cells were treated with increasing amounts of trypsin. Reactions were stopped with PMSF, cells were lysed by addition of SDS loading buffer and probed with appropriate antibodies. As anticipated, these experiments revealed that the surface exposed proteins Ng-MIP, MtrE, and AniA were susceptible to digestion by trypsin as their amounts significantly decreased upon incubation with increasing concentrations of protease (Fig. 5D). Similar results were obtained by probing samples with antisera against vaccine candidates BamA, LptD, TamA, NGO2054, and NGO2139. The vast majority of other proteins remained unaltered as evidenced by comparison of protein profiles from trypsin-treated and untreated samples by either immunoblotting analyses of BamD and ObgGC or by staining using colloidal Coomassie G-250 (Fig. 5D and 5E, respectively), which demonstrated the intactness of the cells.
Cumulatively, these data showed that the five vaccine candidates are surface-localized proteins and supported our earlier observation (21) that they are also constituents of naturally released MVs.
Complement-mediated Antibody-dependent Serum Bactericidal Activity
The ability of recombinant antigens to elicit protective antibodies against homologous and heterologous N. gonorrhoeae strains FA1090 (serum resistant) and MS11 (serum-sensitive) was tested using the rabbit anti-BamA, anti-LptD, anti-TamA, anti-NGO2054, and anti-NGO2139 post-immune sera and NHS as the complement source (101). As expected (67), FA1090 was more resistant than MS11 to bactericidal killing by all the sera examined (Fig. 6). All antisera displayed bactericidal activity, and overall, the most potent against both FA1090 and MS11 were anti-BamA, anti-NGO2054, and anti-NGO2139. In particular, the bactericidal50 titers against FA1090 and MS11 were 32 and 512 for anti-BamA, respectively, and 20 and 400 for anti-NGO20154 and anti-NGO2139.
Fig. 6.
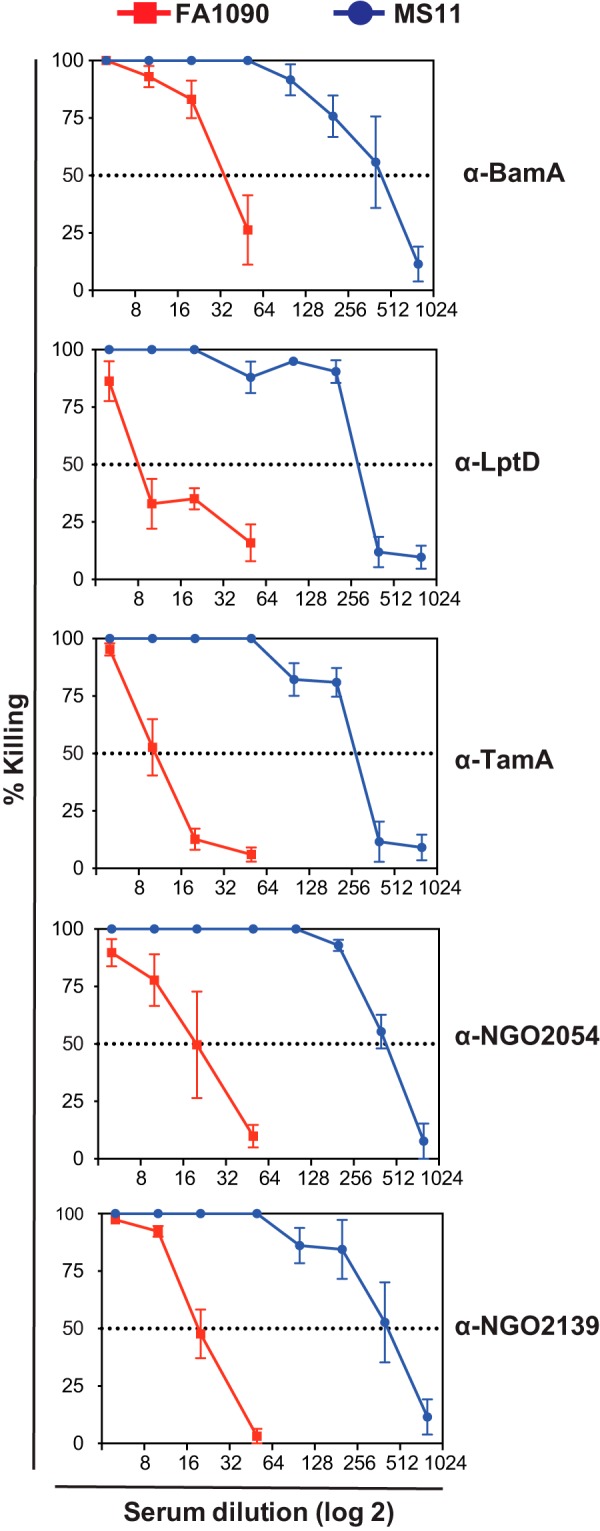
The purified recombinant variants of BamA, LptD, TamA, NGO2054, and NGO2139 elicit bactericidal antibodies. Serum bactericidal assays were conducted against 5 × 103 CFUs/ml of FA1090 (serum resistant) and MS11 (serum sensitive) N. gonorrhoeae strains using the rabbit anti-BamA, anti-LptD, anti-TamA, anti-NGO2054, and anti-NGO2139 post-immune sera and NHS as the complement source. The rabbit sera were heat-inactivated at 56 °C for 30 min. The bacterial cells were pre-sensitized with dilutions of the antigen-specific heat-inactivated sera for 15 min, followed by the addition of NHS at 10% final concentration, and incubation continued for 30 min. The CFUs were determined by plating bacteria on solid media. The average percent killing was determined from at least four independent experiments and was calculated as the number of CFUs in samples incubated with rabbit post-immune sera and NHS to the number of CFUs recovered from samples treated with rabbit post-immune sera and HI-NHS. N. gonorrhoeae viability was not affected in any of the control samples.
Conservation of Vaccine Candidates
The conservation of the selected vaccine candidates (BamA, LptD, TamA, NGO2054, and NGO2139) was examined by analyzing the presence of polymorphic sites at both nucleotide and amino acid levels using the completed genome sequence of strain FA1090 (Gen Bank accession number AE004969), the draft genome sequences of 14 different N. gonorrhoeae strains (downloaded from the Broad Institute http://www.broadinstitute.org/annotation/genome/Neisseria_gonorrhoeae/MultiHome.html, and the 14 WHO 2015 reference strains (10, 28, 29). Overall, these analyses demonstrated that our five vaccine candidates were well conserved among 29 examined gonococcal isolates, with the number of nucleotide/amino acid polymorphic sites ranging from 2 (0.23%)/0 (0%) for NGO2139 to 21 (0.88%)/7 (0.88%) for BamA (Table III).
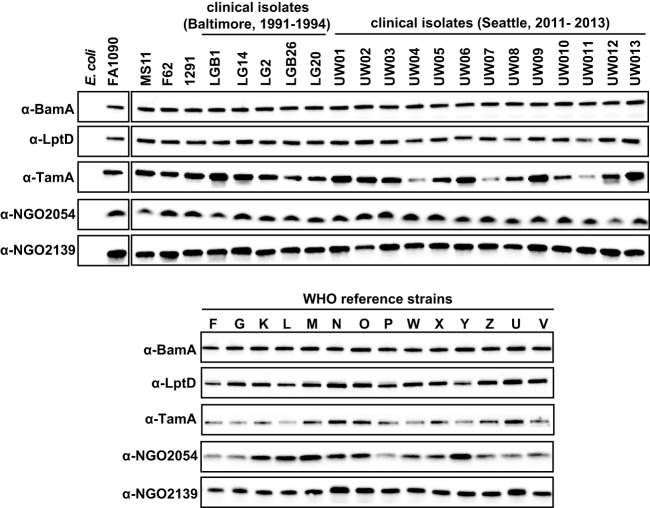
Subsequently, the expression of selected vaccine candidates was assessed using whole-cell lysates derived from a panel of temporally and geographically diverse N. gonorrhoeae strains (Fig. 7, supplemental Table S1). Because BamA and LptD have homologs in other Gram-negative bacteria, the specificities of the antisera were also tested using E. coli. All of the vaccine candidates were expressed by the 36 N. gonorrhoeae isolates; BamA, LptD, and NGO2139 showed similar abundance, whereas TamA and NGO2054 seemed to be expressed at variable levels in some gonococcal strains. In addition, none of the antisera cross-reacted with the E. coli whole-cell extracts (Fig. 7).
Fig. 7.
Expression of potential vaccine targets was evaluated using a panel of 36 temporally and spatially diversified GC isolates. Clinical isolates of N. gonorrhoeae including WHO reference strains (as indicated) were harvested from GCB, matched by equivalent OD600 units and resolved in 4–20% Tris-glycine precast gels. Following transfer onto nitrocellulose membrane and blocking, the proteins were probed with polyclonal rabbit antisera against BamA, LptD, TamA, NGO2054, and NGO2139. An E. coli strain (ER2566) was used as a negative control.
Together, these studies demonstrated that BamA, LptD, TamA, NGO2054, and NGO2139 are conserved and expressed not only in the commonly used laboratory strains (FA1090, MS11, F62, and 1291) but also clinical isolates from Baltimore and Seattle, as well as the 2015 WHO reference strains (10, 28, 29).
Final Remarks
Gonorrhea occurs at a high incidence and has a major impact on reproductive and neonatal health worldwide (2). Alarmingly, with each new antibiotic introduced for gonorrhea, resistance has emerged, including resistance to penicillins, tetracycline, macrolides, fluoroquinolones, and recently the third-generation cephalosporins. Current treatment options are seriously limited, and the development of a gonorrhea vaccine is the only long-term solution to this problem. Progress on gonorrhea vaccines has been slow, in part because of the high number of surface-exposed molecules in N. gonorrhoeae that undergo phase or antigenic variation and a lack of understanding of protective responses. Gonorrhea vaccine development can therefore benefit from a comprehensive, unbiased approach for antigen discovery. The application of comparative proteomics described in this study, together with previous comprehensive analyses of the N. gonorrhoeae cell envelopes and MVs (21), as well as other global proteomic approaches are ideally suited for identifying promising N. gonorrhoeae antigens and to guide future gonorrhea vaccine development (102). These newly identified cell envelope-associated proteins can then be subjected to rigorous evaluation that includes their expression as recombinant proteins in E. coli, examination of their surface localization and conservation among temporally, geographically, and genetically diverse panels of gonococcal strains, assessment of their ability to induce antibodies that react against heterologous N. gonorrhoeae strains and are bactericidal, functional characterization, identification of protective epitopes, testing different combinations of antigens with various adjuvants and routes of immunization, as well as their protective capabilities in the gonorrhea mouse models. These studies could ideally lead to a selection of rational vaccine candidates that should be further tested alone and in different combination(s) in the human male challenge model of gonococcal infection to examine protection against N. gonorrhoeae, as well as the safety and nature of the immune responses generated. Finally, our studies also offer an unbiased overview of the N. gonorrhoeae cell envelope at the proteome level. Accordingly, the new research leads generated could provide novel paths for the scientific community to investigate the mechanisms that underpin N. gonorrhoeae pathophysiology. This, in turn, may drive the rational design of new preventive, diagnostic, and therapeutic interventions.
Supplementary Material
Acknowledgments
We thank Mariagrazia Pizza and Cynthia Cornelissen for generous gifts of anti-Ng-MIP and anti-TbpB antibodies, respectively.
Footnotes
Author contributions: A.E.S. designed research; R.A.Z., I.H.W., B.I.B., P.R.G., O.O.S., and M.U. performed research; K.K.H. and M.U. contributed new reagents or analytic tools; R.A.Z. and A.E.S. analyzed data; A.E.J. and A.E.S. wrote the paper; A.E.S. prepared all figures.
* This work was funded by AES grant R01-AI117235 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The FHCRC Proteomics Facility is funded in part by P30 CA015704 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- GCB
- gonococcal base agar solid medium
- C
- cytoplasmic
- CE
- cell envelopes
- CFU
- colony-forming units
- COG
- Clusters of Orthologous Groups
- GCBL
- gonococcal base liquid medium
- IPTG
- isopropyl-β-D-galactopyranoside
- iTRAQ
- isobaric tagging for relative and absolute quantification
- MVs
- membrane vesicles
- NHS
- normal human serum
- 2D-LC
- two-dimensional liquid chromatography
- SCX
- strong cation exchange
- SGC
- standard growth conditions
- SS
- soluble fractions of supernatants.
REFERENCES
- 1. Fernandez-Romero J. A., Deal C., Herold B. C., Schiller J., Patton D., Zydowsky T., Romano J., Petro C. D., and Narasimhan M. (2015) Multipurpose prevention technologies: the future of HIV and STI protection. Trends Microbiol. 23, 429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newman L., Rowley J., Vander Hoorn S., Wijesooriya N. S., Unemo M., Low N., Stevens G., Gottlieb S., Kiarie J., and Temmerman M. (2015) Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PloS One 10, e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis D. A. (2010) The Gonococcus fights back: is this time a knock out? Sex. Transm. Infect. 86, 415–421 [DOI] [PubMed] [Google Scholar]
- 4. Unemo M., and Shafer W. M. (2014) Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin. Microbiol. Rev. 27, 587–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edwards J. L., and Apicella M. A. (2004) The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17, 965–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westrom L. V. (1994) Sexually transmitted diseases and infertility. Sex. Transm. Dis. 21, S32–S37 [PubMed] [Google Scholar]
- 7. Woods C. R. (2005) Gonococcal infections in neonates and young children. Semin. Ped. Infect. Dis. 16, 258–270 [DOI] [PubMed] [Google Scholar]
- 8. Campbell M. F. (1928) The surgical pathology of epididymitis. Ann. Surg. 88, 98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleming D. T., and Wasserheit J. N. (1999) From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohnishi M., Golparian D., Shimuta K., Saika T., Hoshina S., Iwasaku K., Nakayama S., Kitawaki J., and Unemo M. (2011) Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55, 3538–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. (2012) Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. [Google Scholar]
- 12. Seib K. L., Zhao X., and Rappuoli R. (2012) Developing vaccines in the era of genomics: a decade of reverse vaccinology. Clin. Microbiol. Infect. 18, 109–116 [DOI] [PubMed] [Google Scholar]
- 13. Adamczyk-Poplawska M., Markowicz S., and Jagusztyn-Krynicka E. K. (2011) Proteomics for development of vaccine. J. Proteomics 74, 2596–2616 [DOI] [PubMed] [Google Scholar]
- 14. Heckels J. E., and Williams J. N. (2010) The influence of genomics and proteomics on the development of potential vaccines against meningococcal infection. Genome Med. 2, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delany I., Rappuoli R., and Seib K. L. (2013) Vaccines, reverse vaccinology, and bacterial pathogenesis. Perspectives Med. 3, a012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jerse A. E., Bash M. C., and Russell M. W. (2014) Vaccines against gonorrhea: current status and future challenges. Vaccine 32, 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hadad R., Jacobsson S., Pizza M., Rappuoli R., Fredlund H., Olcen P., and Unemo M. (2012) Novel meningococcal 4CMenB vaccine antigens- prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. APMIS 120, 750–760 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y., Feinen B., and Russell M. W. (2011) New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front. Microbiol. 2, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y., Egilmez N. K., and Russell M. W. (2013) Enhancement of adaptive immunity to Neisseria gonorrhoeae by local intravaginal administration of microencapsulated interleukin 12. J. Infect. Dis. 208, 1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y., Liu W., and Russell M. W. (2014) Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. 7, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zielke R. A., Wierzbicki I. H., Weber J. V., Gafken P. R., and Sikora A. E. (2014) Quantitative proteomics of the Neisseria gonorrhoeae cell envelope and membrane vesicles for the discovery of potential therapeutic targets. Mol. Cell. Proteomics 13, 1299–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Connell T. D., Black W. J., Kawula T. H., Barritt D. S., Dempsey J. A., Kverneland K. Jr., Stephenson A., Schepart B. S., Murphy G. L., and Cannon J. G. (1988) Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol. Microbiol. 2, 227–236 [DOI] [PubMed] [Google Scholar]
- 23. Meyer T. F., Mlawer N., and So M. (1982) Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell 30, 45–52 [DOI] [PubMed] [Google Scholar]
- 24. Apicella M. A., Breen J. F., and Gagliardi N. C. (1978) Degradation of the polysaccharide component of gonococcal lipopolysaccharide by gonococcal and meningococcal sonic extracts. Infect. Immun. 20, 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sparling P. F. (1966) Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J. Bacteriol. 92, 1364–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maness M. J., and Sparling P. F. (1973) Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J. Infect. Dis. 128, 321–330 [DOI] [PubMed] [Google Scholar]
- 27. Garvin L. E., Bash M. C., Keys C., Warner D. M., Ram S., Shafer W. M., and Jerse A. E. (2008) Phenotypic and genotypic analyses of Neisseria gonorrhoeae isolates that express frequently recovered PorB PIA variable region types suggest that certain P1a porin sequences confer a selective advantage for urogenital tract infection. Infect. Immun. 76, 3700–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Unemo M., Fasth O., Fredlund H., Limnios A., and Tapsall J. (2009) Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J. Antimicrob. Chemother. 63, 1142–1151 [DOI] [PubMed] [Google Scholar]
- 29. Unemo M. G., D., Grad Y., Limnios A., Wi T., Lahra M., Harris S. (2015) Phenotypic, genetic and genomic characterisation of the WHO Neisseria gonorrhoeae reference strains for quality assurance of laboratory investigations globally. Sex. Transm. Infect. 91 (Suppl. 2), A111 [Google Scholar]
- 30. Spence J. M., Wright L., and Clark V. L. (2008) Laboratory maintenance of Neisseria gonorrhoeae. Curr. Protoc. Microbiol. Chapter 4, Unit 4A 1 [DOI] [PubMed] [Google Scholar]
- 31. Kellogg D. S. Jr., Peacock W. L. Jr., Deacon W. E., Brown L., and Pirkle D. I. (1963) Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85, 1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen C. J., Sparling P. F., Lewis L. A., Dyer D. W., and Elkins C. (1996) Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infec.t Immun. 64, 5008–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardinale J. A., and Clark V. L. (2000) Expression of AniA, the major anaerobically induced outer membrane protein of Neisseria gonorrhoeae, provides protection against killing by normal human sera. Infect. Immun. 68, 4368–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark V. L., Campbell L. A., Palermo D. A., Evans T. M., and Klimpel K. W. (1987) Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect. Immun. 55, 1359–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zielke R. A., Gafken P. R., and Sikora A. E. (2014) Quantitative proteomic analysis of the cell envelopes and native membrane vesicles derived from gram-negative bacteria. Curr. Protoc. Microbiol. 34, 1F 3 1–1F 3 16 [DOI] [PubMed] [Google Scholar]
- 36. Yi E. C., Lee H., Aebersold R., and Goodlett D. R. (2003) A microcapillary trap cartridge-microcapillary high-performance liquid chromatography electrospray ionization emitter device capable of peptide tandem mass spectrometry at the attomole level on an ion trap mass spectrometer with automated routine operation. Rapid Commun. In Mass Spectrom. 17, 2093–2098 [DOI] [PubMed] [Google Scholar]
- 37. Sikora A. E., Zielke R. A., Lawrence D. A., Andrews P. C., and Sandkvist M. (2011) Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J. Biol. Chem. 286, 16555–16566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saeed A. I., Bhagabati N. K., Braisted J. C., Liang W., Sharov V., Howe E. A., Li J., Thiagarajan M., White J. A., and Quackenbush J. (2006) TM4 microarray software suite. Methods Enzymol. 411, 134–193 [DOI] [PubMed] [Google Scholar]
- 39. Gardy J. L., Laird M. R., Chen F., Rey S., Walsh C. J., Ester M., and Brinkman F. S. (2005) PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21, 617–623 [DOI] [PubMed] [Google Scholar]
- 40. Fukushima T., Arai K., Tomiya M., Mitsuhashi S., Sasaki T., Santa T., Imai K., and Toyo'oka T. (2008) Fluorescence determination of N-acetylaspartic acid in the rat cerebrum homogenate using high-performance liquid chromatography with pre-column fluorescence derivatization. Biomed. Chromatogr. 22, 100–105 [DOI] [PubMed] [Google Scholar]
- 41. Yu C. S., Lin C. J., and Hwang J. K. (2004) Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 13, 1402–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petersen T. N., Brunak S., von Heijne G., and Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 [DOI] [PubMed] [Google Scholar]
- 43. Zielke R. A., Wierzbicki I. H., Baarda B. I., and Sikora A. E. (2015) The Neisseria gonorrhoeae Obg protein is an essential ribosome-associated GTPase and a potential drug target. BMC Microbiol. 15, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu W., Thomas C. E., and Sparling P. F. (2004) DNA immunization of mice with a plasmid encoding Neisseria gonorrhoeae PorB protein by intramuscular injection and epidermal particle bombardment. Vaccine 22, 660–669 [DOI] [PubMed] [Google Scholar]
- 45. Shewell L. K., Ku S. C., Schulz B. L., Jen F. E., Mubaiwa T. D., Ketterer M. R., Apicella M. A., and Jennings M. P. (2013) Recombinant truncated AniA of pathogenic Neisseria elicits a non-native immune response and functional blocking antibodies. Biochem. Biophys. Res. Commun. 431, 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pinne M., and Haake D. A. (2009) A comprehensive approach to identification of surface-exposed, outer membrane-spanning proteins of Leptospira interrogans. PloS One 4, e6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Falsetta M. L., Steichen C. T., McEwan A. G., Cho C., Ketterer M., Shao J., Hunt J., Jennings M. P., and Apicella M. A. (2011) The Composition and Metabolic Phenotype of Neisseria gonorrhoeae Biofilms. Front. Microbiol. 2, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cornelissen C. N. (2008) Identification and characterization of gonococcal iron transport systems as potential vaccine antigens. Future Microbiol. 3, 287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Molloy M. P., Herbert B. R., Slade M. B., Rabilloud T., Nouwens A. S., Williams K. L., and Gooley A. A. (2000) Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 267, 2871–2881 [DOI] [PubMed] [Google Scholar]
- 50. Yun S. H., Choi C. W., Kwon S. O., Park G. W., Cho K., Kwon K. H., Kim J. Y., Yoo J. S., Lee J. C., Choi J. S., Kim S., and Kim S. I. (2011) Quantitative proteomic analysis of cell wall and plasma membrane fractions from multidrug-resistant Acinetobacter baumannii. J. Proteome Res. 10, 459–469 [DOI] [PubMed] [Google Scholar]
- 51. Poetsch A., and Wolters D. (2008) Bacterial membrane proteomics. Proteomics 8, 4100–4122 [DOI] [PubMed] [Google Scholar]
- 52. Papanastasiou M., Orfanoudaki G., Koukaki M., Kountourakis N., Sardis M. F., Aivaliotis M., Karamanou S., and Economou A. (2013) The Escherichia coli peripheral inner membrane proteome. Mol. Cell. Proteomics 12, 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwechheimer C., and Kuehn M. J. (2015) Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiology 13, 605–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yates J. R., Ruse C. I., and Nakorchevsky A. (2009) Proteomics by mass spectrometry: approaches, advances, and applications. Annu. Rev. Biomed. Eng. 11, 49–79 [DOI] [PubMed] [Google Scholar]
- 55. Yu N. Y., Wagner J. R., Laird M. R., Melli G., Rey S., Lo R., Dao P., Sahinalp S. C., Ester M., Foster L. J., and Brinkman F. S. (2010) PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26, 1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Imai K., Asakawa N., Tsuji T., Akazawa F., Ino A., Sonoyama M., and Mitaku S. (2008) SOSUI-GramN: high performance prediction for sub-cellular localization of proteins in gram-negative bacteria. Bioinformation 2, 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jackson L. A., Ducey T. F., Day M. W., Zaitshik J. B., Orvis J., and Dyer D. W. (2010) Transcriptional and functional analysis of the Neisseria gonorrhoeae Fur regulon. J. Bacteriol. 192, 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stohl E. A., Criss A. K., and Seifert H. S. (2005) The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol. Microbiol. 58, 520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Isabella V. M., and Clark V. L. (2011) Deep sequencing-based analysis of the anaerobic stimulon in Neisseria gonorrhoeae. BMC Genomics 12, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu H. J., Seib K. L., Srikhanta Y. N., Edwards J., Kidd S. P., Maguire T. L., Hamilton A., Pan K. T., Hsiao H. H., Yao C. W., Grimmond S. M., Apicella M. A., McEwan A. G., Wang A. H., and Jennings M. P. (2010) Manganese regulation of virulence factors and oxidative stress resistance in Neisseria gonorrhoeae. J. Proteomics 73, 899–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ducey T. F., Carson M. B., Orvis J., Stintzi A. P., and Dyer D. W. (2005) Identification of the iron-responsive genes of Neisseria gonorrhoeae by microarray analysis in defined medium. J. Bacteriol. 187, 4865–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turner P. C., Thomas C. E., Stojiljkovic I., Elkins C., Kizel G., Ala'Aldeen D. A. A., and Sparling P. F. (2001) Neisserial TonB-dependent outer-membrane proteins: detection, regulation and distribution of three putative candidates identified from the genome sequences. Microbiology 147, 1277–1290 [DOI] [PubMed] [Google Scholar]
- 63. Stevenson P., Williams P., and Griffiths E. (1992) Common antigenic domains in transferrin-binding protein 2 of Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae type b. Infect. Immun. 60, 2391–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Forng R. Y., Ekechukwu C. R., Subbarao S., Morse S. A., and Genco C. A. (1997) Promoter mapping and transcriptional regulation of the iron-regulated Neisseria gonorrhoeae fbpA gene. J. Bacteriol. 179, 3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beucher M., and Sparling P. F. (1995) Cloning, sequencing, and characterization of the gene encoding FrpB, a major iron-regulated, outer membrane protein of Neisseria gonorrhoeae. J. Bacteriol. 177, 2041–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Biswas G. D., Anderson J. E., and Sparling P. F. (1997) Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB and exbD genes. Mol. Microbiol. 24, 169–179 [DOI] [PubMed] [Google Scholar]
- 67. Price G. A., Masri H. P., Hollander A. M., Russell M. W., and Cornelissen C. N. (2007) Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine 25, 7247–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hoehn G. T., and Clark V. L. (1992) The major anaerobically induced outer membrane protein of Neisseria gonorrhoeae, Pan 1, is a lipoprotein. Infect. Immun. 60, 4704–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Trees D. L., and Spinola S. M. (1990) Localization of and immune response to the lipid-modified azurin of the pathogenic Neisseria. J. Infect. Dis. 161, 336–339 [DOI] [PubMed] [Google Scholar]
- 70. Householder T. C., Fozo E. M., Cardinale J. A., and Clark V. L. (2000) Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect. Immun. 68, 5241–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Turner S. M., Moir J. W., Griffiths L., Overton T. W., Smith H., and Cole J. A. (2005) Mutational and biochemical analysis of cytochrome c', a nitric oxide-binding lipoprotein important for adaptation of Neisseria gonorrhoeae to oxygen-limited growth. Biochem. J. 388, 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hopper A., Tovell N., and Cole J. (2009) A physiologically significant role in nitrite reduction of the CcoP subunit of the cytochrome oxidase cbb3 from Neisseria gonorrhoeae. FEMS Microbiol. Lett. 301, 232–240 [DOI] [PubMed] [Google Scholar]
- 73. Hopper A. C., Li Y., and Cole J. A. (2013) A critical role for the cccA gene product, cytochrome c2, in diverting electrons from aerobic respiration to denitrification in Neisseria gonorrhoeae. J. Bacteriol. 195, 2518–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Knapp J. S., and Clark V. L. (1984) Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46, 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Clark V. L., Knapp J. S., Thompson S., and Klimpel K. W. (1988) Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb. Pathog. 5, 381–390 [DOI] [PubMed] [Google Scholar]
- 76. Hagen T. A., and Cornelissen C. N. (2006) Neisseria gonorrhoeae requires expression of TonB and the putative transporter TdfF to replicate within cervical epithelial cells. Mol. Microbiol. 62, 1144–1157 [DOI] [PubMed] [Google Scholar]
- 77. Stork M., Bos M. P., Jongerius I., de Kok N., Schilders I., Weynants V. E., Poolman J. T., and Tommassen J. (2010) An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 6, e1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Phillips N. J., Steichen C. T., Schilling B., Post D. M., Niles R. K., Bair T. B., Falsetta M. L., Apicella M. A., and Gibson B. W. (2012) Proteomic analysis of Neisseria gonorrhoeae biofilms shows shift to anaerobic respiration and changes in nutrient transport and outermembrane proteins. PloS One 7, e38303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Selkrig J., Mosbahi K., Webb C. T., Belousoff M. J., Perry A. J., Wells T. J., Morris F., Leyton D. L., Totsika M., Phan M. D., Celik N., Kelly M., Oates C., Hartland E. L., Robins-Browne R. M., Ramarathinam S. H., Purcell A. W., Schembri M. A., Strugnell R. A., Henderson I. R., Walker D., and Lithgow T. (2012) Discovery of an archetypal protein transport system in bacterial outer membranes. Nat. Structural Mol. Biol. 19, 506–510, S501 [DOI] [PubMed] [Google Scholar]
- 80. Mercante A. D., Jackson L., Johnson P. J., Stringer V. A., Dyer D. W., and Shafer W. M. (2012) MpeR regulates the mtr efflux locus in Neisseria gonorrhoeae and modulates antimicrobial resistance by an iron-responsive mechanism. Antimicrob. Agents Chemother. 56, 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stork M., Grijpstra J., Bos M. P., Manas Torres C., Devos N., Poolman J. T., Chazin W. J., and Tommassen J. (2013) Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 9, e1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pan W., and Spratt B. G. (1994) Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol. Microbiol. 11, 769–775 [DOI] [PubMed] [Google Scholar]
- 83. Hagman K. E., Pan W., Spratt B. G., Balthazar J. T., Judd R. C., and Shafer W. M. (1995) Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141 (Pt 3), 611–622 [DOI] [PubMed] [Google Scholar]
- 84. Lee E. H., and Shafer W. M. (1999) The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33, 839–845 [DOI] [PubMed] [Google Scholar]
- 85. Hagan C. L., Silhavy T. J., and Kahne D. (2011) beta-Barrel membrane protein assembly by the Bam complex. Annu. Rev. Biochem. 80, 189–210 [DOI] [PubMed] [Google Scholar]
- 86. Bos M. P., Robert V., and Tommassen J. (2007) Functioning of outer membrane protein assembly factor Omp85 requires a single POTRA domain. EMBO Rep. 8, 1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Volokhina E. B., Beckers F., Tommassen J., and Bos M. P. (2009) The beta-barrel outer membrane protein assembly complex of Neisseria meningitidis. J. Bacteriol. 191, 7074–7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Volokhina E. B., Grijpstra J., Beckers F., Lindh E., Robert V., Tommassen J., and Bos M. P. (2013) Species-specificity of the BamA component of the bacterial outer membrane protein-assembly machinery. PloS One 8, e85799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Noinaj N., Kuszak A. J., Gumbart J. C., Lukacik P., Chang H., Easley N. C., Lithgow T., and Buchanan S. K. (2013) Structural insight into the biogenesis of beta-barrel membrane proteins. Nature 501, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Su Y. C., Wan K. L., Mohamed R., and Nathan S. (2010) Immunization with the recombinant Burkholderia pseudomallei outer membrane protein Omp85 induces protective immunity in mice. Vaccine 28, 5005–5011 [DOI] [PubMed] [Google Scholar]
- 91. Mehr I. J., Long C. D., Serkin C. D., and Seifert H. S. (2000) A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154, 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Braun M., and Silhavy T. J. (2002) Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol. Microbiol. 45, 1289–1302 [DOI] [PubMed] [Google Scholar]
- 93. Bos M. P., Tefsen B., Geurtsen J., and Tommassen J. (2004) Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc. Natl. Acad. Sci. U.S.A. 101, 9417–9422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ruiz N., Kahne D., and Silhavy T. J. (2009) Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat. Rev. Microbiology 7, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shen H. H., Leyton D. L., Shiota T., Belousoff M. J., Noinaj N., Lu J., Holt S. A., Tan K., Selkrig J., Webb C. T., Buchanan S. K., Martin L. L., and Lithgow T. (2014) Reconstitution of a nanomachine driving the assembly of proteins into bacterial outer membranes. Nat. Commun. 5, 5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kovacs-Simon A., Titball R. W., and Michell S. L. (2011) Lipoproteins of bacterial pathogens. Infect. Immun. 79, 548–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Barondess J. J., and Beckwith J. (1990) A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature 346, 871–874 [DOI] [PubMed] [Google Scholar]
- 98. Yang X., Wu Z., Wang X., Yang C., Xu H., and Shen Y. (2009) Crystal structure of lipoprotein GNA1946 from Neisseria meningitidis. J. Struct. Biol. 168, 437–443 [DOI] [PubMed] [Google Scholar]
- 99. Chahrour O., Cobice D., and Malone J. (2015) Stable isotope labelling methods in mass spectrometry-based quantitative proteomics. J. Pharm. Biomed. Anal. 113, 2–20 [DOI] [PubMed] [Google Scholar]
- 100. Leuzzi R., Serino L., Scarselli M., Savino S., Fontana M. R., Monaci E., Taddei A., Fischer G., Rappuoli R., and Pizza M. (2005) Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol. Microbiol. 58, 669–681 [DOI] [PubMed] [Google Scholar]
- 101. Elkins C., Carbonetti N. H., Varela V. A., Stirewalt D., Klapper D. G., and Sparling P. F. (1992) Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol. Microbiol. 6, 2617–2628 [DOI] [PubMed] [Google Scholar]
- 102. Baarda B. I., and Sikora A. E. (2015) Proteomics of Neisseria gonorrhoeae: the treasure hunt for countermeasures against an old disease. Front. Microbiol. 6, 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dai K., Xu Y., and Lutkenhaus J. (1996) Topological characterization of the essential Escherichia coli cell division protein FtsN. J. Bacteriol. 178, 1328–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Legrain M., Mazarin V., Irwin S. W., Bouchon B., Quentin-Millet M. J., Jacobs E., and Schryvers A. B. (1993) Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 130, 73–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.