Abstract
Lung cancer is the leading cause of cancer-related death worldwide. Both diagnostic and prognostic biomarkers are urgently needed to increase patient survival. In this study, we identified/quantified 1763 proteins from paired adenocarcinoma (ADC) tissues with different extents of lymph node (LN) involvement using an iTRAQ-based quantitative proteomic analysis. Based on a bioinformatics analysis and literature search, we selected six candidates (ERO1L, PABPC4, RCC1, RPS25, NARS, and TARS) from a set of 133 proteins that presented a 1.5-fold increase in expression in ADC tumors without LN metastasis compared with adjacent normal tissues. These six proteins were further verified using immunohistochemical staining and Western blot analyses. The protein levels of these six candidates were higher in tumor tissues compared with adjacent normal tissues. The ERO1L and NARS levels were positively associated with LN metastasis. Importantly, ERO1L overexpression in patients with early-stage ADC was positively correlated with poor survival, suggesting that ERO1L overexpression in primary sites of early-stage cancer tissues indicates a high risk for cancer micrometastasis. Moreover, we found that knockdown of either ERO1L or NARS reduced the viability and migration ability of ADC cells. Our results collectively provide a potential biomarker data set for ADC diagnosis/prognosis and reveal novel roles of ERO1L and NARS in ADC progression.
Lung cancer is one of the most common human cancers and the leading cause of cancer deaths worldwide (1). Nonsmall cell lung cancer (NSCLC)1, including squamous cell carcinoma, adenocarcinoma (ADC), large-cell carcinoma, and some rare subtypes, is the most common type of lung cancer, representing ∼80% of all cases (2, 3). Lung ADC is the predominant histological type of lung cancer, comprising ∼40% of NSCLC cases (4). Notably, ADC is the major cell type of lung carcinoma in female patients, and its proportion increased (from 61.9% to 77.8%) between 1991 and 1999 in Taiwan. ADC has emerged as a greater problem than other histological types of lung carcinoma (5). The persistently poor survival rate of lung cancer patients is largely attributable to the delayed diagnosis of this type of cancer because 75% of lung cancer patients are found in advanced stages (stages III and IV) at the time of diagnosis (6). Based on the TNM system, lung cancer patients are classified into different stages (stages I (A/B), II (A/B), III (A/B) and IV) through an assessment of primary tumors (T descriptor: tumor size and associated local invasion), regional lymph node (LN) involvement (N descriptor: pN0, no nodes involved; pN1, ipsilateral peribronchial/interlobar/hilar LN metastasis; pN2, ipsilateral mediastinal LN metastasis; pN3, contralateral mediastinal or hilar and supraclavicular LN metastasis) and occurrence of distant metastasis or malignant effusion (M descriptor) (7). The stage of lung cancer is highly correlated to prognosis and mortality; specifically, the degree of cancer spread to the LN is the determining factor for accurate staging and the basis for surgery and adjuvant treatments. However, because of the limited diagnostic approaches, the diagnosis and prognosis of NSCLC patients have improved only minimally in the past decade. The 5-year survival rates of patients with clinical stages I, II, IIIA, IIIB, and IV are 66∼82%, 47∼52%, 36%, 19%, and less than 10%, respectively (7, 8). Notably, ∼60% of patients with clinical stage I have cancer recurrences presumably because of extrathoracic micrometastatic involvement at presentation, which is not currently detectable with the existing diagnostic modalities (9). Thus, it is necessary to identify good biomarkers for the early detection of cancer and metastasis to increase patient survival.
Proteomic technologies have been widely used in global analyses of lung cancer for biomarker discovery (10). Serum is one of the less-invasive types of sample for marker discovery, but serum proteins exhibit a highly dynamic range of relative abundances that might mask the signals of potential low-abundance markers (11, 12). An alternative strategy for biomarker discovery is the use of tissue specimens, which may both reflect the progression of cancers in vivo and serve as prognostic markers. Several groups have successfully used tissue proteomic technologies to identify potential biomarkers in lung cancer (13–25). Recently, Kikuchi et al. performed an in-depth proteomic analysis of 3621 proteins from squamous cell carcinoma and ADC tissues using label-free quantitative proteomic approaches (14). As expected, these researchers identified diagnostic proteins that could be used to discriminate between these two types of NSCLC, and their findings support the powerful use of global proteomic analyses of tissue samples for biomarker discovery.
In this study, we aimed to identify potential markers for the diagnosis of early-stage lung ADC without LN metastasis using isobaric tags for relative and absolute quantification (iTRAQ) labeling combined with 2D-LC-MS/MS. Accordingly, paired lung ADC tissues with different extents of LN involvement (pN0, pN1, pN2 or M) and adjacent normal lung tissues (Nor) were included in the discovery phase. Based on the results from pathway and network analyses, protein expression profiles released in the public Human Protein Atlas database, literature search and novelty, six candidates were selected for validation through immunohistochemistry (IHC) staining and Western blot. Additionally, the clinical and biological significance of two novel candidates, ERO1L and NARS, was further analyzed. Collectively, our results provide a useful biomarker data set for lung ADC diagnosis/prognosis and provide new insights into ERO1L-and NARS-mediated tumorigenesis.
EXPERIMENTAL PROCEDURES
Patient Populations and Clinical Specimens
Lung tissue samples were collected consecutively from 2008 to 2013 at Chang Gung Memorial Hospital, Linkou, Tao-Yuan, Taiwan, with Institutional Review Board approval and written informed consent from each patient. The lung ADC samples with their paired adjacent normal tissues from different stages were selected retrospectively. All of the resected tissues used in the current study were collected using the same protocol by one clinical physician, Professor Yi-Cheng Wu. After surgical resection, the tissues were stored immediately in an ice bucket, transferred to the laboratory and washed twice with phosphate-buffered saline containing a protease inhibitor mixture (Roche, Mannheim, Germany). The tissues were then cut into small pieces (sized ∼0.3 × 0.3 × 0.2 cm3) and stored at −80 °C until use. The entire process was completed within 1 h after tumor resection.
Experimental Design and Statistical Rationale
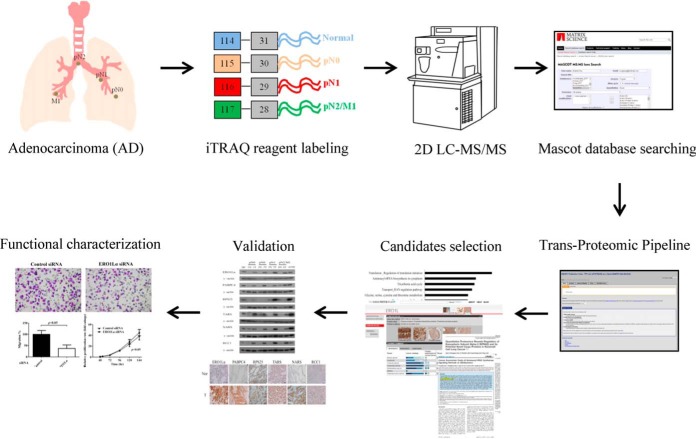
To identify potential diagnostic and prognostic biomarkers from lung cancer tissues, we performed an iTRAQ-based quantitative proteomic analysis of ADC and adjacent normal lung tissues (Fig. 1). Four groups of pooled tissue extracts (normal [Nor], pN0, pN1, and pN2/M1) were included and labeled with 4-plex iTRAQ reagents of varying masses (114–117). For the discovery phase, we used 14 paired tissues, including five stage I tissues with pN0, five stage II tissues with pN1, two stage III tissues with pN2, and two stage IV tissues with M1. Clinical information on these 14 patients and the corresponding iTRAQ labeling results are summarized in Table I. We did not perform any replicates for the mass spectrometry analysis. To examine the differential expression of potential biomarkers in ADC tissues through IHC, an independent cohort with ten lung ADC tissues (three stage I samples with pN0, two stage II samples with pN1, two stage III samples with pN2 and three stage IV samples with pN2 or M1) was included (supplemental Table S1). For further validation of the six marker candidates in tissues by Western blot, 48 paired ADC tissue samples, including 22 pN0, seven pN1, and 19 pN2 or M1 (pN2/M1) samples, were used. There were no significant differences in gender, age or smocking status between any two of these three groups of patients (p > 0.05, Kruskal-Wallis test). The demographic characteristics of these 48 patients are shown in supplemental Table S2. Because of the limited amount of tissue proteins obtained from each individual, only three tissue samples used in the discovery stage (14 patients) were included in the validation stage (48 patients).
Fig. 1.
Experimental workflow used to identify potential lung ADC biomarkers from cancer tissues with different extents of LN involvement. Total proteins extracted from lung tissues with different extents of LN involvement (pN0, pN1, and pN2/M1) and from adjacent normal tissue (Nor) were digested and labeled with 4-plex iTRAQ reagents (114–117) as indicated. The labeled peptides were mixed in equal amounts and analyzed using 2D LC-MS/MS analysis. The Mascot search engine and Trans-Proteomic Pipeline were used to identify and quantify the proteins. Based on a MetacoreTM pathway analysis, the protein expression profiles released in the public Human Protein Atlas database, literature search and novelty, the differentially expressed proteins were selected and validated in clinical specimens through immunohistochemical staining and Western blot analyses. The potential biomarkers were functionally characterized using lung cancer cell lines.
Table I. Clinical characteristics of 14 patients used to establish differential tissue proteomes from ADC tissues using iTRAQ—labeling and mass spectrometry.
| Patient No. | Gender | Age | TNM stage | Tumor stage | Histology | iTRAQ labeling |
|---|---|---|---|---|---|---|
| 1 | Male | 76 | T1N0M0 | IA | Adjacent normal | 114 |
| Adenocarcinoma | 115 | |||||
| 2 | Male | 50 | T1N0M0 | IA | Adjacent normal | 114 |
| Adenocarcinoma | 115 | |||||
| 3 | Female | 46 | T1N0M0 | IA | Adjacent normal | 114 |
| Adenocarcinoma | 115 | |||||
| 4 | Female | 32 | T1N0M0 | IA | Adjacent normal | 114 |
| Adenocarcinoma | 115 | |||||
| 5 | Male | 71 | T2N0M0 | IB | Adjacent normal | 114 |
| Adenocarcinoma | 115 | |||||
| 6 | Female | 67 | T1N1M0 | IIA | Adjacent normal | 114 |
| Adenocarcinoma | 116 | |||||
| 7 | Male | 71 | T2N1M0 | IIB | Adjacent normal | 114 |
| Adenocarcinoma | 116 | |||||
| 8 | Male | 62 | T2N1M0 | IIB | Adjacent normal | 114 |
| Adenocarcinoma | 116 | |||||
| 9 | Male | 53 | T2N1M0 | IIB | Adjacent normal | 114 |
| Adenocarcinoma | 116 | |||||
| 10 | Female | 35 | T2N1M0 | IIB | Adjacent normal | 114 |
| Adenocarcinoma | 116 | |||||
| 11 | Male | 69 | T2N2M0 | IIIA | Adjacent normal | 114 |
| Adenocarcinoma | 117 | |||||
| 12 | Female | 68 | T2N2M0 | IIIA | Adjacent normal | 114 |
| Adenocarcinoma | 117 | |||||
| 13 | Male | 66 | T2N1M1 | IV | Adjacent normal | 114 |
| Adenocarcinoma | 117 | |||||
| 14 | Female | 63 | T4N2M1 | IV | Adjacent normal | 114 |
| Adenocarcinoma | 117 |
Lung Tissue Extraction
The lung tissues were dissolved in lysis buffer (20 mm Tris-HCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 50 mm NaF, 20 mm Na4P2O7, 1 mm Na3VO4, and protease inhibitor mixture) and extracted with Precellys Homogenizer (Bertin Technologies, Saint Quentin en Yvelines Cedex, France). After homogenization, the tissue extracts were centrifuged (13,000 rpm at 4 °C for 20 min), and the supernatants were collected. The protein concentrations were determined using a Bradford protein assay (Bio-Rad Laboratories, Inc., Hercules, CA), and the protein samples were stored at −80 °C until use.
In-Solution Digestion of Protein and iTRAQ Labeling
The lung tissue extracts were reduced with 5 mm tris-(2-carboxyethyl) phosphine at 60 °C and then alkylated with 10 mm methyl methanethiosulfonate (MMTS) for 30 min at room temperature. After the proteins were digested overnight at 37 °C with sequencing-grade modified porcine trypsin (Promega, Madison, WI), the peptides were dried in a SpeedVac and stored at −80 °C until further use. For iTRAQ labeling, the tryptic peptides were reconstituted in iTRAQ reagent buffer, and the four groups of tissue extracts (normal [Nor], pN0, pN1, and pN2/M1) were separately labeled with four different iTRAQ labeling reagents (Nor: 114, pN0: 115, pN1: 116, and pN2/M1: 117), according to the manufacturer's instructions (Applied Biosystems Inc., Foster City, CA).
2D LC-MS/MS
The iTRAQ-labeled peptide mixtures were separated and analyzed using the two-dimensional liquid chromatography-tandem mass spectrometry (2D LC-MS/MS) technique with an offline reversed-phase separation and a reversed-phase 18 (RP18) nanoscale liquid chromatography system coupled with a LTQ-Orbitrap Discovery mass spectrometer (Thermo Fisher, San Jose, CA). Briefly, the peptides were dissolved in buffer A (10 mm NH3·H2O, pH 10) for fractionation through reversed-phase chromatography using an Ettan MDLC system (GE Healthcare, Piscataway, NJ). For peptide fractionation, the iTRAQ-labeled peptides were loaded onto a 4.6-mm × 150-mm Gemini column containing 3-μm particles with a pore size of 110 Å (Phenomenex, Torrance, CA). The peptides were eluted at a flow rate of 350 μl/min with a gradient of 2% buffer B (100% acetonitrile, pH 10) for 5 min, 2–25% buffer B for 35 min, 25–50% buffer B for 20 min, 50–75% buffer B for 10 min, 75–100% buffer B for 2.5 min, and 100% buffer B for 7.5 min. The elution was monitored by the absorbance at 220 nm, and fractions were collected every 1 min. Following this procedure, 70 fractions were separated and collected. The first 15 fractions were pooled into five fractions, respectively (three fractions per pool), and the resultant 60 fractions were vacuum-dried and resuspended in buffer C (0.1% formic acid) for further desalting and concentration using a ZipTip packed in-house with C18 resin (5–20 μm, LiChroprep RP-18, Merck Millipore).
To analyze the iTRAQ-labeled peptide mixtures, each peptide fraction was reconstituted in buffer C, loaded onto a trap column (Zorbax 300SB-C18, 0.3 mm × 5 mm, Agilent Technologies, Wilmington, DE) at a flow rate of 10 μl/min in buffer C, and separated on a resolving 10-cm analytical C18 column (inner diameter, 75 μm) with a 15-μm tip (New Objective, Woburn, MA). The separation was performed using the Thermo Finnigan Surveyor MS Pump Plus system with a linear gradient of 2–30% buffer D (acetonitrile containing 0.1% formic acid) for 63 min, 30–45% buffer D for 5 min, and 45–95% buffer D for 2 min at a flow rate of 0.25 μl/min through the analytical column.
The setup of the LC was equipped with a linear ion trap mass spectrometer LTQ-Orbitrap Discovery (Thermo Fisher, San Jose, CA) and operated with Xcalibur 2.0 software (Thermo Fisher). Intact peptides could be detected at a resolution of 30,000. The lock mass internal calibration was m/z 445.120025 of the (Si(CH3)2O)6H+ ion signal. A data-dependent acquisition protocol in which one MS scan was followed by three MS/MS scans for the three most abundant precursor ions in the MS survey scan was applied. The MS/MS analysis was performed using the pulsed Q collision-induced dissociation (PQD) mode with a normalized collision energy of 27%, and the fragment ions were detected with an LTQ system. The m/z values selected for MS/MS were dynamically excluded for 180 s. An electrospray voltage of 1.8 kV was applied. The MS and MS/MS spectra analyses were acquired using four microscans with a maximum full-time of 1000 ms and 100 ms for the MS and MS/MS analyses, respectively. Automatic gain control prevented the over-filling of the ion trap, and 5 × 104 ions were accumulated in the ion trap to generate the PQD spectra. For MS scans, the m/z scan range was 350 to 2000 Da.
Database Search and Protein Quantification Pipeline
The MS/MS spectra were searched using the MASCOT searching engine (Matrix Science, London, UK; version 2.2.04) against a nonredundant Swiss-Prot database (released in January 2010; Homo sapiens, 20,367 entries). For protein identification, we set thresholds of 10 ppm for intact peptide tolerance masses and 0.5 Da for PQD fragment ions. The analysis allowed for one missed cleavage from the trypsin digest, and iTRAQ (N-terminal, +144 Da), iTRAQ (Lys, +144 Da), oxidized methionine (+16 Da), and MMTS (Cys, +46 Da) were set as potential variable modifications. The results of the MASCOT database search for each reversed-phase elution were further analyzed using the Trans-Proteomic Pipeline (TPP, version 4.0), which included the PeptideProphet, ProteinProphet, and Libra programs (26). Protein and peptide identifications with ProteinProphet probabilities of at least 0.8 and PeptideProphet probabilities of at least 0.5, respectively, were accepted. We also searched the spectra against a decoy database to estimate that the false-discovery rate of our identified peptides was 1.2%.
Proteins were quantified with the Libra program using the default settings (http://tools.proteomecenter.org/wiki/index.php?title=Software:Libra). Briefly, the quantification of a protein was derived from a group of peptides associated with the protein. Each peptide integrated intensity was normalized by the sum of its channel intensities, the normalized channels were averaged over all peptides of a protein, and the standard deviation of the mean was determined for each normalized channel of a peptide. The choice of peptides used for quantification should fit the following two criteria. First, the integrated intensity for a peptide channel lower than 30 counts represents a poor signal-to-noise spectrum, and these peptides were thus removed. Second, the normalized channels of a peptide greater than 2 sigma from the mean were removed. In addition, we performed a global normalization for the quantified peptides to obtain the fold-changes in the protein levels in ADC cancer tissues compared with adjacent normal tissues.
Pathway and Network Analyses
The proteins identified as up-regulated in pN0 cancer tissues compared with adjacent normal tissues (more than a 1.5-fold change) were converted to Swiss-Prot accession numbers, and these numbers along with the iTRAQ ratios were uploaded to MetaCore (GeneGo, St. Joseph, MI) for biological pathway and network analyses, which were performed as previously described (27).
Antibodies
The commercially available primary antibodies used in this study included the following: interferon-induced GTP-binding protein Mx1 (Mx1; EPITOMICS, Burlingame, CA; S2143), Serpin H1 (SERPH; Enzo Life Sciences, Ann Arbor, MI; M16.10A1), beta-actin (Millipore, Temecula, CA; MAB1501), gamma-actin (Santa Cruz Biotechnology, Santa Cruz, CA; sc-65638), ERO1-like protein alpha (ERO1L; Abnova, Taipei, Taiwan; H00030001-M01), polyadenylate-binding-protein 4 (PABPC4; Novus Biologicals, Littleton, CO; NB100–74593), 40S ribosomal protein S25 (RPS25; Proteintech, Rosemont, IL; 23599–1-AP), asparagine-tRNA ligase, cytoplasmic (NARS; Abcam, Cambridge, CA; ab129162), and regulator of chromosome condensation (RCC1; Santa Cruz Biotechnology, sc1161). To generate an anti-human threonine-tRNA ligase (TARS) polyclonal antibody, a New Zealand White rabbit was immunized with a BSA-conjugated synthetic peptide corresponding to residues 101–116 of TARS (TPYQIACGISQGLADN). The anti-TARS antibody was purified with TARS peptide-conjugated Sepharose S4B (GE Healthcare, Björkgatan, Uppsala, Sweden).
Immunohistochemical Staining
Immunohistochemical (IHC) staining was performed using an automatic immunohistochemical staining device following the manufacturer's instructions (BondTM, Vision Biosystems, Mount Waverley, Victoria, Australia), as previously described (28). Briefly, consecutive sections (5 μm thick) of formalin-fixed, paraffin-embedded specimens from ADC patients were stained with various antibodies using the Envision kit (Dako Corp., Carpinteria, CA). IHC staining was performed using specific antibodies against ERO1L (1:500), PABPC4 (1:50), RPS25 (1:100), TARS (1:100), NARS (1:100), and RCC1 (1:50). The intensities and percentages of the stained sections were scored by pathologists. The staining intensity was then graded, with 0 representing a negative stain and 1, 2, and 3 indicating weak, moderate, and strong staining, respectively. The IHC scores were calculated by multiplying the percentages of positively stained cells by their corresponding intensities to obtain the final protein expression score. Four groups of protein expression scores were obtained: negative staining (scores of 0), weak staining (scores of 1–50), moderate staining (scores of 51–150), and strong staining (scores of >150).
Western blot Analysis
The protein concentrations of the extracted lung tissues were determined, and 50 μg of protein from each sample was separated on an SDS-PAGE gel and transferred to a PVDF membrane (Merck Millipore, Darmstadt, Germany). The membranes were then blocked with 5% milk in Tris-buffered saline (TBS) and incubated overnight with the primary antibody in 5% milk in TBS containing 0.1% Tween-20 (TBST). After washing with TBST, the membranes were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody and developed using a chemiluminescent HRP substrate (Merck Millipore, Darmstadt, Germany).
Cell Culture
CL1–0 and CL1–5cells, which were established from a 64-year-old man with poorly differentiated lung adenocarcinoma, were kindly provided by Dr. P.C. Yang (Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan, Republic of China). A transwell invasion chamber was used to progressively select more invasive cancer cell populations from the parental CL1 cells. CL1–5 is a subline with higher metastatic and invasive potential compared with CL1–0 (29). CL1–0 and CL1–5 cells were maintained in RPMI 1640 with 10% FBS plus antibiotics at 37 °C in a humidified atmosphere of 95% air/5% CO2 as described previously (30).
Migration Assay
For the cell migration assay, CL1–0 or CL1–5 cells (5 × 104 cells) transfected with control siRNA, ERO1L or NARS siRNA were suspended in 200 μl of OPTI-MEM medium and seeded into the upper transwell chamber (8.0-μm pore size filter; Corning, Canton, NY). Six hundred microliters of OPTI-MEM containing 10 μl/ml fibronectin (Sigma Aldrich, Shanghai, China), a chemoattractant, was then added to a 24-well plate. After 6 h of incubation at 37 °C, the cells that migrated into the lower chamber membrane were fixed with methanol and subjected to Giemsa staining. The cells in the lower chamber membrane were counted microscopically (six fields per filter).
Cell Viability Assays
The cell viability was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenytetrazolium bromide (MTT) colorimetric growth assay. Cells transfected with control siRNA, ERO1L siRNA, or NARS siRNA were trypsinized, resuspended in complete medium, and seeded into 24-well plates. At the indicated time intervals, MTT solution (5 mg/ml) was added, and the cells were incubated at 37 °C for 1 h. The supernatant was then aspirated, the cells were treated with dimethyl sulfoxide, and the absorbance was measured at 540 nm using a Multiskan FC ELISA reader (Thermo Fisher Scientific, San Jose, CA).
Gene Knockdown of ERO1L and NARS with Small Interfering RNA
For the gene knockdown of ERO1L and NARS, 25 nucleotide RNA duplexes targeting human ERO1L and NARS were synthesized by Invitrogen (Thermo Fisher Scientific Inc., Waltham, MA). CL1–0 cells were transfected with control siRNA and pooled siRNA for ERO1L (GGGCUUUAUCCAAAGUGUUACCAUU, CAGGAACUUGUUACAGAAUAUUCAU, and GGCUUCUGGUCAAGGGACAAGUGAA) or NARS (GAGAGACUGAUGACAGACACCAUUA, GGUGUUGCGAGAUGGUACAGGUUAU, and GGCCAUGAGCUGAGUUGUGACUUCU) using Lipofectamine RNAiMAX reagents (Invitrogen, Grand Island, NY) according to the manufacturer's protocol. To confirm the knockdown efficiency, the expression of ERO1L and NARS at 48 h after transfection was determined by Western blot analysis.
Tissue Transcriptome Data set of Lung ADC
The Oncomine database (https://www.oncomine.org/resource/login.html) was used to search the mRNA expression profiles of ADC tissues and perform the analysis of Kaplan-Meier plots. The Okayama data set from the Oncomine database contains differential gene profiles generated from 226 stage I-II lung ADC samples, and the ERO1L gene expression levels were selected for further analysis of the overall survival of patients in the current study.
Statistical Analysis
All of the data were processed using SPSS 12.0 (SPSS Inc., Chicago, IL), and all of the continuous variables are expressed as the mean ± standard deviation (S.D.). To compare the protein levels between paired normal and tumor tissues, a paired t test was used. To compare the protein levels between tumors with different pN statuses, we used the nonparametric Mann-Whitney U test. For cell viability assay, two-way ANOVA was used. A p value less than 0.05 was considered statistically significant. Survival rates were obtained using the Kaplan-Meier method and were compared using the log-rank test.
RESULTS
Generation of Quantitative Lung Tissue Proteomes from ADC and Adjacent Normal Lung Tissues
Based on the experimental design shown in Fig. 1, a iTRAQ-based quantitative lung tissue proteomic data set was obtained using 2D LC-MS/MS, a database search, and TPP and Libra analyses. Accordingly, we identified 1857 proteins and quantified 1763 proteins from these four groups of tissue samples. The full raw data have been deposited into the ProteomeXchange consortium via the PRIDE partner repository with the data set identifier PXD004077. Among these proteins, 1261 proteins were identified with a minimum of two unique peptides, and 1208 proteins were quantified with a minimum of two quantified spectra. Details of these 1261 identified proteins and 1208 identified/quantified proteins are provided in supplemental Table S3A and supplemental Table S3B, respectively. The iTRAQ ratio values were calculated based on reporter ion intensities of 114 (Nor), 115 (pN0), 116 (pN1) and 117 (pN2/M1) using the Libra program. For example, the fold-change distributions (115/114) of the quantified proteins in the pN0 cancer tissues and adjacent normal tissues are shown in supplemental Fig. S1. We did not include the biological and analytical replicates in the iTRAQ-based quantitative proteomics analysis. However, before selecting the biomarker candidates for further verification in the current study, we analyzed two independent cohorts (14 and 12 individuals were used in the discovery and validation phases, respectively) via a Western blot analysis to validate the reliability of our quantitative proteomic analysis and evaluate the feasibility of using this data set as a source for ADC biomarker discovery. As shown in Fig. 2 and supplemental Fig. S2, we detected the expression levels of three proteins (Mx1, ERO1L, and SERPH) in two independent cohorts via Western blot and observed a consistent expression profile from our MS-based quantitative analysis and the two independent immunodetection analyses. To identify potential markers for the early diagnosis of lung ADC, we used an iTRAQ ratio of 115/114 ≥ 1.5 as the criterion and selected 133 proteins as potential biomarkers of cancer tissues without LN metastasis compared with adjacent normal tissues. Details of these 133 proteins are provided in supplemental Table S4.
Fig. 2.
Validation of the differentially expressed proteins obtained from iTRAQ-based proteomic analysis by Western blot. The lung cancer tissues used in the discovery phase were prepared for Western blot analysis (50 μg/lane) using anti-Mx1, anti-ERO1L and anti-SERPH antibodies as indicated. γ-actin was used as the loading control. The MS spectra of reporter ions (114, 115, 116, and 117), peptide sequences, and quantified protein ratios of Mx1, ERO1L and SERPH are shown. The relative intensities of the quantified Mx1, ERO1L, and SERPH peptides are shown for comparison.
Pathway Analysis of 133 Proteins Up-regulated in Cancer Tissues Without LN Metastasis Compared with Adjacent Normal Tissues
To clarify potential pathways or network processes occurring in cancer tissues without LN metastasis, the 133 proteins that were up-regulated in stage I tissues compared with adjacent normal tissues were further analyzed using the MetaCore bioinformatics tool. The pathway analysis revealed the top three pathways in which these 133 up-regulated proteins were involved: translation_regulation of translation initiation, aminoacyl-tRNA biosynthesis in cytoplasm, and tricarbonic acid cycle (Fig. 3A and supplemental Table S5). The process network analysis also showed that the 133 up-regulated proteins were highly associated with translation_translation initiation, translation_ elongation-termination, and protein folding_ER and cytoplasm (Fig. 3B and supplemental Table S6). This analysis suggests that these 133 up-regulated proteins were highly associated with protein translation and folding. Specifically, 28 proteins were identified as ribosomal proteins that play essential roles in protein synthesis and are involved in cancer tumorigenesis (31–33). In addition, four are aminoacyl-tRNA synthases that are implicated in several noncanonical functions, such as angiogenesis and tumorigenesis (34, 35). Therefore, the combination of proteomic and bioinformatics analyses of differentially expressed proteins in tissues improves our understanding of the potential molecules involved in the early stages of cancer tumorigenesis and provides valuable information for lung cancer biomarker discovery.
Fig. 3.
Functional classification of 133 proteins up-regulated in lung tissues without LN metastasis compared with adjacent normal tissues. The top ten pathway maps (A) and process networks (B) deduced from the 133 up-regulated proteins (115/114 ≥ 1.5) in the lung tissue proteome data set were analyzed using MetaCoreTM software, as described in the Experimental Procedures. The proteins were linked to at least one annotation term within the (A) and (B) categories.
Selection and Validation of Candidates by IHC Staining and Western Blot
To select potential ADC biomarkers for further validation, we set several criteria, including functional classification based on our bioinformatics analysis, the protein expression profiles obtained by IHC and deposited in the public Human Protein Atlas database, literature search, and novelty (supplemental Table S4). First, the integration of the proteins involved in the top ten pathway map and process network analyses allowed us to select 66 candidates from the 133 proteins up-regulated in cancer tissues without LN metastasis. According to a search of the Human Protein Atlas database, 37 proteins were detected in lung tissues via IHC and exhibited high to moderate expression patterns in cancer tissues (protein expression levels [high + medium] > 50%). We then obtained 32 from these 37 candidates based on literature search and their novelty (supplemental Table S4). We also validated the expressions of eight candidates (ERO1L, GARS, PABPC4, NARS, RCC1, RPS9, RPS25, and TARS) in cancer tissues by IHC (n = 3) and/or Western blot and ultimately selected six candidates (ERO1L, PABPC4, NARS, RCC1, RPS25, and TARS) for further validation based on the performance of the IHC and Western blot analyses. Fig. 4 shows a representative staining pattern obtained from the analysis of ten paired lung ADC tissues that showed overexpression of the candidates in tumor sections (T) compared with adjacent normal tissues (Nor). The IHC scores from ten cancer patients were converted into four different intensities according to their staining scores and are represented as different colors, and the T/N ratio, which was determined via MS-based analysis, is shown for comparison. With the exception of RCC1, five of the six candidates were significantly overexpressed in all ten cancer tissues (Fig. 4B). We then confirmed this result by examining these six candidates by Western blot in 48 paired lung ADC tissues with different extents of LN involvement (supplemental Fig. S3). Fig. 5A shows that the expression levels of the six candidates were higher in four tumor sections than in the adjacent normal sections. γ-actin was used as the loading control. Notably, one pooled tissue protein (QC) and one A549 cell lysate served as quality and internal controls for the Western blot analyses. The signals of the six candidates in 48 paired tissue samples (supplemental Fig. S3) were quantified, and the results demonstrated that these six candidates had higher expression levels in cancer tissues than in the Nor tissues, regardless of the LN metastasis (Figs. 5B and 5C and Table II). Interestingly, the expression levels of ERO1L and NARS were significantly increased in the cancer tissues with LN metastasis (pN1 and pN2/M1, pN≥1) compared with the pN0 cancer tissues (Fig. 5C). The clinicopathologic characteristics analysis of ADC patients revealed that the ERO1L and NARS expression levels were significantly associated with cancer and LN metastasis (Table III, p < 0.001). There was no apparent correlation between the candidates' protein levels and patient age, smoking, or cell differentiation, but there was a significant correlation between ERO1L expression and gender. These observations collectively demonstrated the consistency of the results obtained from our quantitative proteomics and immunological detection of potential tumor markers in ADC. In addition, our results suggest that ERO1L and NARS are potential protein markers for lung ADC diagnosis.
Fig. 4.
Detection of six marker candidates in lung tissue by IHC staining. A, Representative IHC staining of six biomarker candidates (ERO1L, PABPC4, RPS25, TARS, NARS, and RCC1) in lung ADC tissues (T) paired with adjacent normal tissues (Nor). The scale bar is 100 μm. B, IHC staining scores for six marker candidates in 10 paired ADC tissue sections. A T>N frequency of 7/10 indicates that the candidate was overexpressed in seven of the ten tumors compared with the adjacent normal tissues. The IHC scores were converted into four different intensities and are represented as different colors. The T/N ratio determined via the MS-based analysis is shown for comparison.
Fig. 5.
Validation of six marker candidates in 48 paired lung ADC tissues by Western blot. A, Proteins prepared from paired lung ADC tumors (T) and the corresponding adjacent normal tissues (Nor) were subjected to Western blot analysis using specific antibodies (ERO1L, PABPC4, RPS25, TARS, NARS and RCC1) as indicated. γ-actin was used as the loading control. One pooled tissue protein (QC) and one A549 cell lysate served as quality and internal controls, respectively, for each Western blot analysis. B, and C, Quantitative analysis of protein expression levels obtained from Western blots of 48 paired tissue samples. Each protein signal detected via Western blot was acquired, quantified and normalized to the corresponding γ-actin signal. The protein level detected in the QC sample was also used for the normalization of the protein expression levels in each gel. B, All six marker candidates were overexpressed in lung ADC tissues (T) compared with the adjacent normal tissues (Nor). The horizontal line represents the mean value. *, a p value < 0.05 obtained from paired t test indicates statistical significance. C, The levels of ERO1L and NARS were significantly increased in tissue samples with LN metastasis (pN ≥ 1) compared with tissue samples without LN metastasis (pN0). *, a p value < 0.05 obtained from paired t test (pN0 versus Nor) or Mann-Whitney U test (pN0 versus pN ≥ 1) indicates statistical significance.
Table II. Expression levels of six marker candidates in 48 ADC tissue samples.
| Fold change (mean ± S.D.) |
||||||
|---|---|---|---|---|---|---|
| T/Nor (n = 48) | p valuea | pN0/Nor (n = 22) | p valuea | pN ≥ 1/pN0 (n = 26 vs. 22) | p valueb | |
| ERO1L | 3.43 ± 2.62 | <0.0001 | 2.41 ± 1.71 | 0.025 | 1.54 ± 2.68 | 0.029 |
| PABPC4 | 3.35 ± 5.18 | 0.0004 | 3.95 ± 5.67 | 0.025 | 1.97 ± 12.86 | 0.605 |
| RPS25 | 3.03 ± 3.57 | <0.0001 | 2.87 ± 3.64 | 0.009 | 1.21 ± 3.85 | 0.192 |
| TARS | 3.57 ± 2.84 | <0.0001 | 2.82 ± 2.05 | 0.0005 | 1.24 ± 2.85 | 0.605 |
| NARS | 2.72 ± 2.15 | <0.0001 | 2.64 ± 1.87 | 0.0007 | 1.76 ± 3.35 | 0.021 |
| RCC1 | 2.72 ± 2.79 | 0.0002 | 2.33 ± 2.69 | 0.028 | 0.90 ± 2.31 | 0.780 |
a The p values were determined by paired t-test.
b The p values were determined by the Mann-Whitney U test.
Table III. Relationships between the protein levels of two potential biomarkers and clinical characteristics of 48 ADC patients.
| Characteristics | Number | ERO1L |
NARS |
||
|---|---|---|---|---|---|
| Protein levela (mean ± S.D.) | p valueb | Protein levela (mean ± S.D.) | p valueb | ||
| Gender | 0.001 | 0.701 | |||
| Male | 27 | 2.11 ± 1.44 | 1.69 ± 1.41 | ||
| Female | 21 | 1.04 ± 0.63 | 1.64 ± 1.26 | ||
| Age | 0.343 | 0.217 | |||
| <60 | 19 | 1.67 ± 0.89 | 1.95 ± 1.45 | ||
| ≥60 | 29 | 1.63 ± 1.48 | 1.48 ± 1.24 | ||
| Smoker | 0.388 | 0.47 | |||
| No | 32 | 1.59 ± 1.36 | 1.56 ± 1.15 | ||
| Yes | 16 | 1.75 ± 1.11 | 1.90 ± 1.66 | ||
| Cell type | |||||
| Adjacent normal | 48 | 0.48 ± 0.77 | <0.001 | 0.61 ± 0.63 | <0.001 |
| Tumor | 48 | 1.64 ± 1.27 | 1.67 ± 1.33 | ||
| Cell differentiation | 0.893 | 0.392 | |||
| Well | 25 | 1.54 ± 0.82 | 1.82 ± 1.36 | ||
| Poor/Moderate | 23 | 1.75 ± 1.64 | 1.50 ± 1.32 | ||
| LN metastasis | 0.028 | 0.020 | |||
| No (pN0) | 22 | 1.27 ± 0.93 | 1.18 ± 0.86 | ||
| Yes (pN ≥ 1) | 26 | 1.96 ± 1.44 | 2.08 ± 1.53 | ||
a Protein expression level relative to level of γ-actin.
b A p value less than 0.05 obtained from the Mann-Whitney U test indicates statistical significance.
Correlation of Patient Survival with ERO1L or NARS Overexpression in Lung ADC
The protein expression levels obtained from the Western blot analysis were used to evaluate whether ERO1L or NARS overexpression was correlated with the survival of 34 patients with lung ADC (stage I, II, or IIIA) undergoing complete standardized treatment with regular follow-up. The long-term disease-free survival rates for the patient subgroups stratified by low and high expression of ERO1L were 61.8% and 44.4%, respectively. This difference in disease-free survival was statistically significant according to the log-rank test (p = 0.0058, Fig. 6A). Although the high expression levels of ERO1L or NARS proteins in these 34 ADC tissue were positively associated with total stage and LN metastasis (supplemental Table S7), our disease-free survival analysis did not demonstrate a significant difference when the patients were stratified by the tissue levels of NARS protein (p = 0.41, Fig. 6A). Based on the univariate analysis with the Cox proportional regression model, we found that both ERO1L overexpression and LN metastasis, but not other clinical characteristics (gender, age, and smoker), were significant predictors of poor disease-free survival (supplemental Table S8). The multivariate analysis further revealed that patients with ERO1L overexpression had significantly lower disease-free survival (hazard ratio: 6.925, p = 0.017; supplemental Table S8), confirming that ERO1L overexpression serves as an independent prognostic factor for disease-free survival. Because 32 of 34 ADC patients were alive at the completion of this study, we did not analyze the correlations between protein levels and the overall survival of these ADC patients. To further explore the clinical application of ERO1L overexpression, we examined the expression of ERO1L in 60 early-stage lung ADC (stages I and II) tissue sections by IHC. The IHC scores were then used to evaluate the correlation between ERO1L overexpression and the overall survival of these 60 patients. As shown in Fig. 6B, the overall survival rates of the patient subgroups stratified by low and high expression of ERO1L were 72% and 47.5%, respectively. This difference in overall survival was statistically significant according to the log-rank test (p = 0.026, Fig. 6B). The univariate analysis with a Cox proportional regression model also showed that patients with ERO1L overexpression had significantly lower overall survival (hazard ratio: 2.946, p = 0.034; supplemental Table S9). Furthermore, to investigate whether the ERO1L gene level is also associated with patient survival, we analyzed the ERO1L mRNA expression level using the Okayama Lung data set (36), which is based on 226 lung ADC samples (stages I and II). Consistently, we found that ERO1L gene overexpression was significantly correlated with poor overall survival of ADC patients (p = 0.0011 for ERO1L, log-rank test, Fig. 6C). These observations support the hypothesis that ERO1L overexpression in primary sites of early-stage cancer tissues indicates a high risk for cancer micrometastasis, which is correlated to patients' poor outcomes and forms the basis for surgery and adjuvant treatments. These results collectively suggest a potential clinical application of the ERO1L gene and protein expression levels in lung ADC diagnosis and prognosis.
Fig. 6.

Association between ERO1L or NARS expression and patient survival. A, The protein levels obtained from the Western blot analysis were used to analyze the disease-free survival of 34 patients with lung ADC (stage I/II/IIIA). The high and low expression of marker candidates were stratified according to the mean values of the relative protein levels (cutoff for ERO1L: 1.65; NARS: 1.66) obtained from 34 ADC patients. The Kaplan-Meier plot shows that high ERO1L expression was significantly associated with poor disease-free survival of lung ADC patients. B, The protein levels of ERO1L obtained from IHC were used to analyze the overall survival of 60 patients with early-stage (stages I and II) lung ADC. The high and low expression of marker candidates were stratified according to the mean value of the IHC scores (cutoff: 186) obtained from 60 ADC patients. C, Association between high ERO1L gene expression and poor patient survival. The mRNA levels obtained from the Okayama Lung data set were used to analyze the overall survival of 226 patients with early-stage (stages I and II) lung ADC. The high and low expression of marker candidates were stratified according to the mean value of the mRNA level (cutoff: 1009) obtained from 226 ADC patients. The Kaplan-Meier plot shows that ERO1L overexpression was significantly associated with poor overall survival of lung ADC patients. A p value < 0.05 obtained from the log rank test indicates statistical significance.
Both ERO1L and NARS are Involved in the Migration Ability and Viability of Lung Cancer Cells
Based on our immunodetection findings, we hypothesized that both ERO1L and NARS play important roles in lung cancer progression. To test this possibility, we applied the siRNA approach to knock down ERO1L or NARS expression in lung ADC cells and then performed survival and transwell migration assays. A Western blot analysis showed that the protein levels of ERO1L and NARS were significantly reduced in CL1–0 and CL1–5 cells transfected with ERO1L or NARS siRNA (Figs. 7A and 7B). The transwell migration assay (6 h of incubation) revealed that the migration abilities in ERO1L- and NARS- knockdown CL1–0 cells were reduced to 37 and 9% of the control values, respectively. Cell viability was slightly suppressed after ERO1L knockdown and was reduced after NARS knockdown in CL1–0 cells (Fig. 7A). Similar results were observed in ERO1L- or NARS- knockdown CL1–5 cells: the migration abilities of ERO1L- and NARS- knockdown cells were reduced to 58 and 29% of the control values, respectively (Fig. 7B). The cell viability was reduced in the ERO1L- knockdown CL1–5 cells, but 48 h after the knockdown of NARS in CL1–5 cells, severe cell death was observed, and the MTT assay could therefore not be accurately used for this cell line. These results suggest that both ERO1L and NARS play roles in the positive regulation of the cell migration ability and viability of lung cancer cells.
Fig. 7.
ERO1L and NARS are involved in the migration ability of NSCLC cells. CL1–0 (A) and CL1–5 (B) cells were transfected with control siRNA, ERO1L siRNA or NARS siRNA as indicated. After transfection for 48 h, the cells were harvested for an MTT viability assay, and protein expression was detected by Western blot analysis. Simultaneously, photographs of migrating cells were acquired during the migration assay, and the cell numbers were calculated. Because NARS knockdown caused severe CL1–5 cell death after transfection for 48 h, the MTT assay could not be performed, and the migration assay was conducted 24 h after transfection with NARS siRNA. The data are presented as the mean ± S.D. from three independent migration assays. A p value < 0.05 obtained from the Mann-Whitney U test (migration assay) or two-way ANOVA (cell viability assay) indicates statistical significance.
DISCUSSION
This study used, for the first time, iTRAQ labeling technology combined with 2D LC-MS/MS to establish a comparative tissue proteome data set from frozen paired lung tumors with different extents of LN involvement and adjacent normal tissues. Pathway and network analyses of the identified differentially expressed proteins elucidated potential oncogenic mechanisms that occur in the early stages of lung cancer progression. Six candidate markers with functions in the synthesis and quality control of proteins, such as numerous ribosomal proteins, aminoacyl-tRNA synthases, and molecules involved in protein folding and transport RAN regulation, attracted our attention because of their high ranks in the lists obtained from the bioinformatics analysis and their novelty. We confirmed the dysregulation of these six candidates in cancer tissues and examined the clinical and biological significance of two marker candidates, ERO1L and NARS, in lung ADC. The current study not only provides a useful database for lung ADC biomarker discovery but also provides new insights into ERO1L- or NARS-mediated cancer progression.
Aminoacyl-tRNA synthetases (ARSs) are essential enzymes responsible for catalyzing the ligation of amino acids to their cognate tRNAs (37). In addition to serving this canonical function, higher eukaryotic ARSs have been implicated in a variety of noncanonical functions, including inflammation (38), angiogenesis (39), translation regulation (40), gene-specific translational silencing (41), apoptosis and angiogenesis (40–45). Specifically, recent studies reported that ARSs are implicated in tumorigenesis through the interactions between different regulators and new domains of the ARSs (46). NARS, a class II ARS, was identified as an up-regulated protein in early-stage tumor tissues in this study. Our findings demonstrate that NARS is involved in cell survival, which is consistent with previous studies suggesting that NARS may be correlated with the survival rate of glioblastoma and is an important mediator of FGF2-induced survival signaling in osteoblasts (47, 48). Moreover, the current study demonstrated a novel role of NARS in promoting the migration ability of lung cancer cells.
ERO1L is a human protein distributed in the endoplasmic reticulum (ER) and involved in disulfide bond formation, which is necessary for protein folding and function (49, 50). Many chaperones and enzymes participate in the process of protein folding, and protein disulfide isomerase is a key enzyme that promotes protein disulfide formation, isomerization or reduction (51). ERO1L has been identified as a prognostic gene through gene expression profiling of pulmonary adenocarcinoma (52) and has been implicated to be involved in cancer through an HIF-1-mediated pathway to improve disulfide bond formation and vascular endothelial growth factor secretion (53). In the current study, we show that ERO1L was overexpressed in lung ADC and that ERO1L overexpression was associated with poor disease-free and overall survival among lung ADC patients. Future research is warranted to develop less-invasive methods, such as an enzyme-linked immunosorbent assay and mass spectrometric multiple-reaction monitoring-based strategy, for determining the ERO1L level in bodily fluids (e.g. plasma or serum). We also demonstrated that ERO1L plays a novel role in regulating the migration ability and viability of lung cancer cells. Our results collectively suggest that ERO1L is a potential diagnostic and prognostic marker for lung ADC, although a larger clinical sample size is necessary to confirm this assumption in the near future.
Many researchers have applied quantitative proteomic approaches or combined proteomic and genomic analyses for the systematic discovery of potential lung cancer biomarkers (13, 14, 22–25). We compared the 133 potential lung cancer biomarkers identified in the current study with six lung cancer biomarker-related proteome data sets. The overlapping proteins identified in other publications and our data are summarized in supplemental Fig. S4 and supplemental Table S10. Recently, Li et al. identified 1240 proteins that were up-regulated in NSCLC primary tumors compared with matched normal lung tissues (22), and 77 proteins were found in our list of candidate markers. Kikuchi et al. established a comprehensive tissue proteome data set comprising 3621 proteins from an analysis of pooled human samples of squamous cell carcinoma, ADC, and control specimens using a label-free proteomic approach (14). Among the 143 up-regulated proteins in the ADC compared with control tissues, 11 proteins (AGR2, THBS2, TXNDC17, CRABP2, TARS, GFPT1, PRPF8, MUC5B, MYO6, EIF5A, and PDIA4) were identified in our list of candidate markers. Moreover, Kawamura et al. used a shotgun proteomic approach to establish a cancer stage-related proteome comprising more than 500 proteins from formalin-fixed, paraffin-embedded, stage IA and IIIA lung ADC tissue sections (13). Among 81 stage IA- or IIIA-related proteins, four proteins (SFN, AGR2, RPS9, and HSPA5) were also found in our identified list of marker candidates. The partial overlap of potential biomarkers between our study and previous reports supports the feasibility of using iTRAQ technology combined with 2D LC-MS/MS to discover protein markers from lung ADC samples with different extents of LN involvement. However, the limitation of the current study is that we performed the iTRAQ-based proteomic analysis using an aging mass spectrometer, LTQ-Orbitrap Discovery. This machine does not offer higher-energy collisional dissociation (HCD) fractionation of peptides. Instead of HCD, we analyzed the peptide fragments using the PQD mode, which is not preferred for peptide fractionation and is not sufficiently powerful because it is hampered by the high coefficient of variations of reporter ions in the discovery experiment (54, 55).
We also compared our 133 candidates with potential biomarkers secreted by lung cancer cells. Birse et al. identified 179 candidate lung cancer biomarkers across three discovery platforms (lung cancer cell lines, conditioned medium, and fresh resected tissue) (23), and one protein (THY1) was identified in our list of candidates. Clark et al. found 62 proteins that were commonly increased in abundance in NSCLC exosomes compared with HBE4 exosomes (24), and one protein (MUC5B) was found in our tissue proteome data set. This decreased overlap between our list of candidates and a secretome or exosome data set may be because of the missed detection of these secreted proteins in our tissue samples. Additionally, Stewart et al. identified 137 differentially expressed proteins between squamous cell carcinoma (SCC) and ADC tumor samples (25). Only one protein (FKBP10) was identified in our biomarker candidate list, likely because of the histological specificity of biomarkers in NSCLC, such as ADC and SCC.
In conclusion, our study identified and validated six potential biomarkers for lung ADC. We provide evidence that ERO1L and NARS have the potential to promote tumor metastasis and growth. Furthermore, our results show that ERO1L is significantly associated with the survival of ADC patients. Further research should focus on the detailed molecular mechanisms underlying ERO1L- and/or NARS- mediated oncogenesis. For clinical practice, the development of a method to detect the serum or plasma levels of these marker candidates in cancer patients is worthwhile. The specificity of these markers for use in the diagnosis and prognosis of lung ADC also needs to be clarified by further clinical validations using multiple cancer types. Collectively, our study provides a potential useful biomarker data set for lung ADC and reveals novel roles of ERO1L and NARS in lung cancer progression.
Supplementary Material
Footnotes
Author contributions: C.J.Y. designed research; C.H.H., C.W.H., and C.C.L. performed research; C.L.W., Y.C.W., C.C.W., J.S.Y., and Y.S.C. contributed new reagents or analytic tools; C.H.H., C.H.W., C.H., and C.L.W. analyzed data; C.H.H. and C.J.Y. wrote the paper.
* This work was supported by grants from the Ministry of Science and Technology, Taiwan, R.O.C. (https://www.most.gov.tw/; 101-2320-B-182-035-MY3 and 104-2320-B-182-027), the Chang Gung Medical Research Fund (CMRPD3E0081-2, CMRPD1C0092, CLRPD190015 and CLRPD190016) and the Ministry of Education, Taiwan, R.O.C. (EMRPD1E1401).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- NSCLC
- nonsmall cell lung cancer
- ADC
- adenocarcinoma
- LN
- lymph node
- iTRAQ
- isobaric tags for relative and absolute quantification
- 2D LC-MS/MS
- two-dimensional liquid chromatography-tandem mass spectrometry
- ACN
- acetonitrile
- ERO1L
- ERO1-like protein alpha
- NARS
- asparaginyl-tRNA synthetase, cytoplasmic
- SD
- standard deviation.
REFERENCES
- 1. Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., and Parkin D. M. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., and Thun M. J. (2008) Cancer statistics, 2008. CA Cancer J. Clin. 58, 71–96 [DOI] [PubMed] [Google Scholar]
- 3. Charloux A., Quoix E., Wolkove N., Small D., Pauli G., and Kreisman H. (1997) The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int. J. Epidemiol. 26, 14–23 [DOI] [PubMed] [Google Scholar]
- 4. Hoffman P. C., Mauer A. M., and Vokes E. E. (2000) Lung cancer. Lancet 355, 479–485 [DOI] [PubMed] [Google Scholar]
- 5. Chen K. Y., Chang C. H., Yu C. J., Kuo S. H., and Yang P. C. (2005) Distribution according to histologic type and outcome by gender and age group in Taiwanese patients with lung carcinoma. Cancer 103, 2566–2574 [DOI] [PubMed] [Google Scholar]
- 6. Molina J. R., Yang P., Cassivi S. D., Schild S. E., and Adjei A. A. (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 83, 584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstraw P., Crowley J., Chansky K., Giroux D., Groome P., Rami-Porta R., Postmus P., Rusch V., and Sobin L. (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Throac. Oncol. 2, 706. [DOI] [PubMed] [Google Scholar]
- 8. Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W. E., Nicholson A. G., Groome P., Mitchell A., Bolejack V., International Association for the Study of Lung Cancer, S., Prognostic Factors Committee, A. B., Participating, I., International Association for the Study of Lung Cancer, S., Prognostic Factors Committee Advisory, B., and Participating, I. (2016) The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 11, 39–51 [DOI] [PubMed] [Google Scholar]
- 9. Silvestri G. A., Gould M. K., Margolis M. L., Tanoue L. T., McCrory D., Toloza E., Detterbeck F., and American College of Chest Physicians (2007) Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 132, 178S–201S [DOI] [PubMed] [Google Scholar]
- 10. Kisluk J., Ciborowski M., Niemira M., Kretowski A., and Niklinski J. (2014) Proteomics biomarkers for non-small cell lung cancer. J. Pharm. Biomed. Anal. 101, 40–49 [DOI] [PubMed] [Google Scholar]
- 11. Liu T., Qian W. J., Mottaz H. M., Gritsenko M. A., Norbeck A. D., Moore R. J., Purvine S. O., Camp D. G. 2nd, and Smith R. D. (2006) Evaluation of multiprotein immunoaffinity subtraction for plasma proteomics and candidate biomarker discovery using mass spectrometry. Mol. Cell. Proteomics 5, 2167–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Issaq H. J., Xiao Z., and Veenstra T. D. (2007) Serum and plasma proteomics. Chem. Rev. 107, 3601–3620 [DOI] [PubMed] [Google Scholar]
- 13. Kawamura T., Nomura M., Tojo H., Fujii K., Hamasaki H., Mikami S., Bando Y., Kato H., and Nishimura T. (2010) Proteomic analysis of laser-microdissected paraffin-embedded tissues: (1) Stage-related protein candidates upon non-metastatic lung adenocarcinoma. J. Proteomics 73, 1089–1099 [DOI] [PubMed] [Google Scholar]
- 14. Kikuchi T., Hassanein M., Amann J. M., Liu Q., Slebos R. J., Rahman S. M., Kaufman J. M., Zhang X., Hoeksema M. D., Harris B. K., Li M., Shyr Y., Gonzalez A. L., Zimmerman L. J., Liebler D. C., Massion P. P., and Carbone D. P. (2012) In-depth proteomic analysis of nonsmall cell lung cancer to discover molecular targets and candidate biomarkers. Mol. Cell. Proteomics 11, 916–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng G. Q., Zhang P. F., Deng X., Yu F. L., Li C., Xu Y., Yi H., Li M. Y., Hu R., Zuo J. H., Li X. H., Wan X. X., Qu J. Q., He Q. Y., Li J. H., Ye X., Chen Y., Li J. Y., and Xiao Z. Q. (2012) Identification of candidate biomarkers for early detection of human lung squamous cell cancer by quantitative proteomics. Mol. Cell. Proteomics 11, M111.013946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang P. F., Zeng G. Q., Hu R., Li C., Yi H., Li M. Y., Li X. H., Qu J. Q., Wan X. X., He Q., Li J. H., Chen Y., Ye X., Li J. Y., Wang Y. Y., Feng X.P., and Xiao Z. Q. (2012) Identification of flotillin-1 as a novel biomarker for lymph node metastasis and prognosis of lung adenocarcinoma by quantitative plasma membrane proteome analysis. J. Proteomics 77, 202–214 [DOI] [PubMed] [Google Scholar]
- 17. Tan F., Jiang Y., Sun N., Chen Z., Lv Y., Shao K., Li N., Qiu B., Gao Y., Li B., Tan X., Zhou F., Wang Z., Ding D., Wang J., Sun J., Hang J., Shi S., Feng X., He F., and He J. (2012) Identification of isocitrate dehydrogenase 1 as a potential diagnostic and prognostic biomarker for non-small cell lung cancer by proteomic analysis. Mol. Cell. Proteomics 11, M111.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang P. F., Zeng G.Q., Yi L. Z., Liu J. P., Wan X.X., Qu J. Q., Li J. H., Li C. C., Tang C.E., Hu R., Ye X., Chen Y., Chen Z. C., and Xiao Z. Q. (2013) Identification of integrin β1 as a prognostic biomarker for human lung adenocarcinoma using 2D-LC-MS/MS combined with iTRAQ technology. Oncol. Rep. 30, 341–349 [DOI] [PubMed] [Google Scholar]
- 19. Li Y., Wang X., Ao M., Gabrielson E., Askin F., Zhang H., and Li Q. K. (2013) Aberrant Mucin5B expression in lung adenocarcinomas detected by iTRAQ labeling quantitative proteomics and immunohistochemistry. Clin. Proteomics 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pernemalm M., De Petris L., Branca R. M., Forshed J., Kanter L., Soria J. C., Girard P., Validire P., Pawitan Y., van den Oord J., Lazar V., Pahlman S., Lewensohn R., and Lehtio J. (2013) Quantitative proteomics profiling of primary lung adenocarcinoma tumors reveals functional perturbations in tumor metabolism. J. Proteome Res. 12, 3934–3943 [DOI] [PubMed] [Google Scholar]
- 21. Okayama A., Miyagi Y., Oshita F., Nishi M., Nakamura Y., Nagashima Y., Akimoto K., Ryo A., and Hirano H. (2014) Proteomic analysis of proteins related to prognosis of lung adenocarcinoma. J. Proteome Res. 13, 4686–4694 [DOI] [PubMed] [Google Scholar]
- 22. Li L., Wei Y., To C., Zhu C. Q., Tong J., Pham N. A., Taylor P., Ignatchenko V., Ignatchenko A., Zhang W., Wang D., Yanagawa N., Li M., Pintilie M., Liu G., Muthuswamy L., Shepherd F. A., Tsao M. S., Kislinger T., and Moran M. F. (2014) Integrated omic analysis of lung cancer reveals metabolism proteome signatures with prognostic impact. Nat. Commun. 5, 5469. [DOI] [PubMed] [Google Scholar]
- 23. Birse C. E., Lagier R. J., FitzHugh W., Pass H. I., Rom W. N., Edell E. S., Bungum A. O., Maldonado F., Jett J. R., Mesri M., Sult E., Joseloff E., Li A., Heidbrink J., Dhariwal G., Danis C., Tomic J. L., Bruce R. J., Moore P. A., He T., Lewis M. E., and Ruben S. M. (2015) Blood-based lung cancer biomarkers identified through proteomic discovery in cancer tissues, cell lines and conditioned medium. Clin. Proteomics 12, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark D. J., Fondrie W. E., Yang A., and Mao L. (2016) Triple SILAC quantitative proteomic analysis reveals differential abundance of cell signaling proteins between normal and lung cancer-derived exosomes. J. Proteomics 133, 161–169 [DOI] [PubMed] [Google Scholar]
- 25. Stewart P. A., Parapatics K., Welsh E. A., Muller A. C., Cao H., Fang B., Koomen J. M., Eschrich S. A., Bennett K. L., and Haura E. B. (2015) A pilot proteogenomic study with data integration identifies MCT1 and GLUT1 as prognostic markers in lung adenocarcinoma. PLoS ONE 10, e0142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keller A., Eng J., Zhang N., Li X. J., and Aebersold R. (2005) A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol. Syst. Biol. 1, 2005.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang C. I., Chien K. Y., Wang C. L., Liu H. P., Cheng C. C., Chang Y. S., Yu J. S., and Yu C. J. (2012) Quantitative proteomics reveals regulation of karyopherin subunit alpha-2 (KPNA2) and its potential novel cargo proteins in nonsmall cell lung cancer. Mol. Cell. Proteomics 11, 1105–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang C. I., Wang C. L., Wang C. W., Chen C. D., Wu C. C., Liang Y., Tsai Y. H., Chang Y. S., Yu J. S., and Yu C. J. (2011) Importin subunit alpha-2 is identified as a potential biomarker for non-small cell lung cancer by integration of the cancer cell secretome and tissue transcriptome. Int. J. Cancer 128, 2364–2372 [DOI] [PubMed] [Google Scholar]
- 29. Chu Y. W., Yang P. C., Yang S. C., Shyu Y. C., Hendrix M. J., Wu R., and Wu C. W. (1997) Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am. J. Respir. Cell Mol. Biol. 17, 353–360 [DOI] [PubMed] [Google Scholar]
- 30. Wang C. I., Wang C. L., Wu Y. C., Feng H. P., Liu P. J., Chang Y. S., Yu J. S., and Yu C. J. (2015) Quantitative proteomics reveals a novel role of karyopherin alpha 2 in cell migration through the regulation of vimentin-pErk protein complex levels in lung cancer. J. Proteome Res. 14, 1739–1751 [DOI] [PubMed] [Google Scholar]
- 31. Zhou X., Liao W. J., Liao J. M., Liao P., and Lu H. (2015) Ribosomal proteins: functions beyond the ribosome. J. Mol. Cell. Biol. 7, 92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takada H., and Kurisaki A. (2015) Emerging roles of nucleolar and ribosomal proteins in cancer, development, and aging. Cell. Mol. Life Sci. 72, 4015–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang W., Nag S., Zhang X., Wang M. H., Wang H., Zhou J., and Zhang R. (2015) Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med. Res. Rev. 35, 225–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim D., Kwon N. H., and Kim S. (2014) Association of aminoacyl-tRNA synthetases with cancer. Top. Curr. Chem. 344, 207–245 [DOI] [PubMed] [Google Scholar]
- 35. Guo M., and Schimmel P. (2013) Essential nontranslational functions of tRNA synthetases. Nat. Chem. Biol. 9, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okayama H., Kohno T., Ishii Y., Shimada Y., Shiraishi K., Iwakawa R., Furuta K., Tsuta K., Shibata T., Yamamoto S., Watanabe S., Sakamoto H., Kumamoto K., Takenoshita S., Gotoh N., Mizuno H., Sarai A., Kawano S., Yamaguchi R., Miyano S., and Yokota J. (2012) Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 72, 100–111 [DOI] [PubMed] [Google Scholar]
- 37. Park S. G., Ewalt K. L., and Kim S. (2005) Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem. Sci. 30, 569–574 [DOI] [PubMed] [Google Scholar]
- 38. Park S., Kim H., Min Y., Choi E., Shin Y., Park B., Lee S., and Kim S. (2005) Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response. Proc. Natl. Acad. Sci. U.S.A. 102, 6356–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ray P. S., and Fox P. L. (2007) A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 26, 3360–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wakasugi K., and Schimmel P. (1999) Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–151 [DOI] [PubMed] [Google Scholar]
- 41. Sampath P., Mazumder B., Seshadri V., Gerber C. A., Chavatte L., Kinter M., Ting S. M., Dignam J. D., Kim S., Driscoll D. M., and Fox P. L. (2004) Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell 119, 195–208 [DOI] [PubMed] [Google Scholar]
- 42. Halwani R., Cen S., Javanbakht H., Saadatmand J., Kim S., Shiba K., and Kleiman L. (2004) Cellular distribution of Lysyl-tRNA synthetase and its interaction with Gag during human immunodeficiency virus type 1 assembly. J. Virol. 78, 7553–7564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martinis S. A., Plateau P., Cavarelli J., and Florentz C. (1999) Aminoacyl-tRNA synthetases: a new image for a classical family. Biochimie 81, 683–700 [DOI] [PubMed] [Google Scholar]
- 44. Ko Y. G., Kim E. Y., Kim T., Park H., Park H. S., Choi E. J., and Kim S. (2001) Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J. Biol. Chem. 276, 6030–6036 [DOI] [PubMed] [Google Scholar]
- 45. Javanbakht H., Halwani R., Cen S., Saadatmand J., Musier-Forsyth K., Gottlinger H., and Kleiman L. (2003) The interaction between HIV-1 Gag and human lysyl-tRNA synthetase during viral assembly. J. Biol. Chem. 278, 27644–27651 [DOI] [PubMed] [Google Scholar]
- 46. Guo M., and Yang X. L. (2014) Architecture and metamorphosis. Top. Curr. Chem. 344, 89–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim Y. W., Kwon C., Liu J. L., Kim S. H., and Kim S. (2012) Cancer association study of aminoacyl-tRNA synthetase signaling network in glioblastoma. Plos one 7, e40960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park S. J., Kim S. H., Choi H. S., Rhee Y., and Lim S. K. (2009) Fibroblast growth factor 2-induced cytoplasmic asparaginyl-tRNA synthetase promotes survival of osteoblasts by regulating anti-apoptotic PI3K/Akt signaling. Bone 45, 994–1003 [DOI] [PubMed] [Google Scholar]
- 49. Cabibbo A., Pagani M., Fabbri M., Rocchi M., Farmery M. R., Bulleid N. J., and Sitia R. (2000) ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 275, 4827–4833 [DOI] [PubMed] [Google Scholar]
- 50. Braakman I., Helenius J., and Helenius A. (1992) Role of ATP and disulphide bonds during protein folding in the endoplasmic reticulum. Nature 356, 260–262 [DOI] [PubMed] [Google Scholar]
- 51. Gething M. J., and Sambrook J. (1992) Protein folding in the cell. Nature 355, 33–45 [DOI] [PubMed] [Google Scholar]
- 52. Endoh H., Tomida S., Yatabe Y., Konishi H., Osada H., Tajima K., Kuwano H., Takahashi T., and Mitsudomi T. (2004) Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J. Clin. Oncol. 22, 811–819 [DOI] [PubMed] [Google Scholar]
- 53. May D., Itin A., Gal O., Kalinski H., Feinstein E., and Keshet E. (2005) Ero1-L alpha plays a key role in a HIF-1-mediated pathway to improve disulfide bond formation and VEGF secretion under hypoxia: implication for cancer. Oncogene 24, 1011–1020 [DOI] [PubMed] [Google Scholar]
- 54. Wu W. W., Wang G., Insel P. A., Hsiao C. T., Zou S., Martin B., Maudsley S., and Shen R. F. (2012) Discovery- and target-based protein quantification using iTRAQ and pulsed Q collision induced dissociation (PQD). J. Proteomics 75, 2480–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. DiFonzo A., S. P., and Manousidou T. (2011) Evaluation of two separation techniques, SCX and OFFGEL and of two fragmentation methods, CAD and PQD, to asses iTRAQ quantitation efficiency. J. Biomol. Tech. 22, S67 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.