Introduction
We are entering an important new chapter in the story of hepatitis C virus (HCV) infection. There are clear challenges and opportunities. On the one hand, new HCV infections are still occurring, and an estimated 185 million have already been infected worldwide. Most HCV-infected persons are unaware of their status yet are at risk for life-threatening diseases like cirrhosis and hepatocellular carcinoma (HCC) whose incidences are predicted to rise in the coming decade. On the other hand, new HCV infections can be prevented, and those that have already occurred can be detected and treated; viral eradication is even possible. How the story ends will largely be determined by the extent to which these rapidly-advancing opportunities overcome the growing challenges, and that by the vigor of the public health response.
HCV infection and the related diseases
Most of the morbidity and mortality of HCV occurs from chronic infection
When HCV infection first occurs, hepatitis, jaundice and even fulminant hepatic failure can occur1. However, fulminant hepatic failure is rare and most HCV infections occur with no symptoms2–4. Cox and coworkers studied typical infections occurring in Baltimore injection drug users. By monthly serologic testing before and during acute HCV infection, 62 persons with acute HCV infection were detected, but none of these individuals reported symptoms of sufficient severity to seek medical care3. In 10–40% infection is self-limited; HCV antibodies but not RNA are detected in blood, and there are no recognized long-term complications5.
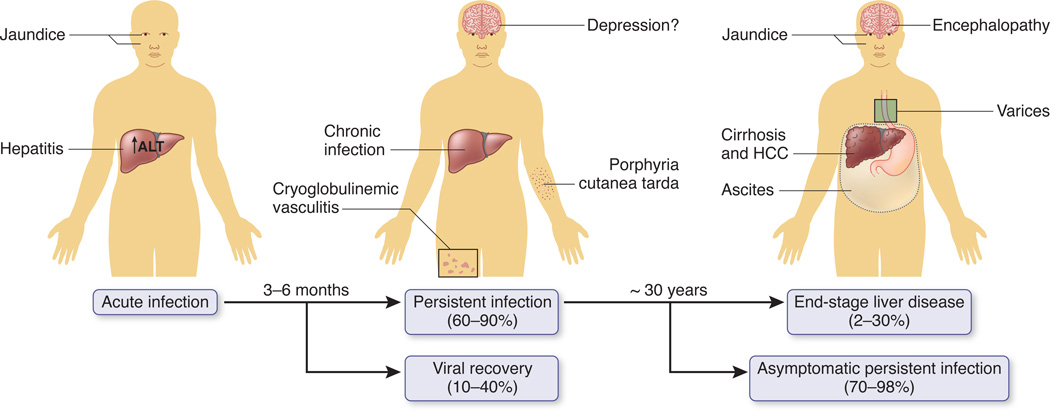
In the majority of persons, HCV infection persists and can cause an array of outcomes including cirrhosis and HCC. Cirrhosis can cause ascites, encephalopathy, and/or variceal bleeding, a constellation of events referred to as end-stage liver disease or liver failure (Fig.1); HCC can cause those same symptoms and weight loss, jaundice, or fever. HCC typically occurs in those with cirrhosis6. Without treatment, cirrhosis and HCC can be fatal and explain most of the mortality directly attributed to HCV infection.
Figure 1. Outcomes associated with HCV infection.
After acute infection, most persons develop viral persistence. Fatigue, joint pain, depression and a variety of skin manifestations can occur but are uncommonly diagnostic. In otherwise-healthy persons infected at young ages, after decades some develop cirrhosis, end state liver disease or hepatocellular cancer (HCC). There are wide ranges of the frequencies of these outcomes due to difference in host factors such as race, age, HIV infection, and for progression to end stage disease, alcohol use (see text).
Cirrhosis and/or HCC occur in a wide range (2–30%) of all persons with chronic HCV infection over 30 years7. The risks of cirrhosis and HCC increase with the infection duration and are higher in persons who are coinfected with human immunodeficiency virus 1 (HIV), infected at >40 years of age, or consume more than recommended amounts of alcohol 8–10. In a study of 1667 HCV antibody-positive injection drug users with an estimated median duration of infection of 14 years followed over 12,737 person years, only 40 had end-stage liver disease or HCC, an incidence of 3.1/1000 person years. However, the incidence was 3.6 times higher for those who consumed more than 260g of alcohol per week and 3.7 times higher for those who were 38 years of age or more at enrollment compared to the reference groups9. On the other extreme, of 39 patients followed an average of 9.7 years after acquiring HCV from blood transfusion during cardiac surgery, end-stage liver disease developed in 12% and cirrhosis in 20% in whom the mean age of infection was 58 years11. Additional examples of even more rapid progression have been reported, often in persons infected at ≥ 60 years of age or with immunosuppression 12,13. As the risks of cirrhosis and HCC increase progressively with age and duration of infection, future incidences are expected to rise (Box 1).
Box 1. Dynamic course of HCV infection.
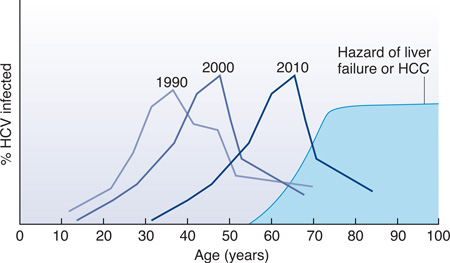
In most regions of the world the full impact of HCV infection is projected to rise as the age cohorts disproportionately affected cross thresholds of infection duration/age that increase disease risk. The most detailed modeling has occurred for the US20,56—at least two-thirds of persons with HCV infection in the United States were born between 1945 and 1965 and probably acquired HCV infection 25–45 years ago51. The first national survey was done near 1990 and the peak age of infected persons was 30–39 years (Figure, Box 1). When the survey was repeated ten years later on another randomly selected segment of the population, the mean age of HCV-infected persons was then 40–49 years. Assuming that cirrhosis and HCC incidences start to climb after 30 years of infection and as persons become more than 60 years of age, it is anticipated that both forms of disease will sharply increase in the next decade.
Davis and coworkers built a model that predicted 29,090 HCV liver-related deaths from 1980 to 1989, 56,377 from 1990 to 1999, 145,667 from 2000 to 2009, 254,550 from 2010 to 2019, and peaking at 283,378 from 2020 to 2029. As the model accurately predicted what was actually observed before 2009, the projections seem reliable. Another group predicted the same trend but with a later peak and lower rates: 38,600 new cases of end-stage liver disease; 3200 referrals for transplant; and 36,100 deaths between the years 2030 and 203595. Others have added commensurate projections for the escalating economic impact of HCV infection96.
Likewise, in Egypt, HCV infection is projected to produce 127,821 deaths from cirrhosis and 117,556 deaths from HCC over the next 20 years, prematurely ending 32.86 million years of life compared to a non-infected cohort.97 In France, HCV HCC related mortality is projected to increase through 202098. With few exceptions99, most countries have yet to experience the peak burden of HCV infection. Fortunately, these projections do not account for improved efforts to control morbidity and mortality by prevention and treatment.
Extra-hepatic diseases also cause morbidity
Persons with chronic HCV infection also have an increased prevalence of multiple extra-hepatic conditions compared to age-matched, general-population controls. For example, chronic HCV infection is strongly associated with mixed cryoglobulinemia vasculitis, a syndrome in which cryoglobulin-containing immune complexes deposit in small and medium sized blood vessels causing inflammation in skin, kidney, and/or other tissues14. There are also complex associations between HCV and other conditions like diabetes15, thyroid disease, lichen planus, and an array of neuro-psychiatric conditions like depression (Fig. 1).
Quality of life is reduced in HCV-infected persons
Collectively, the hepatic, extra-hepatic, and other related conditions are associated with a lower health-related quality of life for HCV-infected persons. In a systematic review, Spiegel and coworkers found that HCV-infected persons scored lower than controls on all scales of the validated Short Form-36 survey16. The largest difference in the HCV-infected group was in the role-physical scale where there was a mean difference of −15.8 points, followed by the role-emotional (−13.0) and general health (−12.6) scales. Stigmatization is also reported by HCV-infected persons. In one Australian study of 70 HCV-infected persons, fear and anxiety about stigma and discrimination were uniformly reported17. Stigmatization undoubtedly contributes to underreporting of HCV infection, and negative societal attitudes might constrain resource allocation beyond what would be justified by the public health impact.
There is an economic impact
Several investigators have tried to estimate the economic impact of HCV infection, but there are remarkably few studies of real costs. Leigh and coworkers estimated that in 1997 a total of $5.46 billion was spent on HCV infection, one-third on direct costs18. Another study calculated that the total, all-cause costs by managed care plans for HCV-positive individuals ($20,961) was substantially higher than for HCV-negative members ($5451); HCV-related costs themselves accounted for $6,864, nearly one-third of the total19. Most HCV control measures including HCV testing and treatment are considered cost-effective based on typical metrics 20,21.
Global disease burden
Data on the global burden of disease reveal both the urgency of control measures as well as the strategies most likely to succeed. For example, the leading transmission routes are salient targets for preventive interventions (primary prevention) while data on the distribution and dynamics of existing infections are crucial to design strategies to manage disease (secondary prevention). These epidemiological data are presented first then applied to specific control measures.
HCV transmission routes provide targets for prevention
HCV can be transmitted by percutaneous inoculation of contaminated blood, sexual intercourse (especially between men) and from a mother to her infant. In most instances the source of virus is blood. While HCV RNA has been amplified from semen, saliva, tears, and urine, there is less evidence those fluids are important sources of transmitted virus, possibly because there are too few intact viruses and/or percutaneous exposure to those fluids is uncommon22.
The size of the inoculum appears to matter. HCV infection is nearly universal when a unit of contaminated blood is transfused but occurs in only 1–5/100 single needlestick exposures and almost never when HCV RNA cannot be recovered from donor blood containing HCV antibodies23–25. There is also a correlation between the concentration of virus in the exposed blood and the likelihood of transmission. For example, HCV is more likely to be transmitted from a mother to her infant when the mother is also HIV-infected and has higher HCV RNA levels26–28. The virus also appears to be relatively stable in environmental conditions such as in syringes, gauze, or water bottles29,30. In one study, a high HCV inoculum remained infectious in cell culture after storage in bottled water for 3 weeks30.
Although percutaneous exposures cause most HCV infections, permucosal infection also occurs. Permucosal transmission has been reported when HCV contaminated blood is splashed into the eyes31, but more often refers to transmission by sexual intercourse or during intranasal cocaine use32,33. There is some controversy regarding the efficiency of sexual transmission. On the one hand, HCV infection is associated with high-risk sexual exposures, and there are outbreaks reported among men who report no other risk than having sex with other men32,34,35. On the other hand, HCV transmission is rare among long-term heterosexual partners of HCV-infected persons36,37. These disparate findings could be reconciled by enhancement of transmission by permucosal tears that occur when men have intercourse with other men (but are uncommon in long-term monogamous heterosexual relationships). HCV also could be more transmissible in the acute phase of infection, which might lead to sexual spread early after infection occurs but be missed in studies of monogamous, long-term relationships 38.
Worldwide, the frequencies of HCV transmission by various routes differ, and major changes have occurred in some regions of the world. In economically-developed regions where injection drug use occurs, it is the dominant transmission route. For example, in the last decade in the US, injection drug use accounted for more than two-thirds of HCV infections39. Moreover, the incidence of HCV infection is 5–30 per 100 person years in US illicit injection drug users3,40. Transfusions once were major forms of HCV transmission accounting for almost one-quarter of cases. However, transfusion transmission of HCV has been nearly eliminated in regions of the world where donations are tested for HCV antibodies and RNA 41.
HCV transmission has also been clearly demonstrated from percutaneous exposures in the context of traditional and some nontraditional medical care. As with transmission by transfusion, transmission of HCV in medical practice has declined in many regions probably because of expanded recognition of blood-borne pathogens and adoption of universal precautions. Where those measures are observed, exposure to medical care is not reported statistically more often by persons with acute HCV infection compared to controls. However, sporadic transmission is still well documented when there are breaches in optimal infection control practices42,43. For example, between 1998 and 2005 recent exposure to a medical practice was the only exposure reported by 67% of 109 persons in Spain with new HCV infection43. Moreover, in economically-developing regions of the world, it is likely that this form of HCV transmission remains the predominant route of spread. In economically-developing regions, the World Health Organization (WHO) estimated that there are an average of 3.4 injections per person per year, 39% performed with reused medical equipment, causing 2 million new HCV infections44.
Likewise, other percutaneous practices like tattoos, scarification rituals, circumcisions, and acupuncture have caused HCV infections in the past, and when proper sterilization techniques are not observed, they continue to be important45. As discussed below (Control), the challenges are the resources and education needed to eliminate these unsafe procedures.
Infection prevalence is global but uneven
A major challenge to control of HCV infection is the large number of persons already infected and their location in some of the poorest regions of the world. In many cases, the estimates are based on very limited data. WHO recently revised their estimates such that in 2005 there were more than 185 million HCV-antibody positive persons, or 2.8% of the human population, up from 122 million and 2.3% in 1990 46; 130–170 million are believed to be chronically infected. However, there are marked differences in HCV prevalence between regions (Fig. 2) and between age and risk groups within regions. Egypt seems to have the highest HCV prevalence, up to 50% in persons born before 196047, and the history of HCV infection in Egypt is exemplary of global transmission patterns. From the 1950s to the 1980s, there was a campaign to eradicate schistosomiasis infection by intravenously administering tartar emetic to millions of residents48. Without proper sterilization procedures, HCV was transmitted extensively, and HCV prevalence is 15–50% among persons alive during that campaign while being 1–2% in those born later. Similar age cohorts have been described elsewhere in the world and linked to unsafe infections or percutaneous practices that no longer occur 49,50.
Figure 2. Global prevalence of HCV antibodies.
Based on meta-analysis of 232 studies published 1997–2007, point prevalences are calculated using regional population weights46. GBD-Global Burden of Diseases
The best data on HCV prevalence in the Unites States come from the serial National Health and Nutrition Examination Surveys (NHANES)51. By testing blood collected from a subset of persons representing households in the United States around 1990, it was estimated that 4.1 million individuals had been infected with HCV, or 1.6% of the general population. Omitted from this survey were nearly 2 million incarcerated persons in the United States, who probably represent another 500,000 HCV-infected persons52. The survey was repeated a decade later in a new sample of the population, and the same age cohort was evident, now ten years older (Fig. Box 1)51. Based on these data, it is estimated that more than three-quarters of all HCV-infected persons in the US were born between 1945 and 1965, an epidemiological observation that has had direct impact on strategies for control (discussed below).
Figure Box 1. Dynamic course of HCV infection in the US.
As the cohort born between 1945 and 1965 ages, a greater fraction will have HCV infection for sufficient time at old enough ages to develop liver failure and HCC.
Infection prevalence is higher among racial minorities than in Caucasian Americans. In the 2000 NHANES report, in non-Hispanic Blacks 40–49 years of age, the HCV prevalence was 14% compared to a general population prevalence of 1.6%. HCV infection is also strongly linked to injection drug use, and 50–80% of injection drug users have HCV infection 39,53. Thus, it appears that just as injections for schistosomiasis in the 1950s–1980s caused a cohort of HCV-infected persons in Egypt, in approximately the same period in the US, expansion of the illicit drug use, unsafe medical practices, and contaminated transfusions, created the 1945–1965 age cohort of HCV infection.
Cirrhosis and HCC prevalence
There are no reliable estimates of the number of persons living with HCV-related cirrhosis or HCC and even mortality figures are imprecise. The WHO estimated that in 2002 there were 783,000 deaths from cirrhosis, and 27% of all cases were attributed to HCV infection54. The highest percent of cirrhosis attributed to HCV (62%) was reported from a Western Pacific region that included Japan and Australia while the lowest percent (16%) derived from sub-Saharan Africa. In another comprehensive review of global mortality, deaths from HCV-related cirrhosis were estimated at 287,400 in 2010, a 35.6% increase from 199055. HCV-related cirrhosis prevalence has also been modeled using estimates of the number of infected persons and distribution of disease cofactors. For example, Davis and coworkers constructed various models of cirrhosis in the US based on an assumption of 3.49 million persons infected in 1994 and published estimates of the time to develop cirrhosis56. In 2006 they estimated 20% of those with persistent HCV infection had cirrhosis, or approximately 650,000 persons; the number with cirrhosis in the US is expected to rise (Figure Box 1).
Data on HCC are better than on cirrhosis because most primary liver cancer is due to HCC, and there is a high fatality rate. In 2002, WHO estimated that there 619,000 deaths from HCC and attributed 25% to HCV infection54. In another comprehensive review of global mortality, deaths from HCV-related HCC were estimated at 195,700 in 2010, a 73.3% increase from 199055. Marked global differences in HCV-related HCC incidence and the proportion of HCC due to HCV infection are explained by differences in HCV and hepatitis B virus (HBV) prevalences and the age/duration of infections. For example, HCV is estimated to cause just ~15% of HCC in Taiwan, where HBV infection is the dominant cause57. In contrast, the highest rates of HCC are reported among HCV-infected persons in Japan, where HCV infection also accounts for the highest percent of HCC58. While genetic and environmental factors may play a role, there is increasing evidence that the older age of HCV-infected persons in Japan is largely responsible59. If so, this observation would suggest a similar epidemic of HCV-related HCC will occur in Europe and the USA when the average of infected persons approaches that in Japan.
Hepatitis C can be controlled
The two major approaches to controlling HCV infection are prevention of new infections (primary prevention) and management of existing infections (secondary prevention)(Table 1). The strategies differ but the results are complementary. Since treatment eliminates infection and humans are the only source of virus, treatment can also be a form of prevention. Likewise, since reinfection negates the benefits of treatment, prevention can be a form of treatment.
Table 1.
Key elements in HCV control program
| Prevent infection (primary prevention) |
| Test blood supply for HCV antibodies and RNA |
| Counseling infected persons to eliminate transmission-prone practices |
| Universal precautions |
| Reduce illicit drug use and improve safety |
| Vaccination (investigational) |
| Prevent disease (secondary prevention) |
| Testing to detect infection |
| Vaccination for hepatitis A virus and hepatitis B virus |
| Reduce or eliminate alcohol use |
| Antiviral treatment |
Infection can be prevented
As with any infectious disease, the optimal method of HCV control is prevention. HCV transmission by transfusion has virtually been eliminated by testing donations for HCV antibodies and nucleic acid60. Likewise, implementation of universal precautions and standard infection control procedures prevent HCV transmission by medical practices. There is also evidence that the incidence of HCV infection is declining among injection drug users. For example, Mehta and coworkers reported that HCV infection incidence declined in consecutive Baltimore injection drug using cohorts from 1988 through 200861.
Hepatitis C can be treated
HCV treatment efficacy is rapidly improving
The success of HCV treatment is chiefly measured by the proportion of persons in whom HCV RNA can no longer be detected in blood by the end of treatment and six months after treatment is stopped, called a sustained virologic response (SVR). Because relapse of infection is rare more than a few months after treatment is stopped, SVR is often used interchangeably with cure. Since interferon alfa was first approved by the US Food and Drug Administration for treatment of HCV infection in 1991, all licensed therapies have included some form of interferon alfa injection. Responses to interferon alfa were improved in 1998 by combining interferon alfa with oral ribavirin and then in 2001–2002 by linking the interferon molecule with polyethylene glycol producing peginterferon alfa62–64. For genotype 1 HCV infection, an additional advance came in 2011 with approval of HCV protease inhibitors telaprevir and boceprevir65,66. Accordingly, there has been progressive improvement in HCV treatment efficacy. The SVR rate for the most difficult to treat genotype 1 HCV infection was ~40% in 201067, ~66% in 201165,66, and is anticipated to be >75% by 2013.
Successful treatment improves outcomes
There is clear evidence that most of the morbidity and mortality associated with HCV infection can be controlled by treatment. Successful treatment of HCV infection is associated with a reduced incidence of liver disease progression including cirrhosis, end stage liver disease, and HCC68–71. A study of persons with advanced fibrosis or cirrhosis (Ishak fibrosis stage 4–6) who received interferon-based HCV treatment between 1990 and 2003 followed 530 patients over a median of 8.4 years72. The ten year mortality rate among persons with SVR was 8.9% compared to 26.0% among those without SVR. The ten-year liver-related mortality/transplant rate was 1.9% with SVR compared to 27.4% without SVR. Another study of US veterans who started HCV treatment between January 2001 and June 2007 found a clear survival benefit to those who achieved an SVR73. Among more than 17,000 persons, SVR was associated with a reduced hazard of death of 0.51 to 0.70, depending on the HCV genotype. Whereas these findings may reflect greater treatment response in persons with less pretreatment disease (rather than a treatment effect), adjustments were made for pretreatment fibrosis stage and placebo-controlled studies are not ethical. A study of Moreover, a recent systematic review found clear evidence that therapy improved clinical outcomes74.
Similarly, successful treatment of HCV infection can improve quality of life75,76. Bernstein and coworkers evaluated the change in health-related quality of life reports from baseline to end of follow-up for 1441 persons from international studies of HCV treatment75. Improvements in quality of life were strongly associated with successful treatment. Likewise, another study reported better quality of life results, fewer absences from work, and greater involvement in volunteer and household activities among HCV-infected persons with successful treatment responses compared to those whose treatment was unsuccessful76. Thus, successful treatment has the capacity to reduce many of the complications of HCV infection.
There are major challenges to control
Challenges to control by prevention
Although there is evidence HCV infection can be prevented, there are abundant examples that all prevention efforts need to be intensified. Vaccination is a major tool for prevention of many infectious diseases. However, HCV is genetically and antigenically diverse, and the immunologic correlates of protective immunity are only incompletely understood. These, and other factors, have constrained HCV vaccine development. (See review in this series by Liang 77). Thus, HCV infection is prevented mainly by eliminating exposures or making them safer.
Worldwide, the leading route of HCV transmission remains unsafe medical procedures44,46,47,49,50,78. As mentioned above, the WHO estimates there are 2 million new HCV infections each year as a result of unsafe infection44. Even in Europe and the United States, HCV transmission during routine health procedures continues when there are breaks in infection control practices43,78. Clearly education about avoiding unnecessary injections is key, as is information on universal precautions and resources to implement them.
The second leading global route of HCV transmission is probably illicit drug use. Although in some settings, HCV incidence has declined among injection drug users, new infections still occur at incidences of 5–20% per year61,79. Measures like needle exchange may have contributed to the reduced incidence in some places80 but have not been sufficient and, from a global perspective, have had minimal impact on HCV transmission. Data demonstrating the stability of virus in syringes and drug use equipment need to be translated into practices that eliminate transmission risk when drug use itself cannot be stopped.
Likewise, there remain major challenges to prevent HCV transmission in other contexts. Multiple outbreaks of new HCV infections have been reported among male homosexual populations,81 and as with injection drug users, in some instances these infections have negated the benefits of prior successful treatment82. Methods to prevent transmission from a mother to her infant (such as Cesarean section, maternal treatment, and infant vaccination) that have worked for other infectious diseases have either not worked for HCV or are not yet possible83. In this regard, development of HCV treatment that is not toxic to the fetus is particularly promising but not currently available. Thus, the available evidence demonstrates that infection can be (and is) prevented but that current control efforts have to be intensified in these key settings.
Challenges to control by treatment
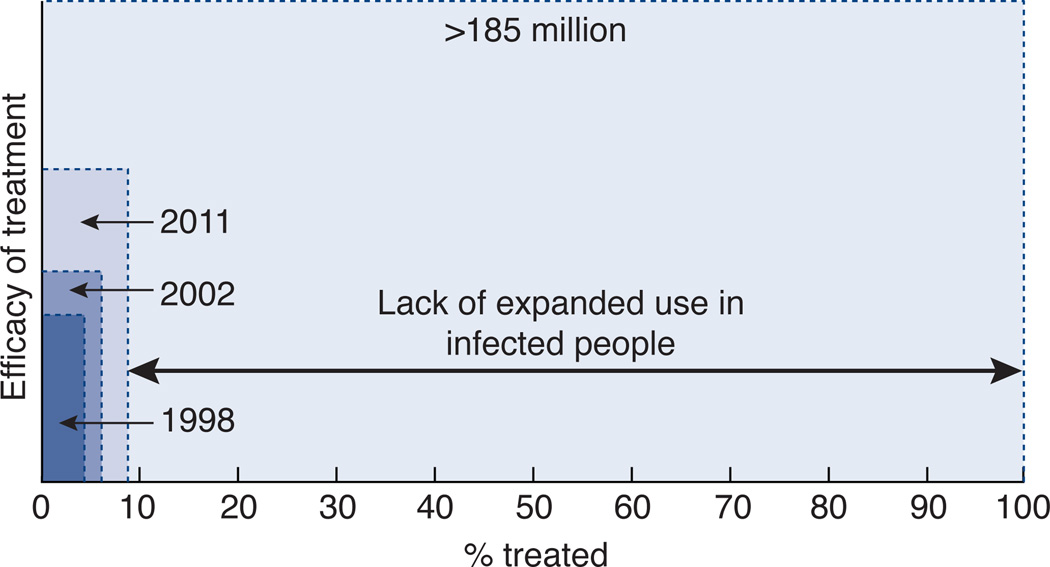
Lack of recognition of infection is a major impediment to controlling existing infection. In the United States, it is estimated that less than half of HCV-infected individuals are aware of their infection. In the 2001–2008 NHANES survey of US households, 393 HCV antibody positive persons were identified and 170 were contacted after being notified of their HCV status through the study84. Only 49.7% were already aware of their HCV infection before being notified by the study personnel. Interestingly, only 3.7% had been tested because their doctor followed the “risk based” testing strategy in effect since 1998. Since this study excluded inmates, the homeless, and other populations at highest risk of unrecognized HCV infection, the true prevalence of unrecognized infection may be more than 60%. Even treatments of 100% efficacy will have no impact on unrecognized infection (Fig. 3).
Figure 3. Importance of expanded testing and treatment to impact the global prevalence of HCV infection.
Because the % cured is a function of the distribution of many variables such as genotype, age, and race, the y axis arbitrarily shows the efficacy of treatment, which is increasing but having little impact on the overall burden of disease represented by the total area of the figure. 130–170 million are estimated to have chronic HCV infection, while >185 million were estimated to have HCV antibodies in 200546.
Rates of treatment uptake among those with recognized infection are also low. In Europe, by 2006 it was estimated that chronic hepatitis C treatment was provided for 308,000 persons who comprised no more than 16% of the HCV-infected persons in any individual country85. In the USA, from 2002 to 2007 an estimated 663,000 of the ~4 million HCV-infected persons were treated86. In one analysis of nearly 100,000 US veterans with chronic hepatitis C, only 11.6% received and 6.4% completed treatment 87.
Effective HCV control measures exceed the available resources in many regions of the world and segments of society where infection burden is greatest (see Prevalence). In the US, the average wholesale cost of existing HCV treatment for genotype 1 HCV infection can exceed $100,000 for medications alone. This is certainly an extreme example, and in other regions of the world generic drugs can be obtained for a fraction of that price. Nonetheless, peginterferon alfa is necessary for all forms of HCV treatment but is not currently on the WHO List of Essential Medications. In addition, beyond the drug costs, there are other issues such as the availability of laboratory testing, refrigeration of drug, and monitoring of treatment contribute additional costs and complexities that make it extremely challenging to initiate widespread treatment of HCV infection in some regions of the world.
HCV control challenges can be overcome
Expanded testing is crucial
No more than 15% of the >185 million HCV-infected persons is aware of their status, despite the availability for more than 20 years of an accurate screening test. Thus, one of the most important elements in a successful control program has to be expanded HCV testing. Methods are now available for rapid testing for HCV antibodies at the point of care88. Since in some societies there is stigma around HCV infection, anonymous testing options also should be made available. With all screening, it is important to add HCV RNA testing for persons in whom HCV antibodies are detected by screening. Unfortunately, RNA testing requires a specialized laboratory.
In August 2012, the US CDC has recommended a new HCV screening strategy focused on all individuals born between 1945 through 1965, the cohort with the highest HCV prevalence89. As mentioned above, this cohort is believed to comprise at least two-thirds of all HCV-infected persons in the USA. In a recent analysis of the cost effectiveness of this approach, the CDC estimated that 1.9 million persons from this age group had chronic HCV infection in 2006 and saw a health care provider at least once20. Their models suggest birth cohort testing would identify 1,070,840 new cases of chronic hepatitis C, a measure that would reduce the morbidity and mortality predicted in this cohort in the next 10–20 years. Given current treatment efficacy, full implementation of this testing recommendation might prevent 121,000 deaths and be cost effective compared to most accepted practices such as colorectal cancer screening. Similar methods should be employed elsewhere, even prior to the widespread availability of treatments, since those infected can benefit from counseling to delay progression of disease by limitation of alcohol ingestion, to be vaccinated against hepatitis A virus and HBV, and to prevent secondary transmission.
Treatment will be expanded with interferon-sparing regimens
Lok and coworkers published proof that interferon-free treatments are possible90, and even more encouraging results have already been reported for phase 2 studies. For example, Poordad and coworkers reported that 93–95% of 33 HCV genotype 1-infected persons achieved an SVR 12 weeks after stopping treatment with various doses of an inhibitor of the HCV NS3 serine protease (ABT-450 coformulated with ritonavir, ABT-450/r), along with a nonnucleoside inhibitor of the NS5b HCV polymerase (ABT-333), and ribavirin91. ABT-450/r has since been coformulated with an inhibitor of HCV NS5A (ABT-267) and moved into phase 3 testing to examine the safety and efficacy with ABT-333 and ribavirin. Likewise, Gane and coworkers reported that 21 of 25 genotype 1-infected persons and 10 of 10 HCV genotype 2 or 3-infected persons achieved an SVR when treated with a nucleotide inhibitor of the HCV polymerase (sofosbuvir), along with oral ribavirin for 12 weeks92. In another study, sofosbuvir was combined with an inhibitor of NS5A (GS5885) and ribavirin and produced SVR at least 4 weeks after treatment was stopped in 25 of 25 genotype 1 infected persons 93. The compounds sofosbuvir and GS5885 were coformulated into a once-daily, fixed-dose medication that has moved into phase 3 testing of one pill once a day for treatment of genotype 1 HCV infection with or without ribavirin. Also noteworthy are the adverse event profiles of these medications, which appear safer than the currently-approved, peginterferon-based treatments. Thus, coming rapidly are oral medications that can cure HCV more often, in less time, and with fewer side effects.
While interferon-sparing HCV treatments are not yet approved for clinical use, there are obvious ways in which they will improve HCV control. Persons who are intolerant to interferon or for whom interferon-based therapies were not efficacious will now have additional treatment options. In addition, it is possible that a growing perception of greater treatment safety and efficacy will encourage more patients to opt for therapy. Simpler, safer, and more efficacious treatments might also make primary care providers expand testing, a critical need given <50% are aware of their status. Since HCV is not transmissible after SVR, more effective HCV treatment should also reduce new infections. Prevention of transmission by treatment has worked to varying extent for other infections such as HIV, tuberculosis and syphilis. However, the degree to which HCV treatment will reduce transmission within populations or in particular settings such as from a mother to her infant remains to be demonstrated.
Hepatitis C eradication is the goal
The example of small pox makes it clear that eradication is the most successful method of controlling infectious diseases. Notably, many attributes that make an infection a candidate for eradication exist for HCV infection (Table 1). Blood-born infections are much easier to control than those transmitted by aerosols or food. In addition, existing therapies can permanently eliminate infection. Since there is no natural nonhuman reservoir, elimination of infection from individuals effectively prevents subsequent transmission. Thus, even though many infected populations such as injection drug users do not actively seek medical care, treatment efforts only need to be sustained for finite periods of time to cure a given host. Treatment of enough individuals could eliminate transmission once the HCV reservoir drops below what is necessary to sustain the epidemic, even if risk practices themselves cannot be controlled.
On May 21, 2010, the World Health Assembly passed a resolution that called for WHO to develop a comprehensive approach to control chronic hepatitis. In July 2012, WHO announced a framework for global action for the prevention and control of viral hepatitis infection (http://www.who.int/csr/disease/hepatitis/Framework/en/index.html). However, by September, 2012 we still have very little information on the number of infected persons, proportion with cirrhosis or HCC, or fraction with knowledge of infection. These observations are in striking contrast to the information available for HIV, malaria and other public health priorities. In the US, although HCV causes more mortality and infects 3–4 times as many individuals as HIV, appropriations for HCV in the United States are less than 2% for research and for medical care94. This level of funding is clearly insufficient to drive the type of public health impact that will be possible in the coming years when safer and more efficacious HCV treatments are approved. At a global scale, the projections are even worse, which is of considerable concern given that sustained political and societal will is a key determinant to the success of measures to control infection. Thus, there is no question that HCV will be eliminated increasingly from populations and settings where treatment is provided. Penetrance of testing and treatment into the rest of the world will depend on global commitment.
Conclusion
HCV infection causes cirrhosis and HCC worldwide; however, for most of the duration of infection, and notably during the interval when treatment is possible, the infection is silent and either unrecognized or ignored by most persons. Improvements in treatment seem to be coming just as the impact of infection begins to rise. However, from a public health perspective, these treatment advances are not likely to have major impact without substantial changes in the methods of control. Efforts to prevent transmission can (and do) work but must be intensified. Treatments can (and do) cure infection but are uncommonly received. In the next decade, there is no doubt that HCV infection will be eradicated from many individuals. The question remains to what extent these exciting new breakthroughs reach the populations most affected. With sufficient investment, infection could be eradicated from segments of the population and, in principle, the world.
Table 2.
Attributes of an infectious disease that can be eradicated*
| Attribute | Relevance to HCV |
|---|---|
| Can be cleared from host | True |
| Control transmission | True |
| No natural non-human reservoir | True |
| Adequate public health infrastructure | ? |
| Sufficient funding | ? |
| Sustained political and societal will | ? |
adapted from Dowdle and Cochi, Vaccine 2011.
Box 2 Breaking tolerance is critical to control of HCV infection.
There is an interesting parallel between the manner in which HCV has evolved mechanisms to replicate in hepatocytes and the persistence of HCV infection in society. At the cellular level, Gale, Lemon and others have characterized the elegant mechanisms through which the HCV protease silences the cellular sensors of RNA replication such as the retinoic acid inducible gene I and toll like receptor 3 100. Although these and other molecules recognize pathogen associated molecular patterns on HCV RNA, the virus dampens downstream signaling cascades and net immune responses sufficiently to persist in most persons.
Likewise, HCV replication is uncommonly recognized by the host. Acute infection rarely causes sufficient symptoms to lead an infected person to seek medical care. Clinically-silent acute infections are more likely to cause long-term complications than symptomatic ones, both because they are more likely to persist and because persons with unrecognized infections cannot be treated or cautioned to prevent further transmission2,101. During the vast majority of its subsequent natural history, replication continues without production of sufficient symptoms to provoke a person to seek medical help. The virus also disproportionately affects segments of society less apt to receive medical treatments, further enhancing its odds of persisting.
Control of infection both within a cell and within society requires that this tolerance for HCV replication be broken. Medications have been developed that penetrate into hepatocytes and stop replication within that cellular compartment. Whereas, these medications are clearly able to eradicate infection, they have not yet penetrated into most segments of society where replication continues unchecked. From the virus’ perspective, prisoners, illicit drug users, and all individuals with unrecognized HCV infection represent protected replication sanctuaries.
All projections call for a sharp rise in the incidence of HCV related cirrhosis and HCC. It is necessary for the danger of this threat to signal society to expanded HCV testing and treatment access to prevent these outcomes. The degree of success is likely to be commensurate to the vigor with which the silence is overcome.
References
- 1.Hoofnagle JH. Hepatitis C: The clinical spectrum of disease. Hepatology. 1997;26:15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 2.Villano SA, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–914. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- 3.Cox AL, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 4.Mosley JW, et al. Viral and host factors in early hepatitis C virus infection. Hepatology. 2005;42:86–92. doi: 10.1002/hep.20742. [DOI] [PubMed] [Google Scholar]
- 5.Lee MH, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469–477. doi: 10.1093/infdis/jis385. [DOI] [PubMed] [Google Scholar]
- 6.Bruno S, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: A prospective study. Hepatology. 1997;25:754–758. doi: 10.1002/hep.510250344. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398. vi. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Goedert JJ, et al. End-stage liver disease in persons with hemophilia and transfusion- associated infections. Blood. 2002;100:1584–1589. [PubMed] [Google Scholar]
- 9.Thomas DL, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 10.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 11.Di Bisceglie AM, et al. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–974. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 12.Farci P, et al. Profibrogenic chemokines and viral evolution predict rapid progression of hepatitis C to cirrhosis. Proc Natl Acad Sci U S A. 2012;109:14562–14567. doi: 10.1073/pnas.1210592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fierer DS, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–686. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in Type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 15.Mehta SH, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Hepatology. 2001;33:1554. doi: 10.1053/jhep.2001.0103306le01. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel BM, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41:790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- 17.Conrad S, et al. Living with chronic hepatitis C means 'you just haven't got a normal life any more'. Chronic Illn. 2006;2:121–131. doi: 10.1177/17423953060020020701. [DOI] [PubMed] [Google Scholar]
- 18.Leigh JP, et al. Costs of hepatitis C. Arch Intern Med. 2001;161:2231–2237. doi: 10.1001/archinte.161.18.2231. [DOI] [PubMed] [Google Scholar]
- 19.Davis KL, et al. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45:e17–e24. doi: 10.1097/MCG.0b013e3181e12c09. [DOI] [PubMed] [Google Scholar]
- 20.Rein DB, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, et al. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156:279–290. doi: 10.1059/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendel I, et al. Detection and genotyping of the hepatitis C RNA in tear fluid from patients with chronic hepatitis C. J Med Virol. 1997;51:231–233. doi: 10.1002/(sici)1096-9071(199703)51:3<231::aid-jmv15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Esteban JI, et al. High rate of infectivity and liver disease in blood donors with antibodies to hepatitis C virus. Ann Intern Med. 1991;115:443–449. doi: 10.7326/0003-4819-115-6-443. [DOI] [PubMed] [Google Scholar]
- 24.Seeff LB. Hepatitis C from a needlestick injury. Ann Intern Med. 1991;115:411. doi: 10.7326/0003-4819-115-5-411_1. [DOI] [PubMed] [Google Scholar]
- 25.Dore GJ, Kaldor JM, McCaughan GW. Systematic review of role of polymerase chain reaction in defining infectiousness among people infected with hepatitis C virus. Br Med J. 1997;315:333–337. doi: 10.1136/bmj.315.7104.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas DL, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Journal of Infectious Diseases. 1998;177:1480–1488. doi: 10.1086/515315. [DOI] [PubMed] [Google Scholar]
- 27.Mast EE, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880–1889. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- 28.Zanetti AR, et al. Mother-to-infant transmission of hepatitis C virus. Lancet. 1995;345:289–291. doi: 10.1016/s0140-6736(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 29.Paintsil E, et al. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis. 2010;202:984–990. doi: 10.1086/656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doerrbecker J, et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis. 2013;207:281–287. doi: 10.1093/infdis/jis677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartori M, et al. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis. 1993;25:270–271. doi: 10.3109/00365549309008497. [DOI] [PubMed] [Google Scholar]
- 32.Thomas DL, et al. Sexual transmission of hepatitis C virus among patients attending sexually transmitted diseases clinics in Baltimore: an analysis of 309 sex partnerships. J Infect Dis. 1995;171:768–775. doi: 10.1093/infdis/171.4.768. [DOI] [PubMed] [Google Scholar]
- 33.Conry-Cantilena C, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996;334:1691–1696. doi: 10.1056/NEJM199606273342602. [DOI] [PubMed] [Google Scholar]
- 34.Akahane Y, et al. Hepatitis C virus infection in spouses of patients with type C chronic liver disease. Ann Intern Med. 1994;120:748–752. doi: 10.7326/0003-4819-120-9-199405010-00005. [DOI] [PubMed] [Google Scholar]
- 35.van de Laar TJ, et al. Sexual transmission of hepatitis C virus in human immunodeficiency virusnegative men who have sex with men: a series of case reports. Sex Transm Dis. 2011;38:102–104. doi: 10.1097/OLQ.0b013e3181ec9de5. [DOI] [PubMed] [Google Scholar]
- 36.Terrault NA, et al. Sexual transmission of HCV among monogamous heterosexual couples: The HCV partners study. Hepatology. 2012:10. doi: 10.1002/hep.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandelli C, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855–859. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 38.Magiorkinis G, et al. Integrating phylodynamics and epidemiology to estimate transmission diversity in viral epidemics. PLoS Comput Biol. 2013;9:e1002876. doi: 10.1371/journal.pcbi.1002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams IT, et al. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Arch Intern Med. 2011;171:242–248. doi: 10.1001/archinternmed.2010.511. [DOI] [PubMed] [Google Scholar]
- 40.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15:543–549. doi: 10.1097/01.ede.0000135170.54913.9d. [DOI] [PubMed] [Google Scholar]
- 41.Schreiber GB, et al. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 42.Thompson ND, et al. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998-2008. Ann Intern Med. 2009;150:33–39. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Bauer E, et al. Hospital admission is a relevant source of hepatitis C virus acquisition in Spain. J Hepatol. 2008;48:20–27. doi: 10.1016/j.jhep.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 44.Hauri AM, Armstrong GL, Hutin YJ. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 45.Ko YC, et al. Tattooing as a risk of hepatitis C virus infection. J Med Virol. 1992;38:288–291. doi: 10.1002/jmv.1890380411. [DOI] [PubMed] [Google Scholar]
- 46.Hanafiah KM, et al. Global epidemiology of hepatitis C virus infection: New estimates of agespecific antibody to hepatitis C virus seroprevalence. Hepatology. 2012:10. [Google Scholar]
- 47.Frank C, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 48.Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43:915–922. doi: 10.1002/hep.21173. [DOI] [PubMed] [Google Scholar]
- 49.Guadagnino V, et al. Hepatitis C virus infection in an endemic area of Southern Italy 14 years later: Evidence for a vanishing infection. Dig Liver Dis. 2012:10. doi: 10.1016/j.dld.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi J, et al. Transmission of hepatitis C virus by health care workers in a rural area of Japan. Am J Gastroenterol. 1995;90:794–799. [PubMed] [Google Scholar]
- 51.Armstrong GL, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 52.Baillargeon J, et al. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–48. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 53.Thomas DL, et al. Correlates of hepatitis C virus infections among injection drug users in Baltimore. Medicine. 1995;74:212–220. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Perz JF, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobson IM, et al. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8:924–933. doi: 10.1016/j.cgh.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 57.Liao SF, et al. Fifteen-year population attributable fractions and causal pies of risk factors for newly developed hepatocellular carcinomas in 11,801 men in Taiwan. PLoS ONE. 2012;7:e34779. doi: 10.1371/journal.pone.0034779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka Y, et al. Molecular tracing of the global hepatitis C virus epidemic predicts regional patterns of hepatocellular carcinoma mortality. Gastroenterology. 2006;130:703–714. doi: 10.1053/j.gastro.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber GB, et al. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 61.Mehta SH, et al. Changes in blood-borne infection risk among injection drug users. J Infect Dis. 2011;203:587–594. doi: 10.1093/infdis/jiq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McHutchison JG, et al. Interferon Alfa-2b Alone or in Combination with Ribavirin as Initial Treatment for Chronic Hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 63.Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 64.Fried MW, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 65.Poordad F, et al. Boceprevir for Untreated Chronic HCV Genotype 1 Infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobson IM, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 67.McHutchison JG, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 68.Shiratori Y, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 69.Veldt BJ, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 70.Cardoso AC, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–657. doi: 10.1016/j.jhep.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 71.Berenguer J, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–413. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 72.van der Meer AJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 73.Backus LI, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Chou R, et al. Screening for hepatitis C virus infection in adults: a systematic review for the u.s. Preventive services task force. Ann Intern Med. 2013;158:101–108. doi: 10.7326/0003-4819-158-2-201301150-00574. [DOI] [PubMed] [Google Scholar]
- 75.Bernstein D, et al. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 76.John-Baptiste AA, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol. 2009;104:2439–2448. doi: 10.1038/ajg.2009.346. [DOI] [PubMed] [Google Scholar]
- 77.Liang J. Nat Med. [[xxxx]] [Google Scholar]
- 78.Patel PR, et al. Hepatitis C virus infections from a contaminated radiopharmaceutical used in myocardial perfusion studies. JAMA. 2006;296:2005–2011. doi: 10.1001/jama.296.16.2005. [DOI] [PubMed] [Google Scholar]
- 79.Hagan H, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201:378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 80.Hagan H, et al. Reduced risk of hepatitis B and hepatitis C among injection drug users in the Tacoma syringe exchange program. American Journal of Public Health. 1995;85:1531–1537. doi: 10.2105/ajph.85.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van de Laar T, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–1617. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grebely J, et al. Reinfection with hepatitis C virus following sustained virological response in injection drug users. J Gastroenterol Hepatol. 2010;25:1281–1284. doi: 10.1111/j.1440-1746.2010.06238.x. [DOI] [PubMed] [Google Scholar]
- 83.Cottrell EB, Chou R, Wasson N, Rahman B, Guise J-M. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. doi: 10.7326/0003-4819-158-2-201301150-00575. (published online 27 November 2012) [DOI] [PubMed] [Google Scholar]
- 84.Denniston MM, et al. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lettmeier B, et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J Hepatol. 2008;49:528–536. doi: 10.1016/j.jhep.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 86.Volk ML, et al. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009 doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 87.Kramer JR, et al. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–325. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 88.Shivkumar S, et al. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558–566. doi: 10.7326/0003-4819-157-8-201210160-00006. [DOI] [PubMed] [Google Scholar]
- 89.Smith BD, et al. Hepatitis C Virus Testing of Persons Born During 1945 to 1965: Recommendations From the Centers for Disease Control and Prevention. Ann Intern Med. 2012 doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lok AS, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 91.Poordad F, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [DOI] [PubMed] [Google Scholar]
- 92.Gane EJ, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 93.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. High rate of sustained virologic response with the all-oral combination of daclatasvir plus sofosfobir, with or without ribavirin in treatment-naïve patients chronically infected with genotypes 1, 2, or 3. Abstract LB-2. AASLD. Boston. 2012 [Google Scholar]
- 94.Edlin BR. Perspective: test and treat this silent killer. Nature. 2011;474M:S18–S19. doi: 10.1038/474S18a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rein DB, et al. Forecasting the morbidity and mortality associated with prevalent cases of precirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 96.Wong JB, et al. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lehman EM, Wilson ML. Epidemic hepatitis C virus infection in Egypt: estimates of past incidence and future morbidity and mortality. J Viral Hepat. 2009;16:650–658. doi: 10.1111/j.1365-2893.2009.01115.x. [DOI] [PubMed] [Google Scholar]
- 98.Deuffic S. Modeling the hepatitis C virus epidemic in France. Hepatology. 1999;29:1596–1601. doi: 10.1002/hep.510290528. [DOI] [PubMed] [Google Scholar]
- 99.Mariano A, et al. Estimating the incidence, prevalence and clinical burden of hepatitis C over time in Italy. Scand J Infect Dis. 2009;41:689–699. doi: 10.1080/00365540903095358. [DOI] [PubMed] [Google Scholar]
- 100.Foy E, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acidinducible gene-I signaling. Proc Natl Acad Sci U S A. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barrett S, et al. The natural course of hepatitis C virus infection after 22 years in a unique homogenous cohort: spontaneous viral clearance and chronic HCV infection. Gut. 2001;49:423–430. doi: 10.1136/gut.49.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]