Abstract
Objective(s):
In nuclear medicine studies, gallium-68 (8Ga) citrate has been recently known as a suitable infection agent in positron emission tomography (PET). In this study, by applying an in-house produced 68Ge/68Ga generator, a simple technique for the synthesis and quality control of 68Ga-citrate was introduced; followed by preliminary animal studies.
Methods:
68GaCl3 eluted from the generator was studied in terms of quality control factors including radiochemical purity (assessed by HPLC and RTLC), chemical purity (assessed by ICP-EOS), radionuclide purity (evaluated by HPGe), and breakthrough. 68Ga-citrate was prepared from eluted 68GaCl3 and sodium citrate under various reaction conditions. Stability of the complex was evaluated in human serum for 2 h at 370C, followed by biodistribution studies in rats for 120 min.
Results:
68Ga-citrate was prepared with acceptable radiochemical purity (>97 ITLC and >98% HPLC), specific activity (4-6 GBq/mM), chemical purity (Sn, Fe<0.3 ppm and Zn<0.2 ppm) within 15 min at 500C. The biodistribution of 68Ga-citrate was consistent with former reports up to 120 minutes.
Conclusion:
This study demonstrated the possible in-house preparation and quality control of 68Ga-citrate, using a commercially available 68Ge/68Ga generator for PET imaging throughout the country.
Keywords: 68Ge/68Ga Generator, 68Ga-Citrate, Biodistribution, Quality Control
Introduction
Considering the intriguing physical properties and availability of gallium-68 as a generator, this isotope has become an interesting nuclide for developing new positron emission tomography (PET) tracers (1). In nuclear medicine, the increasing trend in the production and use of PET radionuclides has provided new opportunities for researchers to focus on the production of new 68Ga radiopharmaceuticals, considering the availability and commercialization of 68Ge/68Ga generators.
68Ge decays via pure electron capture (EC) to the ground state of 68Ga with a half-life of 270.95 days (2). 68Ga in turn decays with a half-life of 67.71 min by a combination of EC and positron emission to the ground state of zinc-68 (68Zn). 68Ga also decays to an excited state at 1077 keV (with a probability of about 3%) and some higher energy-excited states (with a combined probability of <0.4 %).
For decades, 67Ga-citrate has been known as an imaging agent for the detection of infections and inflammations (3). The preparation, quality control, and significance of 67Ga-citrate for the evaluation of various infections have been previously investigated (4). After the development of 68Ga generators, the application of 68Ga-citrate was not promising since most of 68Ga images were obtained far beyond the physical half-life of 68Ga; as a result, preparation and application of 68Ga-citrate were discarded for some time.
However, after the implementation of few clinical trials in various centers, researchers became interested in 68Ga infection studies. The preliminary data confirmed the possible role of 68Ga-citrate in the diagnosis of bone infections (5). These reports initiated various studies on infectious animals, and different production routes were reported (6-10). Also, some researchers have revealed the application of this tracer for the detection of atherosclerotic plaques in animal models (11).
The area of research is now open to clinical researchers for the evaluation of this tracer in already-confirmed applications of its SPECT homolog (i.e. 67Ga-citrate) for conditions such as fever of unknown origin, severe lymphocytic inflammation, autoimmune-based inflammations, chronic pancreatitis, idiopathic pulmonary fibrosis, pulmonary Wegener’s granulomatosis, chronic bronchial asthma, and sarcoidosis (12). On the other hand, the short half-life of 68Ga-citrate, which is considered an advantage from a dosimetric point of view, can be a drawback at the same time since it does not allow long uptake duration, which is typical of 67Ga-citrate scintigraphy.
In this study, we report the development of an in-house generator, the quality assurance and quality control of eluted 68GaCl3, and a simple 68Ga-citrate production method for any stand-alone PET center, utilizing a 68Ga generator. The preclinical evaluation of the 68Ga-citrate complex in wild-type rats was also reported (Figure 1).
Figure 1.
The chemical structure of 68Ga-citrate
Methods
The prototype 68Ge/68Ga generator (50 mCi/day) was obtained from Pars Isotope Co. Karaj, Iran. Chemicals were purchased from Aldrich chemical Co., Germany. Normal saline, sodium citrate, acetone, and sodium acetate, used for radiolabeling, had high purities and were filtered by 0.22 μm Cativex filters.
Instant thin-layer chromatography (ITLC) was performed by counting Whatman filter papers (No. 2), using a thin-layer chromatography scanner (Bioscan AR2000, Bioscan Europe Ltd., France). Analytical high-performance liquid chromatography (HPLC), used to determine the specific activity, was performed by a Shimadzu LC-10AT pump, equipped with two detector systems, a flow scintillation analyzer (Packard-150 TR), and an ultraviolet–visible spectrophotometer (Shimadzu), using a Whatman Partisphere C-18 column (250×4.6 mm) (Whatman, NJ, USA).
Analytical HPLC was also used to determine the specific radioactivity of the labeled compound. Biodistribution data were acquired by counting normal saline-washed tissues after weighing on a Canberra™ high-purity germanium (HPGe) detector (model GC1020-7500SL). Radionuclidic purity was assessed using the same detector.
For activity measurement of samples, a CRC Capintec Radiometer (NJ, USA) was applied. All calculations and ITLC counting were based on the 511 keV peak. Animal studies were performed in accordance with the Guidelines on the Use of Living Animals in Scientific Investigations (2nd edition) by the United Kingdom Biological Council.
68Ge/68Ga generator
The preparation of the prototype generator has been formerly described in detail; however, few modifications were made to enhance the efficacy of this generator (13). In brief, for a 50 mCi 68Ge/68Ga generator, metallic gallium powder was melted, transferred to niobium capsules, and then sealed. The target was irradiated at 29 MeV in a 30 MeV IBA cyclotron (effective 22 MeV protons on the target material).
Following irradiation, the target material was dissolved in 12 M H2SO4, while being gently heated and stirred. For increasing the solubility, 30% hydrogen peroxide was added to the mixture, followed by the addition of 2 M HCl (30 ml). After the completion of dissolution, carbon tetrachloride (150 ml) was added to the mixture. The organic 68Ge-containing layer was extracted from 68Ga, containing aqueous solution. 68Ge was then back-extracted into the aqueous solution, using 0.05 M HCl. The final solution was used for the production of a SnO2-based generator.
Quality control of the product
Radionuclide purity
Gamma spectroscopy of the final sample was carried out using an HPGe detector, coupled to a Canberra™ multi-channel analyzer for 1000 sec. Breakthrough was measured by counting the same sample 48 hours after the first test for the detection of small amounts of 68Ge in the sample.
Chemical purity
This step was carried out to ensure that the values of Sn, Zn, Fe, Ge, and Ga ions, resulting from the target material and presenting in the final product, were within the international limits. Chemical purity was assessed using Inductively-Coupled Plasma Optical Emission Spectrometry (ICP-OES) method. The detection limit of our system was 0.1 ppm for all cations.
Preparation of 68Ga-citrate
The acidic solution of 68GaCl3 (3-5 mCi in 150µl), eluted by 0.6 M HCl (metal free and ultrapure), was transferred to a 5 ml borosilicate vial and heated to dryness, using a flow of N2 gas at 50-60 °C, followed by the addition of sodium citrate solution (300 μL, 0.1 M); the mixture was vortexed at 50 °C for 10-15 min and controlled for radiochemical purity. The active solution with acceptable radiochemical purity was sterile filtered, using a 0.22 micron membrane; also, pH was adjusted to 5.5-7.
Quality control of 68Ga-citrate
Radio thin-layer chromatography
A 5 μl sample of the final fraction was spotted on Whatman No. 2 paper and/or silica gel-coated plates, using various mobile phases.
HPLC
HPLC was performed with a flow rate of 1 ml/min under 130 kgF/cm2 pressure for 20 min. HPLC was performed on the final preparation, using a mixture of water and acetonitrile with a ratio of 3:2 (v/v) as the eluent, using reversed-phase Whatman Partisphere C18 column (4.6×250 mm).
Stability tests
The stability of the complex was assessed using conventional ITLC method (14). A sample of 68Ga-citrate (37 MBq) was kept at room temperature for up to 2 hours, while being checked by ITLC at different time intervals in order to check the stability of the final product, using the above-mentioned chromatography system.
In vitro stability of 68Ga-citrate in the presence of human serum
The final solution (200 µCi in 50 µL) was incubated in the presence of freshly prepared human serum (300 µL) (purchased from Iranian Blood Transfusion Organization, Tehran) and kept at 37 °C for 2 hrs. Every 30 min, trichloroacetic acid (10%, 100 µL) was added to a portion of the mixture (50 μl) and the mixture was centrifuged at 3000 rpm for 5 min, followed by decanting the supernatant from the debris. Stability was determined by performing frequent ITLC analysis of supernatant, using the ITLC system.
Biodistribution in wild-type rats
The distribution of the radiolabelled complex, as well as free 68Ga cation, was determined in rat tissues. The total amount of radioactivity injected into each rat was measured by evaluating the 1 ml syringe before and after the injection in a dose calibrator with fixed geometry. The animals were sacrificed by CO2 asphyxiation at selected time intervals after injection (n=3 for each time interval). The tissues (blood, heart, lung, brain, intestine, feces, skin, stomach, kidneys, liver, muscle and bone) were weighed and rinsed with normal saline and their specific activities were determined as the percentage of injected dose per gram of tissues, using an HPGe detector, equipped with a sample-holder device.
Results
Quality control of 68Ga generator
Radiochemical separation of 68Ge from irradiated natural Ga was performed via a two-step extraction method in an organic solution, followed by back extraction with a 96% yield. The presence or absence of 68Ga and 68Ge was assessed at each step, using the HPGe detector (Figure 2).
Figure 2.
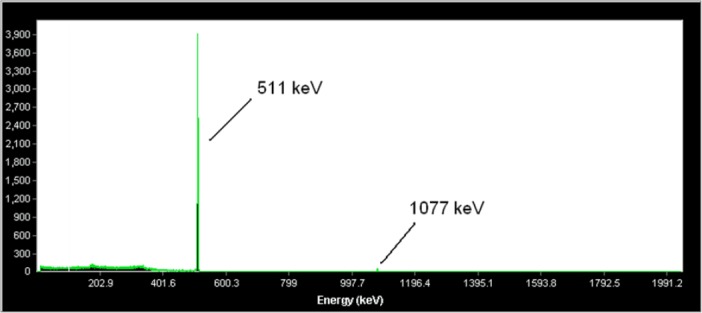
Gamma spectrum for 68GaCl3 solution, eluted from the generator, used in radiolabeling
Radionuclidic control indicated the presence of 511 and 1077 keV energies, which originated from 68Ga and showed a radionuclidic purity higher than 99% (E.O.S.). For the quality control of the 68GaCl3 solution, a time-activity study was performed on the eluted sample after more than 10 half-lives of 68Ga in order to assess the 68Ge breakthrough. The data were recorded up to 8 days after elution. Calculations showed that the 68Ge/68Ga activity ratio was 1.6600×10-5 at the time of elution.
The concentrations of tin (from the generator material), iron (from the sealing parts and acid impurities), zinc (as a decay product), and gallium (as the target material) were determined, using the ICP-OES method (Table 1).
Table 1.
ICP-OES mass data on various elutions of prototype generator in our study
| Element | Generator 1 (mg/L) | Generator 2 (mg/L) | Generator 3 (mg/L) |
|---|---|---|---|
| Fe | 0.557 | 0.432 | 0.230 |
| Sn | 0.340 | <0.1 | <0.1 |
| Zn | 0.284 | 0.110 | <0.1 |
| Ga | <0.1 | <0.1 | <0.1 |
| Ge | <0.1 | <0.1 | <0.1 |
The radiochemical purity of 68GaCl3 solution was evaluated in two solvents. In 10 mmol*L-1 diethylenetriaminepentaacetic acid (DTPA) aqueous solution (solvent 1), free 68Ga3+ was coordinated to more lipophilic moiety as 68Ga (DTPA)2- and migrated to a higher Rf value (Figure 3). The small radioactive fraction remaining at the origin could be associated with colloids, since in the presence of a strong complexing agent (i.e., DTPA), existence of other ionic species other than 68Ga (DTPA)2- is rare. On the other hand, a 1:1 ratio of 10% ammonium acetate to methanol mixture (solvent 2) was used for the determination of radiochemical purity.
Figure 3.
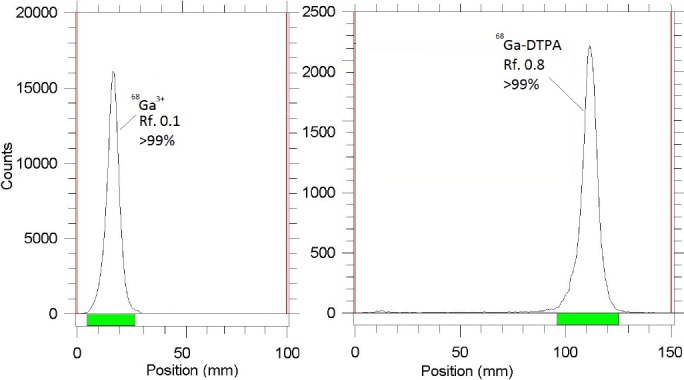
ITLC chromatograms of 68GaCl3 solution in 10% ammonium acetate: methanol (1:1) (left) on Silica gel sheets and in 10 mM DTPA solution (pH~4) on Whatman No. 1 paper (right)
The fast-eluting species were possibly 68Ga and other ionic forms of 68Ga including 68GaCl4- (if existed), which remained at the origin (Rf= 0), and colloids (not detected) (Figure 3). The differences in the impurity peaks in the two chromatograms could be to some extent related to the presence of colloidal impurity, which was insignificant. Additionally, the insignificant amount of activity (about <1%) could be attributed to other ionic impurities.
Radiolabeling
Considering the limited half-life of 68Ga, in order to obtain the best reaction yields in the shortest possible time, optimization studies were conducted, using various citrate sources (i.e., sodium citrate and/or citric acid) and citrate anion concentrations at different temperatures. In order to select the best solid phase/eluent for fast and efficient radiochemical purity control, various solvent mixtures and stationary phases (silica gel and Whatman paper) were employed.
Table 2: demonstrates the Rf for various solvent mixtures, as well as the stationary phases for radiolabeling reactions. However, most of the systems did not provide reproducible and/or clear results for Ga cation and the labeled compound. The best system was considered a mixture of acetone and glacial acetic acid (3:1) in both silica gel and Whatman paper in the stationary phase. Finally, due to better peak distinction and rapid elution time, Whatman paper was selected (Figure 4).
Table 2.
Chromatographic properties of systems used for the determination of 68Ga-citrate radiochemical purity
| Mobile phase | Stationary phase | 68Ga-citrate (Rf) | 68Ga-chloride (Rf) |
|---|---|---|---|
| Methanol/ammonium acetate 10% (1:1) | Whatman | 0.45 | 0.70 |
| Sodium citrate (0.1 M) | Whatman | 0.63 | 0.70 |
| DTPA (1 mM) pH=5 | Whatman | 0.75 | 0.80 |
| DTPA (10 mM) pH=5 | Whatman | 0.80 | 0.85 |
| Methanol/glacial acetic acid (9:1) | Whatman | 0.26 | 0.50 |
| Sodium acetate (1.5 g)+ acetic acid (0.58 ml) in 100 ml of water | Silica gel | 0.40 | 0.50 |
| Sodium acetate (1.5 g)+ acetic acid (0.58 ml) in 100ml of water | Whatman | 0.70 | 0.40 |
| Acetone/glacial acetic acid (3:1) | Whatman | 0.10 | 0.45 |
| Acetone/glacial acetic acid (3:1) | Silica gel | 0.15 | 0.07 |
| Sodium acetate (0.16 M) | Whatman | 0.80 | 0.53 |
| Sodium acetate (0.16 M) | Silica gel | 0.15 | 0.07 |
Figure 4.
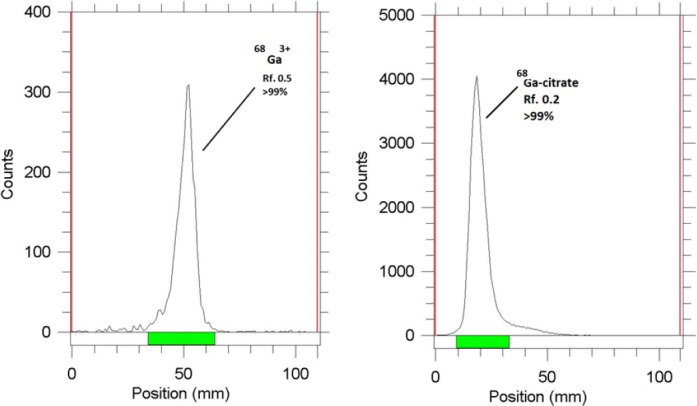
ITLC chromatograms of 68GaCl3 (left) and 68Ga-citrate (right) solutions in acetone/glacial acetic acid (3:1) on Whatman paper
Although the ITLC studies approved the production of the radiolabeled compound, HPLC studies demonstrated the existence of radiolabeled species, using a scintillation detector. A more fast-eluting compound at 3.17 min (using the scintillation detector) was observed for free gallium-68 cation, while for 68Ga-Citrate, a second peak was eluted at minute 4.93, using the scintillation detector (Figure 5).
Figure 5.
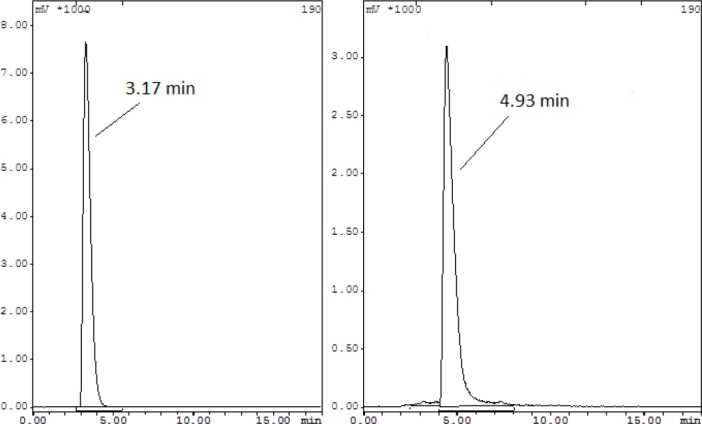
HPLC chromatogram of 68Ga chloride (left) and 68Ga-citrate (right) solutions on a reversed-phase column, using acetonitrile+0.1% trifluoroacetic acid/water+0.1% trifluoroacetic acid (90:10)
Both species were ionic and possibly, using an ion chromatographic column would be of great value for characterization. However, in our setup, at 5-6 min elution time, the two peaks were distinguishable, using the mentioned system.
Biodistribution
Biodistribution study was performed for 68Ga-citrate. The percentages of injected dose per gram (%ID/g) are summarized in Figure 6. As previously reported, 68Ga was excreted majorly from the gastrointestinal tract with high blood content due to transferrin binding at early time intervals (3.2% ID/g at minute 15). Moreover, significant lung, bone, and stomach activities were observed; however, kidney was not a significant accumulation site.
Figure 6.
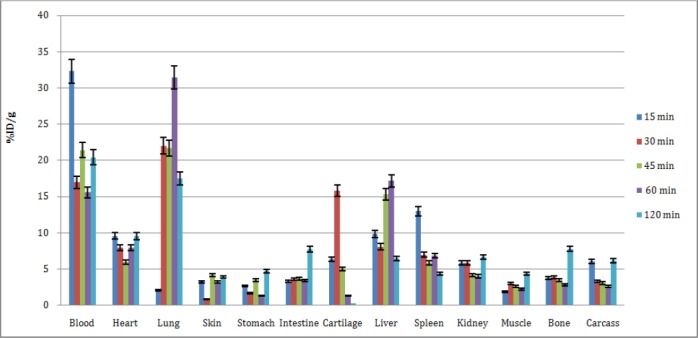
The ID/g percentages of 68Ga-citrate in the tissues of mice at 15-120 min after the injection
Liver was a major accumulation site for transferrin and many radiolabeled proteins. With respect to the ferric ion-mimicking of Ga cation in the body, the liver could be a major accumulation site within 60 min (17% ID/g). Lung and spleen were two important reticuloendothelial organs in which macrophage cells were located. The mechanism of Ga accumulation in WBCs and macrophage cells has been well documented (15), indicating high lung uptake (33% ID/g at 15 min). Significant cardiac uptake was also attributed to ferric cation homology (10% ID/g at 15 and 120 minutes).
Discussion
The results showed better chemical purities for all cation impurities during the development of generators with different particle sizes and packing procedures. As a radiolabeling source for 68Ga-based radiopharmaceuticals, especially ligands with very low molarities (including peptides), this chemical purity is crucial and sufficient for the procedure.
The United States Pharmacopeia has not yet approved the 68Ga generator; however, the European Pharmacopoeia published a monograph on 68Ga edotreotide injection in 2011, covering all aspects of this radiopharmaceutical without pointing out metal impurities (16). On the other hand, other available publications on generator production only focus on the breakthrough of 68Ge and its half-life. In recent works, the best chemical yields have been reported to be less than 0.1 ppm. So far, no pharmacopeial limit for 68Ga generators has been reported in pharmacopoeial references.
One important issue in the elution process was determining the radiochemical form of 68Ga. In highly acidic elutions, the formation of other ionic complexes from the generator including 68GaCl4- was possible. In many cases, this species, present in the solution, did not participate in the complexation process. Dilution and/or pH changes were also not possible due to the formation of volatile species and/or high ionic strength of the solution, which is not recommended for radiopharmaceuticals.
As it was shown, although the reaction would take place in the presence of various citrate sources, the best yields were obtained by using sodium citrate. On the other hand, the reaction was completed within an hour at room temperature; however, applying gentle heat in a water bath at 50 °C provided acceptable radiochemical yields within 10-15 min.
For the best performance, the generator must be eluted daily (even if not used every day) in order to remove the unwanted decay or radiation-induced impurities. Also, when starting the preparation procedure, the first 1-2 ml fractions can be discarded. At a typical run, the 3-5 fractions show the best specific activities daily available.
Rizzello et al. reported a radiochemical yield of 64% for 68Ga-citrate production with an average activity of about 970 MBq available and 98% radiochemical purity (8). On the other hand, our data demonstrated a significant enhanced radiochemical yield of 72% with almost the same radiochemical purity (>98% HPLC) and high specific activity (4-6 GBq/mM).
In many reports, the biodistribtion of free gallium cation has been reported in a 72 h time span, using 67Ga (17). 67Ga is excreted majorly from the gastrointestinal tract; thus, colon and stool uptakes are significant, while blood stream activity is not major in the urinary system. However, for Ga-citrate, data are acquired in a shorter time span. Since Ga as a homolog of ferric cation is bound to plasma proteins, the blood content is high up to 2 hours. Also, the fate of transferrin and similar proteins is determined in the liver, which is the final accumulation site as already seen in case of 68Ga.
Conclusion
In this study, 68Ga-citrate was prepared with acceptable radiochemical purity (>97 ITLC and >98% HPLC), specific activity (4-6 GBq/mM), and chemical purity (Sn, Fe <0.3 ppm and Zn<0.2 ppm) within 15 min at 50 °C. The biodistribution of 68Ga-citrate was consistent with former reports up to 120 min. This study demonstrated possible in-house preparation and quality control of 68Ga-citrate, using commercially available 68Ge/68Ga generator for PET imaging throughout the country.
Acknowledgements
The authors wish to thank Mr. Mazidi for performing animal tests and Ms. Moradkhani for the instrumental support.
References
- 1.Firestone RB, Shirley VS, Baglin CM, Zipkin J. 8th edition. New York: John Wiley and Sons; 1996. Table of isotopes; p. 1447. [Google Scholar]
- 2.DDEP, Decay Data Evaluation Project Data. 2008. http://www.nucleide.org/DDEP_WG/DDEPdata.htm .
- 3.Burleson RL, Johnson MC, Head H. Scintigraphic demonstration of experimental abscesses with intravenous 67Ga citrate and 67Ga labeled blood leukocytes. Ann Surg. 1973;178(4):446–52. doi: 10.1097/00000658-197310000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalilian AR, Novinrooz A, Motamedi-Sedeh F, Moradkhani S, Rajamand AA, Solati J. Evaluation of [67Ga]Citrate in The Detection of Various Microorganism Infections in Animal Models. Iran J Nucl Med. 2009;17(2):34–41. [Google Scholar]
- 5.Nanni C, Errani C, Boriani L, Fantini L, Ambrosini V, Boschi S, et al. 68Ga-citrate PET/CT for evaluating patients with infections of the bone: preliminary results. J Nucl Med. 2010;51(12):1932–6. doi: 10.2967/jnumed.110.080184. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Boddeti DK, Evans SG, Angelides S. (68)Ga-Citrate-PET for diagnostic imaging of infection in rats and for intra-abdominal infection in a patient. Curr Radiopharm. 2012;5(1):71–5. doi: 10.2174/1874471011205010071. [DOI] [PubMed] [Google Scholar]
- 7.Jensen SB, Nielsen KM, Mewis D, Kaufmann J. Fast and simple one-step preparation of 68Ga citrate for routine clinical PET. Nucl Med Commun. 2013;34(8):806–12. doi: 10.1097/MNM.0b013e328363142f. [DOI] [PubMed] [Google Scholar]
- 8.Rizzello A, Di Pierro D, Lodi F, Trespidi S, Cicoria G, Pancaldi D, et al. Synthesis and quality control of 68Ga citrate for routine clinical PET. Nucl Med Commun. 2009;30(7):542–5. doi: 10.1097/MNM.0b013e32832b9ac8. [DOI] [PubMed] [Google Scholar]
- 9.Vorster M, Mokaleng B, Sathekge MM, Ebenhan T. A modified technique for efficient radiolabeling of 68Ga-citrate from a SnO2-based 68Ge/68Ga generator for better infection imaging. Hell J Nucl Med. 2013;16(3):193–8. [PubMed] [Google Scholar]
- 10.Kumar V, Boddeti DK, Evans SG, Angelides S. (68)Ga-Citrate-PET for diagnostic imaging of infection in rats and for intra-abdominal infection in a patient. Current Radiopharmaceut. 2011;5(1):71–5. doi: 10.2174/1874471011205010071. [DOI] [PubMed] [Google Scholar]
- 11.Silvola JM, Laitinen I, Sipilä HJ, Laine VJ, Leppänen P, Ylä-Herttuala S, et al. Uptake of gallium-68 in atherosclerotic plaques in LDLR-/-ApoB100/100 mice. EJNMMI Res. 2011;1(1):14. doi: 10.1186/2191-219X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalilian AR, Bineshmarvasti M, Sardari S. Application of radioisotopes in Inflammation. Curr Med Chem. 2006;13(8):959–65. doi: 10.2174/092986706776361049. [DOI] [PubMed] [Google Scholar]
- 13.Fazaeli Y, Jalilian AR, Ardaneh K, Rahiminejad A, Bolourinovin F, Moradkhani S, et al. Development of a 68Ga-fluorinated porphyrin complex as a possible PET imaging agent. Nucl Med Mol Imaging. 2012;46(1):20–6. doi: 10.1007/s13139-011-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jalilian AR, Rowshanfarzad P, Sabet M, Novinrooz A, Raisali G. Preparation of [66Ga]Bleomycin Complex as a Possible PET Radiopharmaceutical. J Radioanal Nucl Chem. 2005;264:617–21. [Google Scholar]
- 15.Kamada M. Distribution of 67Ga-citrate in tumor tissues and various organs. Radioisotopes (Tokyo) 1978;27(7):390–396. doi: 10.3769/radioisotopes.27.7_390. [DOI] [PubMed] [Google Scholar]
- 16.Gallium (68Ga) endotreotide injection. Pharmeuropa. 2011;23(2):310–3. [Google Scholar]
- 17.Jalilian AR, Yousefnia H, Zolghadri S, Khoshdel MR, Bolourinovin F, Rahiminejad A. Development of radiogallium–ethylenecysteamine cysteine complex as a possible renal imaging agent. J Radioanal Nucl Chem. 2010;284:4–54. [Google Scholar]


