Abstract
This study describes the organic synthesis of 5-(2-amimo-4-styryl pyrimidine-4-yl)-4-methoxy benzofuran-6-ol (SPBF) as an example of a benzofuran derivative used as a new series of amyloid imaging agents. These benzofuran derivatives may be useful amyloid imaging agents for detecting B-amyloid plagues in the brain of Alzheimer’s disease. The precursor is 1-[6-hydroxy-4-methoxybenzofuran-5-yl]-phenyl butadiene ketone, which react with guanidine hydrochloride. The purification process was done via crystallization using solvent ethanol. The overall yield was 75% and the structure of the synthesized compound was confirmed by correct analytical and spectral data. Also, The synthesized compound was labeled with radioactive iodine -125 via electrophilic substitution reaction, in the presence of iodogen as an oxidizing agent, the labeling process was carried out at 95°C for 20min. The radiochemical yield was determined by using a thin layer chromatography and the yield was equal to 80%. Preliminary an in-vivo study examined normal mice after intravenous injection through the tail vein and the data showed the labeling compound was quickly cleared from most body organs. The radioiodinated compound showed high brain uptake. The results of this study suggest that radioiodinated (SPBF) may be useful as a brain imaging agents.
Keywords: Benzofuran derivatives, Iodine -125, imaging agents, Tissues distribution
INTRODUCTION
During the past decades, compounds bearing nitrogen containing heterocyclic rings have received much attention due to their increased chemotherapeutic value and brain penetrating abilities for the development of novel antimicrobials, anthelmintic (1), anti-depressant and agents used in the diagnosis of Alzheimer’s disease (2). Thus, pyridine derivatives continue to attract great interest due to the wide variety of interesting biological activities observed for these compounds, such as anticancer, analgesic, antimicrobial and antidepressant activities (3-5). Heterocyclic molecules can act as highly functionalized scaffolds and are known pharmacophores of a number of biologically active and medicinally useful molecules (6, 7). Cytotoxic drugs remain the mainstay of cancer chemotherapy and are administered with novel ways of therapy such as signal inhibitors (8). It is therefore important to discover novel cytotoxic agents with spectra of activity and toxicity that differ from current agents (8). Also the cytotoxic activity of some benzofuran derivatives were evaluated against HEPG2 (human liver carcinoma cell line) in comparison with 5-fluorouracil (9). Benzofuran derivatives possess a wide range of biological activities. They have been reported to possess antimicrobial (10-14), antitumor (13, 15-17), anti-inflammatory (15) activity etc. Benzothiazoles play a significant role as antibacterial (13, 15-17) and antifungal agents and it has been known that the benzofuran ring system incorporated with different heterocyclic moieties has a wide spectrum of anticancer activity against different types of carcinomas (18-23). There are also some derivatives of pyrazole used as receptors that have important biological effects like tetrahydro and tetrahydrocannabinol (THC), that exert many of their effects on the brain cannabinoid CB1 receptor. A ligand labeled with a radionuclide suitable for positron emission tomographic (PET) or single photon emission computed tomographic (SPECT) imaging could be used to examine the distribution of cannabinoid receptors in the living human brain. However, previous attempts to study THC, in which tetrahydro cannabinol was labeled in the omega position of the alkyl side chain, was only partially successful, probably because of a combination of high lipophilicity and low affinity (24, 25). In this study a simple method is described to prepare 125I-ASPMBF by direct iodination of SPBF with Auger-electron emitter iodine-125 using several oxidizing agents and the optimum conditions required to produce high labeling yield. Preliminary, an in vivo study of 125I-ASPMBF in normal mice was done to elucidate the biological behavior of this labeled compound.
Materials and Methods
All melting points were uncorrected and in degree Celsius (MPA100 melting point Apparatus). The IR spectra were recorded on a pyeunicam sp-11100 spectrophotometer. Mass spectras were performed by a shimadzu Gc-MS-QP 100 Ex (shimadzu, Japan). Elemental analysis was carried out by the Micro analytical Research Center, Faculty of Science, Cairo University. Radioactivity was measured by the means of a gamma counter (Nucleus Model 2010) connected with a well type NaI (Tl) crystal. All other chemicals were purchased from Merck Co. Radioactive iodine-125 was purchased from the Institute of Isotopes Co. Ltd. (IZOTOP) Budapest, Hungary.
Procedures
1-[6-hydroxy-4-methoxy benzofuran-5-yl]-5-phenylpenta-2,4-dien-1-one(II)
To a solution of compound (I) (3.2 gm, 0.01 mol) and cinamaldehyde (0.01 mol) in ethanol (30 ml) 10% alcoholic sodium hydroxide (5 ml) was added and the reaction mixture was stirred at room temperature for 30 min. The reaction mixture was acidified with hydrochloric acid and the resulting solid was washed with water and crystallized by ethanol to give compound (II) (Table 1).
Table 1.
Characteristics data for the prepared compounds

5-(2-amimo-4-styryl pyrimidine-4-yl)-4-methoxy benzofuran-6-ol
A mixture of compound (II) (3.2 gm, 0.01 mol) guanidine hydrochloride (0.59 gm, 0.01 mol) and potassium hydroxide (0.5 gm) in ethanol (50 ml) was refluxed for 4 hours, then allowed to cool. The solid product was collected and crystallized from ethanol to produce compound (III).
Iodination
The iodination process was achieved using two oxidizing agents, chloramine-T and iodogen. The ASPMBF compound was labeled with 125I using the chloramine–T method. Briefly, 0.2 mg of the compound was dissolved in 80µl of glacial acetic acid and to this solution 10µl of sodium 125I iodide (50 µCi) was added, followed by 20 µl of 0.1% chloramine-T solution in 0.05 M phosphate buffer (pH 7.4). After 2 min, the reaction mixture was quenched with 20 µl of 0.2% sodium metabisulfite in 0.05 M phosphate buffer (7.4). After adding 50 µl of saturated sodium bicarbonate, the radiochemical purity of labeled compound was checked by TLC, paper chromatographic methods and paper electrophoresis.
Preparing iodogen coated tubes and coated glass frit
20 µl of iodogen (1 mM, 668.10-6 g/l) was dissolved in chloroform and transferred to glass tubes. CHCl3 was evaporated by dry N2 gas and iodogen was deposited on the wall of the glass tube as a thin film. The other method was carried out by dissolving 334×10-6 g/l of iodogen in a glass tube containing glass frit and chloroform; this system was then allowed to dry under dry N2 gas. These tubes were stored at 0°C until use.
Determination of radiochemical yield and purity
Radiochemical yield and purity of the radiodinated compound was determined by
TLC chromatographic method
This technique was done using a thin layer silica gel coated on an aluminum sheet (20 cm×20 cm). It was cut into strips, each strip was 1.5 cm wide and 13 cm long; the spotted point was placed 2 cm above the edge. The solvent used for development was a methylene chloride : hexane mixture (4:1, v/v), radioiodide 125I remained near the origin (Rf=0-0.1), while the labeled compounds (125I-SPBF) moved to the solvent front ((Rf=0.9). the radiochemical yield (%) at the time (t), were calculated as the percent ratio of activity on the TLC-strip according to the following equation: Radiochemical yield (%)= Activity of labeled product×100 / total activity.
Paper chromatographic method
This technique was done using strips of Whatman paper. On a 1 cm wide and 13 cm long strip, 1-2 µl of the reaction mixture was placed 2 cm above the lower edge and allowed to evaporate spontaneously. For development, a fresh mixture of chloroform: ethanol (9:1, v/v) was used. After complete development, the paper sheet was removed, dried, and cut into strips, each strip was 1 cm wide, strips were then counted in a well type gamma counter.
Paper electrophoresis
Radiochemical yield was further confirmed by paper electrophoresis. On a Whatman paper sheet (2cm width and 47 cm length), 1-2 µl of the reaction mixture was placed 12cm above the lower edge and allowed to evaporate spontaneously. Electrophoresis was carried out for 1 h at a voltage of 300 V using normal saline (0.9% w/v NaCl solution) as an electrolytes source. After complete development, the paper was removed, dried, and cut into strips, each strip was 1 cm wide, and the strip were counted in a well type gamma counter. The percentage of radiochemical yield was estimated as the ratio of the radioactivity of radioiodinated compounds to the total activity multiplied by 100.
Octanol distribution
Synthesized compound was mixed with a 1:1 (wt /wt) mixture of 1-octanol and 0.1 M phosphate buffer (pH 7.4), in a centrifuge tube. The mixture was vortexed at room temperature for 1 min and then centrifuged at 5,000 rpm for 5 min. Subsequently 100 µl samples from the 1-octanol and aqueous phases were pipetted into other test tubes and counted in a gamma counter. The measurement was repeated three times. The partition coefficient (P) was calculated as the ratio of optical density in the organic phase to the optical density in the aqueous phase (26-28).
Biodistribution studies
Animals
Albino type mice, weighing 20-25g were used for the biological distribution study.
Method
This experiment was done by diluting the neutral solution of labeled 125I-ASPMBF with 3ml of saline for injection and the resulting solution was filtered through a 0.22 µm Millipore filter into a sterile sealed vial.100 µl (100-150 MBq) was injected in the tail vein of Albino white mice (3 groups each with 3 mice, with approximate weight of 25 g). The mice were maintained on a normal diet in metabolic cage. The mice were sacrificed at 30min and 1 h post- injection. Samples of fresh blood, bone and muscle were collected in pre-weighted vials and counted. The different organs were removed, counted and compared to a standard solution of the 125I-ASPMBF.
The in-vitro stability
The stability assessment of the labeled product was performed by TLC, using a methylene chloride : hexane mixture (4:1, v/v) as a developing solvent. The study continued for up to 36 h and the data was recorded at pre-planned time intervals.
RESULTS AND DISCUSSION
Chemical synthesis
Scheme 1 outlines the synthesis of benzofuran derivative. 1-[6-hydroxy-4-methoxy benzofuran-5-yl] ethanone (I) reacted with cinnamaldehyde forming 1-[6-hydroxyl-4-methoxybenzofuran-5-yl]-5-phenylpenta-2,4-diene-1-one(II) (Scheme 1). Compound (II) was established by correct analytical and spectral data. The mass spectrum afforded a molecular ion peak at m/z 320 [M+, 50%] with a base peak at 190 and the following observed peaks at 227 (20.2%), 230 (16.3%), 164 (38.04%), 148 (20.6%) and 117 (1603%) which were compatible with the molecular formula C20H16O4 (Chart 1).
Scheme 1.

Chart 1.

Furthermore, the reaction of compound (II) with a binucleophilic reagent was investigated. Interaction of compound (II) with guanidine hydrochloride in the presence of potassium hydroxide resulted in a pyrimidine derivative or its possible isomer (IIIa-IIIb) (scheme 1). The reaction continued via Michael addition followed by intermolecular cyclization followed by water elimination. IR spectrum of (III) showed the disappearance of the carbonyl group which was found in the parent compound and showed bands at 3124 & 3164 for NH2 and 3413 for the OH groups. Radiochemical purity of 125I-ASPMBF
The radiochemical purity of the 125I-ASPMBF was determined using paper chromatography where radioiodide (-I) remained near the origin (Rf = 0 - 0.1), while the 125I-ASPMBF moved to the solvent front (Rf=0.9). Radiochemical purity was further confirmed by paper electrophoresis where the radioiodide, 125I-ASPMBF moved to different distances away from the spotting point towards the cathode depending on the molecular weight of each one (distance from spotting point=12, 10 and 8 cm, respectively) as in (Figure 1).
Figure 1.

Electrophoresis radiochromatogram of 125I-ASPMBF
Effect of the oxidizing agents
The study was carried out to optimize the synthesis of 125I-ASPMBF using different oxidizing agents, including chloramines-T and iodogen. Chloramine-T (CAT) was used in the range of 1-7 nmol, and the labeling yield increased by rising the CAT concentration. A labeling yield of 90± 1% was obtained at 7 nmol of CAT as shown in table 2. Also, higher concentration of iodogen did not produce a high yield of 125I-ASPMBF and it took a long time (20 min) to obtain 75± 0.5% yield of 125I-ASPMBF with iodogen
Table 2.
Radiochemical yield from labeling of 125I-ASPMBF with different concentrations of oxidizing agents

Effect of pH
The hydrogen ion concentration of the reaction mixture was found to be critical. The effect of the pH on the labeling of SPBF was investigated with pH ranging from 1-10 for both oxidizing reagents (Figure 2). A yield of 90±1 was obtained at pH value equal to 7 when CAT was used as an oxidizing agent. This is due to the fact that cleavage of the aryl compounds is electrophilic in nature and is accelerated by acid. This finding is in complete agreement with the statement by El-Zahar et al (29).
Figure 2.

Radiochemical yield of 125I-SPBF as a function of pH Reaction condition: 0.2 mg of ASPMBF +10 µl Na125I (3.7 MBq) + 20 µl oxidizing agent at different pH values, reaction time was 20 min at room temperature
Effect of reaction time
The labeling yield of 125I-ASPMBF was strongly dependent on reaction time. The time points for this experiment ranged between 10 sec to 60 min and it is clear from Fig 3 that the yield was significantly increased with increase of reaction time. The results indicate that the reaction was very fast. After 2-3 min, the radiochemical yield was the same for both oxidizing agent. At 20 min the maximum radiochemical labeling (90 ± 2.3 %), (70 ± 1.5) was obtained for Chloramines-T and iodogen, respectively.
Figure 3.

Radiochemical yield of 125I-SPBF as a function of reaction time. Reaction condition: 0.2 mg of substrate +10 µl Na125I + 20 µl oxidizing agent at pH value of 7 for different time intervals at room temperature.
Effect of the amount of substrate:
The quantity of the substrate precursor played a role in the labeling of ASPMBF with iodine-125, but this was not as significant as the effect of pH of the reaction mixture as shown in Figure 4. Twenty micrograms of the precursor was not found sufficient to produce high yields of 125I-ASPMBF. Multiplying this quantity by the factor of 2.5 or 3 gives a radiochemical yield of 98 ± 1 when CAT was used as an oxidizing agent.
Figure 4.

Radiochemical yield of 125I-SPBF as a function of substrate concentration. Reaction condition: x mg of ASPMBF +10 µl Na125I + 20 µl (CAT) at pH value of 7.5 for 20 min at room temperature.
The in-vitro stability
The in-vitro stability of the labeled 125I-ASPMBF was studied in order to determine the suitable injection for avoiding the formation of the undesired products, which resulted from the radiolysis of the labeled compounds. These undesired radioactive products might be toxic or accumulate in undesired organ. The data presented in table 3 shows that the tracer is stable for up to 5h and can be injected without any precaution, due to the formation of by products, which may be formed from the radiolysis of the labeled products.
Table 3.
Stability of 125I-ASPMBF

Biological study
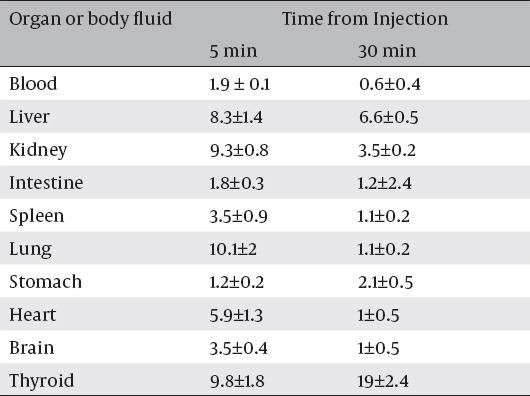
The biological pathway of 125I was done for three healthy mice, to elucidate its normal uptake by mice organs. The data of this study are presented in tables 4 and 5. The thyroid gland is the normal trapping site of iodine, as iodide combines with triiodothyronine (T3) changing it to tetraiodothyronine (T4). Our data showed that the activity of the thyroid was more than 19 % at 30 min post injection. Benzofuran showed a high and fast brain uptake and a fast washout from the brain in the normal mice. The 125I-ASPMBF could be used as an imaging or a therapeutic agent. To follow up the biological distribution of the 125I-ASPMBF, it was injected in normal albino mice via the tail vein, and the organs uptake was determined at different time intervals. The results of this study is summarized in table 4, the 125I-SPBF tracer shows early high uptake in the stomach and heart equal to 6.2 % ± 0.2 and 6.9 % ± 1.3 at 5 min post injection, respectively.
Table 4.
Bio-distribution of radioactivity after intravenous administration of 125I-ASPMBF in mice. (% ID/g ± SD, n=5)

Table 5.
Bio-distribution of iodine-125 in normal mice (Vial content: 100 µl). (% ID/g ± SD)
References
- 1.Watanabe H, Ono M, Kimura H, Kagawa S, Nishii R, Fuchigami T, et al. A dual fluorinated and iodinated radiotracer for PET and SPECT imaging of beta-amyloid plaques in the brain. Bioorganic & Medicinal Chemistry Letters. 2011;21(21):6519–22. doi: 10.1016/j.bmcl.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 2.Cheng Y, Ono M, Kimura H, Kagawa S, Nishii R, Saji H. A novel 18F-labeled pyridyl benzofuran derivative for imaging of β-amyloid plaques in Alzheimer’s brains. Bioorganic & Medicinal Chemistry Letters. 2010;20(20):6141–4. doi: 10.1016/j.bmcl.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Nofal ZM, Soliman EA, Abd El-Karim SS, El Zahar MI, Srour AM, Sethumadhavan S, et al. Novel benzimidazole derivatives as expected anticancer agents. Acta poloniae pharmaceutica. 2011;68(4) [PubMed] [Google Scholar]
- 4.Bednarczyk-Cwynar B, Zaprutko L, Marciniak J, Lewandowski G, Szulc M, Kaminska E, et al. The analgesic and anti-inflammatory effect of new oleanolic acid acyloxyimino derivative. European Journal of Pharmaceutical Sciences. 2012;47(3):549–55. doi: 10.1016/j.ejps.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Judge V, Narasimhan B, Ahuja M, Sriram D, Yogeeswari P, Clercq E, et al. Isonicotinic acid hydrazide derivatives: synthesis, antimicrobial activity, and QSAR studies. Medicinal Chemistry Research. 2012;21(7):1451–70. doi: 10.2174/157340613804488404. [DOI] [PubMed] [Google Scholar]
- 6.Kumar P, Narasimhan B, Yogeeswari P, Sriram D. Synthesis and antitubercular activities of substituted benzoic acid N-(substituted benzylidene/furan-2-ylmethylene)-N-(pyridine-3-carbonyl)-hydrazides. European Journal of Medicinal Chemistry European Journal of Medicinal Chemistry. 2010;45(12):6085–9. doi: 10.1016/j.ejmech.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Guilbaud N, Kraus-Berthier L, Meyer-Losic F, Malivet V, Chacun C, Jan M, et al. Marked antitumor activity of a new potent acronycine derivative in orthotopic models of human solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7(8):2573–80. [PubMed] [Google Scholar]
- 8.Manna K, Agarwal YK, Srinivasan KK. Synthesis and biological evaluation of new benzofuranyl isoxazoles as antitubercular, antibacterial and antifungal agents. INDIAN JOURNAL OF HETEROCYCLIC CHEMISTRY. 2008;18(1):87–8. [Google Scholar]
- 9.El-Zahar MI, Adb El-Karim SS, Haiba ME, Khedr MA. Synthesis, antitumor activity and molecular docking study of novel benzofuran-2-yl pyrazole pyrimidine derivatives. Acta poloniae pharmaceutica. 2011;68(3) [PubMed] [Google Scholar]
- 10.Kumar DBA, Prakash GK, Kumaraswamy MN, Nandeshwarappa BP, Sherigara BS, Mahadevan KM. Synthesis and antimicrobial investigation of some novel phenyl pyrazole, azetidinone and diazenyl ethanone derivatives of benzofurans. Indian journal of chemistry Section B, Organic including medicinal. 2007;46(2):336. [Google Scholar]
- 11.Benkli K, Demirayak S, Gundogdu-Karaburun N, Kiraz N, Iscan G, Ucucu U. Synthesis and Antimicrobial Activities of Some Imidazole Substituted Indoles. ChemInform ChemInform. 2004;35(17) [Google Scholar]
- 12.Karaburun AC, Gundogdu-Karaburun N, Ucucu U, Demirayak S. Synthesis and Antifungal Activities of Some Aryl Naphthofuran Ketoximes. lett drug des discov Letters in Drug Design & Discovery. 2011;8(8):758–62. [Google Scholar]
- 13.Hayakawa I, Shioya R, Agatsuma T, Furukawa H, Naruto S, Sugano Y. 4-Hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as potent anti-tumor agents. Bioorganic & medicinal chemistry letters. 2004;14(2):455–8. doi: 10.1016/j.bmcl.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Urzúa A, Echeverría J, Rezende MC, Wilkens M. Antibacterial properties of 3 H-spiro[1-benzofuran-2,1′-cyclohexane] derivatives from Heliotropium filifolium. Molecules. 2008;13(10):2385–93. doi: 10.3390/molecules13102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galal SA, Abd El-All AS, Abdallah MM, El-Diwani HI. Synthesis of potent antitumor and antiviral benzofuran derivatives. Bioorganic & Medicinal Chemistry Letters Bioorganic & Medicinal Chemistry Letters. 2009;19(9):2420–8. doi: 10.1016/j.bmcl.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 16.Galal SA, Abd El-All AS, Hegab KH, Magd-El-Din AA, Youssef NS, El-Diwani HI. Novel antiviral benzofuran-transition metal complexes. European Journal of Medicinal Chemistry. 2010;45(7):3035–46. doi: 10.1016/j.ejmech.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Jang DS, Park EJ, Kang Y-H, Su B-N, Hawthorne ME, Vigo JS, et al. Compounds obtained fromSida acuta with the potential to induce quinone reductase and to inhibit 7,12-dimethylbenz-[a]anthracene-induced preneoplastic lesions in a mouse mammary organ culture model. Arch Pharm Res Archives of Pharmacal Research. 2003;26(8):585–90. doi: 10.1007/BF02976704. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa I, Shioya R, Agatsuma T, Sugano Y. Synthesis and evaluation of 3-methyl-4-oxo-6-phenyl-4,5,6,7-tetrahydrobenzofuran-2-carboxylic acid ethyl ester derivatives as potent antitumor agents. Chemical & pharmaceutical bulletin. 2005;53(6):638–40. doi: 10.1248/cpb.53.638. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M, Hayakawa I, Hayashi N, Agatsuma T, Oda Y, Tanzawa F, et al. Synthesis and biological evaluation of benzothiazole derivatives as potent antitumor agents. Bioorganic & medicinal chemistry letters. 2005;15(14):3328–32. doi: 10.1016/j.bmcl.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 20.Lee SK, Cui B, Mehta RR, Kinghorn AD, Pezzuto JM. Cytostatic mechanism and antitumor potential of novel 1H-cyclopenta[b]benzofuran lignans isolated from Aglaia elliptica. Chemico-Biological Interactions Chemico-Biological Interactions. 1998;115(3):215–28. doi: 10.1016/s0009-2797(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 21.Pautus S, Aboraia AS, Bassett CE, Brancale A, Coogan MP, Simons C. Design and synthesis of substituted imidazole and triazoleN-phenylbenzo[d]oxazolamine inhibitors of retinoic acid metabolizing enzyme CYP26. Journal of Enzyme Inhibition and Medicinal Chemistry Journal of Enzyme Inhibition and Medicinal Chemistry. 2009;24(2):487–98. doi: 10.1080/14756360802218334. [DOI] [PubMed] [Google Scholar]
- 22.Pautus S, Yee SW, Jayne M, Coogan MP, Simons C. Synthesis and CYP26A1 inhibitory activity of 1-[benzofuran-2-yl-(4-alkyl/aryl-phenyl)-methyl]-1H-triazoles. Bioorganic & Medicinal Chemistry. 2006;14(11):3643–53. doi: 10.1016/j.bmc.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Saberi MR, Vinh TK, Yee SW, Griffiths BJN, Evans PJ, Simons C. Potent CYP19 (Aromatase) 1-[(Benzofuran-2-yl)(phenylmethyl)pyridine, -imidazole, and -triazole Inhibitors:ÂSynthesis and Biological Evaluation. J Med Chem Journal of Medicinal Chemistry. 2006;49(3):1016–22. doi: 10.1021/jm0508282. [DOI] [PubMed] [Google Scholar]
- 24.Charalambous A, Marciniak G, Shiue CY, Dewey SL, Schlyer DJ, Wolf AP, et al. PET studies in the primate brain and biodistribution in mice using (-)-5’-18F-delta 8-THC. Pharmacology, biochemistry, and behavior. 1991;40(3):503–7. doi: 10.1016/0091-3057(91)90354-5. [DOI] [PubMed] [Google Scholar]
- 25.Charalambous A, Lin S, Marciniak G, Banijamali A, Friend FL, Compton DR, et al. Pharmacological evaluation of halogenated delta 8-THC analogs. Pharmacology, biochemistry, and behavior. 1991;40(3):509–12. doi: 10.1016/0091-3057(91)90355-6. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Cao X, He W, Song J, Dai Z, Zhang B, et al. Monitoring of tumor response to cisplatin by subsurface fluorescence molecular tomography. J Biomed Opt Journal of Biomedical Optics. 2012;17(4):040504. doi: 10.1117/1.JBO.17.4.040504. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Luo L, Wei Y, Wang W, Li B, Yan L, et al. A functional NQO1 609C>T polymorphism and risk of hepatocellular carcinoma in a Chinese population. Tumor Biol Tumor Biology. 2012;(5 Suppl.2) doi: 10.1007/s13277-012-0509-x. [DOI] [PubMed] [Google Scholar]
- 28.Liu F-y, Luo K-w, Yu Z-m, Co N-n, Wu S-h, Wu P, et al. Suillin from the mushroom Suillus placidus as potent apoptosis inducer in human hepatoma HepG2 cells. Chemico-biological interactions. 2009;181(2):168. doi: 10.1016/j.cbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 29.el-Zahar MI, Kamel MM, Anwar MM. New tetrahydronaphthyl thiazole derivatives. Die Pharmazie. 1994;49(8):616–7. [PubMed] [Google Scholar]


