Abstract
Objective(s):
Recently, bone-avid radiopharmaceuticals have been shown to have potential benefits for the treatment of widespread bone metastases. Although 177Lu-triethylene tetramine hexa methylene phosphonic acid (abbreviated as 177Lu-TTHMP), as an agent for bone pain palliation, has been evaluated in previous studies, there are large discrepancies between the obtained results. In this study, production, quality control, biodistribution, and dose evaluation of 177Lu-TTHMP have been investigated and compared with the previously reported data.
Methods:
TTHMP was synthesized and characterized, using spectroscopic methods. Radiochemical purity of the 177Lu-TTHMP complex was determined using instant thin-layer chromatography (ITLC) and high performance liquid chromatography (HPLC) methods. The complex was injected to wild-type rats and biodistribution was studied for 7 days. Preliminary dose evaluation was investigated based on biodistribution data in rats.
Results:
177Lu was prepared with 2.6-3 GBq/mg specific activity and radionuclide purity of 99.98%. 177Lu-TTHMP was successfully prepared with high radiochemical purity (>99%). The complex showed rapid bone uptake, while accumulation in other organs was insignificant. Dosimetric results showed that all tissues received almost insignificant absorbed doses in comparison with bone tissues.
Conclusion:
Based on the obtained results, this radiopharmaceutical can be a good candidate for bone pain palliation therapy in skeletal metastases.
Keywords: Bone pain palliation, Dosimetry, Lu-177, TTHMP
Introduction
Bone metastasis is a common and severe complication in advanced stages of cancer (1, 2). It develops in up to 70% of patients with prostate cancer and breast cancer, and in nearly 30% of those with lung, bladder, and thyroid cancers (3, 4).
Standard treatments for this condition include systemic therapies (e.g., analgesics, chemotherapy, hormonal therapy, and bis-phosphonates) and local control (e.g., radiation therapy, radiofrequency ablation, and surgical stabilization of the affected site) (5).
The proven efficacy and minimal toxicity of radiation therapy make it very suitable for pain palliation in cancer patients (3). Radionuclide therapy, by using specific tumor-seeking radiopharmaceuticals, can be applied as a treatment for bone metastases. Recently, various phosphonate ligands, labeled with β-emitting radionuclides, have been effective in metastatic bone pain palliation (6, 7).
Successful bone pain palliation is based on selective concentration and prolonged retention of the radiopharmaceutical at skeletal lesions, while keeping the bone marrow absorbed dose as low as possible (8); for this purpose, low energy β-particles are recommended.
Recently, radiopharmaceuticals with radio-nuclides such as 32P, 89Sr, 186Re, 188Re, 153Sm, 166Ho, and 177Lu have been developed for the treatment of painful metastases. Among these radioisotopes, 177Lu [t1/2=6.73 d, Eβ(max) = 497 keV, Eγ=112 keV (6.4%), 208 keV (11%)] has notable characteristics. The significant advantage of utilizing 177Lu is its β-particle energies which are adequately low; therefore, bone marrow suppression is minimum when it accumulates in skeletal lesions (9, 10). Various bone-avid agents with 177Lu have been developed and used for pain palliation including 177Lu-DOTMP (11) and 177Lu-EDTMP (12).
Improved cancer treatments and increased emphasis on the quality of life of cancer patients have led to a search for effective pain palliation for clinically active bone metastases, with fewer short-term and long-term side-effects. Therefore, various 177Lu complexes including PYP (13), DTPMP, TTHMP (14), MDP (15), HEDP (16), and CTMP (17) have been developed and evaluated.
Among these radiopharmaceuticals, 177Lu-triethylene tetramine hexa methylene phosphonic acid (abbreviated as 177Lu-TTHMP) has been shown to have favorable characteristics. This complex accumulates massively in the bone, while its accumulation in other critical organs is very slow. Previous studies on 177Lu-TTHMP have shown that this complex is comparable to other radiopharmaceutical complexes such as 188Re-HEDP and 177Lu-EDTMP, used in clinical trials; however, the results of these studies are inconsistent. Furthermore, despite the direct relationship between the absorbed dose and the effect of radiopharmaceuticals in disease management, the absorbed dose of this complex has not been reported, so far.
Accordingly, in this study, TTHMP was synthetized and labeled with 177Lu. The chemical structure of the ligand is demonstrated in Figure 1. The parameters affecting radiochemical purity such as ligand concentration, pH of the reaction mixture, and incubation time were investigated. Biodistribution of the complex in wild-type rats was surveyed for better comparison with the reported studies. Finally, the preliminary dose evaluation in different human organs was investigated based on distribution data in rats.
Figure 1.
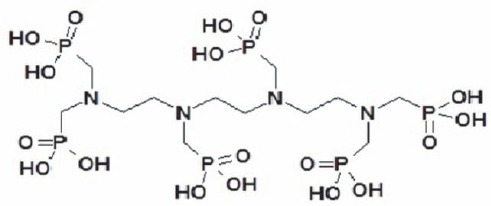
The chemical structure of TTHMP
Methods
177Lu was produced by irradiation of natural Lu2O3 target at a thermal neutron flux of approximately 4×1013 n/cm2/s for 5 days at Tehran Research Reactor (TRR). Whatman No. 3 paper was obtained from Whatman, UK.
Radiochromatography was performed using a Bioscan AR-2000 radio-TLC scanner (Bioscan, France). Analytical high performance liquid chromatography (HPLC) method was applied to determine specific activity of the complex by a Shimadzu LC-10AT, equipped with two detector systems, flow scintillation analyzer (Packard-150 TR), and UV-visible spectrophotometer (Shimadzu), using Whatman Partisphere C-18 column, 250×4.6 mm (Whatman Co., NJ, USA).
A high-purity germanium (HPGe) detector, coupled with a Canberra™ multichannel analyzer (model GC1020-7500SL, Canberra Industries Inc., CT, U.S.A.) and a dose calibrator ISOMED 1010 (Elimpex-Medizintechnik, Austria) was used for measuring the distributed activity in rat organs. All other chemical reagents were purchased from Merck, Germany.
Calculations were based on the 112 kev peak for 177Lu. All values were expressed as mean ± standard deviation, and the data were compared using Student’s T-test. Animal studies were carried out in accordance with the United Kingdom Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations (2nd edition). The approval of NSTRI Ethical Committee was obtained for conducting this research.
The wild-type rats (NMRI), weighing 180-200g, were purchased from Pasteur Institute of Karaj, Iran, and were acclimatized to proper rodent diet.
Production and quality control of 177LuCl3 solution
Lutetium-177 was produced by neutron irradiation of 1 mg of natural Lu2O3 (99.999% from Aldrich Co., UK), according to the reported procedures (18) at Tehran Research Reactor (TRR). The irradiated target was dissolved in 200 µL of 1.0 M hydrochloric acid (HCl) to prepare 177LuCl3; then, it was diluted to the appropriate volume with ultrapure water to produce a stock solution with the final volume of 5 ml (0.04 mol/l).
The mixture was filtered through a 0.22 µm filter for sterilization (Waters, U.S.A). The radionuclidic purity of the solution was tested for the presence of other radionuclides, using HPGe spectroscopy for the detection of various interfering gamma-emitting radionuclides. The radiochemical purity of 177LuCl3 was checked using 2-solvent systems for instant thin-layer chromatography (ITLC) [A: 10 mmol/l diethylene triamine pentaacetic acid (DTPA) at pH 5 and B: 10% ammonium acetate-methanol (1:1)].
Synthesis of TTHMP
The experimental procedure for the synthesis of TTHMP ligand was in accordance with other bisphosphonates, as reported (14). Briefly, a quantity of 0.48 g (3.3 mM) of triethylenetetramine was dissolved in 0.75 ml of concentrated HCl and a concentrated aqueous solution of 1.62 g phosphorous acid (20 mM). The resulting solution was heated to the reflux temperature and 3.2 ml of 37% aqueous formaldehyde solution (40 mM) was added dropwise during 1 h to the refluxing solution, and the refluxing was continued for another 1 h. The result of the reaction was a precipitated ethanol of a white product from the concentrated reaction solution.
Radio-labeling of TTHMP with 177LuCl3
A stock solution of TTHMP was prepared by dissolution in 1 N NaOH) and was diluted to the appropriate volume with ultrapure water through dissolving a specific amount of ligand in 1.5 ml NaOH (2 N) and 3.5 ml distilled H2O (pH=12). Then, 0.3 ml of this solution was added to 200 µl 177LuCl3 (210.9 MBq) and pH was adjusted to 7, using a phosphate buffer.
The reaction mixtures were incubated by stirring at room temperature for 1 h. Various parameters such as ligand concentration, pH of the reaction mixture, and incubation time were optimized to achieve maximum complexation yield. The radiolabeling yield of the ligand was determined with paper chromatography, using Whatman No. 3 paper by sampling 5 µl of the reaction mixture on the paper strip, developed in NH4OH:MeOH:H2O (1:10:20) mixture.
Stability studies
Stability of the complexes stored at room temperature (22°C) and in human serum (37°C) was studied at different intervals by determining the radiochemical purity of complexes, using paper chromatography in NH4OH:MeOH:H2O (1:10:20) system.
In vitro protein binding of 177Lu-TTHMP in presence of human serum
In vitro protein binding of the complex was carried out in human blood by protein precipitation. One ml of the labeled complex was mixed with 3 ml of fresh human plasma, and incubated for 1 h at 37°C. Content of the tube was centrifuged at 3000 rpm for 10 min in order to separate the serum from blood cells. After mixing an approximately equal volume of 10% trichloroacetic acid (TCA), the mixture was centrifuged at 3000 rpm for 10 min. Residue was separated from supernatant and both layers were counted for radioactivity in a well-type gamma counter. Protein binding of the complex was expressed as a fraction of protein-bound radioactivity in percentage of total radioactivity.
Biodistribution of 177Lu-TTHMP in wild-type rats
Final 177Lu-TTHMP solution of 3.7 MBq in 50-100 μl was injected intravenously to the rats through their tail vein. The animals were sacrificed at specified time intervals (2, 4, 24, 48, 72, and 168 h), and specific activity of different organs was calculated as the percentage of injected dose per gram (%ID/g), using HPGe detector.
Dosimetric studies
The absorbed dose of each human organ was calculated by medical internal radiation dosimetry (MIRD) method, based on biodistribution data in wild-type rats. For this purpose, first the accumulated source activity was calculated by plotting the clearance curves for each organ and computing the area under the curves. Then, the accumulated activity in animals was extrapolated to the accumulated activity in humans by the proposed method of Sparks et al. (equation 1) (19).

In order to extrapolate the accumulated activity to human, the mean weight of each organ for standard human were considered. Finally, the absorbed radiation dose was calculated by MIRD formulation (20):

Where D(rk) is the absorbed dose of the target organ and S(rk) is defined as the mean absorbed dose to the target region rk per unit accumulated activity in the source region rh. The S factors have been obtained from OLINDA software (21)
Results
Radionuclide production
The radionuclide was prepared in the range of 2.6-3 GBq/mg specific activity for radiolabeling use. After counting the samples on the HPGe detector for 5 h, two major photons (6.4% of 0.112 MeV and 11% of 0.208 MeV) were observed.
The radiochemical purity of the 177Lu solution was checked in the 2 solvents. In 10 mmol/l DTPA aqueous solution (solvent 1), free Lu3+ cation was complexed to more lipophilic Lu-DTPA form and migrated to higher Rf. The small radioactive fraction, which remained at the origin, could be related to other Lu ionic species, which were not involved in forming Lu-DTPA complex, such as LuCl4- and/or colloids. On the other hand, 10% ammonium acetate-methanol mixture (1:1) (solvent 2) was used for the determination of radiochemical purity.
Ligand synthesis
The TTHMP ligand was synthesized and its structure was determined using Proton Nuclear Magnetic Resonance (1H NMR), mass, and infrared (IR) methods.
TTHMP: [m.p. 90-92°C, 1H-NMR (D2O, δ [ppm]): 3.02-3.25(m, 12 H, >N-CH2CH2-N<), 3.37-3.47(m, 12 H, -NCH2-PO3H2)].
Mass: m/z (%), 710 (M+, % 26), 370 (%55), 233 (%44), 116 (%63), 110 (%100).
Labeling optimization studies
In order to obtain maximum complexation yields, several experiments were carried out by various reaction parameters such as ligand concentration, pH, and reaction time. The effect of pH variation on complexation yield of all ligands at room temperature was also studied by varying the pH of reaction mixture from 2 to 12, using 1 M HCl or 2 M NaOH solution. Maximum yield of 99% was observed at pH=7-8 for complexes. The effect of pH on complexation yield is shown in Figure 2.
Figure 2.
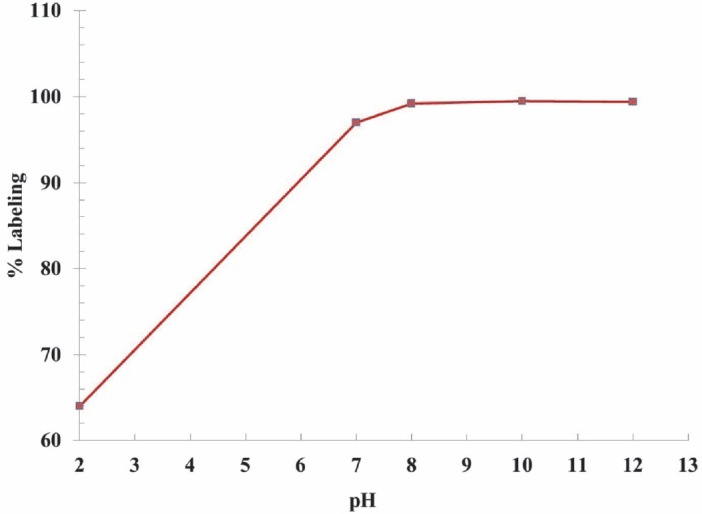
Effect of pH variation on complexation yield of 177Lu-TTHMP at room temperature
Ligand concentration was varied within a wide range of 10 to 50 mg/ml for each ligand. It was observed that at room temperature, complexation >99% was achieved with 50:1 ligand-molar ratio.
The reaction mixture was incubated at room temperature for different time periods and 60 min incubation was found to be adequate to
yield maximum complexation. The best ITLC mobile phase was Whatman 3 MM paper, using NH4OH: MeOH: H2O (1:10:20), as shown in Figure 3.
Figure 3.
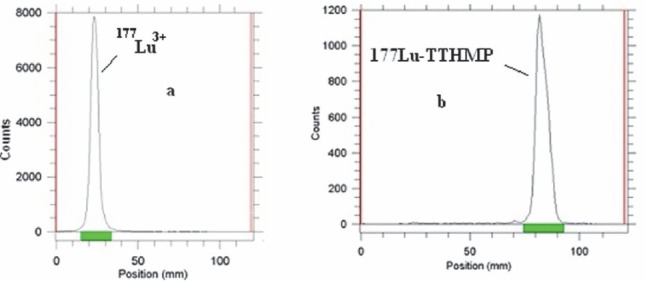
(a) ITLC chromatograms of 177LuCl3 solution, and (b) 177Lu-TTHMP in a NH4OH: MeOH: H2O (0.2:2:4) solution, using Whatman 3 MM
Although the ITLC studies confirmed the production of the radiolabeled compound, HPLC studies demonstrated the existence of one radiolabeled species, utilizing both UV and scintillation detectors.
Stability studies
The stability of 177Lu-TTHMP, prepared under optimized reaction conditions, was studied, and the complex showed excellent stability both in human serum at 37°C and at room temperature after 72 h.
In vitro protein binding of the complex was carried out in human blood by protein precipitation. Protein binding of the complex was expressed as a fraction of protein-bound radioactivity in percentage of total radioactivity. The complex protein binding was approximately 58–60%.
Biodistribution of 177Lu-TTHMP in wild-type rats
The tissue uptake of the complex was calculated as the percentage of area under the curve of the related photopeak per gram of tissue (% ID/g) (Figure 4). The major radioactivity was accumulated in bones, as expected for bone-avid radiopharmaceuticals; the complex was also excreted through the kidneys.
Figure 4.
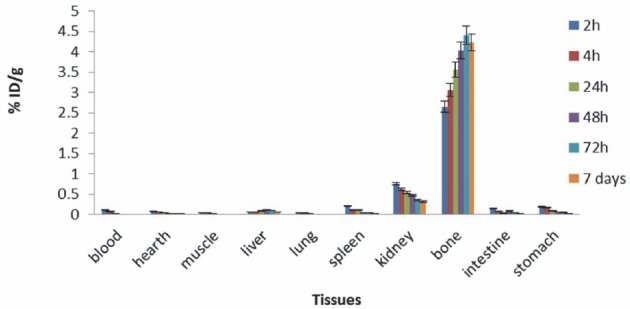
Biodistribution of 177Lu-TTHMP in different organs of wild-type rats
Dosimetric studies
Preliminary dosimetric evaluation in human organs was performed by MIRD method, based on biodistribution data in rat organs. First, the area under the clearance curve was calculated for each organ (Figure 5). Then, the absorbed dose was calculated according to the s factors in OLINDA software. The absorbed doses in each organ after 177Lu-TTHMP injection are shown in Table 1.
Figure 5.
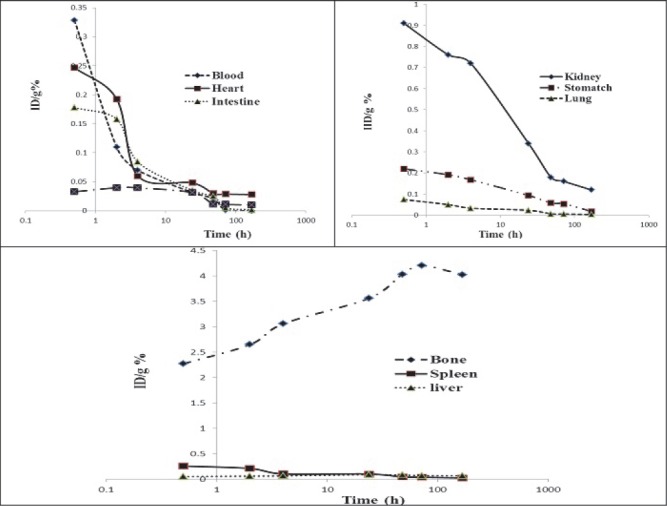
The clearance curves of each organ of rats
Table 1.
Absorbed dose in each organ after the injection of 177Lu-TTHMP
| Organ | Absorbed dose (mSv/MBq) | Organ | Absorbed dose (mSv/MBq) |
|---|---|---|---|
| Adrenal glands | 0.032 | Ovaries | 0.019 |
| Brain | 0.036 | Pancreas | 0.020 |
| Breasts | 0.009 | Red Mar. | 2.538 |
| GB Cont. | 0.013 | Cort Bone Sur. | 6.049 |
| LLI Cont. | 0.035 | Trab. Bone Sur. | 7.731 |
| SI Cont. | 0.017 | Cort Bone Vol. | 1.441 |
| Stom. Cont. | 0.018 | Trab. Bone Vol. | 3.716 |
| ULI Cont. | 0.014 | Spleen | 0.031 |
| Heart Cont. | 0.023 | Testes | 0.011 |
| Heart Wall | 0.034 | Thymus | 0.014 |
| Kidneys | 0.114 | Thyroid | 0.023 |
| Liver | 0.048 | UB Cont | 0.011 |
| Lungs | 0.022 | Uterus | 0.014 |
| Muscle | 0.028 | Tot. Body | 0.496 |
Discussion
The radiolabeled 177Lu-TTHMP was prepared with high radiochemical purity (>99%, ITLC) and by specific activity of 2.6-3 GBq/mg. 177Lu-TTHMP demonstrated a great stability at room temperature. The final product was administered to wild-type rats and biodistribution of the radiopharmaceutical was checked 2-168 h later. The results showed massive accumulation in the bone tissue, which increased up to 7 days (4.22 %ID/g). The complex was rapidly cleared of blood and no significant uptake was observed in critical organs. 20177Lu-TTHMP has been prepared and reported in previous studies (14, 16). In these studies, significant accumulation was reported in the bone, while accumulation in other organs was negligible. However, in this study, the complex pharmacokinetics in the bone followed a pattern similar to previously reported research, although the amount of radioactivity in the bone (%ID/g) was considerably different.
Lungu et al. investigated the biodistribution of 177Lu-TTHMP in rats, demonstrating a rapid uptake in bone 4 h after the injection (79 %ID/g). Accumulation in the bone increased until 72 h after the injection and reached about 93 %ID/g. In the study by Chakraborty et al., the bone uptake was reported as 6.58 %ID/g, 3 h, which increased up to 7.82 %ID/g 96 h after the injection. In the current study, the maximum bone uptake of 4.40 %ID/g was obtained 72 h after the injection, which is in agreement with the findings of Chakraborty and colleagues (14).
According to Mitterhauser’s findings (22), radiopharmaceutical preparation conditions strongly influence bone binding. Therefore, the differences in bone accumulation of 177Lu-TTHMP, reported in various studies, may be due to variations in preparation conditions of the complex. The optimum conditions for radiolabeling of the complex in this research and previous studies are provided in Table 2.
Table 2.
Optimum conditions for radiolabeling of 177Lu-TTHMP
In addition to the bone, low accumulations were observed in the liver, kidney, and spleen. The amount of radioactivity (%ID/g) in these tissues is demonstrated in Table 3. According to the results of Chakraborty et al., maximum liver uptake (0.15 %ID/g) was reported 3 h after the injection, while the best liver uptake was obtained after 48 h in the current study (0.10 %ID/g). Except the liver uptake, the pharmacokinetics of the complex in kidney and spleen followed a similar pattern, demonstrating the uptake reduction of kidney and spleen with time.
Table 3.
Comparison of liver, kidney, and spleen uptake for 177Lu-TTHMP complex in normal rats (%ID/g)
In this study, the absorbed dose of the complex was investigated for the first time. As expected, the highest absorbed dose for this complex was observed in the bone surface with 7.731 mSv/MBq.
Conclusion
According to the results, 177Lu-TTHMP massively accumulated in the bone, while no significant accumulation was observed in other organs. Also, the dosimetric results demonstrated that the bone surface/red marrow dose ratio was approximately 3, while this ratio was 1.5 for 166Ho-DOTMP (6). Considering these desirable characteristics, this radiopharmaceutical can be a good candidate for bone pain palliation therapy in skeletal metastases.
References
- 1.Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001;42:895–906. [PubMed] [Google Scholar]
- 2.Pandit-Taskar N, Batraki M, Divgi CR. Radiopharmaceutical Therapy for Palliation of Bone Pain from Osseous Metastases. J Nucl Med. 2004;45:1358–65. [PubMed] [Google Scholar]
- 3.Austria, Vienna: IAEA; 2007. Criteria for Palliation of Bone Metastases – Clinical Applications, IAEA-TECDOC-1549. [Google Scholar]
- 4.Lipton A. Pathophysiology of Bone Metastases: How This Knowledge May Lead to Therapeutic Intervention. J Support Oncol. 2004;2:205–13. [PubMed] [Google Scholar]
- 5.Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain Palliation in Patients with Bone Metastases Using MR-Guided Focused Ultrasound Surgery: A Multicenter Study. Ann Surg Oncol. 2009;16:140–6. doi: 10.1245/s10434-008-0011-2. [DOI] [PubMed] [Google Scholar]
- 6.Rajendran JG, Eary JF, Bensinger W, Durack LD, Vernon C, Fritzberg A. High-Dose 166Ho-DOTMP in Myeloablative Treatment of Multiple Myeloma: Pharmacokinetics, Biodistribution, and Absorbed Dose Estimation. J Nucl Med. 2002;43:1383–90. [PubMed] [Google Scholar]
- 7.Farhanghi M, Holmes RA, Volkert WA, Logan KW, Singh A. Samarium- 153-EDTMP: Pharmacokinetic, Toxicity and Pain Response Using an Escalating Dose Schedule in Treatment of Metastatic Bone Cancer. J NucI Med. 1992;33:1451–8. [PubMed] [Google Scholar]
- 8.Hosain F, Spencer RP. Radiopharmaceuticals for palliation of metastatic osseous lesions: biologic and physical background. Semin Nucl Med. 1992;22:11–6. doi: 10.1016/s0001-2998(05)80152-7. [DOI] [PubMed] [Google Scholar]
- 9.Deligny CL, Gelsema WJ, Tji TG, Huigen YM, Vink HA. Bone seeking radiopharmaceuticals. Nucl Med Biol. 1990;17:161–179. doi: 10.1016/0883-2897(90)90144-p. [DOI] [PubMed] [Google Scholar]
- 10.Lewington VJ. Targeted radionuclide therapy for bone metastases. Eur J Nucl Med. 1993;20:66–74. doi: 10.1007/BF02261248. [DOI] [PubMed] [Google Scholar]
- 11.Breitz H, Wendt R, Stabin M, Bouchet L, Wessels B. Dosimetry of high dose skeletal targeted radiotherapy (STR) with177Lu-DOTMP. Cancer Biother Radiopharm. 2003;18:225–30. doi: 10.1089/108497803765036391. [DOI] [PubMed] [Google Scholar]
- 12.Bahrami-Samani A, Anvari A, Jalilian AR, Shirvani-Arani S, Yousefnia H, Aghamiri MR, et al. Production, quality control and pharmacokinetic studies of 177Lu-EDTMP for human bone pain palliation therapy trials. Iran J Pharmaceut Res. 2012;11:137–44. [PMC free article] [PubMed] [Google Scholar]
- 13.Abbasi IA. Preliminary studies on 177Lu-labeled sodium pyrophosphate (177Lu-PYP) as a potential bone-seeking radiopharmaceutical for bone pain palliation. Nucl Med Biol. 2012;39:763–9. doi: 10.1016/j.nucmedbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty S, Das T, Unni PR, Sarma HD, Samuel G, Banerjee S, et al. 177Lu labeled polyaminophosphonates as potential agents for bone pain palliation. Nucl Med Commun. 2002;23:67–74. doi: 10.1097/00006231-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi IA. Studies on 177Lu-labeled methylene diphosphonate as potential bone-seeking radiopharmaceutical for bone pain palliation. Nucl Med Biol. 2011;38:417–25. doi: 10.1016/j.nucmedbio.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Lungu V, Niculae D, Bouziotis P, Pirmettis I, Podina C. Radiolabeled phosphonates for bone metastases therapy. J Radioanalytical Nucl Chem. 2007;273:663–7. [Google Scholar]
- 17.Das T, Chakraborty S, Unni PR, Banerjee S, Samuel G, Sarma HD, et al. 177Lu-labeled cyclic polyaminophosphonates as potential agents for bone pain palliation. Appl Radiat Isotopes. 2002;57:177–84. doi: 10.1016/s0969-8043(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 18.Austria, Vienna: IAEA; 2003. Manual For Reactor Produced Radioisotopes, IAEA-TECDOC-1340. [Google Scholar]
- 19.Sparks RB, Aydogan B. Proceeding of the sixth International Radiopharmaceutical Dosimetry Symposium. Oak Ridge. TN: Oak Ridge Associated Universities; 1996. Comparison of the effectiveness of some common animal data scaling techniques in estimating human radiation dose; pp. 705–16. [Google Scholar]
- 20.Bevelacqua JJ. Internal Dosimetry Primer. Radiat Prot Manage. 2005;22:7–17. [Google Scholar]
- 21.OLINDA - Organ Level Internal Dose Assessment Code (Version 1.1), copyright Vanderbilt University. 2007 [Google Scholar]
- 22.Mitterhauser M, Toegel S. What to consider in the development of new bone seekers: mechanistic and tracer-related aspects. Nucl Med Biol. 2008;35:817–24. doi: 10.1016/j.nucmedbio.2008.09.003. [DOI] [PubMed] [Google Scholar]


