Abstract
Objective(s):
The aim of this research was the development of 111In-labeled porphyrins as possible radiopharmaceuticals for the imaging of tumors.
Methods:
Ligands, 5, 10, 15, 20-tetrakis (3, 5-dihydroxyphenyl) porphyrin) (TDHPP), 5, 10, 15, 20-tetrakis (4-hydroxyphenyl) porphyrin (THPP) and 5, 10, 15, 20-tetrakis (3,4-dimethoxyphenyl) porphyrin) (TDMPP) were labeled with 111InCl3 (produced from proton bombardment of natCd target) in 60 min at 80 ºC. Quality control of labeled compounds was performed via RTLC and HPLC followed by stability studies in final formulation and presence of human serum at 37 ºC for 48 h as well as partition coefficient determination. The biodistribution studies performed using tissue dissection and SPECT imaging up to 24h.
Results:
The complexes were prepared with more than 99% radiochemical purity (HPLC and RTLC) and high stability to 48 h. Partition coefficients (calculated as log P) for 111In-TDHPP, 111In-THPP and 111In-TDMPP were 0.88, 0.8 and 1.63 respectively.
Conclusion:
Due to urinary excretion with fast clearance for 111In-TDMPP, this complex is probably a suitable candidate for considering as a possible tumor imaging agent.
Keywords: Hydroxy phenyl porphyrins, 111In, Biodistribution, Radiolabeling, SPECT
Introduction
Porphyrins can be appropriate ligands for designing metal complex including radio-pharmaceuticals because porphyrins and their related compounds are used as tumor seeking drugs, especially as photosensitizers in the photodynamic therapy (PDT) of cancer (1, 2) where the combination of light and photosensitizer, generates active oxygen species near the tumor, to damage the malfunctioned tissues. Varieties of porphyrins including anionic porphyrins (3), hematoporphyrins (4), cationic porphyrins (5) and phethalocyanines (6) have been successfully used for tumor treatment.
Porphyrins taken into the blood circulation as tumor localizing agents are transported to target tissue by human serum albumin (HSA) and other plasma proteins such as low and high-density lipoproteins (7). Other investigators have suggested that porphyrin accumulation in the tumor is related to low-density lipoprotein (LDL) uptake that occurs through receptor-mediated endocytosis (8).
Meso tetrakis (4-hydroxyphenyl) porphyrin (THPP) has been used as a potential molecule in PDT with high photosensitivity for cancer treatment, leading to the destruction of intrahepatic tumors with better efficacy and fewer side effects (9).
Several investigators have reported the synthesis and radiolabeling of wide varieties of porphyrin derivatives with various types of peripheral moieties with several important medical radionuclides for developing an ideal tumor localizing agent (10-15). However, none of these radiolabeled porphyrins have succeeded as a popular regular product. In-111 is a cyclotron produced radionuclide, decaying by electron capture (EC) with subsequent emission of gamma photons of 173 and 247 keV (89% and 94% intensity, respectively), is widely used in gamma scintigraphy. It is reported that 111In-labeled porphyrins exhibit specific accumulation in tumor tissues (16, 17).
Our attempt was to prepare a series of water soluble suitable radiolabeled porphyrins for tumor diagnosis, due to the availability of In-111. Structures of ligands used in this study are shown in Figure 1.
Figure 1.
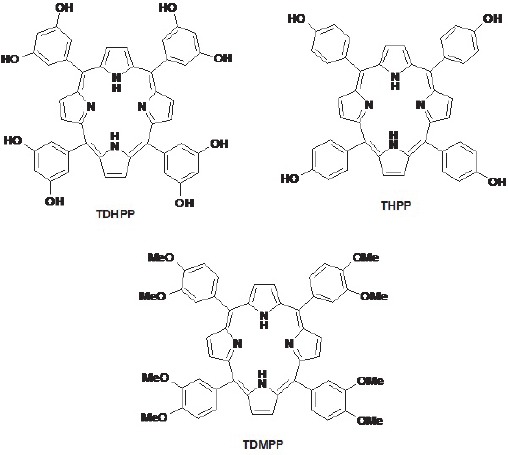
Chemical structures for ligands used in this work
In this research, synthesis, radiolabeling, partition coefficient, quality control and bio-distribution studies using SPECT and scarification of 111In-TDHPP, 111In-THPP and 111In-TDMPP in wild-type rats are reported.
Methods
In-111 was produced at the Agricultural, Medical and Industrial Research School (AMIRS), 30 MeV cyclotron (Cyclone-30, IBA) using natCd(p,x)111In reaction. Natural cadmium sulfate with a purity of more than 99% was obtained from Merck Co. Germany. All chemicals were purchased from Sigma-Aldrich Chemical Co. U.K. Radio-chromatography was performed by Whatman paper using a thin layer chromatography scanner, Bioscan AR2000, Paris, France. Analytical HPLC to determine the specific activity was performed by a Shimadzu LC-10AT, armed with two detector systems, flow scintillation analyzer (Packard-150 TR) and UV-visible (Shimadzu) using Whatman Partisphere C-18 column 250 × 4.6 mm (Whatman Co. NJ, USA). Calculations were based on the 172 keV peak for In-111. All values were expressed as mean ± standard deviation (Mean ± SD) and the data were compared using student T-test. Animal studies were performed in accordance with the United Kingdom Biological Council's Guidelines on the Use of Living Animals in Scientific Investigations, second edition.
Electroplating of the natural Cd targets:
In order to prepare Cd targets for the production, cadmium electroplating was performed over a copper surface was performed according to the previously reported method (18). Cadmium was electroplated over the copper backing according to the method given in the literature (19). A mixture of CdSO4.8/3H2O, KCN, BrijTM detergent solution and traces of hydrazine hydrate with a final volume of 450 ml double-distilled water (DDH2O) at pH=13 was used as the electroplating bath (constant current: 320 mA, stirring rate 780 rpm, time 0.5 h). After the deposition of an about 500 mg cadmium layer, the targets were wrapped in Parafilm® coatings to avoid atmospheric oxygen exposure. Finally, the target was sent for irradiation.
Production and quality control of 111InCl3 solution
Indium-111 chloride was prepared by 22 MeV proton bombardment of the cadmium target at a 30 MeV cyclotron, with a current of 100 μA for 48 min (80 μAh). After dissolution of the irradiated target by conc. HBr, the solution was passed through a cation exchange dowex 50×8 resin, pre-conditioned by 25 ml of conc. HBr. The resin was then washed by HBr conc. solution (50 ml). In order to remove the undesired impurities of Cd and Cu, the resin was totally washed with DDH2O. Indium-111 was eluted with 1 N HCl (25 ml) as 111InCl3 for labeling use.
Quality control of the product
Control of Radionuclide purity: Gamma spectroscopy of the final sample was carried out counting in an HPGe detector coupled to a Canberra multi-channel analyzer for 1000 seconds.
Chemical purity control: This step was carried out to ensure that the amounts of cadmium and copper ions resulting from the target material and backing in the final product are acceptable regarding internationally accepted limits. Chemical purity was checked by differential-pulsed anodic stripping polaro-graphy. The detection limit of our system was 0.1 ppm for both cadmium and copper ions.
Preparation and quality control of radiolabeled porphyrins
Complexion of In-111 with porphyrins was carried out by using acidic solution of 111InCl3, acetate buffer and porphyrins in absolute ethanol. The reaction was performed by adding acidic solution (100 µl) of 111InCl3 (185 MBq, 5 mCi) to a 5 mL-borosilicate vial. The solution was heated under a flow of nitrogen till it was dried at 50ºC. A volume (100 µl) of porphyrin dissolved in absolute ethanol (20 mg/ml, 290-300 nmol) was transferred to the vial. The pH was adjusted to 5.5-7 by adding 2000 µl of acetate buffer. Resulting solution was stirred at 25 °C for 30–60 min at pH=5. Radiochemical purity of the solution was measured by RTLC and HPLC. Radio thin layer chromatography was done with 10% ammonium acetate: methanol (1:1) mixture as mobile phase and chromatography Whatman No. 2 paper as stationary phase. For high performance liquid chromatography, a mixture of acetonitrile: water (40:60), used as elution and reversed phase column Whatman Partisphere C18 4.6 × 250 mm, used as stationary phase. HPLC was performed with a flow rate of 1 ml/min, pressure: 130 kgF/cm2 for 20 min. Thereafter, the solution was filtered through a 0.22 µm filter.
Determination of partition coefficient
For calculation of partition coefficient (log p), P (ratio of specific activities of organic and aqueous phases) was determined. 37 MBq of the radiolabeled indium complex was added to a mixture of 1 ml of 1-octanol and 1 ml of isotonic acetate buffered saline (pH=7) and then the solution was stirred at 37 °C and kept for 5 min. After that the solution was centrifuged at 1200 g for 5 min. Finally 500 µl of the octanol phase and aqueous phases were sampled and the specific activity in both phases was calculated (20).
Stability tests
For evaluation of stability test, ITLC method was done. ITLC was carried out for 2 samples and then compared to each other which one is 37 MBq of 111In-porphyrin complexes that was kept at room temperature for 2 days and another is a mixture of 500 µl freshly collected human serum and 36.1 MBq (976 µCi) of 111In-porphyrin complexes that was incubated at 37 °C for 48 h.
Biodistribution of labeled compound in wild-type rats
Biodistribution of 111In-porphyrin complexes among tissues of wild-type rats were determined. Animals were sacrificed by CO2 asphyxiation after injection (2, 4 and 24 h). Dose calibrator with fixed geometry counted the total amount of radioactivity which injected into each rats. The final activity of the radiopharmaceutical for injection was 50 µCi. After that the tissues (blood, heart, lung, brain, intestine, faces, skin, stomach, kidneys, spleen, bone, liver and muscle) were weighed and washed with normal saline. For calculation of percentage of injected dose per gram of tissues, HPGe detector armed with a sample holder device used to determine specific activity of percentage of injected dose per gram of tissues, HPGe detector armed with a sample holder device used to determine specific activity of tissues.
Imaging of 111In-porphyrin complexes in wild-type rats
Images were taken 2, 4 and 24 hours after administration of the radiopharmaceutical by a dual-head SPECT system. The mouse-to-high energy septa distance was 12 cm. Images were taken from both normal and tumor bearing mice. The useful field of view (UFOV) was 540 mm × 400 mm.
Results and Discussion
Radionuclide production
Indium-111, in form of InCl3, was prepared by 22 MeV proton bombardment of the enriched Cd-112 target at Cyclone-30 on a regular basis. Radionuclidic control showed the presence of 172 and 245 keV gamma energies, all originating from 111In and showed a radionuclidic purity higher than 99% (E.O.S.). The concentrations of cadmium (from target material) and copper (from target support) were determined using polarography and shown to be below the internationally accepted levels, i.e. 0.1 ppm for Cd and Cu (21, 22).
The radioisotope was dissolved in acidic media as a starting sample and was further diluted and evaporated for obtaining the desired pH and volume followed by sterile filtering. The radiochemical purity of the In-111 solution was checked in two solvent systems, in 1 mM DTPA, free In3+ cation is converted to more lipophilic In-DTPA form and migrates to higher Rf=0.8 while any small radioactive fraction remaining at the origin could be related to other Indium-111 ionic species, not forming In-DTPA complex, such as InCl4-, etc. and/or colloids (not observed).
On the other hand, 10 % ammonium acetate:methanol mixture was also used to determine the radiochemical purity. The fast eluting species was possibly the ionic In-111 cations other than In3+ (not observed) and the remaining fraction at Rf=0 was a possible mixture of In3+ and/or colloids (Figure 2).
Figure 2.
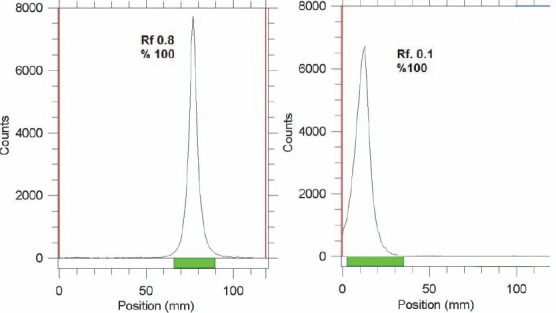
ITLC chromatograms of 111InCl3 solution in DTPA solution (pH=5) (left) and 10 % ammonium acetate : methanol (1:1) solution (right) using Whatman no. 2 paper
The radiolabeling process was checked by ITLC using 10 % ammonium acetate:methanol (1:1) solution as shown in Figure 3. In all cases radiolabeled porphyrins migrate to higher Rf due to higher lipophilicity compared to free indium cation. Among the radiolabeled porphyrins In-TDMPP demonstrated higher Rf due to more lipophilicity compared to two other phenolic derivatives. Dihydroxy complex (111In-TDHPP) showed more hydrophilicity compared to the mono hydroxyl compound. ITLC studies approved the production of a single radiolabeled compound in each case.
Figure 3.
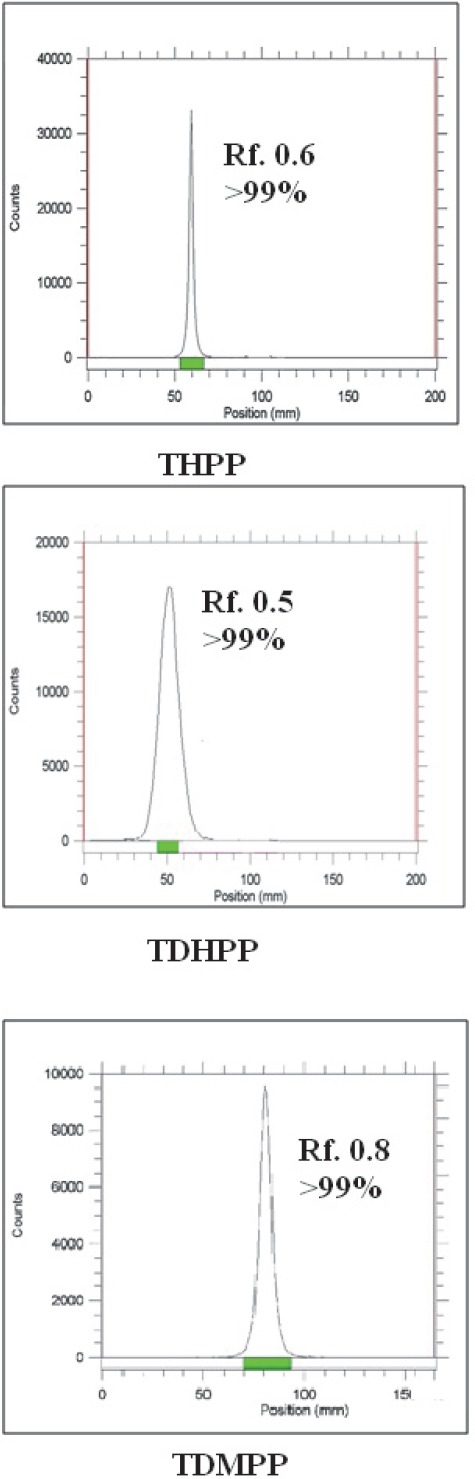
RTLC chromatograms of 111In-porphyrins using 10 % ammonium acetate:methanol (1:1) solution (right) on Whatman no. 2
HPLC studies also demonstrated the existence of only one radiolabeled species using both UV and scintillation detectors. In all cases a more fast-eluting compound observed with scintillation detector was almost time- coincident with a related peak of UV detector, demonstrating a more lipophilic compound compared to 111In cation (Figure 4).
Figure 4.
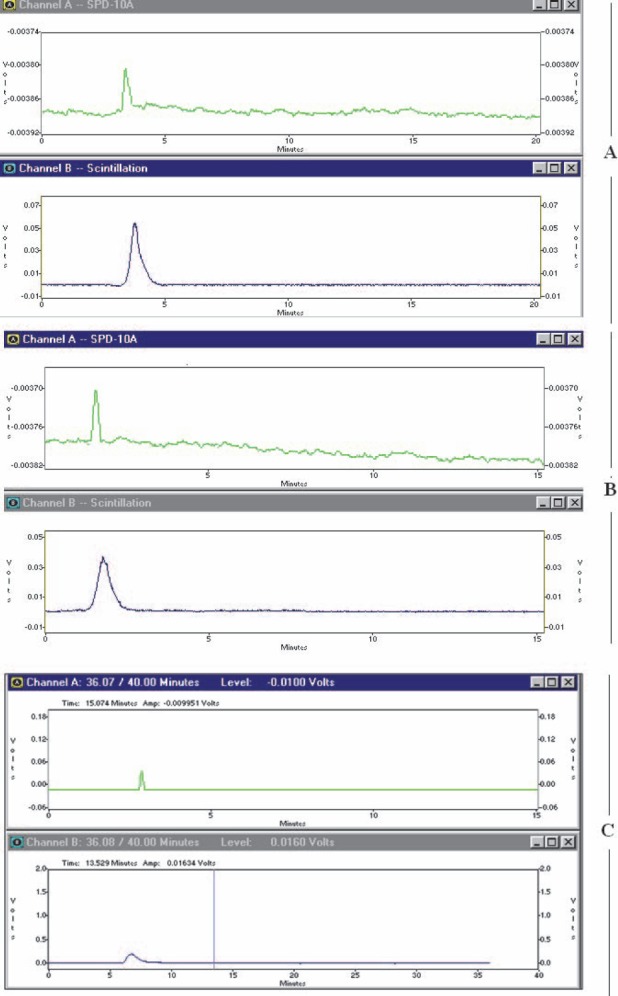
HPLC chromatograms of 111In-THPP (section A), 111In-TDHPP (section B), 111In-TDMPP (section C) on a reversed phase column using acetonitrile:water 40:60, (in all sections; upper: UV chromatogram, scintillation chromatogram; below)
Stability studies
Studies on 111In-porphyrins showed that stability of the labeled compounds was high enough to carry out further studies. ITLC showed no loss of In-111 from labeled compound after incubation of 111In-porphyrins in freshly prepared human serum for 2 days at 37 °C. At physiologic conditions the radiochemical purity of labeled compound remained more than 99% for 2 days.
Biodistribution studies
Free indium cation: For better comparison biodistribution study was performed for free In3+. The %ID/g data are summarized in Figure 5.
Figure 5.
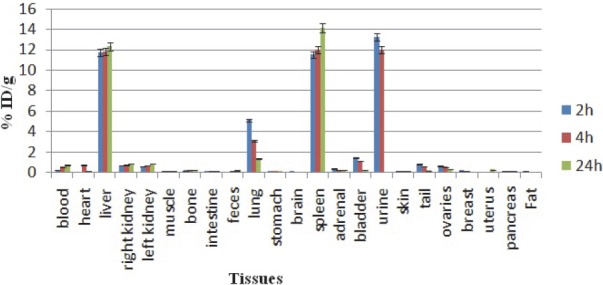
Biodistribution of 111InCl3 (1.85 MBq, 50 μCi) in wild-type rats 2, 4 and 24 h after injection via tail vein (ID/g%: percentage of injected dose per gram of tissue) (n=5)
Indium cation similarity with ferric ion is important in the development of indium radiopharmaceuticals, since iron is an essential element in the human body and a number of iron binding proteins, such as transferrin (in blood), which used in transporting and storing iron in vivo (23).
As shown in Figure 5, indium cation almost mimics the ferric cation behavior and is rapidly removed from the circulation and is accumulated in the liver, also a major fraction is excreted rapidly through the kidney leading to high accumulation in urine in 2-24 h post injection as a water soluble cation.
Radiolabeled porphyrins
111In-THPP: Biodistribution data of 111In-THPP (Figure 6), 2 h after injection the highest activity was seen in and blood pool, while this amount is reduced due to other tissue's uptake. Due to the presence of polar groups in the structure of the compound, a major portion of the radioactive compound was concentrated in kidneys, 2-24 h after injection. Although liver is another accumulation site due to lipoprotein transport into this organ leading to significant feces activity content.
Figure 6.
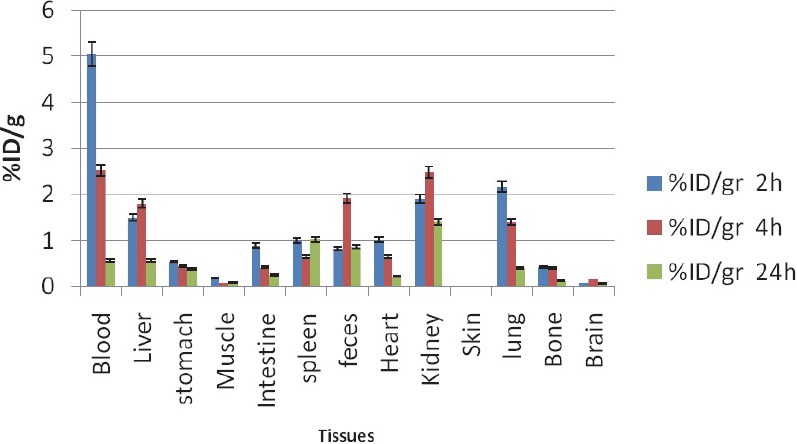
Biodistribution of 111In-THPP (50 µCi) in wild type rats 2,4 and 24 h after iv injection via tail vein (ID/g%: percentage of injected dose per gram of tissue calculated based on the area under curve of 245 keV peak in gamma spectrum) (n=3)
111In-TDMPP: despite the presence of two CH3O- groups in TMPP complex and low water solubility leading to higher log P, kidneys are the major accumulation sites of excretion while liver stayed a minor excretion route. Lung and spleen also show significant activity (Figure 7).
Figure 7.
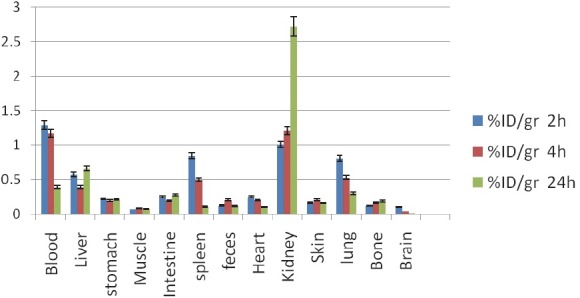
Biodistribution of 111In-TDMPP (50 µCi) in wild type rats 2,4 and 24 h after iv injection via tail vein (ID/g%: percentage of injected dose per gram of tissue calculated based on the area under curve of 245 keV peak in gamma spectrum) (n=3)
111In-TDHPP: Similar to 111In-THPP, di-hydroxy compound is also accumulated majorly in the liver and kidneys which are typical accumulation sites for porphyrins. Due to oxidative/reductive enzymes present in lungs a significant uptake is also observed (Figure 8).
Figure 8.
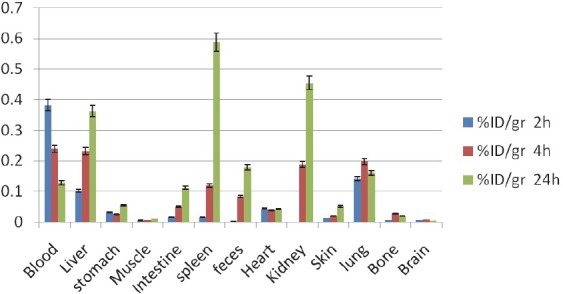
Biodistribution of 111In-TDHPP (50 µCi) in wild type rats 2,4 and 24 h after iv injection via tail vein (ID/g%: percentage of injected dose per gram of tissue calculated based on the area under curve of 245 keV peak in gamma spectrum) (n=3)
Due to the importance of low liver accumulation compared to the kidneys excretion, a kidney: liver uptake ratio can be suggested as a suitable criterion for rapid clearance of the radiolabeled complexes as tumor imaging agents, while imposing less irradiation dose as shown in Table 1.
Table 1.
The kidney: liver uptake ratio of the radiolabeled complexes in 2, 4 and 24 h post injection (n=3)
| Time/complex | 111In-TDHPP | 111In-THPP | 111In-TDMPP |
|---|---|---|---|
| 2 h | 1.46 | 1.27 | 1.74 |
| 4 h | 2.08 | 1.37 | 3.08 |
| 24 h | 1.65 | 2.47 | 4.13 |
As shown in Table 1, TDMPP complex demonstrates the best kidney:liver uptake ratio among the three complexes at all time intervals despite the high lipophilic behavior observed in partition coefficient and chromatographic studies.
It can be proposed that a metabolic pathway leading to the formation of more water soluble metabolites exists for di-methoxy complex. Thus 111In-TDMPP complex is probably a suitable candidate for considering as a possible tumor imaging agent.
Imaging of wild-type rats
The In-111 labeled porphyrin imaging in the wild-type rats showed a distinct accumulation of the radiotracer in the chest region all the time after injection. Most of the activity is washed out from the body after 24 h and the picture contrast weakened. Figure 9, demonstrates the liver and spleen uptake of 111In-THPP complex among the rat tissues at all time intervals, however a minor kidney uptake is also observed. These findings are in full agreement with the dissection studies shown in Figure 6.
Figure 9.
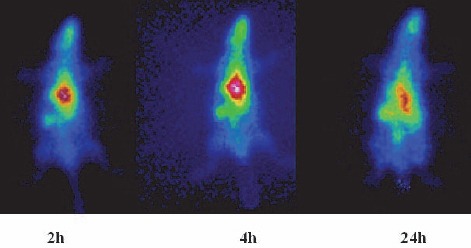
SPECT images of 111In-THPP (70 μCi) in wild-type rats 2, 4 and 24 h post injection
In case of 111In-TDMPP, kidneys are the significant excreting organs although lung, liver and spleen uptakes are observed in a single signal (Figure 10). These findings are in full agreement with the dissection studies shown in Figure 7.
Figure 10.
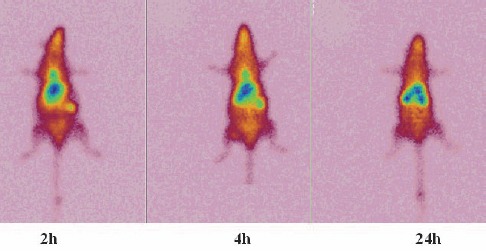
SPECT images of 111In-TDMPP (70 μCi) in wild-type rats 2, 4 and 24 h post injection
Figure 11, also demonstrates the liver and spleen uptake of 111In-TDHPP complex among the rat tissues at all time intervals. A significant kidney uptake is also observed. These findings are in agreement with the dissection studies shown in Figure 8.
Figure 11.
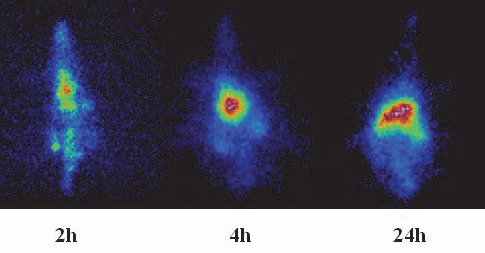
SPECT images of 111In-TDHPP (70 μCi) in wild-type rats 2, 4 and 24 h post injection
All porphyrins are transferred in the body as bonded to the lipoproteins, thus any possible targets of lipoproteins are the accumulation site, and among many other porphyrins this is the major drawback.
Conclusion
111In-TDHPP, 111In-THPP and 111In-TDMPP were prepared using 111InCl3 (produced from proton bombardment of natCd target) in 60 min at 80 ºC. The complexes were prepared with more than 99% radiochemical purity (HPLC and RTLC) and high stability to 48h. Partition coefficients (calculated as log P) for 111In-TDHPP, 111In-THPP and 111In-TDMPP were 0.88, 0.8 and 1.63 respectively. Biodistribution studies using SPECT imaging and tissue dissection demonstrated significant urinary excretion with fast clearance for 111In-TDMPP, while the two other complexes demonstrated liver uptake and longer retention in animal tissues. Thus 111In-TDMPP complex is probably a suitable candidate for considering as a possible tumor imaging agent.
References
- 1.Lang K, Mosinger J, Wagnerová D. Photophysical properties of porphyrinoid sensitizers non-covalently bound to host molecules;models for photodynamic therapy. Coordination chemistry reviews. 2004;248:321–50. [Google Scholar]
- 2.Bonnett R, Martínez G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron. 2001;57:9513–47. [Google Scholar]
- 3.Andrade SM, Costa S. Spectroscopic studies on the interaction of a water soluble porphyrin and two drug carrier proteins. Biophys J. 2002;82:1607–19. doi: 10.1016/S0006-3495(02)75512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenbaum M, Akande S, Bonnett R, Kaur H, Ioannou S, White R, et al. Meso-Tetra (hydroxyphenyl) porphyrins, a new class of potent tumour photosensitisers with favourable selectivity. Br J Cancer. 1986;54:717. doi: 10.1038/bjc.1986.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrechts SA, Aalders MC, Verbraak FD, Lagerberg JW, Dankert JB, Schuitmaker JJ. Effect of albumin on the photodynamic inactivation of microorganisms by a cationic porphyrin. J Photochem Photobiol B. 2005;79:51–7. doi: 10.1016/j.jphotobiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Gantchev TG, Ouellet R, van Lier JE. Binding interactions and conformational changes induced by sulfonated aluminum phthalocyanines in human serum albumin. Arch Biochem Biophys. 1999;366:21–30. doi: 10.1006/abbi.1999.1174. [DOI] [PubMed] [Google Scholar]
- 7.An W, Jiao Y, Dong C, Yang C, Inoue Y, Shuang S. Spectroscopic and molecular modeling of the binding of meso-tetrakis (4-hydroxyphenyl) porphyrin to human serum albumin. Dyes and Pigments. 2009;81:1–9. [Google Scholar]
- 8.Allison BA, Pritchard PH, Levy JG. Evidence for low-density lipoprotein receptor-mediated uptake of benzoporphyrin derivative. Br J Cancer. 1994;69:833–9. doi: 10.1038/bjc.1994.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovers J, Saarnak A, Molina A, Schuitmaker J, Sterenborg H, Terpstra O. Effective treatment of liver metastases with photodynamic therapy, using the second-generation photosensitizer meta-tetra (hydroxyphenyl) chlorin (mTHPC), in a rat model. Br J Cancer. 1999;81:600–8. doi: 10.1038/sj.bjc.6690736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hambright P, Fawwaz R, Valk P, McRae J, Bearden A. The distribution of various water soluble radioactive metalloporphyrins in tumor bearing mice. Bioinorg Chem. 1975;5:87–92. doi: 10.1016/s0006-3061(00)80224-0. [DOI] [PubMed] [Google Scholar]
- 11.Whelan HT, Kras LH, Ozker K, Bajic D, Schmidt MH, Liu Y, et al. Selective incorporation of111In-labeled PHOTOFRIN™by glioma tissue in vivo. J Neurooncol. 1994;22:7–13. doi: 10.1007/BF01058350. [DOI] [PubMed] [Google Scholar]
- 12.Bhalgat MK, Roberts JC, Mercer-Smith JA, Knotts BD, Vessella RL, Lavallee DK. Preparation and biodistribution of copper-67-labeled porphyrins and porphyrin-A6H immunoconjugates. Nucl Med Biol. 1997;24:179–85. doi: 10.1016/s0969-8051(96)00215-6. [DOI] [PubMed] [Google Scholar]
- 13.Subbarayan M, Shetty S, Srivastava T, Noronha O, Samuel A. Evaluation studies of technetium-99m-porphyrin (T3, 4BCPP) for tumor imaging. J Porphyrins Phthalocyanines. 2001;5:824–8. [Google Scholar]
- 14.Kavali RR, Chul Lee B, Seok Moon B, Dae Yang S, Soo Chun K, Woon Choi C, et al. Efficient methods for the synthesis of 5-(4-[18F] fluorophenyl)-10, 15, 20-tris (3-methoxyphenyl) porphyrin as a potential imaging agent for tumor. J Label Compd Radiopharm. 2005;48:749–58. [Google Scholar]
- 15.Das T, Chakraborty S, Sarma H, Banerjee S. A novel [109Pd] palladium labeled porphyrin for possible use in targeted radiotherapy. Radiochimica Acta. 2008;96:427–33. [Google Scholar]
- 16.Yamazaki K, Hirata S, Nakajima S, Kubo Y, Samejima N, Sakata I. Whole-body Autoradiography of Tumor-bearing Hamsters with a New Tumor Imaging Agent, Indium-111-labeled Porphyrin. Jpn J Cancer Res. 1988;79:880–4. doi: 10.1111/j.1349-7006.1988.tb00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quastel M, Richter A, Levy J. Tumour scanning with indium-111 dihaematoporphyrin ether. Br J Cancer. 1990;62:885. doi: 10.1038/bjc.1990.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeghpour H, Jalilian AR, Akhlaghi M, Kamali-dehghan M, Mirzaii M. Preparation and Biodistribution of [111In]-rHuEpo for Erythropoietin Receptor Imaging. J Radioanal Nucl Chem. 2008;278:117–122. [Google Scholar]
- 19.Mirzaii M, Afarideh H, Haji-Saied SM, Ardaneh K. Production of 111 In by irradiation of natural cadmium with deuterons and protons in NRCAM cyclotron. Energy (MeV) 1998;6:16. [Google Scholar]
- 20.Sangster J. Washington DC: American Chemical Society and the American Institute of Physics for the National Institute of Standards and Technology; 1989. Octanol-water partition coefficients of simple organic compounds. [Google Scholar]
- 21.United States Pharmacopoeia 28, 2005, NF 23. :1009. [Google Scholar]
- 22.United States Pharmacopoeia 28 2005, NF 23. 1895 [Google Scholar]
- 23.Bernard C. Chemistry of Metal Radionuclides (Rb, Ga, In, Y, Cu and Tc) [Google Scholar]


